- 1Laboratory of Traditional Chinese Veterinary Medicine, College of Veterinary Medicine, Northwest A&F University, Yangling, China
- 2Department of Neurosurgery, The First Hospital of Jilin University, Changchun, China
- 3University of Illinois at Urbana–Champaign, Urbana, IL, United States
- 4Department of Neurosciences, University of California, San Diego School of Medicine, La Jolla, CA, United States
Scope: Bleeding, the main drawback of clinically used chemical anti-thrombotic drug is resulted from the unidirectional suppression of platelet activity. Therefore, dual-directional regulatory effect on platelet is the main preponderance of Panax notoginseng over these drugs. The dual-directional regulatory effect should be ascribed to the resourceful Panax notoginseng saponins (PNS). Clarifying the mechanism of main PNS in both inhibiting and promoting platelet aggregation will give a full outlook for the dual-directional regulatory effect. The present study is aimed at explaining the mechanism of Notoginsenoside Fc (Fc), a main PNS, in inhibiting platelet aggregation.
Methods: In the in vitro study, after incubating platelets with Fc and m-3M3FBS, platelet aggregation was triggered by thrombin, collagen or ADP. Platelet aggregation was measured by aggregometer. Phospholipase Cγ2 (PLCγ2) and protein kinase C (PKC) activities were studied by western blotting. Diacylglycerol (DAG), thromboxane B2 (TXB2) and 1,4,5-inositol trisphosphate (IP3) concentrations were measured by corresponding ELISA kits. Calcium concentrations ([Ca2+]) were estimated through the fluorescence intensity emitted from Fluo-4. In the in vivo study, thrombus model was induced by FeCl3. The effect of Fc on thrombosis was evaluated by measurement of protein content and observation of injured blood vessel.
Results: thrombin, collagen and ADP induced platelet aggregation were all suppressed by incubating platelets with Fc. Platelet PLCγ2 and subsequent DAG-PKC-TXA2 and IP3 were down-regulated by Fc as well. However, the basal [Ca2+] in platelet was not altered by Fc. Nevertheless, thrombin triggered activation of PLCγ2 and subsequent DAG-PKC-TXA2 and IP3-[Ca2+] were all abolished by Fc. Fc also attenuated platelet aggregation and PLCγ2 signaling activation induced by PLC activator, m-3M3FBS. In the in vivo study, FeCl3 induced thrombosis in rat femoral artery was significantly alleviated by administration of Fc.
Conclusion: The results above suggested the antiplatelet and antithrombotic effects of Fc are carried out through oppression of PLCγ2 and subsequent DAG-PKC-TXA2 and IP3-[Ca2+]. The present study provided theoretical support for new anti-thrombotic drug exploitation by Panax notoginseng.
Introduction
Platelets are cells in mammal blood, formed from the cytoplasm of bone marrow megakaryocytes (Hartwig and Italiano, 2003). They play important role in a serious of physiological and pathological processes, such as hemostasis, inflammation responses and thrombosis. In normal condition, the main function of platelet is hemostasis. But in the pathological conditions, platelet aggregation was excessively triggered by a series of stimulators in the vascular microenvironment, which may result in thrombosis (Ruggeri and Mendolicchio, 2007). Thrombosis, the foremost precipitating factor for cardiovascular disease, threatened a great many people’s lives during the past decades. Platelet is the primary target for treatment of thrombotic diseases (Mackman, 2008).
Many antiplatelet drugs have been used in clinic for treatment of thrombotic diseases. The dominating defect for the clinically used chemical antiplatelet drugs is the drawback of bleeding, which result from the unidirectional inhibition of platelet aggregation. During antiplatelet therapy, bleeding threatens people’s lives more serious than thrombus itself (Meadows and Bhatt, 2007; Généreux et al., 2015). Although, attempts have been done for discovering new targets and new compounds for antiplatelet drug development, by simply down-regulate platelet aggregation, the defect of bleeding is still ineluctable (Mackman, 2008; Zhang et al., 2015).
Panax notoginseng, a plant mainly produced from Yunnan province of China, has been used as a Traditional Chinese Medicine for 100s of years because of its amazing stasis dispersing and hemostatic effects. Traditionally, the medicinal part of the plant is dried root and rhizome (under-ground part), which called “Sanqi.” According to the Traditional Chinese Medicinal theory, thrombosis implies the syndrome of blood stasis. As a result, stasis dispersing and hemostatic drugs are the most appropriate for treatment of thrombosis (Liao, 2000). Sanqi, the best-known stasis dispersing and hemostatic drug, has amazing dual-directional regulatory effect on platelets. Therefore, the distinctive advantage of Sanqi over chemical antiplatelet drugs is removing stasis without bleeding. In addition, Sanqi have a powerful capacity in inhibiting platelet aggregation, which is superior to aspirin (Wang et al., 2016). As a result, Sanqi have good potential to be explored for anti-thrombotic therapy.
However, the price of Sanqi is so high that any drug developed from it would be hardly afforded for most patients. Meanwhile, more than 90% of the over-ground parts of Panax notoginseng were abolished, despite the leaves and flowers of Panax notoginseng also showed fantastic stasis dispersing and hemostatic effects (Ke et al., 2010). In Yunnan, the over-ground parts of Panax notoginseng are more popular for indigenes. The leaves and flowers were made into tea, food and wine. Recent years, since the leaves and flowers of Panax notoginseng attracted attentions of researchers, they have been made into nourishment, toothpaste and so on.
Preventive treatment is quite advocated by Traditional Chinese Medicine. It implies preventing disease from occurring and preventing disease from exacerbating (Liang and Yin, 2010). Compared with take drugs, prevent disease by daily food and tea is the easiest way for preventive treatment. Because the price of Sanqi is quite high, utilization of the over-ground part of Panax notoginseng instead is a good way for reducing the cost. Pharmacodynamics studies have demonstrated similar functions of Panax notoginseng leaves and flowers with Sanqi, including anti-thrombosis, wound healing, anti-hyperlipidemia, anti-depression, anti-inflammation and so on. By drinking Panax notoginseng tea made from leaves and flowers, thrombus formation was prevented and the symptoms of thrombotic diseases were relieved a lot. Therefore, food, tea and nourishment made from the leaves and flowers of Panax notoginseng will be good resource for thrombotic diseases preventive treatment.
The dual-directional regulatory effect of Panax notoginseng on platelets should be ascribed to the resourceful PNS (Wang et al., 2004; Yuan et al., 2011; Gao et al., 2014). Until now, over 70 saponins have been isolated from Panax notoginseng. Among them, Fc, Ginsenoside Rg1, Rg2, Rg3, Rh2, Re, and Rd were proved capable in inhibiting platelet aggregation. On the other hand, Ft1, Notoginsenoside Fe and protopanaxadiol are effective in promoting platelet aggregation (Gao et al., 2014). Compared with Sanqi, the leaves and flowers are richer in PNS. Furthermore, the content of most effective antiplatelet compound Fc (Figure 1) is the richest in leaves and flowers of Panax notoginseng, compared with in roots (Zhou et al., 2017).
