- 1Departamento de Ciências Patológicas, Universidade Estadual de Londrina, Londrina, Brazil
- 2Department of Pharmacology, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil
- 3Departamento de Ciências Farmacêuticas, Universidade Estadual de Londrina, Londrina, Brazil
Gouty arthritis is characterized by an intense inflammatory response to monosodium urate crystals (MSU), which induces severe pain and reduction in the life quality of patients. Trans-Chalcone (1,3-diphenyl-2-propen-1-one) is a flavonoid precursor presenting biological activities such as anti-inflammatory and antioxidant proprieties. Thus, the aim of this work was to evaluate the protective effects of trans-Chalcone in experimental gout arthritis in mice. Mice were treated with trans-Chalcone (3, 10, or 30 mg/kg, per oral) or vehicle (Tween 80 20% plus saline) 30 min before intra-articular injection of MSU (100 μg/knee joint, intra-articular). We observed that trans-Chalcone inhibited MSU-induced mechanical hyperalgesia, edema, and leukocyte recruitment (total leukocytes, neutrophils, and mononuclear cells) in a dose-dependent manner. Trans-Chalcone also decreased inflammatory cell recruitment as observed in Hematoxylin and Eosin (HE) staining and the intensity of fluorescence of LysM-eGFP+ cells in the confocal microscopy. Trans-Chalcone reduced MSU-induced oxidative stress as observed by an increase in the antioxidant defense [Glutathione (GSH), Ferric Reducing (FRAP), and 2,2’-Azinobis-3-ethylbenzothiazoline 6-sulfonic acid (ABTS assays)] and reduction in reactive oxygen and nitrogen species production [superoxide anion (NBT assay) and nitrite (NO assay)]. Furthermore, it reduced in vivo MSU-induced interleukin-1β (IL-1β), Tumor necrosis factor-α (TNF-α), and IL-6 production, and increased Transforming growth factor-β (TGF-β) production. Importantly, trans-Chalcone reduced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and thereby the mRNA expression of the inflammasome components Nlrp3 (cryopyrin), Asc (apoptosis-associated speck-like protein containing a CARD), Pro-caspase-1 and Pro-IL-1β. In vitro, trans-Chalcone reduced the MSU-induced release of IL-1β in lipopolysaccharide (LPS)-primed macrophages. Therefore, the pharmacological effects of trans-Chalcone indicate its therapeutic potential as an analgesic and anti-inflammatory flavonoid for the treatment of gout.
Introduction
Gout is a painful inflammatory disease caused by the over-production of uric acid, which forms deposits and crystallizes as monosodium urate (MSU) in articular and periarticular tissues (Rees et al., 2014; Fattori et al., 2016). MSU crystals trigger an intense inflammatory response and increase the recruitment of neutrophils to the joint (Rees et al., 2014; Fattori et al., 2016). Management of gout arthritis lies on the use of urate-lowering therapies and the control of acute flares. The main reason for patient seeking of medical care is the intense pain in acute flares (Rees et al., 2014). Gout flares in humans are self-limited and last for nearly 10 days with intense and debilitating pain despite anti-inflammatory treatment (Martin and Harper, 2010). Nevertheless, if left untreated, continuing MSU crystal deposition causes repetitive flares (Rees et al., 2014; Dalbeth et al., 2016). More importantly, irreversible joint damage with chronic symptoms and disability can be observed. In fact, advanced cases of gout are characterized by chronic joint pain, movement limitation, and recurrent flares (Dalbeth et al., 2016). Currently, the management of gout flares lies on the use of non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, corticoids, or biological agents (Rees et al., 2014; Dalbeth et al., 2016). However, the use of these drugs lack safety in patients with comorbidities (NSAIDs), often cause severe side effects (NSAIDs, colchicine, and corticoids), present high cost (biological agents), or possess non-satisfactory analgesic effects in some patients (Rees et al., 2014; Dalbeth et al., 2016).
Monosodium urate crystals trigger an inflammatory response that is dependent on Nacht, LRR, and Pyd domains-containing protein 3 (NLRP3) inflammasome assembly and production of mature interleukin-1β (IL-1β) (Martinon et al., 2006; Amaral et al., 2012). In addition to its hyperalgesic effect, IL-1β also induces the recruitment of neutrophils that further produce hyperalgesic mediators, such as endothelin-1 and prostaglandin E2 (PGE2) (Popa-Nita et al., 2007; Cunha et al., 2008; Amaral et al., 2012; Fattori et al., 2016). In neutrophils, IL-1β induces PGE2 production, which is a molecule responsible for sensitization of nociceptor sensory neurons (Cunha et al., 2008). Thus, the advances in the understanding of the pathophysiology of pain and inflammation in gout raised the possibility of more effective analgesic therapies.
Flavonoids are natural compounds and represent a major subset of polyphenols widely distributed in vegetables, fruits, and herbs (Verri et al., 2012). In general, there is evidence that flavonoids present broad biological activity such as antioxidant, antitumoral, antimicrobial, anti-inflammatory, and analgesic (Verri et al., 2012). Trans-Chalcone (1,3-diphenyl-2-propen-1-one) is an open-chain flavonoid and a major precursor of other flavonoids in plants, which also possesses anti-inflammatory and antioxidant properties (Lamoke et al., 2011; Singh et al., 2016; Martinez et al., 2017a,b). Chalcones have a good pharmacological profile and low toxicity (Karimi-Sales et al., 2018) supporting these molecules as good therapeutic candidates for chronic inflammation such as the observed in rheumatic diseases.
Evidence shows that trans-Chalcone reduces UV-induced skin inflammation (Martinez et al., 2016, 2017b), ameliorates carbon tetrachloride (CCl4)-induced liver damage (Singh et al., 2016), and prevents pathological neovascularization in the ischemic retina (Lamoke et al., 2011). However, to the date, there is no study addressing the analgesic effect of trans-Chalcone or even its effectiveness in gout arthritis. Given the evidence on the biological activities of trans-Chalcone and lack of data evaluating its analgesic effect, we aimed at evaluating the efficacy of this flavonoid in a model of MSU-induced gout arthritis in mice.
Materials and Methods
Animals and Ethics Statement
Male Swiss (25–30 g, 8 weeks) and C57BL/6 (20–25 g, 8 weeks) mice from the Universidade Estadual de Londrina (Londrina, Brazil), LysM-GFP mice (20–25 g, 8 weeks) and C57/BL6 from the Ribeirão Preto Medical School – University of São Paulo (Ribeirão Preto, São Paulo, Brazil) were used in this study. Mice were housed in standard clear plastic cages with free access to food and water with a light/dark cycle of 12/12 h temperature of 21 ± 1°C. All behavioral testing was performed between 9 a.m. and 5 p.m. in a temperature-controlled (21 ± 1°C) room. Animal care and handling procedures were approved by the Ethics Committee of the Universidade Estadual de Londrina (process number 14600.2013.73). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Experimental Procedures
Mice were treated with trans-Chalcone (TC; 3, 10, or 30 mg/kg, p.o.) or vehicle (Tween 80 20% plus saline) 30 min before intra-articular stimulus with MSU (100 μg/10μL, i.a.). trans-Chalcone (at 95% purity was purchased from Santa Cruz Biotechnology, #CAS 614-47-1, Dallas, TX, United States) dose range was selected upon the doses previously used in the literature (Lamoke et al., 2011; Jalalvand et al., 2015; Singh et al., 2016; Karimi-Sales et al., 2018). The negative control of stimulus received only intraarticular injection of 10 μl of saline. In experiments that the effect of trans-Chalcone was higher than the control responses, the effect of trans-Chalcone per se was also investigated.
Mechanical hyperalgesia and edema were evaluated 1, 3, 5, 7, and 15 h after MSU or saline injection. Fifteen hours after MSU injection, knee joint wash was collected to determine leukocyte recruitment. Based on these previous experiments, the dose of 30 mg/kg was chosen for the following experiments: histopathological analysis (HE staining), neutrophil migration to the knee joint wash using LysM-eGFP mice (confocal microscopy), oxidative stress, determination of the expression of genes sensitives to oxidative stress by RT-qPCR (gp91phox, Nrf2, Ho-1), cytokine measurement by ELISA, NF-κB activation (total p65/phosphorylated p65 ratio) by ELISA, and inflammasome components expression Nlrp3, Asc, Pro-caspase-1, and Pro-Il-1β mRNA expression by RT-qPCR. In all in vivo analysis, samples were collected 15 h after MSU injection. Serum samples were also collected 15.5 h after trans-Chalcone or vehicle treatment to evaluate renal and hepatic toxicity considering the same time of protocol of the all experiments with trans-Chalcone. The negative control received only intraarticular injection of 10 μl of saline. As a positive control for liver injury (hepatic toxicity) and for kidney injury (renal toxicity), acetaminophen (650 mg/kg,) and diclofenac (200 mg/kg) were used, respectively. One protocol evaluated the effect of trans-Chalcone (30 mg/kg, p.o) or vehicle (Tween 80 20% plus saline) treatment 30 min after MSU injection on mechanical hyperalgesia and knee edema.
For in vitro analysis, to assess the effect of trans-Chalcone on NF-κB activation, bone marrow derived macrophages (BMDMs) were pre-treated with 0.1–3 μM before 500 ng/mL of LPS (i.e., priming) and supernatants were collected 5 h after MSU (activation) stimulation and IL-1β levels were quantitated by ELISA. A selected concentration of trans-Chalcone using the same protocol was also used to test the flavonoid effect over Nrf2 and Ho-1 mRNA expression. Next, it was also evaluated the effect of trans-Chalcone at 0.1–3 μM on BMDMs 30 min before MSU stimulation (i.e., after priming with LPS/before activation with MSU) to address if TC could interfere directly with the activation stimulus to prevent IL-1β secretion and inflammasome activation. The vehicle (Tween 80 20% plus saline) treatment did not alter the responses. All experiments were performed in Swiss mice except for the experiments presenting LysM-eGFP analysis and BMDM cell culture.
