- 1Department of Pharmacodynamics, Semmelweis University, Budapest, Hungary
- 2MTA-SE Neuropsychopharmacology and Neurochemistry Research Group, Hungarian Academy of Sciences, Semmelweis University, Budapest, Hungary
- 3Department of Genetics, Cell- and Immunobiology, Semmelweis University, Budapest, Hungary
- 4SE-NAP 2 Genetic Brain Imaging Migraine Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary
- 5NAP-2-SE New Antidepressant Target Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary
The active ingredient of ecstasy, ±3,4-methylenedioxymethamphetamine (MDMA), in addition to its initial reinforcing effects, induces selective and non-selective brain damage. Evidences suggest that the hippocampus (HC), a central region for cognition, may be especially vulnerable to impairments on the long-run, nevertheless, transcription factors that may precede and regulate such chronic changes remained uninvestigated in this region. In the current study, we used gene-set enrichment analysis (GSEA) to reveal possible transcription factor candidates responsible for enhanced vulnerability of HC after MDMA administration. Dark Agouti rats were intraperitoneally injected with saline or 15 mg/kg MDMA. Three weeks later HC gene expression was measured by Illumina whole-genome beadarrays and GSEA was performed with MSigDB transcription factor sets. The number of significantly altered genes on the genome level (significance < 0.001) in up/downregulated sets was also counted. MDMA upregulated one, and downregulated 13 gene sets in the HC of rats, compared to controls, including Pax4, Pitx2, FoxJ2, FoxO1, Oct1, Sp3, AP3, FoxO4, and vitamin D receptor (VDR)-regulated sets (q-value <0.05). VDR-regulated set contained the second highest number of significantly altered genes, including among others, Camk2n2, Gria3, and Grin2a. Most identified transcription factors are implicated in the response to ischemia confirming that serious hypoxia/ischemia occurs in the HC after MDMA administration, which may contribute to the selective vulnerability of this brain region. Moreover, our results also raise the possibility that vitamin D supplementation, in addition to the commonly used antioxidants, could be a potential alternative method to attenuate MDMA-induced chronic hippocampal impairments.
Introduction
Ecstasy is a commonly abused drug worldwide for its stimulating, pro-social and euphoric effects (Green et al., 2003). The active ingredient of ecstasy is 3,4-methylenedioximethamphetamine (MDMA) that acutely releases noradrenaline, serotonin, and dopamine in synaptic clefts (Green et al., 2003; Pazmany et al., 2013). Besides of its initial rewarding effects, the drug can lead to lasting functional consequences: elevated risk of mood disorders and persistent cognitive disturbances were demonstrated in multiple animal and human studies (Parrott et al., 2000; Green et al., 2003; Bond et al., 2004; Kalechstein et al., 2007; Nulsen et al., 2010). A central region of cognitive functions, particularly memory, is the hippocampus (HC) (Sweatt, 2004). Its anatomical structure, in terms of organization of neuronal circuitries and (micro)vasculature makes it especially vulnerable to various homeostatic challenges (Schmidt-Kastner and Freund, 1991). The HC seems to be the most vulnerable brain region also to the toxic effects of MDMA in rats and, with some suggestive evidence, in humans (Adori et al., 2006, 2010; den Hollander et al., 2012; Petschner et al., 2018). MDMA-induced hyperthermia, free radical production, relative ischemia through disruption of cerebral autoregulation (Colado et al., 1999; Green et al., 2003; Ferrington et al., 2006; Capela et al., 2009) and lasting selective serotonergic toxicity (Kirilly et al., 2006; Capela et al., 2009; Adori et al., 2010, 2011b; Ando et al., 2010) are all mechanisms that could contribute to the damage of HC. Translation of these complex pathophysiological processes to molecular mechanisms leading to the damage of the HC and to impairments of learning and memory processes are, however, less explored, although changes in LTP in hippocampal slices after neurotoxic MDMA treatment have been described earlier (Morini et al., 2011). We have demonstrated previously that mRNA levels of key members of the long-term potentiation (LTP) pathway were downregulated in the HC 3-weeks after MDMA administration, in parallel with decreased expression of memory and cognition gene sets (Petschner et al., 2018). These may be important contributors to MDMA-induced cognitive impairments. However, based on the complexity of the pathophysiological processes mentioned above, further molecular mechanisms are presumed to contribute. These probably employ downstream mediators, like transcription factors, which regulate expression of mRNA and protein levels directly, and link initial alterations with long-term consequences. Nevertheless, transcription factors remained uninvestigated after chronic MDMA administration.