Despite the stasis dispersing and hemostatic effect of Panax notoginseng is well-known, the mechanism on how the dual-directional regulatory effect been balanced remains to be clarified. Uncovering the molecular mechanisms of main PNS will be quite helpful for clarifying that. Since Fc is the saponin exerts strongest antiplatelet effect among the PNS, the present study aimed at uncovering the mechanism of Fc in inhibiting platelet aggregation.
Materials and Methods
Materials
Fc standard was acquired from Shanghai Shifeng Biological Technology CO., LTD. (Shanghai, China). Collagen was purchased from Chrono-log (Havertown, PA, United States). Thrombin, ADP, clopidogrel and m-3M3FBS were obtained from Sigma–Aldrich (St. Louis, MO, United States). Fluo-4 AM indicator was obtained from Invitrogen (Carlsbad, CA, United States). Protease inhibitor and phosphatase inhibitor cocktail tablets were from Roche Diagnostics (Indianapolis, IN, United States). Phospho antibody for PLCγ2, Phospho antibody for PKC substrate and β-actin were purchased from Cell Signaling Technology (Beverly, MA). Immobilon western detection reagents, HRP-conjugated anti-rabbit and anti-mouse IgG were from Genshare Biological (Xi’an, Shaanxi, China). DAG, TXB2 and IP3 kits were purchased from R&D Systems (Minneapolis, MN, United States). PierceTM BCA Protein Assay Kit was from Pierce Biotechnology (Rockford, IL, United States). All the chemicals used were purchased from standard suppliers.
Animals
All animal experiments were approved by the Ethics Committee of Northwest A&F University. Male SD rats (5–6 weeks of age) were purchased from Dossy Experimental Animals CO., LTD. (Xi’an, Shaanxi, China) and acclimated for 1 week before the experiments. The laboratory animal facility was maintained at a constant temperature and humidity with a 12 h light/dark cycle. Food and water were provided ad libitum.
Washed Platelets Preparation
The method for WP preparation was the same as before (Liu et al., 2013). Briefly, blood was withdrawn from the abdominal aorta of rats anesthetized with ether. Acid-citrate-dextrose (66.6 mM citric acid, 85 mM trisodium citrate, 111 mM glucose) was used as anticoagulant (Acid-citrate-dextrose: blood = 1: 6). Then, the blood was centrifuged at 150 × g for 10 min. After that, the upper layer platelet rich plasma was centrifuged (150 × g) for another 10 min and washed once with washing buffer (138 mM NaCl, 2.8 mM KCl, 0.8 mM MgCl2, 0.8 mM Na2HPO4, 10 mM HEPES, 0.55 mM glucose, 22 mM trisodium citrate, 0.35% BSA, pH 6.5). Finally, the platelet pellets were suspended in suspension buffer (138 mM NaCl, 2.8 mM KCl, 0.8 mM MgCl2, 0.8 mM Na2HPO4, 10 mM HEPES, 5.6 mM glucose, 1 mM CaCl2, 0.3% BSA, pH 7.4) to a final concentration of 2 × 108 platelets/ml.
Platelet Aggregation Study
Platelet aggregation experiments were performed in a LBY-NJ4 platelet aggregometer (Techlink Biomedical). After treated with testing materials, WP aggregation was induced by different stimulators (thrombin, collagen, ADP, or m-3M3FBS). The stimulators were used in the minimal concentrations inducing submaximal aggregation.
Platelet PLCγ2 and PKC Activity Study
The activity of PLCγ2 and PKC were examined by conventional western blot analysis by suitable antibodies. After treatment with testing materials, platelets were precipitated by centrifugation (12,000 × g, 2 min). Then platelets were lysed by lysis buffer (50 μM HEPES, 50 μM NaCl, 50 μM sucrose, 1% Triton X-100, protease inhibitor cocktail, and phosphatase inhibitor cocktail). Protein contents were measured by a PierceTM BCA Protein Assay Kit from Pierce Biotechnology. The lysates were used as western blotting samples. Western blotting experiment procedures were same as everyone known. The activity of PLCγ2 and PKC were assessed by the phosphorylation of PLCγ2 and a 47 kDa protein of PKC substrate, respectively. Because there is no antibody available for measuring the total protein of PLCγ2 and PKC substrate of rats, β-actin was used as internal reference for protein loaded. Result images were obtained and analyzed with ChemiDoc XRS+ system and Image Lab software (Bio-Rad Laboratories, Hercules, CA, United States).
Platelet Calcium Concentration ([Ca2+]) Study
Intracellular [Ca2+] was studied by Fluo-4 AM with a Live Cell Imaging System equipped with TIRF microscope, EMCCD Andor ultra888 and sCMOS Andor zyla4.2Plus (Andor, Belfast, NIR, ENG). Platelets were incubated in washing buffer containing 1 μM Fluo-4 AM and 1% BSA for 30 min. After washing by centrifugation, platelets were suspended in suspension buffer and treated with testing materials. Pictures were taken by the Live Cell Imaging System in a time dependent order. The intracellular [Ca2+] were evaluated by analyzing the fluorescence intensity of the pictures.
Platelet IP3, DAG and Thromboxane A2 (TXA2) Evaluation
The amount of IP3, DAG and TXA2 were evaluated by IP3, DAG and TXB2 ELISA assay kits from R&D Systems (Minneapolis, MN, United States), respectively. After incubating WP with indicated materials, reaction was stopped in ice bath. IP3, DAG and TXA2 content were measured according to the instruction of the test kits.
In vivo Thrombus Study
The method for in vivo thrombus study was in accordance with Chinatsu Sakata’s study (Sakata et al., 2017). Rats were grouped randomly and i.p. injected with saline, Fc (50 mg/kg) or clopidogrel (5 mg/kg). The in vivo anti-thrombotic effect of Fc was evaluated with a FeCl3 arterial thrombosis rat model. Briefly, the rats were anesthetized by i.p. injection of sodium pentobarbital (30 mg/kg). After detach the femoral artery from the surrounding tissues, a filter paper (1 mm × 1 mm) saturated with 20% FeCl3 was applied to the artery for 20 min. The injured artery was isolated, observed and photographed under a LECIA M165FC Stereo Microscope (LECIA, Solms, Hesse, GER). Then, the thrombus was isolated gently and dissolved in NaOH (0.5 M). The size of a thrombus was evaluated by the protein content, which was measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, United States).
Statistical Analyses
Mean and SEM were calculated for all experimental groups. Data were analyzed by One-way Analysis of Variance followed by Dunn’s test, to determine the statistically significant differences. Statistical analyses were performed by SigmaStat Software Ver. 3.5 (Systat Software, San Jose, CA, United States). P < 0.05 were considered as statistically significant.