Preparation of MSU Crystal
The MSU crystals were prepared as described previously (Ruiz-Miyazawa et al., 2017). Briefly, 800 mg of monosodium urate were dissolved in 155 ml of boiling milli-Q water containing 5 ml of NaOH, and after the pH was adjusted to 7.2, the solution was cooled gradually by stirring at room temperature. The crystals were collected, and then centrifuged at 3,000 g for 2 min at 4°C. The crystals were evaporated and sterilized by heating at 180°C for 2 h and stored in a sterile environment until use. The load of endotoxin present in the MSU crystals was determined using a ToxinSensorTM Single Test Kit (GenScript). The selected dose and concentration of MSU did not present detectable endotoxin levels as previously described (Ruiz-Miyazawa et al., 2018).
Induction of MSU-Induced Knee Joint Inflammation
Joint inflammation was induced by the intra-articular (i.a.) administration of MSU (100 μg/10 μl) into the right knee joint of mice under isoflurane anesthesia. Control animals received an i.a. injection of sterile saline (10 μL) (Ruiz-Miyazawa et al., 2017).
Evaluation of Knee Joint Hyperalgesia
The mechanical hyperalgesia of the femur-tibial joint was evaluated by an electronic von Frey apparatus. Mice were placed in acrylic cages with a wire grid floor, and the stimulations were performed only when the animals were quiet (and with the four paws on the grid floor). This method consists of an electronic pressure-meter, with a force transducer fitted with polypropylene tip (Insight instruments, Ribeirao Preto, Brazil). To evaluate the articular pain, we used a large tip (4.15 mm2), to exclude the subcutaneous effect (Guerrero et al., 2006). An increase perpendicular force was applied to the central area of the plantar surface of the hind paw to induce flexion of the femur-tibial joint followed by the hind paw withdrawal. A digital analgesimeter recorded the intensity of the force applied (in grams) when the paw was withdrawal. The test was performed at the times indicated on figures. The investigators were blinded to the treatment groups.
Knee Joint Edema Evaluation
Knee joint edema was determined with a caliper (Mitutoyo) before (zero time), and after the intra-articular injection with MSU at the times indicated. Knee joint edema was determined for each mouse by the difference between the time points indicated on figures and the zero time (Ruiz-Miyazawa et al., 2017). The edema value is represented as Δmm/joint.
In vivo Leukocyte Migration
Knee joint wash was collected 15 h after MSU injection for determination of leukocyte recruitment (Verri et al., 2010). Briefly, articular cavities were washed three times with 3.3 μL of saline with 1 mM EDTA, then, diluted to a final volume of 50 μL with PBS/EDTA to evaluate leukocyte migration. The total number of leukocytes was determined in a Neubauer chamber diluted in Turk’s solution 1:2 (used to lyse the erythrocytes). Differential cell counts were determined in Rosenfeld stained slices using a light microscope and results are expressed as the number of neutrophils per cavity.
Histopathological Analysis
Knee joint was collected 15 h after MSU injection, fixed with 10% paraformaldehyde in PBS, and then decalcified for 10 days with EDTA and embedded in paraffin for histological analysis. The paraffin sections were stained with hematoxylin and eosin for conventional morphological evaluation. Results are expressed as leukocytes infiltrate (cell/field) counted at the inflammatory foci as indicative of synovitis. A dimension used for the analysis of the slices was 569 × 633 pixels and magnification ×400 (Ruiz-Miyazawa et al., 2017).
Fluorescence Assay
Knee joint wash of LysM-GFP mice was collected in sterile slides 15 h after MSU injection into the knee joints. DAPI fluorescent stain was added to slides for localization of nucleus in each sample. The representative images and quantitative analysis were performed using a confocal microscope (SP8, Leica Microsystems, Mannheim, Germany). The intensity of fluorescence was quantified in randomly selected fields of different groups by an investigator blinded to the treatments. Results are presented as the percentage of GFP fluorescent intensity.
GSH Levels Measurement
Samples of articular joint were collected and maintained at -80°C for at least 48 h. The sample was homogenized with 200 μl of 0.02 M EDTA. The homogenate was mixed with 25 μl of trichloroacetic acid 50% and was homogenized three times over 15 min. The mixture was centrifuged (15 min × 1500 g × 4°C). The supernatant was added to 200 μl of 0.2 M TRIS buffer, pH 8.2, and 10 μl of 0.01 M DTNB. After 5 min, the absorbance was measured at 412 nm (Multiskan GO, Thermo Fisher Scientific) against a blank reagent with no supernatant. A standard GSH curve was formed. The results are expressed as GSH per mg of protein (Ruiz-Miyazawa et al., 2017).
ABTS and FRAP Assays
The ability of samples to resist oxidative damage was determined by their free radical scavenging (ABTS [2,2’-Azinobis-3-ethylbenzothiazoline 6-sulfonic acid] assay) and ferric reducing (FRAP assay) properties. The ABTS is effective in detecting anti-radical activity and FRAP detects reducing potential in tissue samples (Menghini et al., 2018). The tests were adapted to a 96-well microplate format as previously described (Ruiz-Miyazawa et al., 2017). Articular tissue samples were collected 15 h after MSU i.a. injection (100 μg/10 μL) and homogenized immediately in ice-cold KCl buffer (500 μl, 1.15% w/v). The homogenates were centrifuged (200 g × 10 min × 4°C), and the supernatants were used in both assays. Diluted ABTS solution (200 μl) was mixed with 10 μL of sample in each well. After 6 min of incubation at 25°C, the absorbance was measured at 730 nm. For FRAP assay, the supernatants (10 μl) were mixed with the freshly prepared FRAP reagent (150 μl). The reaction mixture was incubated at 37°C for 30 min, and the absorbance was measured at 595 nm (Multiskan GO Thermo Fisher Scientific). The results of ABTS and FRAP assays were equated against a standard Trolox curve (0.02–20 nmol).
Superoxide Anion Production
The measurement of superoxide anion production in tissue homogenates (10 mg/ml in 1.15% KCl) was performed using the nitroblue tetrazolium (NBT) assay adapted to a microplate as described previously (Ruiz-Miyazawa et al., 2017). The NBT reduction was measured at 600 nm (Multiskan GO, Thermo Fisher Scientific). The tissue weight was used for data normalization.
Nitrite Production
Samples from knee joint were collected 15 h after MSU injection, homogenized in 500 μl of saline, and nitrite (NO2-) concentration was determined by the Griess reaction as an indicator of nitric oxide (NO) production (Lima-Junior et al., 2013). Briefly, 100 μl of the homogenate was incubated with 100 μl of Griess reagent for 5 min at 25°C, and NO2- concentration was determined by measuring the optical density at 550 nM (Multiskan GO, Thermo Fisher Scientific) in reference to a standard curve of NaNO2 solution. Results are expressed as μmol of NO2- per mg of tissue.
Preparation of Bone Marrow-Derived Macrophages (BMDMs)
Bone marrow cells from femora and tibiae of C57BL/6 mice (8 weeks old) were aspirated and flushed with a syringe filled with RPMI 1640 to extrude the bone marrow into a 15 ml sterile polypropylene tube. A 5 ml plastic pipette was used to homogenize the bone marrow. The fresh bone marrow cells obtained were cultured with RPMI 1640 media supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 15% of L929 cell conditioned medium, as source of granulocyte/macrophage colony stimulating factor and seeded in non-tissue culture treated Optilux Petri dishes (BD Biosciences) and incubated at 37°C in a 5% CO2 atmosphere for 7 days (Marim et al., 2010).
To obtain the BMDM, the supernatants were discarded and the attached cells were washed with 10 ml of sterile PBS 1×. Ten milliliters of ice-cold PBS were added to each plate and incubated at 4°C for 10 min to detach the cells. The macrophages were then aspirated, counted, seed and cultivated in tissue culture plates for 12 h before further RT-qPCR and ELISA assay.
Reverse Transcription and Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extract from 3 × 106 bone marrow macrophages cells (BMDMs) 5 h after MSU activating stimulus and from knee joints at 15 h after MSU injection by using TRIzol® reagent. The total RNA was isolated according to manufacturer’s directions. The purity of total RNA was measured with a spectrophotometer and the wavelength absorption ratio (260/280 nm) was between 1.8 and 2.0 for all preparations. Reverse transcription of total RNA to cDNA and qPCR were carried out using GoTaq® 2-Step RT-qPCR System (Promega) and specific primers (Applied Biosystems®). The mRNA level of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as reference gene. The primers used were Gapdh forward: CAT ACC AGG AAA TGA GCT TG, reverse: ATG ACA TCA AGA AGG TGG TG; gp91phox, forward: AGC TAT GAG GTG GTG ATG TTA GTG G, reverse: CAC AAT ATT TGT ACC AGA CAG ACT TGA G; Nrf2, forward: TCA CAC GAG ATG AGC TTA GGG CAA, reverse: TAC AGT TCT GGG CGG CGA CTT TAT; Ho-1, forward: CCC AAA ACT GGC CTG TAA AA, reverse: CGT GGT CAG TCA ACA TGG AT; Nlrp3, forward: AGC TAT GAG GTG GTG ATG TTA GTG G, reverse: CAC AAT ATT TGT ACC AGA CAG ACT TGA G; Asc, forward: ATG GGG CGG GCA CGA GAT G, reverse: GCT CTG CTC CAG GTC CAT CAC; Pro-caspase-1: forward: TGG TCT TGT GAC TTG GAG GA, reverse: TGG CTT CTT ATT GGC ACG AT; Pro-Il-1β, forward: GAA ATG CCA CCT TTT GAC AGT G, reverse: TGG ATG CTC TCA TCA GGA CAG. Raw data were normalized to Gapdh expression and were analyzed by the 2-ΔΔCt method by calculating the relative expression to the saline control.