In the present study, we have analyzed our previously obtained data, measured by Illumina whole-genome beadarrays, with gene-set enrichment analysis (GSEA) using the MSigDB transcription factor sets, to identify transcription factors that may be responsible for the long-term cognitive impairments in the vulnerable HC region 3-weeks after a single-dose MDMA administration.
Materials and Methods
For detailed methods see (Petschner et al., 2018). Briefly, 21 approximately 8 weeks old male Dark Agouti (DA) rats (Harlan, Olac Ltd., Shaw’s Farm, Blackthorn, Bicester, United Kingdom) weighing 152 ± 3,58 g (SEM) were used. After random assignment, MDMA [(±)3,4-methylenedioxymethamphetamine (Sanofi-Synthelabo-Chinoin, Hungary, purity >99.5%)] dissolved in 0.9% NaCl (SAL), at an equivalent dose of 15 mg/kg free base, or SAL (for control animals) was administered intraperitoneally in a volume of 1 ml/kg. Three weeks after injections rats were sacrificed and HC regions dissected (dorsal HC: from bregma -2.5 to -4.5 mm). RNA extraction was performed according to the TRIzol method (Tamasi et al., 2014; Petschner et al., 2016). Samples with lowest RNA concentrations were excluded until both MDMA and control groups consisted of eight animals. Randomly selected samples were pooled by two and sent to Service XS (Leiden, Netherlands) for microarray analysis with the Illumina (San Diego, CA, United States) RatRef-12v1 beadarray expression chip. Raw data were analyzed using quantile normalization with beadarray, preprocessCore and puma Bioconductor packages for R (Gentleman et al., 2004; Dunning et al., 2007; Pearson et al., 2009; R Core Team, 2012). These methods created the minimum probability of positive log ratio (MinPplr), a statistical significance value. A gene was considered significant, if MinPplr was below 0.001 [to correct for multiple hypothesis testing (Benjamini and Hochberg, 1995; Petschner et al., 2015)]. Results were validated with rt-PCR (for result see Petschner et al., 2018) and microarray data uploaded in Gene Expression Omnibus (Edgar et al., 2002) under GSE475411. GSEA was performed using GSEA software from the Broad Institute at MIT2. GSEA uses a ranked list of all examined genes and searches for groups of genes (gene sets) that are enriched (overrepresented/underrepresented) in the top/bottom of this list. We used official MSigDB C3 TFT (transcription factor) gene sets for grouping, which classifies genes based on shared regulatory motifs and, hence, represents common transcription factor binding properties. Accordingly, up/downregulation of a given set in GSEA means that genes regulated by a given factor are up/downregulated. Sets containing more than 500/less than 15 genes were excluded, t-test was used for ranking and gene set was chosen as permutation type. Thousand permutations were made with seed of permutation set to 149. Normalized enrichment scores (NES), representing relative enrichment in a given phenotype, nominal p-values and false discovery rates (FDR) were calculated. A gene set was considered significant with FDR q-value below 0.05 (Benjamini and Hochberg, 1995). GSEA calculates results obtained through analysis of every (both significant/non-significant) genes between two groups (in this case MDMA vs. SAL). However, we were also interested in sets that may contain most of the significantly altered genes. Therefore, the number of significantly altered genes (MinPplr < 0.001) was counted in each significant (FDR < 0.05) gene set to identify those transcription factors that may be largest contributors to the most substantial changes in the HC 3 weeks after administration.
All experiments complied with the European Community Council Directive of 24 November 1986 (86/609/EEC), National Institutes of Health Principles of Laboratory Animal Care (NIH Publication 85-23, revised 1985) and national laws (the Hungarian Governmental Regulation on animal studies, 31 December 1998 Act) and were approved by the National Scientific Ethical Committee on Animal Experimentation and permitted by the Food Chain Safety and Animal Health Directorate of the Central Agricultural Office, Hungary (permission number: 22.1/3152/001/2007).
Results
Gene-set enrichment analysis revealed 14 gene sets that were dysregulated with an FDR q-value below 0.05 3 weeks after a single-dose MDMA treatment in the HC compared to the controls (Table 1). Among the gene sets one was upregulated, the other 13 were downregulated with the largest downregulation of the Pax4 set (the only upregulated set possesses no known binding factor). From the 14 dysregulated gene sets three bind forkhead box (Fox) transcription factors, namely FoxO1, FoxO4, and FoxJ1, others could not be grouped into families.