Results
Fc Inhibited Platelet Aggregation Induced by Various Stimulators
The impact of Fc on platelet aggregation was examined. To determine the appropriate incubating time for the study, several time points (3, 5, and 10 min) were tested against thrombin. The anti-platelet effect of Fc (400 μM) was peaked at 5 min (Figure 2A). As a result, 5 min was used in the following study. After that, the concentration dependent antiplatelet effect for Fc was investigated. Treatment of WP with Fc resulted in proportional suppression of thrombin induced platelet aggregation, with an IC50 of 204.38 μM (Figure 2B). Consistent with these results, by pretreatment of WP with increasing concentrations (50, 100, 200, 400, and 800 μM) of Fc, platelet aggregation induced by collagen (Figure 2C) and ADP (Figure 2D) were all inhibited dose-dependently, with IC50 of 379.93 and 295.89 μM, respectively. According to these results, Fc can inhibit various stimulators induced platelet aggregation, and most effective to thrombin.
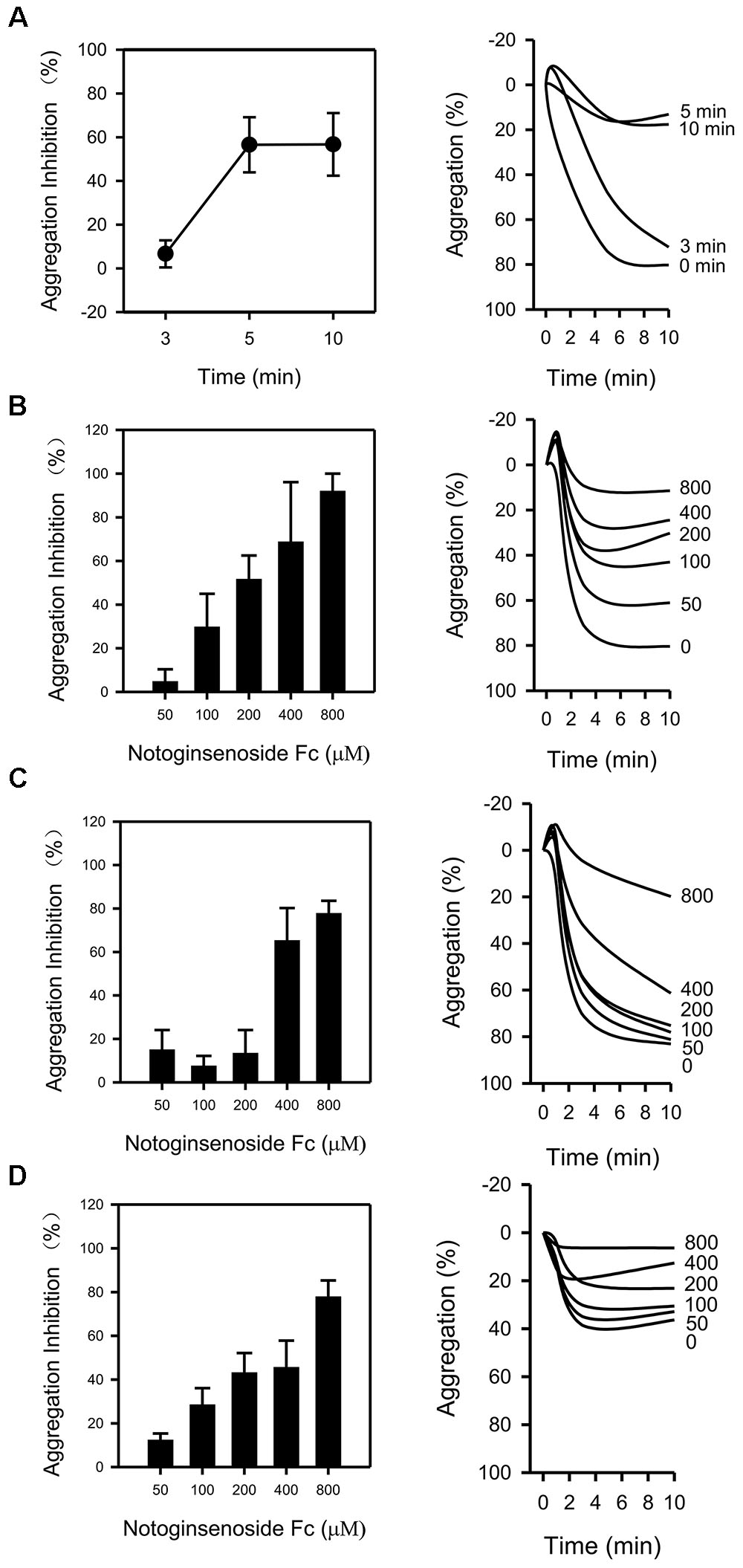
FIGURE 2. Antiplatelet effect of Fc. (A) After incubating WP with Fc (400 μM) for indicated times (3, 5, 10 min), platelet aggregation was induced by thrombin. To determine the dose-dependent antiaggregatory effect of Fc, WP were incubated with indicated concentrations (50, 100, 200, 400, 800 μM) of Fc for 5 min. Platelet aggregation was induced by either thrombin (B), collagen (C), or ADP (D). Tracing graphs represent the percentage of platelet aggregation variation after treated with stimulators. Values are mean ± SEM (n = 3 for A; n = 3∼4 for B and C; n = 3∼5 for D).
Fc Down Regulated the PLCγ2 Cascade in Platelet
To confirm the involvement of the PLCγ2 cascade in the antiplatelet effect of Fc, the following indexes were measured: P-PLCγ2, DAG, IP3, P-P47 (a protein reflect PKC activity), TXB2 (a metabolite of TXA2) and [Ca2+]. By incubating platelets with increasing concentrations of Fc, phosphorylation of PLCγ2 and P47 were reduced dose-dependently (Figure 3A). In addition, platelet IP3, DAG and TXB2 content were also decreased by Fc in a dose-dependent manner (Figures 3B–D). However, the alteration in intra-platelet [Ca2+] was not observed (Figure 4E). This may due to the sensitivity of the fluorescence dye (Fluo-4 AM). Because the basal [Ca2+] in resting platelets was too low to discern a further decrease.

FIGURE 3. Suppression of platelet PLCγ2 cascade by Fc. (A) After incubating WP with different concentrations of Fc, platelet PLCγ2 and PKC activity were evaluated according to the phosphorylation of PLCγ2 and P47. β-actin was used as a loading control. Platelet content of IP3, DAG and TXB2 were measured by ELISA kits. WP was incubated with the indicated concentrations of Fc, and platelet IP3 (B), DAG (C), and TXB2 (D) concentrations were assessed. Values are mean ± SEM (n = 3 for A; n = 5 for B; n = 4 for C,D). ∗P < 0.05 versus control.