Inflammasome Activation/IL-1β Release Assay
In some cultures, BMDMs were seeded at the density of 1 × x 106 cells in 96-well plate. After 24 h, the cells were stimulated with 500 ng/mL of lipopolysaccharide (LPS) from Escherichia coli (Santa Cruz Biotechnology) and 3 h later treated with 450 μg/ml of MSU to stimulate NLRP3 inflammasome activation as described previously (Martinon et al., 2006). To evaluate effect of trans-Chalcone on NF-κB activation in vitro, BMDMs were pre-treated with TC at 0.1–3 μM 30 min before LPS stimulation (priming). Supernatants were collected 5 h after MSU stimulation and IL-1β concentration was quantitated by ELISA. In another experimental set, BMDMs were treated with TC at 0.1–3 μM 30 min after LPS priming and before MSU stimulation, in order to address the direct effect on inflammasome activation. Supernatants were also collected 5 h after MSU stimulation to assess IL-1β concentration. The data are representative of the means of three independent experiments.
Lactate dehydrogenase (LDH) release in the supernatant was used to determine cytotoxicity using LDH Cytotoxicity Assay Kit (Cayman Chemical, MI, United States) according to the manufacturer’s directions. Trypan blue exclusion test of cell viability was also performed.
Cytokine Measurement
Mice were anesthetized and killed, and knee joint was collected and frozen with liquid nitrogen. The samples were then homogenized in 500 μl of buffer containing protease inhibitors, centrifuged (3600 rpm × 4°C × 15 min) and the supernatants were used to determine the levels of TNF-α (#88-7324-76), IL-1β (#88-8014-22), IL-6 (#88-7064-76), TGF-β (#88-8350-76), and IL-10 (#88-7105-76) by an enzyme-linked immunosorbent assay (ELISA) using eBioscience/Thermo Fisher Scientific kits. The levels of IL-1β were also assessed in BMDM culture. The results are expressed as picograms (pg) of cytokine/100 mg of tissue or pg/ml.
NF-κB Activation
Mice were anesthetized and killed, and knee joint was collected and frozen with liquid nitrogen. The samples were then homogenized with a tissue-tearor in 500 μl of ice-cold lysis buffer (Cell Signaling). The homogenates were centrifuged (14000 rpm × 10 min × 4°C), with the supernatants used to assess the levels of total (#7836) and phosphorylated (#7834) NF-κB p65 subunit by ELISA using PathScan kits® (Cell Signaling, Danvers, MA, United States) according to the manufacturer’s directions. Data were expressed as the total NF-κB p65/phospho-NF-κB ratio p65 measured at 450 nm (Multiskan GO, Thermo Fisher Scientific).
Enzymatic Markers of Liver Injury
Blood samples were collected by cardiac puncture 15.5 h after treatment with trans-Chalcone at 30 mg/kg or 10 h after stimulus with acetaminophen at 650 mg/kg and added into microtubes containing anticoagulant (EDTA, 5,000 IU/mL, Sigma Chemical Co., St. Louis, MO, United States). Acetaminophen was used as positive control of liver injury as described previously (Fattori et al., 2017a). The plasma was separated by centrifugation (200 ×g, 10 min, 4°C). Plasma samples were processed according to the manufacturer’s instructions (Labtest Diagnóstica S.A., Lagoa Santa, Brazil) to evaluate ALT and AST levels as indicators of hepatotoxicity. Results are presented as U/L of plasma ALT or AST.
Renal Function Tests
Blood samples were collected by cardiac puncture 15.5 h after treatment with trans-Chalcone at 30 mg/kg or 24 h after stimulus with diclofenac at 200 mg/kg and added into microtubes containing anticoagulant (EDTA, 5,000 IU/mL, Sigma Chemical Co., St. Louis, MO, United States). Diclofenac was used as positive control of kidney injury as described previously (Fattori et al., 2017a). The plasma was separated by centrifugation (200 ×g, 10 min, 4°C). Plasma samples were processed according to the manufacturer’s instructions (Labtest Diagnóstica S.A., Lagoa Santa, Brazil) to evaluate urea and creatinine levels as indicators of nephrotoxicity. Results are presented as mg/dL of plasma urea or creatinine.
Data Analysis
Data were analyzed using GraphPad Prism statistical software (GraphPad Software, Inc., United States-500.288, version 5.0). Results are presented as means ± SEM of measurements made on 12 mice per group that were obtained in two separate experiments with 6 mice per group. For in vitro experiments, 6 wells were performed per group per experiment, and every experiment was repeated three times, and results are the mean of the three experiments. Two-way ANOVA was used to compare the groups and doses at all times when the parameters were measured at different times after the stimulus injection. The analyzed factors were treatments, time, and time versus treatment interaction. One-way ANOVA followed by Tukey’s test was performed for each time-point. P < 0.05 was considered significant.
Results
Trans-Chalcone Inhibits MSU-Induced Mechanical Hyperalgesia and Edema in a Dose-Dependent Manner
Severe pain and swelling around the joint are the most clinical findings after acute gout in humans causing disability to the patients (Amaral et al., 2012; Ruiz-Miyazawa et al., 2017). Considering these features, in the first set of experiments we addressed whether trans-Chalcone would inhibit MSU-induced knee joint mechanical hyperalgesia and edema within 15 h after MSU injection. MSU injection induced mechanical hyperalgesia, which was not reduced by trans-Chalcone at the dose of 3 mg/kg in any evaluated time-points (Figure 1A). Trans-Chalcone at the dose of 10 mg/kg reduced MSU-induced mechanical hyperalgesia between 5–15 h. The dose of 30 mg/kg of trans-Chalcone inhibited MSU-induced mechanical hyperalgesia between 3–15 h, with significant statistical difference compared to the doses of trans-Chalcone of 10 mg/kg in the 15th hour post-MSU (Figure 1A). Only trans-Chalcone at 30 mg/kg inhibited MSU-induced knee joint edema in more than one-time point (5–15 h post-MSU) (Figure 1B). Furthermore, in a post-treatment protocol with trans-Chalcone (Supplementary Figure S1), the dose of 30 mg/kg reduced the MSU-induced knee joint mechanical hyperalgesia at 1, 3, and 15 h post-stimulus (Supplementary Figure S1A). The difference in edema were observed at 7 and 15 post-MSU stimulus (Supplementary Figure S1B).
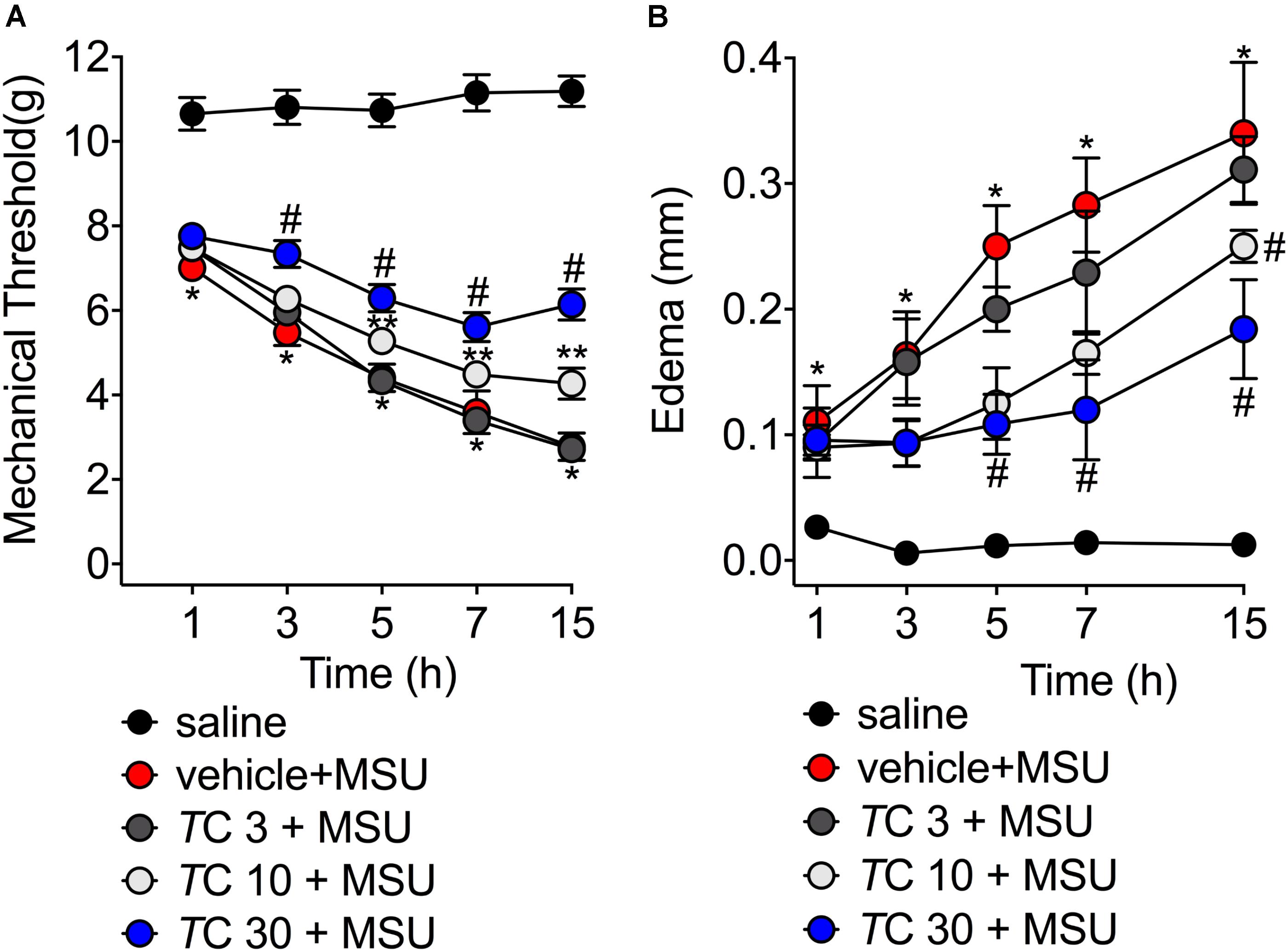
FIGURE 1. Trans-Chalcone inhibits MSU-induced mechanical hyperalgesia and edema in a dose-dependent manner. Mice were treated Trans-Chalcone (TC, 3, 10, or 30 mg/kg, p.o., 100 μl) or vehicle (Tween 80 20% plus saline) 30 min before MSU (100 μg/10 μl/knee) stimulus in the femur-tibial joint of swiss mice. (A) Mechanical hyperalgesia and (B) edema were evaluated 1, 3, 5, 7, and 15 h after MSU injection. Results are expressed as mean ± SEM, data represent a total of 12 mice per group that were obtained in two independent experiment with 6 mice per experiment. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle group, and TC 3 group, two-way ANOVA followed by Tukey’s post-test). ∗∗p < 0.05 vs. vehicle group.