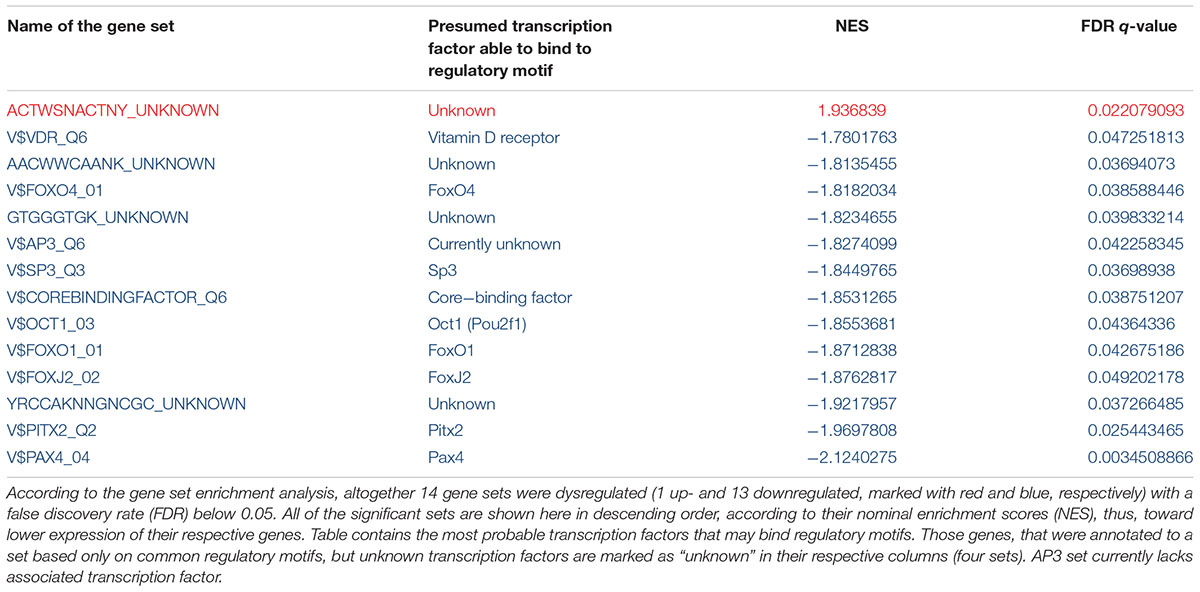
TABLE 1. Up- and downregulated transcription factor gene sets 3 weeks after a single-dose MDMA treatment in the hippocampus of Dark Agouti rats.
To reveal gene sets with the highest number of altered genes, we counted significant gene entries (MinPplr < 0.001) in each significant set (FDR < 0.05). Results showed that AP3 regulated gene set contained the most significantly altered genes with 17 entries, [but AP3 is currently not associated with known transcription factor (see section “Discussion”)], followed by vitamin D receptor (VDR) regulated set with 15 entries. Results with the significant genes are shown in Table 2.
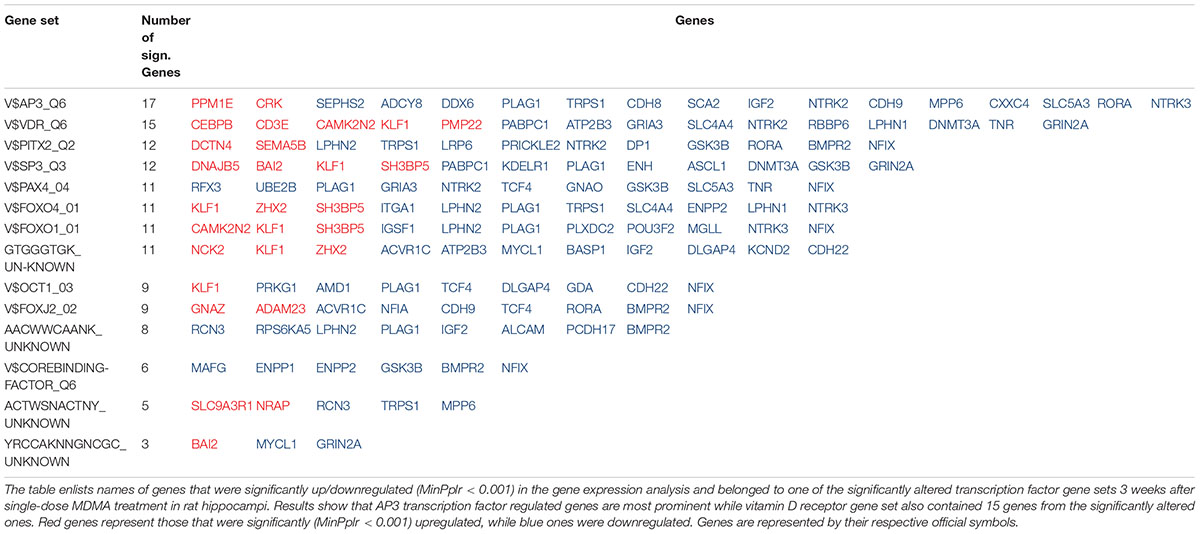
TABLE 2. Number of genes with significantly altered expression 3 weeks after MDMA treatment in the hippocampus in the significantly altered transcription factor gene sets.