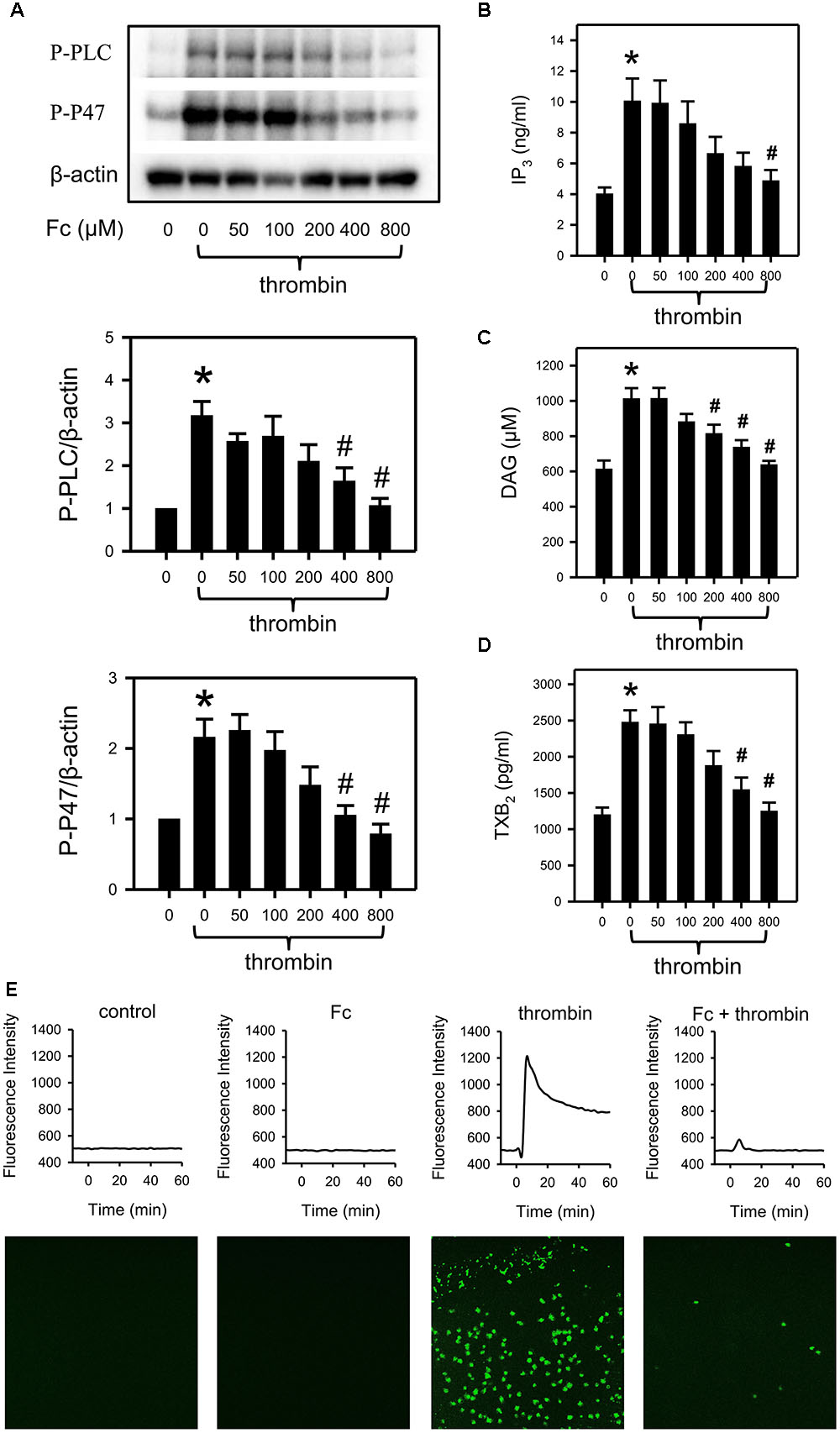
FIGURE 4. Prevention of thrombin induced activation of PLCγ2 cascade by Fc. After incubating with different concentrations of Fc, WP was treated with thrombin for 3 min. (A) The phosphorylation of PLCγ2 and P47 in platelet were measured with western blotting. β-actin was used as a loading control. Platelet IP3 (B), DAG (C) and TXB2 (D) concentrations were assessed by corresponding ELISA kits. (E) Variation in [Ca2+] was assessed by detecting the fluorescence intensity emitted from intracellular Fluo-4. The images are from the peak point of each experiment. Tracings were from representative results in three independent experiments. Values are mean ± SEM (n = 6 for A; n = 4 for B,C; n = 3 for D). ∗P < 0.05 versus control; #P < 0.05 versus thrombin only.
Fc Abolished Thrombin Induced PLCγ2 Cascade Activation
Thrombin induced platelet aggregation was most sensitive to Fc. As a result, we demonstrated the involvement of the PLCγ2 cascade in the anti-platelet effect of Fc against thrombin. In accordance with the previous study, thrombin can activate platelet PLCγ2, P47 (Figure 4A); upregulate IP3 (Figure 4B), DAG (Figure 4C), TXB2 (Figure 4D), and [Ca2+] (Figure 4E). By pre-incubation with increasing concentrations of Fc, thrombin induced platelet aggregation (Figure 2B) and activation of PLCγ2 cascade, including increase in [Ca2+], were downregulated dose-dependently (Figure 4). This proved our conjecture that Fc can decrease platelet [Ca2+] when it high enough to be detected.
Fc Attenuated m-3M3FBS Induced Platelet Aggregation and PLCγ2 Cascade Activation
M-3M3FBS (100, 200, 400 μM), direct PLC activator, activated PLCγ2 (Figure 5B) and induced platelet aggregation (Figure 5A) in a dose-dependent manner. Meanwhile, m-3M3FBS induced PLCγ2 activation was abolished by Fc (Figure 5B). And platelet aggregation was partially restored by pretreatment of Fc (Figure 5A). This demonstrated Fc can inhibit platelet aggregation through preventing m-3M3FBS induced PLCγ2 activation. In addition, m-3M3FBS increased P-P47, IP3, DAG, TXB2 and [Ca2+] were prevented by Fc as well (Figures 5B–F). The above results proved that Fc inhibit platelet aggregation through oppression the activation of PLCγ2 and subsequent DAG-PKC-TXA2 and IP3-Ca2+.