Trans-Chalcone Inhibits MSU-Induced Leukocyte Recruitment to the Knee Joint and Synovitis
Leukocyte recruitment the knee joint, especially of neutrophils, is a hallmark of gout arthritis pathology (Amaral et al., 2012; Fattori et al., 2016; Ruiz-Miyazawa et al., 2017). Thus, the effect of trans-Chalcone on MSU-induced leukocyte recruitment was evaluated. Only trans-Chalcone at the dose of 30 mg/kg reduced MSU-induced total leukocyte (Figure 2A), neutrophil (Figure 2A), and mononuclear cell recruitment (Figure 2A). However, the dose of 10 mg/kg of trans-Chalcone inhibited MSU-induced mononuclear cell recruitment (Figure 2A). Considering the results of Figures 1, 2A, the dose of 30 mg/kg of trans-Chalcone was selected for the next experiments. In agreement with Figures 1, 2A, the histopathological finding shows that trans-Chalcone reduced inflammatory cell recruitment to the knee joint (Figures 2B,C), suggesting a reduction in the synovitis, an inflammation of synovial membrane. Furthermore, by using LysM-eGFP mice as another approach to investigate MSU-induced neutrophil recruitment, we show that trans-Chalcone reduced the infiltration of LysM-eGFP+ cells that includes neutrophils and monocytes/macrophages (Figures 2D,E), as observed by reduced intensity of fluorescence (Figures 2D,E). Importantly, trans-Chalcone at 30 mg/kg did not induce liver injury (Supplementary Figures S2A,B) or kidney injury (Supplementary Figures S2C,D). These side effects are commonly associated with the use of analgesics such as acetaminophen (APAP, paracetamol) (Hohmann et al., 2015) or anti-inflammatory drugs such as diclofenac (Fattori et al., 2017a).
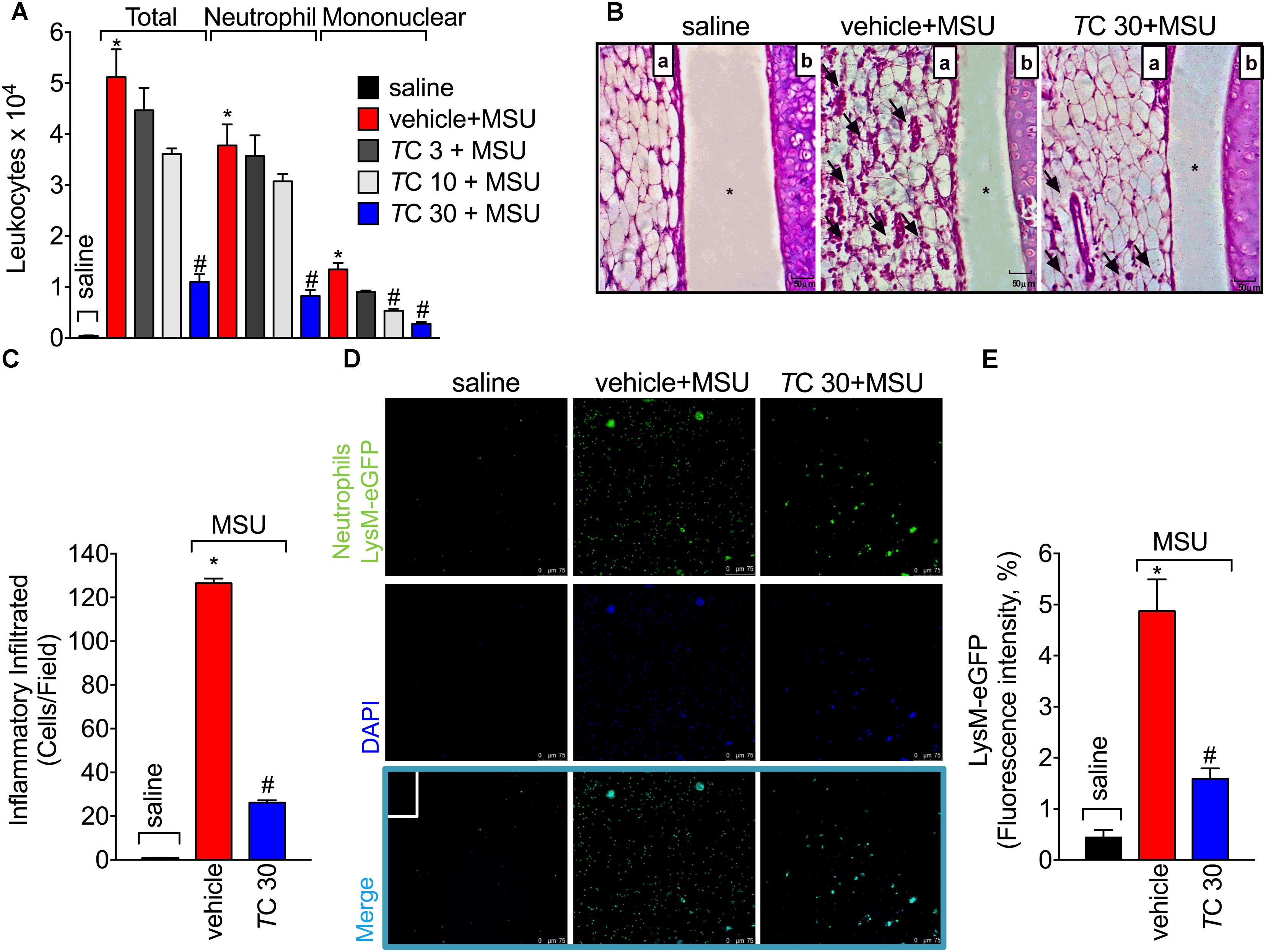
FIGURE 2. Trans-Chalcone inhibits MSU-induced leukocyte recruitment in a dose-dependent manner. Mice were treated Trans-Chalcone (TC, 3, 10, or 30 mg/kg, p.o., 100 μl) or vehicle (Tween 80 20% plus saline) 30 min before MSU (100 μg/10 μl/knee) stimulus in the femur-tibial joint of swiss mice (A) Fifteen hours after MSU, knee joint wash was collected for counting total leukocytes, neutrophils, and mononuclear cells. (B,C) Fifteen hours after MSU, knee joint was collected for histopathological analysis by HE staining. Original magnification image of 400× of groups: saline, vehicle+MSU and TC30+MSU. For panel (B) arrows indicate leukocyte infiltration, (a) synovial tissue, (b) cartilage tissue, and ∗ joint space. Panel (C) shows the total score of inflammatory infiltrate (synovitis). (D,E) Fifteen hours after MSU, knee joint washes of MSU-stimulated LysM-GFP+ mice were collected for the determination of LysM-GFP+ neutrophil recruitment by confocal microscopy. Original magnification 200×. Panel (E) shows the percentagem of LysM-eGFP+ fluorescence. Results are expressed as mean ± SEM, data represent a total of 12 mice per group that were obtained in two independent experiment with 6 mice per experiment. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle, one-way ANOVA followed by Tukey’s post-test).
Trans-Chalcone Inhibits MSU-Induced Oxidative Stress
Given the role of reactive oxygen and nitrogen species in pain processing and inflammation (Gloire and Piette, 2009; Martinon, 2010; Fattori et al., 2016; Grace et al., 2016) and that flavonoids are recognized by their antioxidant activity (Verri et al., 2012), the effect of trans-Chalcone in MSU-induced oxidative stress was investigated. Trans-Chalcone inhibited MSU-induced production of superoxide anion (NBT assay; Figure 3A) and nitric oxide (NO2- assay; Figure 3B) in knee joint samples. In line with these results, trans-Chalcone also restored the endogenous antioxidant capacity of samples by preventing depletion in the GSH levels (Figure 3C) and increasing the FRAP (FRAP assay, Figure 3D) and ABTS free radical scavenging ability (ABTS assay, Figure 3E) that were reduced by MSU injection. ABTS in an effective assay to screen the presence of antioxidant compounds and FRAP assay identifies the reducing ability exerted by antioxidants (Menghini et al., 2018). Thus, both assays were essential tools to demonstrate the in vivo antioxidant effect of trans-chalcone in gouty arthritis. Corroborating the results of oxidative stress status, trans-Chalcone reduced MSU-induced gp91phox mRNA expression (Figure 4A), a NADPH oxidase subunit. Trans-Chalcone also increased the mRNA expression of the antioxidant transcription factor Nrf2 (Figure 4B) and its downstream target HO-1 (Figure 4C). Importantly, the treatment with trans-chalcone in saline group (without MSU stimulus) did not affect the mRNA expression of both genes, pointing that trans-chalcone does not have an effect per se over Nrf2 and HO-1 mRNA expression (Figures 4B,C). Furthermore, in an in vitro system approach using BMDM culture, we also observed that the pre-treatment (30 min) with trans-chalcone in a concentration of 3 μM enhanced Nrf2 as well as Ho-1 mRNA expression under LPS plus MSU stimuli. The treatment of naive BMDM with trans-chalcone did not alter Nrf2 (Figure 4D) and Ho-1 (Figure 4E) mRNA expression, which corroborates the in vivo data. Therefore, trans-Chalcone inhibited MSU-induced pro-oxidant enzymes and increases antioxidant sensitive pathways in the knee joints, confirming an important effect of this flavonoids in the redox biology of gouty arthritis.