Discussion
The present study shows that gene expression alterations 3 weeks after a single-dose MDMA treatment in the HC are probably mediated by 14 different transcription factors, among them hypoxia/ischemia-induced members of the FoxO family, Sp3 and Pax4. The AP3 regulated set contained the most significantly dysregulated genes, but lacks associated transcription factor, while the VDR gene set contained the second most entries. These results suggest that etiologically hypoxia/ischemia may be an important factor in the vulnerability of the HC and vitamin D supplementation may be tested to compensate for the most substantial cognitive effects of the drug.
Among transcription factors regulating the significantly altered sets several could be related to hypoxia/ischemia, a known consequence of MDMA administration (Ferrington et al., 2006). For example, the Pax4 regulated gene set was downregulated after transient hypoxic condition in the fetal pituitary of sheep (Wood et al., 2016). FoxO1 and FoxO4 were implicated in antioxidant mechanisms after brain ischemia (Abbas et al., 2009; Fukunaga and Shioda, 2009; Araujo et al., 2011; Jenwitheesuk et al., 2017). Polymorphisms in Pitx2 associated consistently with ischemic stroke in genome-wide association studies [Traylor et al., 2012; Malik et al., 2016; Ninds Stroke Genetics Network (SiGN) and International Stroke Genetics Consortium (Isgc), 2016]. Significantly decreased Sp3 protein levels were revealed after 7 days of hypobaric hypoxia in HC of rats (Hota et al., 2010). Other transcription factors had wide-scale roles in different processes, e.g., FoxJ2 is an important regulator of dopaminergic neurons (Wijchers et al., 2006) and Oct1 (Pou2f1) polymorphisms associated with Alzheimer’s disease (Taguchi et al., 2005). Core-binding factor is a large complex from Runx1-3 and core-binding factor B, which possesses important transcriptional regulatory properties, and Runx1 was induced after traumatic brain injury (Logan et al., 2013). From the dysregulated gene sets in four, including the only upregulated one, genes are annotated only by common regulatory motifs that are not associated with known transcription factors, indicating that so far unidentified regulatory elements may also contribute to the long-term effects of MDMA. In sum, our results suggest that MDMA employs FoxO1/4, Pitx2, Sp3, and Pax4-dependent mechanisms after its initial vasoactive monoamine release and consequent ischemia/hypoxia and changes in these transcription factors may be responsible for the long-lasting influence on HC processes and the often observed cognitive impairments.