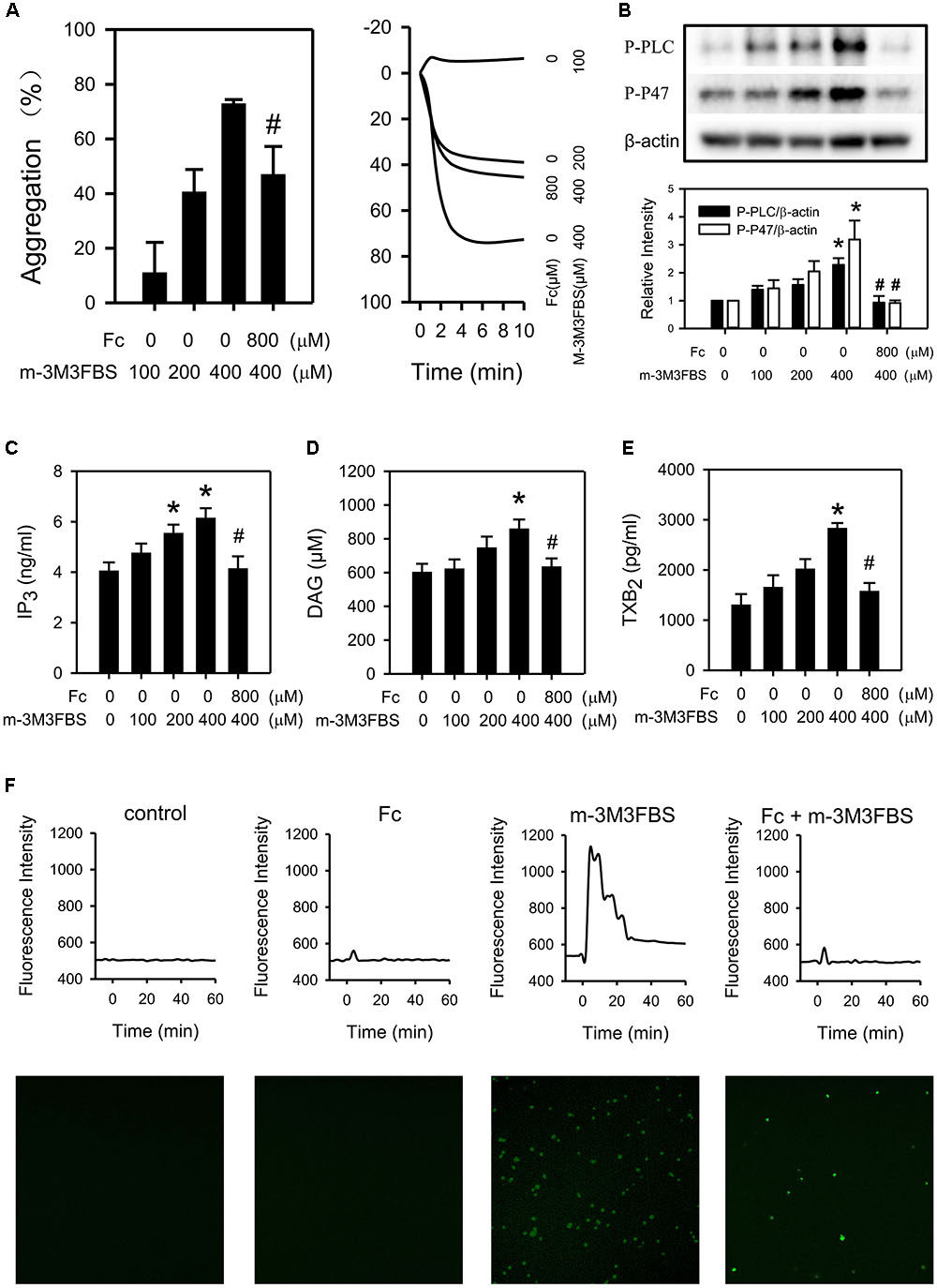
FIGURE 5. Involvement of PLCγ2 cascade in the antiplatelet effect of Fc. WP were treated with either increasing concentrations of m-3M3FBS (100, 200, 400 μM) or m-3M3FBS (400 μM) followed Fc (800 μM). (A) Platelet aggregation was assessed by a 4-channed aggregometer. (B) The phosphorylation of PLCγ2 and P47 of platelet were measured with western blotting. β-actin was used as a loading control. Platelet IP3 (C), DAG (D), and TXB2 (E) concentrations were assessed by ELISA kits. (F) [Ca2+] was assessed by the fluorescence intensity from intracellular Fluo-4. The images are from the peak point of each experiment. Tracings were from representative results in three independent experiments. Values are mean ± SEM (n = 6∼11 for A; n = 3∼4 for B–E). ∗P < 0.05 versus control; #P < 0.05 versus 400 μM m-3M3FBS only.
Fc Alleviated in vivo Thrombus Formation
To evaluate the in vivo anti-thrombotic effect of Fc, a FeCl3 thrombosis model was employed. By i.p. injection of Fc, thrombus protein content was decreased from 647.10 ± 72.30 mg to 406.38 ± 28.77 mg, although not as strong as clopidogrel (Figure 6A). FeCl3 injured blood vessels were also observed under a microscope. The dark part influencing blood vessel’s transparency was thrombus. Fc administration significantly decreased the thickness of thrombus (Figure 6B). This indicated Fc not only can inhibit in vitro platelet aggregation, but also can alleviate in vivo thrombus formation.

FIGURE 6. In vivo anti-thrombotic effect of Fc. Rats were i.p. injected with saline, Fc or clopidogrel. 2 h later, a filter paper saturated with 20% FeCl3 was applied to the isolated femoral artery for 20 min. Then the injured arteries were isolated for observation under microscope and measurement of thrombus protein content. (A) The bar graph indicates the protein contents in FeCl3 induced thrombus of each group. (B) The representative graphs showed the artery condition after injured by FeCl3. Values are mean ± SEM (n = 6 for control and Fc group; n = 3 for clopidogrel group). ∗P < 0.05 versus control.
Discussion
Although antiplatelet drugs have been frequently used in the clinic, the problems of bleeding kept push people to develop new drugs for controlling thrombus growth. In this process, a series of targets for antiplatelet drugs have been suggested, most of which are platelet receptors. Collagen, a stimulator released from damaged blood vessel, induces platelet aggregation through activation of glycoprotein VI and integrin α2β1 receptors. ADP is a platelet activator can be secreted both externally and internally. It activates platelet through P2Y12 and P2Y1 receptors. Thrombin, the most potent platelet activator, predominately activate platelets by protease activator receptor 1 (PAR1) and PAR4 (Coughlin, 2005; Leger et al., 2006). The receptors for thrombin, collagen and ADP are different, but there is a well-known pathway involved in all the three stimulators induced platelet aggregation: PLCγ2 activation improves hydrolyzation of PIP2 into IP3 and DAG, which in turn contribute to Ca2+ release, PKC activation and TXA2 increase (Si-Tahar et al., 1996; Liu et al., 2005; Ragab et al., 2007; Stegner and Nieswandt, 2011). Thrombin, collagen and ADP trigger PLCγ2 cascade activation through PAR, glycoprotein VI and P2Y1 receptors, respectively (Figure 7).