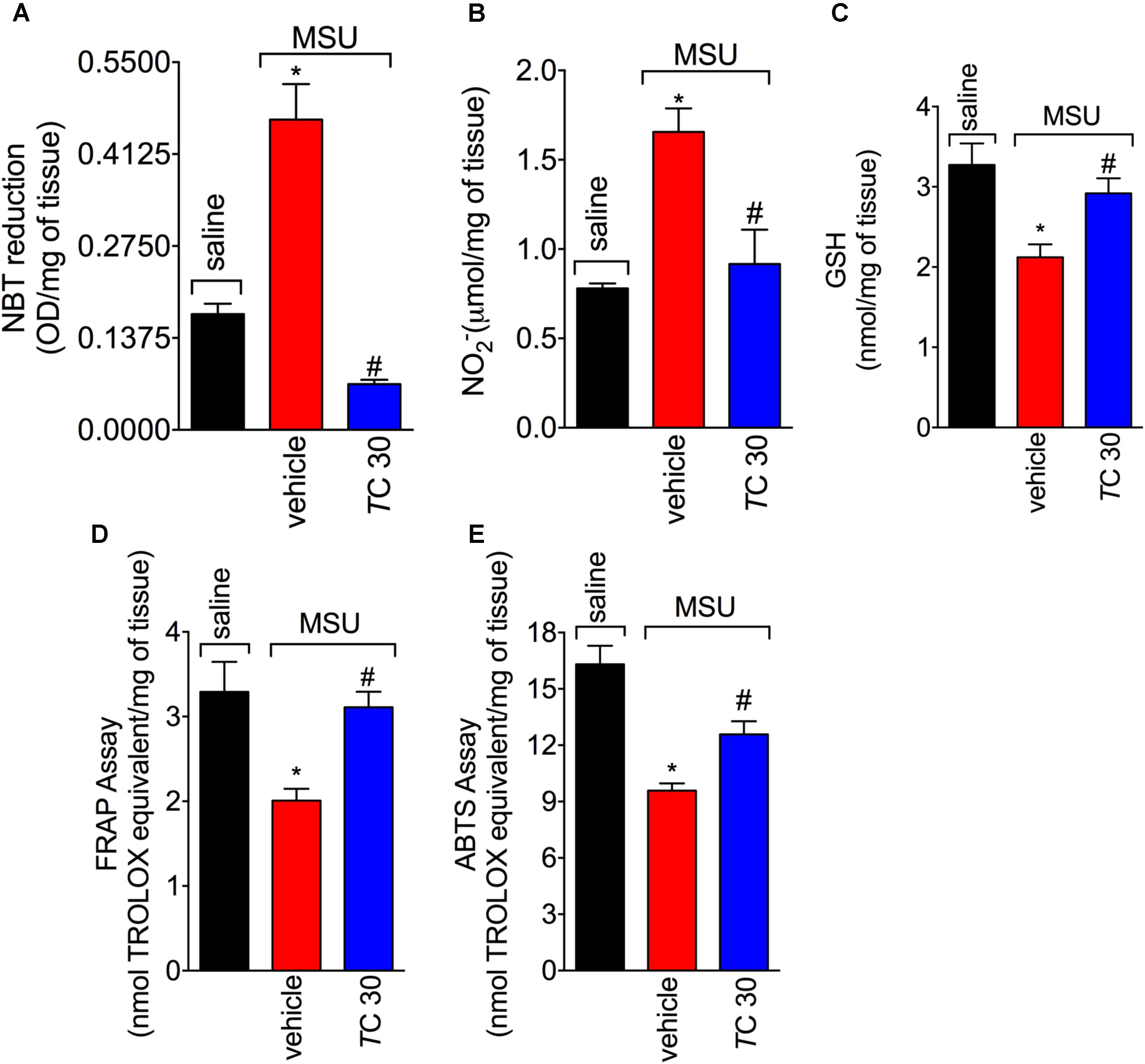
FIGURE 3. Trans-Chalcone inhibits MSU-induced oxidative stress. Mice were treated Trans-Chalcone (TC, 30 mg/kg, p.o., 100 μl) or vehicle (Tween 80 20% plus saline) 30 min before MSU (100 μg/10 μl/knee) stimulus in the femur-tibial joint of swiss mice. Fifteen hours after MSU, knee joint samples were collected and the oxidative stress was assessed by measuring: (A) superoxide anion (NBT assay), (B) NO2- production Griess assay), (C) GSH levels, (D) Ferring reducing power (FRAP assay), and (E) ABTS scavenging ability (ABTS assay). Results are expressed as mean ± SEM, data represent a total of 12 mice per group that were obtained in two independent experiment with 6 mice per experiment. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle, one-way ANOVA followed by Tukey’s post-test).
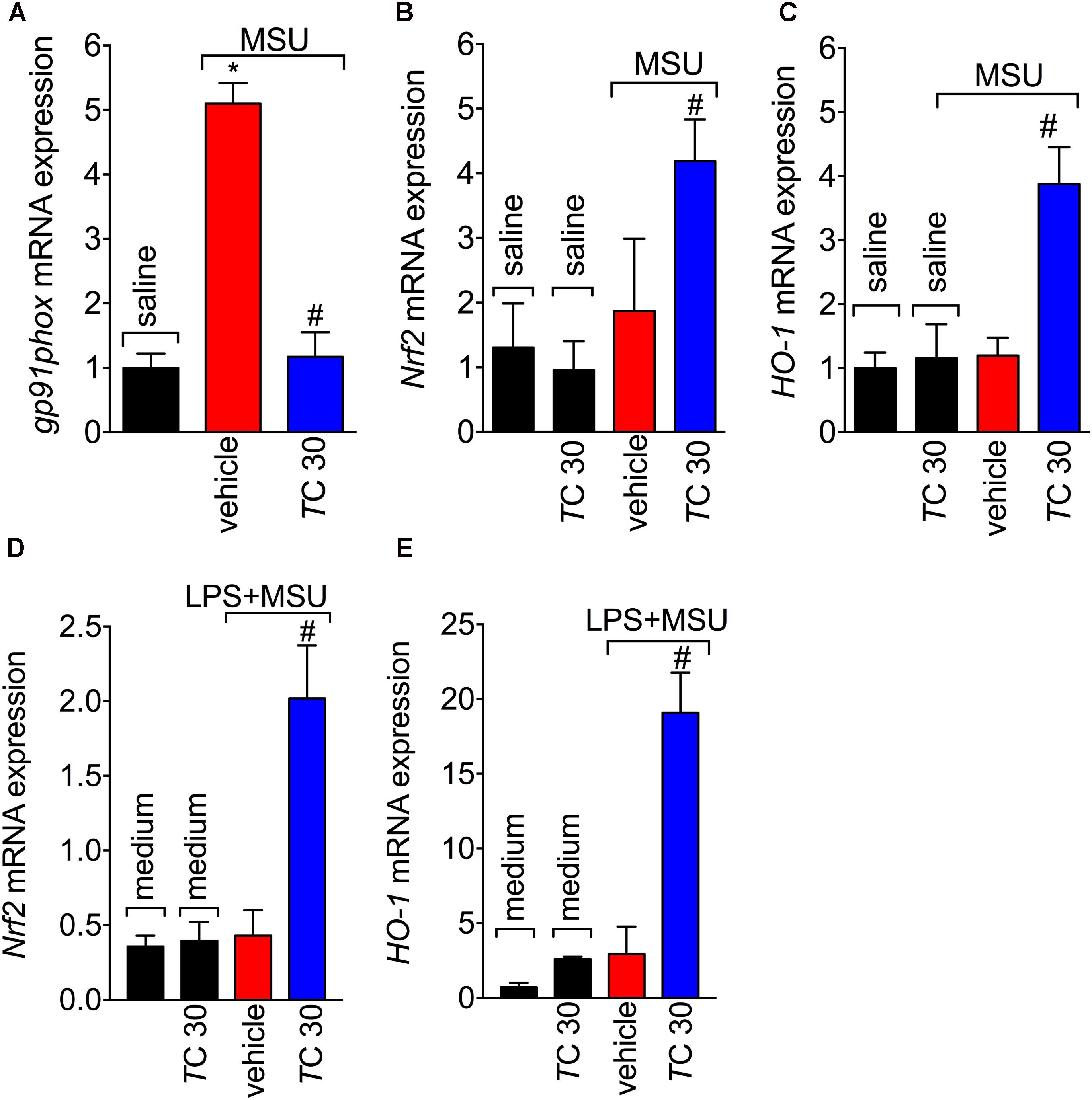
FIGURE 4. Trans-Chalcone inhibits MSU-induced gp91phox mRNA expression and increases Nrf2 and Ho-1 mRNA expression. Mice were treated Trans-Chalcone (TC, 30 mg/kg, p.o., 100 μl) or vehicle (Tween 80 20% plus saline) 30 min before MSU (100 μg/10 μl/knee) or sterile saline (10 μl/knee) stimulus in the femur-tibial joint of swiss mice. Fifteen hours after MSU, knee joint samples were collected for the determination of the mRNA expression of: (A) gp91phox, (B) Nrf2, and (C) HO-1 by RT-qPCR. 3 × 106 BMDMs were seeded in 6 well plate and pre-treated with 3 μM of trans-chalcone 30 min before 500 ng/mL of LPS for priming over 3 h, followed by MSU (450 μg/mL) activation. After 5 h, the cells were collected for determination of mRNA expression of: (D) Nrf2 and (E) HO-1 by RT-qPCR. Results are expressed as mean ± SEM, data represent a total of 12 mice per group that were obtained in two independent experiment with 6 mice per experiment or the mean of 6 wells per group per experiment with three independent experiments for in vitro assays. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle, one-way ANOVA followed by Tukey’s post-test).