From this aspect, perhaps the most interesting result is that most of the VDR regulated genes were downregulated (see Table 2). In fact, the second highest number of genes with significantly altered expressions were among the VDR regulated genes, including those involved in LTP, like upregulated calcium/calmodulin-dependent kinase inhibitor Camk2n2 and downregulated glutamate receptors NMDA2A (Grin2a) and AMPA3 (Gria3). While some of the other transcriptional regulators may also influence genes important for regulation of LTP, none of them offer such straightforward intervention as VDR regulated one in the form of vitamin D supplementation. This is especially tempting for multiple reasons. First, vitamin D exerts its effects via VDR, which after activation binds directly to DNA and influences gene transcription (Whitfield et al., 1995; Haussler et al., 1997; Angelo et al., 2008). Vitamin D was demonstrated to counteract consequences of hypoxia/ischemia via VDR (Won et al., 2015), showed neuroprotective effects in hippocampal neurons after hypoxic insult (Kajta et al., 2009), could influence LTP (Salami et al., 2012), enhanced cognitive performance in dementia (Landel et al., 2016), and protected from the serotonin-depleting effects of methamphetamine, a close relative of MDMA (Cass et al., 2006). Furthermore, a transient decrease in plasma calcitriol level was demonstrated in humans by a metabolomic analysis after acute MDMA administration (Boxler et al., 2018) and ecstasy users promote the use of vitamin D in user-to-user posts3. However, no previous studies investigated vitamin D effects after MDMA use (in PubMed no hit with “MDMA” and “vitamin D” MeSH terms, retrieved on 11th of August, 2018). Harm-reduction techniques, found overall on the internet, target known acute actions of MDMA and suggest application of antioxidants, hydration, and cooling to attenuate the negative effects. Among those cooling was extensively studied and showed limited success (see e.g., Adori et al., 2011a). Nevertheless, based on our results, vitamin D might have the potential to compensate for some of the long-term consequences of MDMA in the highly vulnerable HC.
Conclusion
While our study has to be interpreted with caution due to the indirect examination of the transcription factors and the exclusive investigation of the HC, it provides evidence for an important role of ischemia and subsequent FoxO1/4, Pitx2, Sp3, and Pax4-dependent mechanisms in MDMA-induced hippocampal impairments. In addition, it also raises the possibility of a better, simple, and precision-medicine based harm reduction technique with vitamin D to compensate for the cognitive deficits of the drug.
Author Contributions
PP conducted the GSEA and gene level analysis, interpreted the data, and wrote and drafted the manuscript. NB helped in the GSEA analysis. CA carried out the treatment protocol, dissected the brain samples, and prepared the RNA extractions. VT and SK participated in the interpretation and contributed in drafting the manuscript. GJ helped to draft the manuscript. GB prepared the study design, coordinated the study, and helped in the interpretation and drafting the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by the Hungarian Academy of Sciences (MTA-SE Neuropsychopharmacology and Neurochemistry Research Group), National Hungarian Development Agency (Grant: KTIA-NAP-13-1-2013-0001), Hungarian Brain Research Program (Grant: KTIA-13-NAP-A-II/14), MTA-SE-NAP-B Genetic Brain Imaging Migraine Research Group, Hungarian Academy of Sciences, Semmelweis University (Grant: KTIA_NAP_13-2-2015-0001), Hungarian Brain Research Program 2.0 (Grant: 2017-1.2.1-NKP-2017-00002), János Bolyai Research Scholarship of the Hungarian Academy of Sciences and New National Excellence Program of Ministry of Human Capacities (UNKP-17-4-I-SE-8 and ÚNKP-18-4-SE-127).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^ http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE47541
- ^ http://www.broadinstitute.org/gsea
- ^ www.treato.com
References
Abbas, A. K., Dozmorov, M., Li, R., Huang, F. S., Hellberg, F., Danielson, J., et al. (2009). Persistent LTP without triggered protein synthesis. Neurosci. Res. 63, 59–65. doi: 10.1016/j.neures.2008.10.008
Adori, C., Ando, R. D., Balazsa, T., Soti, C., Vas, S., Palkovits, M., et al. (2011a). Low ambient temperature reveals distinct mechanisms for MDMA-induced serotonergic toxicity and astroglial Hsp27 heat shock response in rat brain. Neurochem. Int. 59, 695–705. doi: 10.1016/j.neuint.2011.06.017
Adori, C., Ando, R. D., Szekeres, M., Gutknecht, L., Kovacs, G. G., Hunyady, L., et al. (2011b). Recovery and aging of serotonergic fibers after single and intermittent MDMA treatment in dark agouti rat. J. Comp. Neurol. 519, 2353–2378. doi: 10.1002/cne.22631
Adori, C., Ando, R. D., Ferrington, L., Szekeres, M., Vas, S., Kelly, P. A., et al. (2010). Elevated BDNF protein level in cortex but not in hippocampus of MDMA-treated Dark Agouti rats: a potential link to the long-term recovery of serotonergic axons. Neurosci. Lett. 478, 56–60. doi: 10.1016/j.neulet.2010.04.061
Adori, C., Ando, R. D., Kovacs, G. G., and Bagdy, G. (2006). Damage of serotonergic axons and immunolocalization of Hsp27, Hsp72, and Hsp90 molecular chaperones after a single dose of MDMA administration in Dark Agouti rat: temporal, spatial, and cellular patterns. J. Comp. Neurol. 497, 251–269. doi: 10.1002/cne.20994
Ando, R. D., Adori, C., Kirilly, E., Molnar, E., Kovacs, G. G., Ferrington, L., et al. (2010). Acute SSRI-induced anxiogenic and brain metabolic effects are attenuated 6 months after initial MDMA-induced depletion. Behav. Brain Res. 207, 280–289. doi: 10.1016/j.bbr.2009.10.011
Angelo, G., Lamon-Fava, S., Sonna, L. A., Lindauer, M. L., and Wood, R. J. (2008). Heat shock protein 90beta: a novel mediator of vitamin D action. Biochem. Biophys. Res. Commun. 367, 578–583. doi: 10.1016/j.bbrc.2007.12.179
Araujo, J., Breuer, P., Dieringer, S., Krauss, S., Dorn, S., Zimmermann, K., et al. (2011). FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3. Hum. Mol. Genet. 20, 2928–2941. doi: 10.1093/hmg/ddr197
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B 57, 289–300.