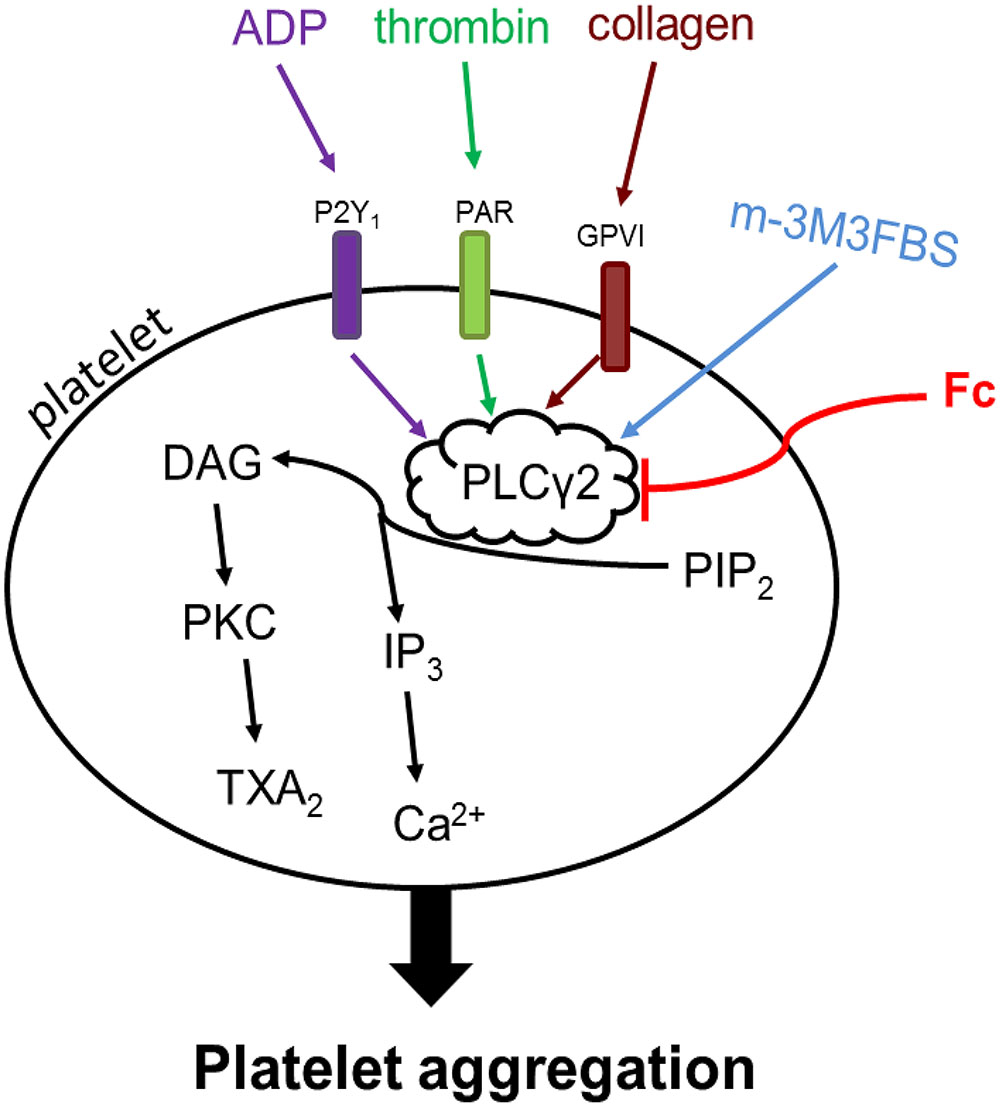
FIGURE 7. Mechanism of Fc inhibits platelet aggregation. Thrombin, collagen and ADP activate PLCγ2 signaling cascade through PAR, GPVI and P2Y1 receptors, respectively. PLCγ2 promotes the hydrolyzation of PIP2 to DAG and IP3 in platelets. The increase in platelet DAG and IP3 subsequently up-regulate PKC activity, TXA2 content and [Ca2+]. m-3M3FBS triggers activation of PLCγ2 and downstream signaling directly. Fc can abolish platelet aggregation induced by thrombin, collagen, ADP, as well as m-3M3FBS, through preventing the activation of PLCγ2 signaling. Arrows indicate the activation of target molecules or stimulation of production. Blunt line means target inhibition. The difference in color demonstrates different stimulators triggered effects.
In the present study, Fc inhibited platelet aggregation induced by thrombin, collagen and ADP (Figure 2). Therefore, it is quite possible that PLCγ2 and its downstream signaling are related with antiplatelet effect. Indeed, by measurement of PLCγ2 and PKC activities, IP3, DAG, PKC and TXA2 concentrations were down-regulated by Fc dose dependently (Figure 3). However, a decrease in [Ca2+] was not observed by Fc treatment. This is because the basal level of [Ca2+] in platelets was not high enough to see a further decrease. This was proved by testing the influence of Fc on thrombin stimulated platelets. According to the result, PLCγ2 and PKC activities, IP3, DAG, PKC, TXA2 and Ca2+ concentrations raised by thrombin were all abolished by pretreatment of Fc (Figure 4).
Although Fc can restore thrombin induced platelet aggregation and activation of PLCγ2 cascade, the relationship between the anti-platelet effect and PLCγ2 cascade is still not clear yet. Since thrombin induced platelet aggregation is quite complicated, PLCγ2 cascade is only part of it. To exclude the interfuse of other pathway, a direct PLC activator, m-3M3FBS, was employed (Bae et al., 2003). As we expected, PLCγ2 activity in platelets were enhanced by m-3M3FBS dose-dependently. And the increase in light transmission detected by aggregometer usually happens in platelet aggregation were triggered by m-3M3FBS as well. However, m-3M3FBS induced platelet aggregation was only partially restored by Fc (Figure 5). This may because of the non-specific effect of m-3M3FBS. Indeed, platelet aggregation induced by m-3M3FBS through activation of PLC is related with the increase in light transmittance of platelets. But it may also occur during platelet apoptosis (Shcherbina and Remold-O’Donnell, 1999; Stivala et al., 2017). It is reported that m-3M3FBS can affect a serious of apoptosis related proteins: up-regulate pro-apoptotic Bax, down-regulate anti-apoptotic Bcl-2, activate caspase and promote release of cytochrome C (Lee et al., 2005). In addition, PLC was classified into 6 families, including PLCβ, PLCγ, PLCδ, PLCε, PLCζ, and PLCη (Nakamura and Fukami, 2017). Except PLCγ2, PLCβ3 also relates with platelet aggregation (Lee et al., 2014; Pradhan et al., 2017). Therefore, PLCβ3 may also contribute to m-3M3FBS induced platelet aggregation. However, specific activator for PLCγ2 is still not available. After all, m-3M3FBS induced activation of PLCγ2 and subsequent DAG-PKC-TXA2 and IP3-[Ca2+] were almost completely abolished by Fc (Figure 5). Therefore, PLCγ2 cascade indeed responsible to the antiplatelet effect of Fc.
By a single administration of Fc, FeCl3 induced thrombosis in rat femoral artery was significantly alleviated (Figure 6). Although the anti-thrombotic potency of Fc is not as strong as clopidogrel, chronic administration may be a safer way for preventive treatment of thrombotic diseases. After all, the key point for new antiplatelet drug development is no longer high potency anymore. As we know, the strong unidirectional antiplatelet effect will result in serious bleeding. Safety is the predominant advantage of Panax notoginseng over clinical antiplatelet drugs (Rao et al., 2005; Bittl et al., 2016; Moon et al., 2018). By treatment of Panax notoginseng, when Fc exerts its effect on platelets, other saponins may provide complementary or eliminatory assistance. For instance, Ft1, another saponin from over-ground part of Panax notoginseng, was proved to have effect in promoting platelet aggregation by activating P2Y12 receptors (Gao et al., 2014). Therefore, a hypothesis was put forward: as main saponins in over-ground part of Panax notoginseng, Ft1 and Fc may be the predominant compositions for the dual-directional regulatory effect on platelet. To clarify the way to balance the dual-directional regulatory effect and comprehensive impact of them on platelet and thrombus, further research is required.