Trans-Chalcone Modulates in vivo and in vitro MSU-Induced Cytokine Production
Next, the effect of trans-Chalcone on MSU-induced pro-inflammatory cytokine production was investigated considering the importance of cytokines to gout arthritis pathology (Dalbeth et al., 2016; Fattori et al., 2016). Treatment with trans-Chalcone reduced in vivo MSU-induced IL-1β (Figure 5A), TNFα (Figure 5B), and IL-6 (Figure 5C) production. Moreover, trans-Chalcone also increased the levels of the anti-inflammatory cytokine TGF-β (Figure 5D) without changing the levels of IL-10 in knee joints (Figure 5E). Regarding TGF-β levels, trans-Chalcone did not alter the levels of this anti-inflammatory cytokine per se (Figure 5D). As IL-1β production in its mature form depends on priming and activation (Martinon et al., 2006) and some flavonoids can potentially inhibit NF-κB activation (Vicentini et al., 2001) and IL-1β maturation (Domiciano et al., 2017), we investigated whether or not trans-Chalcone reduced IL-1β production by targeting LPS-induced NF-κB activation (LPS/priming) and/or interfering with inflammasome activation (MSU/activation). BMDM pre-treatment (before priming with LPS) with trans-Chalcone at the concentrations of 0.3, 1, and 3 μM reduced MSU-induced release of IL-1β in the supernatant of cell culture (Figure 6A). In a post-treatment protocol, after priming the BMDM with LPS and before secondary stimulation with MSU, the treatment with trans-Chalcone at 3 μM also reduced MSU-induced IL-1β release by LPS-primed BMDM (Figure 6B). Thus, these data indicate that trans-Chalcone can either inhibit NF-κB activation (before priming with LPS; Figure 6A) and directly interfere with inflammasome activation in a concentration-dependent manner (activation, treatment after LPS, but before MSU; Figure 6B). However, the effect of trans-Chalcone was more prominent in inhibiting the priming with LPS (lower concentrations of trans-Chalcone) than the activation by MSU (only the highest concentration of trans-Chalcone), which indicates trans-Chalcone presents a more prominent effect in inhibiting NF-κB activation than inflammasome activation as a main mechanism. Importantly, none of the concentrations used herein (up to 3 μM) of trans-Chalcone reduced cell viability as per LDH analysis (Supplementary Figure S3A) and Trypan Blue (Supplementary Figure S3B).
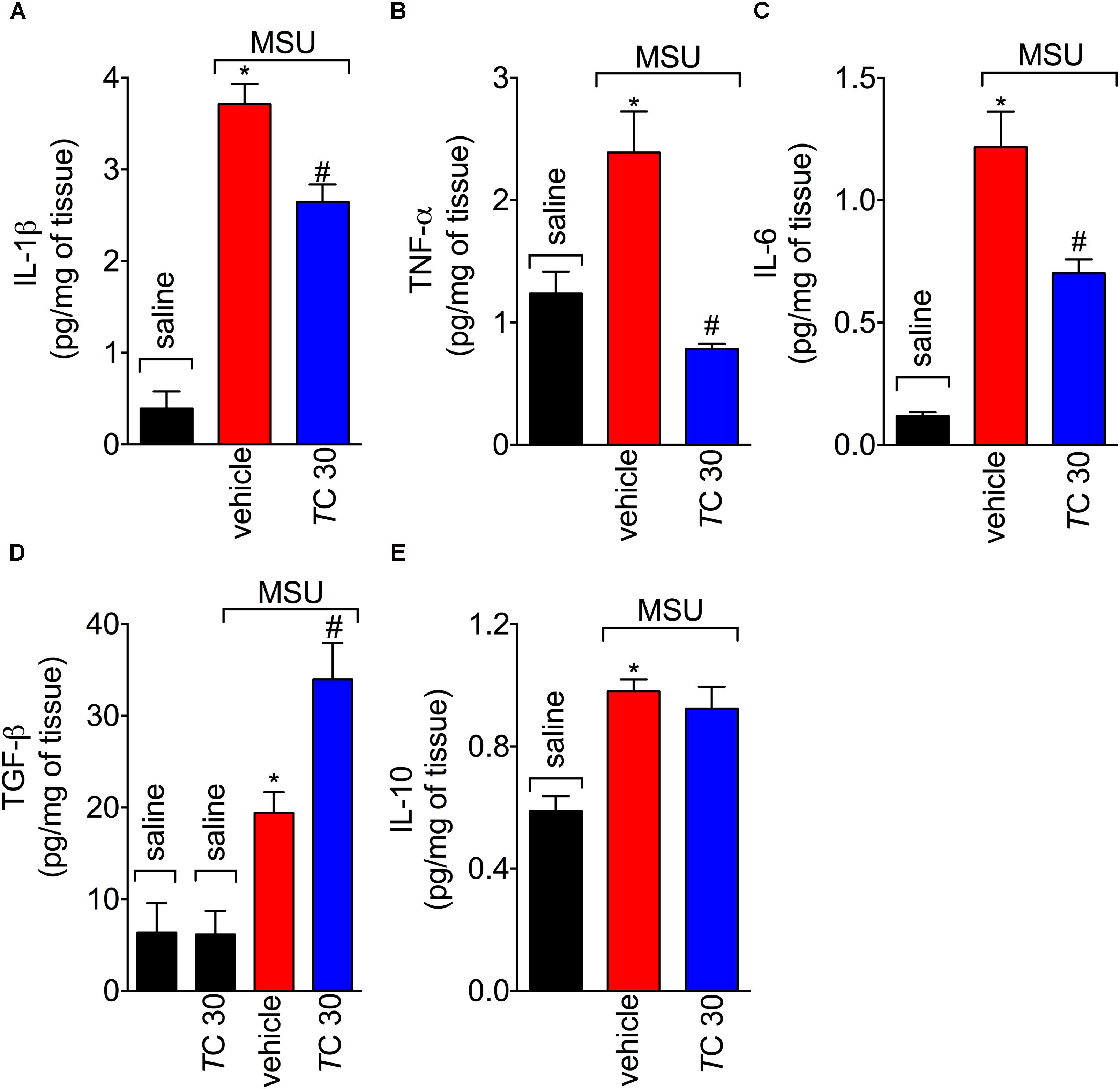
FIGURE 5. Trans-Chalcone modulates MSU-induced cytokine production in vivo. Mice were treated trans-Chalcone (TC, 30 mg/kg, p.o., 100 μl) or vehicle (Tween 80 20% plus saline) 30 min before MSU (100 μg/10 μl/knee) stimulus in the femur-tibial joint of swiss mice. Fifteen hours after MSU, knee joint samples were collected for the determination of (A) IL-1β, (B) TNF-α, (C) IL-6, (D) TGF-β, and (E) IL-10 production by ELISA. Results are expressed as mean ± SEM, data represent a total of 12 mice per group that were obtained in two independent experiment with 6 mice per experiment. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle, one-way ANOVA followed by Tukey’s post-test).