Bond, A. J., Verheyden, S. L., Wingrove, J., and Curran, H. V. (2004). Angry cognitive bias, trait aggression and impulsivity in substance users. Psychopharmacology 171, 331–339. doi: 10.1007/s00213-003-1585-9
Boxler, M. I., Streun, G. L., Liechti, M. E., Schmid, Y., Kraemer, T., and Steuer, A. E. (2018). Human Metabolome changes after a single dose of 3,4-methylenedioxymethamphetamine (MDMA) with special focus on steroid metabolism and inflammation Processes. J. Proteome Res. 17, 2900–2907. doi: 10.1021/acs.jproteome.8b00438
Capela, J. P., Carmo, H., Remiao, F., Bastos, M. L., Meisel, A., and Carvalho, F. (2009). Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol. Neurobiol. 39, 210–271. doi: 10.1007/s12035-009-8064-1
Cass, W. A., Smith, M. P., and Peters, L. E. (2006). Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann. N. Y. Acad. Sci. 1074, 261–271. doi: 10.1196/annals.1369.023
Colado, M. I., Granados, R., O’Shea, E., Esteban, B., and Green, A. R. (1999). The acute effect in rats of 3,4-methylenedioxyethamphetamine (MDEA, “eve”) on body temperature and long term degeneration of 5-HT neurones in brain: a comparison with MDMA (“ecstasy”). Pharmacol. Toxicol. 84, 261–266. doi: 10.1111/j.1600-0773.1999.tb01492.x
den Hollander, B., Schouw, M., Groot, P., Huisman, H., Caan, M., Barkhof, F., et al. (2012). Preliminary evidence of hippocampal damage in chronic users of ecstasy. J. Neurol. Neurosurg. Psychiatry 83, 83–85. doi: 10.1136/jnnp.2010.228387
Dunning, M. J., Smith, M. L., Ritchie, M. E., and Tavare, S. (2007). Beadarray: r classes and methods for illumina bead-based data. Bioinformatics 23, 2183–2184. doi: 10.1093/bioinformatics/btm311
Edgar, R., Domrachev, M., and Lash, A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210. doi: 10.1093/nar/30.1.207
Ferrington, L., Kirilly, E., McBean, D. E., Olverman, H. J., Bagdy, G., and Kelly, P. A. (2006). Persistent cerebrovascular effects of MDMA and acute responses to the drug. Eur. J. Neurosci. 24, 509–519. doi: 10.1111/j.1460-9568.2006.04923.x
Fukunaga, K., and Shioda, N. (2009). Pathophysiological relevance of forkhead transcription factors in brain ischemia. Adv. Exp. Med. Biol. 665, 130–142. doi: 10.1007/978-1-4419-1599-3_10
Gentleman, R. C., Carey, V. J., Bates, D. M., Bolstad, B., Dettling, M., Dudoit, S., et al. (2004). Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80. doi: 10.1186/gb-2004-5-10-r80
Green, A. R., Mechan, A. O., Elliott, J. M., O’Shea, E., and Colado, M. I. (2003). The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol. Rev. 55, 463–508. doi: 10.1124/pr.55.3.3
Haussler, M. R., Haussler, C. A., Jurutka, P. W., Thompson, P. D., Hsieh, J. C., Remus, L. S., et al. (1997). The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J. Endocrinol. 154(Suppl. ), S57–S73.