The effect of PNS on inhibiting platelet aggregation has been mentioned in many studies. PNS can inhibit platelet aggregation through PPAR-γ/PI3K/Akt/eNOS pathway (Shen et al., 2017), [Ca2+], ERK2/p38 (Qi et al., 2016), COX (Wang et al., 2016), FAK, NF-κB (Yuan et al., 2011). Whereas, most of them didn’t mention any single saponin, but only investigated the complex PNS. Which single saponin stimulated the above signals is still unknown. The hemostatic effect of Panax notoginseng is well known, but no systematic study about it yet. Gao et al. (2014) screened several saponins and conclude only Notoginsenoside Ft1, Notoginsenoside Fe and protopanaxadiol are effective in increase ADP induced platelet aggregation. Among them, only Ft1 was demonstrated to activate platelet through P2Y12 receptors. The effect of Notoginsenoside Fe and protopanaxadiol were not further studied (Gao et al., 2014). Based on these studies, it is hard to elaborate the mechanism of the dual-directional regulatory effect of Panax notoginseng. Therefore, our group is trying to investigate the effect and mechanism of more single saponins on platelet. After that, the dual-directional effect of Panax notoginseng can be clarified.
The present study demonstrated the mechanism of the antiplatelet effect of Fc, a main functional saponin in leaves and flowers of Panax notoginseng. This can provide theoretical basis for utilization of over-ground part of the plant. Attentions should be paid to the dual-directional regulatory effect of Panax notoginseng on platelets, which may be a new perspective for thrombotic disease treatment.
Ethics Statement
This study was carried out in accordance with the recommendations of the guideline for the use of Laboratory Animals, the Ethics Committee of Northwest A&F University. The protocol was approved by the Ethics Committee of Northwest A&F University. Male SD rats (5–6 weeks of age) were purchased from Dossy Experimental Animals Co., Ltd. (Xi’an, Shaanxi, China) and acclimated for 1 week before the experiments. The laboratory animal facility was maintained at a constant temperature and humidity with a 12 h light/dark cycle. Food and water were provided ad libitum. After acclimation, rats were randomly divided into 3 groups, and i.p. injected with saline, Fc (50 mg/kg) or clopidogrel (5 mg/kg), respectively. Two hours after i.p. injection, the rats were anesthetized by i.p. injection of sodium pentobarbital (30 mg/kg). After detach the femoral artery from the surrounding tissues, a filter paper (1 mm × 1 mm) saturated with 20% FeCl3 was applied to the artery for 20 min. The injured artery was isolated, observed and photographed under Microscope. Then, the thrombus was isolated gently and dissolved in NaOH (0.5 M). The size of a thrombus was evaluated by the protein content. The rats were sacrificed with diethyl ether after experiment.
Author Contributions
YqL, LC, and XS designed the research. YqL, TL, ZL, YyL, and TH performed the experiments. YqL, KD, WZ, YF, and WM analyzed the data. YqL, LC, and KD wrote the manuscript.
Funding
This work was supported by “National Natural Science Foundation of China” (Grant No. 31802230); “China Postdoctoral Science Foundation” (Grant No. 2016M602882); “Agro-Scientific Research in the Public Interest” (Grant No. 201303040), and “the Fundamental Research Funds for the Central Universities” (Grant No. 2452017297).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was also technically supported by “Life Science Research Core Services (LSRCS) of Northwest A&F University.”
Abbreviations
[Ca2+], calcium concentration; DAG, diacylglycerol; Fc, Notoginsenoside Fc; Ft1, notoginsenoside Ft1; IP3, 1,4,5-inositol trisphosphate; PAR, protease activator receptor; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLCγ2, phospholipase Cγ2; PNS, Panax notoginseng saponins; TXA2, thromboxane A2; TXB2, thromboxane B2; WP, washed platelets.
References
Bae, Y. S., Lee, T. G., Park, J. C., Hur, J. H., Kim, Y., Heo, K., et al. (2003). Identification of a compound that directly stimulates phospholipase C activity. Mol. Pharmacol. 63, 1043–1050. doi: 10.1124/mol.63.5.1043
Bittl, J. A., Baber, U., Bradley, S. M., and Wijeysundera, D. N. (2016). Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 68, 1116–1139. doi: 10.1016/j.jacc.2016.03.512
Coughlin, S. R. (2005). Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x
Gao, B., Huang, L., Liu, H., Wu, H., Zhang, E., Yang, L., et al. (2014). Platelet P2Y(1)(2) receptors are involved in the haemostatic effect of notoginsenoside Ft1, a saponin isolated from Panax notoginseng. Br. J. Pharmacol. 171, 214–223. doi: 10.1111/bph.12435
Généreux, P., Giustino, G., Witzenbichler, B., Weisz, G., Stuckey, T. D., Rinaldi, M. J., et al. (2015). Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J. Am. Coll. Cardiol. 66, 1036–1045. doi: 10.1016/j.jacc.2015.06.1323
Hartwig, J., and Italiano, J. Jr. (2003). The birth of the platelet. J. Thromb. Haemost. 1, 1580–1586. doi: 10.1046/j.1538-7836.2003.00331.x
Ke, Y., Jiang, J. Y., Wang, X. Z., Zeng, X. Y., and Zhu, C. Y. (2010). Effect of total saponins from rhizomes and flowers of Panax notoginseng on tumor cell induced platelet aggregation. Zhong Yao Cai 33, 96–99.
Lee, J. J., Cho, W. K., Kwon, H., Gu, M., and Ma, J. Y. (2014). Galla rhois exerts its antiplatelet effect by suppressing ERK1/2 and PLC beta phosphorylation. Food Chem. Toxicol. 69, 94–101. doi: 10.1016/j.fct.2014.03.032
Lee, Y. N., Lee, H. Y., Kim, J. S., Park, C., Choi, Y. H., Lee, T. G., et al. (2005). The novel phospholipase C activator, m-3M3FBS, induces monocytic leukemia cell apoptosis. Cancer Lett. 222, 227–235. doi: 10.1016/j.canlet.2004.09.017
Leger, A. J., Covic, L., and Kuliopulos, A. (2006). Protease-activated receptors in cardiovascular diseases. Circulation 114, 1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830
Liang, Z. H., and Yin, D. Z. (2010). Preventive treatment of traditional chinese medicine as antistress and antiaging strategy. Rejuv Res. 13, 248–252. doi: 10.1089/rej.2009.0867
Liao, F. (2000). Herbs of activating blood circulation to remove blood stasis. Clin. Hemorheol. Microcirc 23, 127–131.