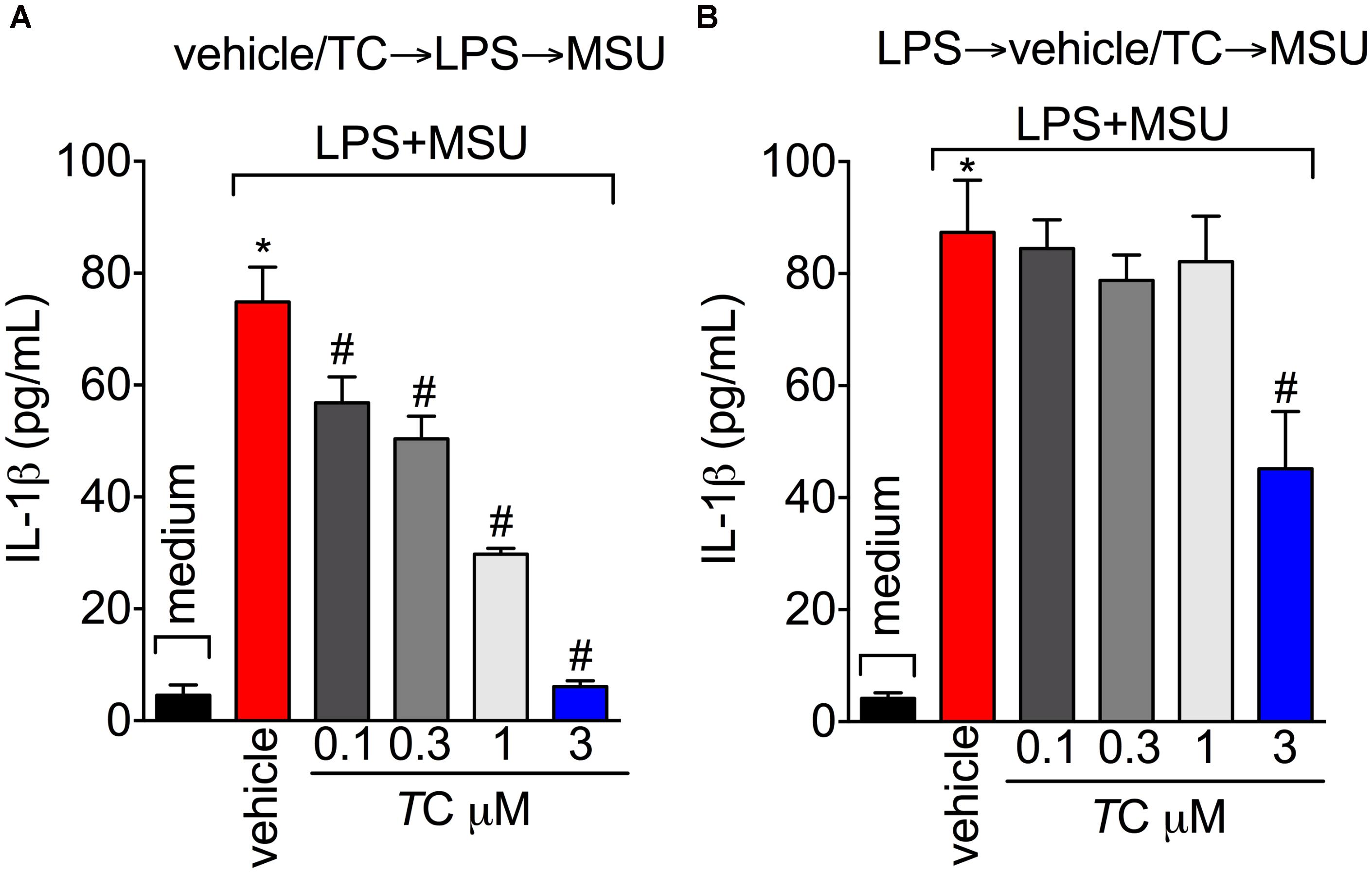
FIGURE 6. Trans-Chalcone reduces MSU-induced IL-1β maturation in vitro. (A) To assess the effect of trans-Chalcone on NF-κB activation, BMDMs were pre-treated with 0.1–3 μM before 500 ng/mL of LPS [i.e., before priming, panel (A) and after 3 h were secondarily stimulated with MSU (450 μg/ml, i.e., activation]. (B) To assess the direct effect of trans-Chalcone on inflammasome activation, LPS-primed BMDMs were treated with 0.1–3 μM 30 min before MSU stimulation (i.e., after priming and before activation). Results are expressed as mean ± SEM, data represent the mean of 6 wells per group per experiment with three independent experiments. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle, one-way ANOVA followed by Tukey’s post-test).
Trans-Chalcone Inhibits MSU-Induced Nlrp3, Asc, Pro-caspase-1, and Pro-Il-1β mRNA Expression, and NF-κB Activation
The assembly of NLRP3 inflammasome is a crucial mechanism of gout arthritis that is dependent on NF-κB activation, which is a transcription factor essential to inducing the expression of inflammasome components and pro-IL-1β (Martinon et al., 2006; Amaral et al., 2012; Dalbeth et al., 2016). Given trans-Chalcone reduced in vivo and in vitro IL-1β production, the effect of trans-Chalcone on the mRNA expression of inflammasome components Nlrp3, Asc, and Pro-caspase-1, and Pro-Il-1β was investigated. Treatment with trans-Chalcone reduced the mRNA expression of all inflammasome components Nlrp3 (Figure 7A), Asc (Figure 7B), Pro-caspase-1 (Figure 7C), and Pro-Il-1β (Figure 7D). Further, MSU induced the activation of NF-κB as observed by a decrease of total-p65/phosphorylated-p65 OD ratio (Figure 7E). The decrease in the ratio demonstrates the increase in the p65 subunit phosphorylation, therefore, indicating activation of NF-κB. In turn, trans-Chalcone treatment inhibited MSU-induced NF-κB activation (Figure 7E). Thus, these data confirmed that the effect of trans-Chalcone in gout arthritis is dependent on inhibiting NF-κB activation, which will then result in reducing inflammasome platform expression.
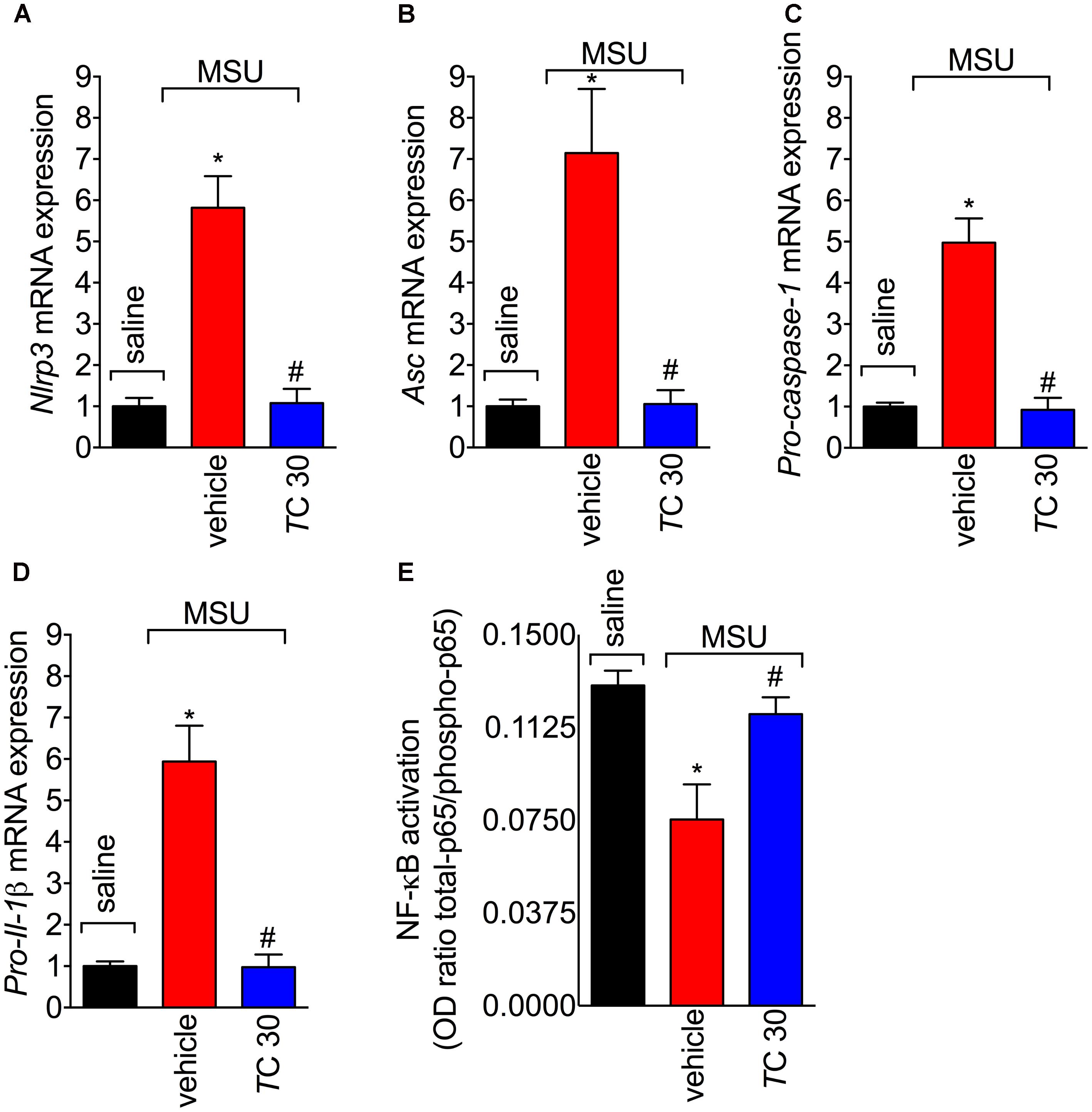
FIGURE 7. Trans-Chalcone decreases MSU-induced Nlrp3, Asc, Pro-caspase-1, and Pro-Il-1β mRNA expression, and NF-κB activation. Mice were treated with trans-Chalcone (TC, 30 mg/kg, p.o., 100 μl) or vehicle (Tween 80 20% plus saline) 30 min before MSU (100 μg/10 μl/knee) stimulus in the femur-tibial joint of swiss mice. Fifteen hours after MSU, knee joint was collected for determination of mRNA expression of (A) Nlrp3, (B), Asc, (C) Pro-caspase-1, (D) Pro-Il- by RT-qPCR, and (E) NF-κB activation by ELISA. Results are expressed as mean ± SEM, data represent a total of 12 mice per group that were obtained in two independent experiment with 6 mice per experiment. (∗p < 0.05 vs. control group; #p < 0.05 vs. vehicle, one-way ANOVA followed by Tukey’s post-test).
Discussion
In this study, we show that the precursor of flavonoids in plants, trans-Chalcone, ameliorates experimental gout arthritis in mice. This effect is related to the reduction of MSU-induced oxidative stress, NF-κB activation and thereby inhibition of inflammasome expression and production of IL-1β in its mature form. Trans-Chalcone also increased the production of the anti-inflammatory cytokine TGF-β. It is also important to highlight that, to our knowledge, this is the first demonstration that trans-Chalcone has analgesic properties and also inhibits MSU-induced joint inflammation.