Hota, S. K., Hota, K. B., Prasad, D., Ilavazhagan, G., and Singh, S. B. (2010). Oxidative-stress-induced alterations in Sp factors mediate transcriptional regulation of the NR1 subunit in hippocampus during hypoxia. Free Radic. Biol. Med. 49, 178–191. doi: 10.1016/j.freeradbiomed.2010.03.027
Jenwitheesuk, A., Boontem, P., Wongchitrat, P., Tocharus, J., Mukda, S., and Govitrapong, P. (2017). Melatonin regulates the aging mouse hippocampal homeostasis via the sirtuin1-FOXO1 pathway. EXCLI J. 16, 340–353. doi: 10.17179/excli2016-852
Kajta, M., Makarewicz, D., Zieminska, E., Jantas, D., Domin, H., Lason, W., et al. (2009). Neuroprotection by co-treatment and post-treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochem. Int. 55, 265–274. doi: 10.1016/j.neuint.2009.03.010
Kalechstein, A. D., De La Garza, R. II, Mahoney, J. J. III, Fantegrossi, W. E., and Newton, T. F. (2007). MDMA use and neurocognition: a meta-analytic review. Psychopharmacology 189, 531–537. doi: 10.1007/s00213-006-0601-2
Kirilly, E., Benko, A., Ferrington, L., Ando, R. D., Kelly, P. A., and Bagdy, G. (2006). Acute and long-term effects of a single dose of MDMA on aggression in Dark Agouti rats. Int. J. Neuropsychopharmacol. 9, 63–76. doi: 10.1017/S146114570500581X
Landel, V., Annweiler, C., Millet, P., Morello, M., and Feron, F. (2016). Vitamin D, cognition and Alzheimer’s disease: the therapeutic benefit is in the D-tails. J. Alzheimers Dis. 53, 419–444. doi: 10.3233/JAD-150943
Logan, T. T., Villapol, S., and Symes, A. J. (2013). TGF-beta superfamily gene expression and induction of the runx1 transcription factor in adult neurogenic regions after brain injury. PLoS One 8:e59250. doi: 10.1371/journal.pone.0059250
Malik, R., Traylor, M., Pulit, S. L., Bevan, S., Hopewell, J. C., Holliday, E. G., et al. (2016). Low-frequency and common genetic variation in ischemic stroke: the metastroke collaboration. Neurology 86, 1217–1226. doi: 10.1212/WNL.0000000000002528
Morini, R., Mlinar, B., Baccini, G., and Corradetti, R. (2011). Enhanced hippocampal long-term potentiation following repeated MDMA treatment in Dark-Agouti rats. Eur. Neuropsychopharmacol. 21, 80–91. doi: 10.1016/j.euroneuro.2010.07.007
NINDS Stroke Genetics Network (SiGN) and International Stroke Genetics Consortium (ISGC). (2016). Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 15, 174–184. doi: 10.1016/S1474-4422(15)00338-5
Nulsen, C. E., Fox, A. M., and Hammond, G. R. (2010). Differential effects of ecstasy on short-term and working memory: a meta-analysis. Neuropsychol. Rev. 20, 21–32. doi: 10.1007/s11065-009-9124-z
Parrott, A. C., Sisk, E., and Turner, J. J. (2000). Psychobiological problems in heavy ’ecstasy’ (MDMA) polydrug users. Drug Alcohol Depend. 60, 105–110.
Pazmany, P., Petschner, P., Adori, C., Kirilly, E., Ando, D. R., Balogh, B., et al. (2013). The cognitive effects of ecstasy. Neuropsychopharmacol. Hung. 15, 214–222.
Pearson, R. D., Liu, X., Sanguinetti, G., Milo, M., Lawrence, N. D., and Rattray, M. (2009). Puma: a Bioconductor package for propagating uncertainty in microarray analysis. BMC Bioinformatics 10:211. doi: 10.1186/1471-2105-10-211
Petschner, P., Bagdy, G., and Tothfalusi, L. (2015). The problem of small “n” and big “P” in neuropsycho-pharmacology, or how to keep the rate of false discoveries under control. Neuropsychopharmacol. Hung. 17, 23–30.