Liu, J., Pestina, T. I., Berndt, M. C., Jackson, C. W., and Gartner, T. K. (2005). Botrocetin/VWF-induced signaling through GPIb-IX-V produces TxA2 in an alpha IIb beta 3- and aggregation-independent manner. Blood 106, 2750–2756. doi: 10.1182/blood-2005-04-1667
Liu, Y., Oh, S. J., Chang, K. H., Kim, Y. G., and Lee, M. Y. (2013). Antiplatelet effect of AMP-activated protein kinase activator and its potentiation by the phosphodiesterase inhibitor dipyridamole. Biochem. Pharmacol. 86, 914–925. doi: 10.1016/j.bcp.2013.07.009
Mackman, N. (2008). Triggers, targets and treatments for thrombosis. Nature 451, 914–918. doi: 10.1038/nature06797
Meadows, T. A., and Bhatt, D. L. (2007). Clinical aspects of platelet inhibitors and thrombus formation. Circ. Res. 100, 1261–1275. doi: 10.1161/01.RES.0000264509.36234.51
Moon, J. Y., Franchi, F., Rollini, F., and Angiolillo, D. J. (2018). The quest for safer antithrombotic treatment regimens in patients with coronary artery disease: new strategies and paradigm shifts. Expert Rev. Hematol. 11, 5–12. doi: 10.1080/17474086.2018.1400378
Nakamura, Y., and Fukami, K. (2017). Regulation and physiological functions of mammalian phospholipase C. J. Biochem. 161, 315–321. doi: 10.1093/jb/mvw094
Pradhan, S., Khatlani, T., Nairn, A. C., and Vijayan, K. V. (2017). The heterotrimeric G protein G beta(1) interacts with the catalytic subunit of protein phosphatase 1 and modulates G protein-coupled receptor signaling in platelets. J. Biol. Chem. 292, 13133–13142. doi: 10.1074/jbc.M117.796656
Qi, H., Huang, Y., Yang, Y., Dou, G., Wan, F., Zhang, W., et al. (2016). Anti-platelet activity of panaxatriol saponins is mediated by suppression of intracellular calcium mobilization and ERK2/p38 activation. BMC Complem. Altern. Med. 16:978034. doi: 10.1186/s12906-016-1160-7
Ragab, A., Séverin, S., Gratacap, M. P., Aguado, E., Malissen, M., Jandrot-Perrus, M., et al. (2007). Roles of the C-terminal tyrosine residues of LAT in GPVI-induced platelet activation: insights into the mechanism of PLC gamma 2 activation. Blood 110, 2466–2474. doi: 10.1182/blood-2007-02-075432
Rao, S. V., O’Grady, K., Pieper, K. S., Granger, C. B., Newby, L. K., Van de Werf, F., et al. (2005). Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am. J. Cardiol. 96, 1200–1206. doi: 10.1016/j.amjcard.2005.06.056
Ruggeri, Z. M., and Mendolicchio, G. L. (2007). Adhesion mechanisms in platelet function. Circ. Res. 100, 1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab
Sakata, C., Suzuki, K. I., Morita, Y., and Kawasaki, T. (2017). Additive antithrombotic effect of ASP6537, a selective cyclooxygenase (COX)-1 inhibitor, in combination with clopidogrel in guinea pigs. Eur. J. Pharmacol. 798, 72–76. doi: 10.1016/j.ejphar.2017.01.015
Shcherbina, A., and Remold-O’Donnell, E. (1999). Role of caspase in a subset of human platelet activation responses. Blood 93, 4222–4231.
Shen, Q., Li, J., Zhang, C., Wang, P., Mohammed, A., Ni, S., et al. (2017). Panax notoginseng saponins reduce high-risk factors for thrombosis through peroxisome proliferator activated receptor-gamma pathway. Biomed. Pharmacother. 96, 1163–1169. doi: 10.1016/j.biopha.2017.11.106
Si-Tahar, M., Renesto, P., Falet, H., Rendu, F., and Chignard, M. (1996). The phospholipase C protein kinase C pathway is involved in cathepsin G-induced human platelet activation: comparison with thrombin. Biochem. J. 313, 401–408. doi: 10.1042/bj3130401
Stegner, D., and Nieswandt, B. (2011). Platelet receptor signaling in thrombus formation. J. Mol. Med. 89, 109–121. doi: 10.1007/s00109-010-0691-5
Stivala, S., Gobbato, S., Infanti, L., Reiner, M. F., Bonetti, N., Meyer, S. C., et al. (2017). Amotosalen/ultraviolet A pathogen inactivation technology reduces platelet activatability, induces apoptosis and accelerates clearance. Haematologica 102, 1650–1660. doi: 10.3324/haematol.2017.164137
Wang, J., Xu, J., and Zhong, J. B. (2004). Effect of radix notoginseng saponins on platelet activating molecule expression and aggregation in patients with blood hyperviscosity syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 24, 312–316.
Wang, M. M., Xue, M., Xu, Y. G., Miao, Y., Kou, N., Yang, L., et al. (2016). Panax notoginseng saponin is superior to aspirin in inhibiting platelet adhesion to injured endothelial cells through COX pathway in vitro. Thromb. Res. 141, 146–152. doi: 10.1016/j.thromres.2016.03.022
Yuan, Z., Liao, Y., Tian, G., Li, H., Jia, Y., Zhang, H., et al. (2011). Panax notoginseng saponins inhibit Zymosan A induced atherosclerosis by suppressing integrin expression, FAK activation and NF-kappaB translocation. J. Ethnopharmacol. 138, 150–155. doi: 10.1016/j.jep.2011.08.066
Zhang, D., Gao, Z. G., Zhang, K., Kiselev, E., Crane, S., Wang, J., et al. (2015). Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520, 317–321. doi: 10.1038/nature14287
Keywords: Panax notoginseng, Notoginsenoside Fc, platelet aggregation, antiplatelet effect, thrombosis, phospholipase Cγ2
Citation: Liu Y, Liu T, Ding K, Liu Z, Li Y, He T, Zhang W, Fan Y, Ma W, Cui L and Song X (2018) Phospholipase Cγ2 Signaling Cascade Contribute to the Antiplatelet Effect of Notoginsenoside Fc. Front. Pharmacol. 9:1293. doi: 10.3389/fphar.2018.01293
Received: 04 May 2018; Accepted: 22 October 2018;
Published: 06 November 2018.
Edited by:
Akio Inui, Kagoshima University, JapanReviewed by:
Munekazu Yamakuchi, Kagoshima University, JapanXu Wang, Wenzhou Medical University, China
Jianxin Deng, Yangtze University, China
Copyright © 2018 Liu, Liu, Ding, Liu, Li, He, Zhang, Fan, Ma, Cui and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Song, c3hweGJubEAxNjMuY29t
 Yingqiu Liu
Yingqiu Liu Tianyi Liu2
Tianyi Liu2