Petschner, P., Juhasz, G., Tamasi, V., Adori, C., Tothfalusi, L., Hokfelt, T., et al. (2016). Chronic venlafaxine treatment fails to alter the levels of galanin system transcripts in normal rats. Neuropeptides 57, 65–70. doi: 10.1016/j.npep.2016.01.010
Petschner, P., Tamasi, V., Adori, C., Kirilly, E., Ando, R. D., Tothfalusi, L., et al. (2018). Gene expression analysis indicates reduced memory and cognitive functions in the hippocampus and increase in synaptic reorganization in the frontal cortex 3 weeks after MDMA administration in Dark Agouti rats. BMC Genomics 19:580. doi: 10.1186/s12864-018-4929-x
R Core Team. (2012). A Language and Environment for Statistical Computing. Vienna: Foundation for Statistical Computing Austria.
Salami, M., Talaei, S. A., Davari, S., and Taghizadeh, M. (2012). Hippocampal long term potentiation in rats under different regimens of vitamin D: an in vivo study. Neurosci. Lett. 509, 56–59. doi: 10.1016/j.neulet.2011.12.050
Schmidt-Kastner, R., and Freund, T. F. (1991). Selective vulnerability of the hippocampus in brain ischemia. Neuroscience 40, 599–636. doi: 10.1016/0306-4522(91)90001-5
Sweatt, J. D. (2004). Hippocampal function in cognition. Psychopharmacology 174, 99–110. doi: 10.1007/s00213-004-1795-9
Taguchi, K., Yamagata, H. D., Zhong, W., Kamino, K., Akatsu, H., Hata, R., et al. (2005). Identification of hippocampus-related candidate genes for Alzheimer’s disease. Ann. Neurol. 57, 585–588. doi: 10.1002/ana.20433
Tamasi, V., Petschner, P., Adori, C., Kirilly, E., Ando, R. D., Tothfalusi, L., et al. (2014). Transcriptional evidence for the role of chronic venlafaxine treatment in neurotrophic signaling and neuroplasticity including also glutamatergic [corrected] - and insulin-mediated neuronal processes. PLoS One 9:e113662. doi: 10.1371/journal.pone.0113662
Traylor, M., Farrall, M., Holliday, E. G., Sudlow, C., Hopewell, J. C., Cheng, Y. C., et al. (2012). Genetic risk factors for ischaemic stroke and its subtypes (the metastroke collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 11, 951–962. doi: 10.1016/S1474-4422(12)70234-X
Whitfield, G. K., Hsieh, J. C., Jurutka, P. W., Selznick, S. H., Haussler, C. A., MacDonald, P. N., et al. (1995). Genomic actions of 1,25-dihydroxyvitamin D3. J. Nutr. 125(Suppl. 6), 1690s–1694s. doi: 10.1093/jn/125.suppl_6.1690S
Wijchers, P. J., Hoekman, M. F., Burbach, J. P., and Smidt, M. P. (2006). Identification of forkhead transcription factors in cortical and dopaminergic areas of the adult murine brain. Brain Res. 1068, 23–33. doi: 10.1016/j.brainres.2005.11.022
Won, S., Sayeed, I., Peterson, B. L., Wali, B., Kahn, J. S., and Stein, D. G. (2015). Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One 10:e0122821. doi: 10.1371/journal.pone.0122821
Keywords: vitamin D, ecstasy, long-term potentiation, gene expression, microarray, hippocampus
Citation: Petschner P, Balogh N, Adori C, Tamasi V, Kumar S, Juhasz G and Bagdy G (2018) Downregulation of the Vitamin D Receptor Regulated Gene Set in the Hippocampus After MDMA Treatment. Front. Pharmacol. 9:1373. doi: 10.3389/fphar.2018.01373
Received: 13 September 2018; Accepted: 08 November 2018;
Published: 03 December 2018.
Edited by:
Francisco López-Muñoz, Universidad Camilo José Cela, SpainReviewed by:
Luis A. Tellez, Universidad Nacional Autónoma de México, MexicoRenato Corradetti, Università degli Studi di Firenze, Italy
Copyright © 2018 Petschner, Balogh, Adori, Tamasi, Kumar, Juhasz and Bagdy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Petschner, cGV0c2NobmVyLnBldGVyQHBoYXJtYS5zZW1tZWx3ZWlzLXVuaXYuaHU=
 Peter Petschner
Peter Petschner Noemi Balogh1
Noemi Balogh1 Gabriella Juhasz
Gabriella Juhasz Gyorgy Bagdy
Gyorgy Bagdy