The management of gout acute flares lies on the use of corticosteroids, AINEs, colchicine, and biological agents (Rees et al., 2014). These single target therapies possess limit analgesic efficacy and when effective often produce several undesirable side effects in gout arthritis, a disease with multiple physiopathological mechanisms (Rees et al., 2014). Flavonoids are widely known for their high spectrum of biological action and low toxicity, which are explained by the fact that flavonoids are multi-target drugs, inhibiting but not abolishing endogenous pathways (Verri et al., 2012). Herein, we show that trans-Chalcone did not induce toxicity in vivo and in vitro, indicating a safe preclinical profile. Although the literature did not define the trans-Chalcone pharmacokinetic profile after oral administration, we could observe that it inhibited gout-induced pain and inflammation as a pre-treatment and post-treatment, which reinforces its therapeutic potential. The antioxidant activity is a hallmark of the activity of flavonoids (Verri et al., 2012) and in fact, the reduction of oxidative and nitrosative stress are important mechanisms by which flavonoids (Anjaneyulu and Chopra, 2003; Al-Rejaie et al., 2015; Ahmed et al., 2016; Pinho-Ribeiro et al., 2016) and other molecules (Fattori et al., 2015, 2017a; Singh and Vinayak, 2015) act. Moreover, injection of a superoxide anion donor, peroxynitrite, or intrathecal delivery of ROS elicits pain behavior in mice (Wang et al., 2004; Fattori et al., 2015, 2017b) indicating that oxidative stress plays important role in pain processing (Grace et al., 2016). Neutrophils produce ROS upon recognition of MSU crystals (Desaulniers et al., 2001) and, in turn, ROS contribute to neutrophil recruitment (Hattori et al., 2010; Sakai et al., 2012). Moreover, nitric oxide quickly react with ROS to produce peroxynitrite and there is a correlation between increased NO generation and gouty arthritis progression (Chen et al., 2004).
The antioxidant capacity of phenolic compounds is related to the ability to generate a stable molecule after donating electrons. An important structural determinant of the antioxidant capacity of flavonoids is attributed to hydroxyl C4 and C3 (Verri et al., 2012). The structure-activity relationship of trans-Chalcone indicates that it does not possess antioxidant properties (Martinez et al., 2017a). In fact, using a cell free in vitro system, trans-Chalcone did not show antioxidant activity in the ABTS (electron transfer), DPPH (hydrogen transfer), iron chelation and superoxide anion (free radical) assays (Martinez et al., 2017a). Thus, electronic transfer of electrons, chelating transition metals or direct scavenging activity are not mechanisms by which trans-Chalcone reduces oxidative stress. However, in vivo data show that trans-Chalcone displays antioxidant effect observed by an increase of antioxidant defense and reduction of ROS (Karkhaneh et al., 2016; Singh et al., 2016; Martinez et al., 2017a,b). We also observed that trans-Chalcone maintained the tissue antioxidant activities such as FRAP reducing and ABTS radical scavenging ability in knee joint samples, and promoted glutathione system improvement, besides inhibiting the production of ROS and reducing nitrite levels. Therefore, the antioxidant effect of trans-Chalcone is not related to a direct chemical antioxidant structure, but rather is dependent on its anti-inflammatory effects and the activation of Nrf2 signaling pathway (Wu et al., 2014; Martinez et al., 2017b). In fact, trans-Chalcone also inhibited gp91phox mRNA expression and induced the mRNA expression of the antioxidant transcription factor Nrf2 and its downstream target HO-1. Our results corroborate other reports showing that trans-Chalcone acts on the antioxidant transcription factor Nrf2 (Wu et al., 2014; Martinez et al., 2017b) and reducing Kelch-like ECH-associated protein 1 (Keap1) activity (Kobayashi et al., 2006). However, it is unlikely that in the present experimental condition trans-Chalcone is acting by increasing Nrf2 and HO-1 per se, but rather, this increase seems to occur only during inflammation.
In addition to antioxidant effects, Nrf2/HO-1 signaling is an important analgesic pathway (Steiner et al., 2001; Carcole et al., 2014). In fact, co-treatment with an HO-1 inducer increases the analgesic effects of opioids and cannabinoids through the activation of cGMP/PKG/ATP-sensitive potassium channel pathway in the CFA-induced inflammatory pain model (Carcole et al., 2014). Thus, the increase of Nrf2/Ho-1 mRNA expression contributed to the reduction of MSU-induced oxidative stress and pain in this study.
The main innate immune event in MSU crystals-induced inflammation is the NLRP3-dependent maturation of IL-1β (Martinon et al., 2006; Amaral et al., 2012). This mechanism accounts for neutrophil recruitment and pain (Amaral et al., 2012). In response to MSU crystals, other macrophages-derived mediators such as TNF-α also drive neutrophil migration toward the tissue (Amaral et al., 2016). Moreover, targeting TNF-α, IL-1β, or IL-33 in rheumatic diseases reduce neutrophil recruitment and pain (Verri et al., 2008, 2010; Amaral et al., 2012, 2016). Trans-Chalcone reduced in vivo MSU-induced TNF-α, IL-1β, and IL-6; and in vitro maturation of IL-1β. Therefore, the reduction of these cytokines certainly contributed to the inhibition of neutrophils recruitment. This is an important finding because neutrophil recruitment is a hallmark of the acute phases of all rheumatic diseases (Fattori et al., 2016) and during gout flares, they are the predominant cell population in the synovial fluid (Mitroulis et al., 2013). After recognition of MSU crystals by neutrophils, they degranulate (Popa-Nita et al., 2007) and undergo NETosis (Mitroulis et al., 2011). Moreover, activated neutrophils produce IL-1β, TNF-α, and PGE2 that contribute to pain (Cunha et al., 2008; Verri et al., 2010; Amaral et al., 2012, 2016). IL-1β and TNF-α also activate nociceptor neuron and thereby producing pain (Jin and Gereau, 2006; Binshtok et al., 2008). Regarding IL-1β, two steps are required for the production and release of its mature form (Martinon et al., 2006). The first step is related to downstream signaling pathways that depend on NF-κB activation or a priming step, whereas the second step is related to inflammasome assembly per se that depends on the phagocytosis of MSU crystals and lysis of phagolysosome releasing cathepsin that activates NLRP3 (Martinon et al., 2006; Amaral et al., 2012; Latz et al., 2013). Thus, naturally occuring molecules (such as flavonoids) that target priming and/or activation signal to inflammasome assembly without the side effect of the current therapies are likely to be highly attractive as an analgesic approach in gout arthritis. In fact, the flavonoid quercetin that inhibits NLRP3 inflammasome and ASC speck formation in mouse vasculitis (Domiciano et al., 2017) and reduces pain in experimental gout arthritis (Ruiz-Miyazawa et al., 2017). In the present study, pre-treated BMDMs (treatment before LPS stimulus, i.e., priming) with trans-Chalcone reduced MSU-induced IL-1β production suggesting that this flavonoid reduced NF-κB activation (priming). In fact, this effect was further confirmed by in vivo data as demonstrated by an increase of NF-κB OD ratio by ELISA. In agreement with the present findings that trans-chacone inhibition of NF-κB activation in gout arthritis, evidence demonstrated that trans-Chalcone reduces NF-κB activation in murine retina (Lamoke et al., 2011). Thus, the inhibition of NF-κB activation explains the reduced levels of the pro-inflammatory cytokines observed. Moreover, trans-Chalcone reduced MSU-induced IL-1β production in the supernatant of LPS-primed BMDMs (that already received priming) indicating that it may also interfere with inflammasome assembly. In fact, other naturally occuring molecules, such as the flavonoids quercetin (Domiciano et al., 2017), apigenin (Zhang et al., 2014), and oroxylin A (Zhou et al., 2017); and the sesquiterpene lactone parthenolide (Juliana et al., 2010) have the ability to interfere with inflammasome assembly. This mecanim is related to the inhibition of ASC speck formation (Zhang et al., 2014; Domiciano et al., 2017; Zhou et al., 2017) or direct targeting of active site of caspase-1 (Juliana et al., 2010). In addition to inhibiting pro-inflammatory cytokines production, trans-Chalcone also increased the level of the anti-inflammatory cytokine TGF-β. This anti-inflammatory cytokine plays an important role in the resolution phase of MSU-induced inflammation (Scanu et al., 2012) by promoting efferocytosis of apoptotic neutrophils (Vieira et al., 2017). Of note, the fact that trans-Chalcone sustained the production of IL-10 and inhibited pro-inflammatory cytokine production is a relevant finding given the anti-hyperalgesic and anti-inflammatory properties of IL-10 and that, in general, anti-inflammatory cytokines are co-released during inflammation to limit the inflammatory response (Verri et al., 2006). Therefore, the reduction of neutrophil recruitment and pro-inflammatory cytokine production, the increase of anti-inflammatory cytokine TGF-β, and the maintenance of anti-hyperalgesic cytokine IL-10 contribute to the analgesic effect of trans-Chalcone.
Concluding, we demonstrated that trans-Chalcone ameliorates MSU-induced pain and inflammation. Trans-Chalcone reduced MSU-induced oxidative stress and thereby demonstrating that this molecule possesses antioxidant effect by modulating Nrf2/HO-1 signaling pathway. We also demonstrated that trans-Chalcone reduced MSU-induced inflammasome assembly and NF-κB activation. As a consequence, it was also observed a reduction of IL-1β, TNF-α, and IL-6 production. Interestingly, trans-Chalcone increased the level of the anti-inflammatory cytokine TGF-β and sustained the levels of the anti-hyperalgesic cytokine IL-10. To our knowledge, this is the first report on the analgesic effect of trans-Chalcone. Therefore, trans-Chalcone displays a safe preclinical profile with analgesic, antioxidants, and anti-inflammatory properties.
Author Contributions
LS-F, KR-M, RC, and WV conceived and designed the experiments. LS-F, KR-M, FP-R, VF, TZ, SB-G, SB, and TC performed the experiments. LS-F, KR-M, FP-R, VF, TZ, SB-G, SB, TC, RC, and WV contributed to collection of data and analysis. JA-F, TC, FC, RC, and WV contributed to reagents, materials, and analysis tools. LS-F, KR-M, VF, and WV wrote the original draft. LS-F, KR-M, VF, RC, and WV contributed to writing-review and editing. All authors contributed to manuscript revision and read and approved the final version of the manuscript.
Funding
This work was supported by Programa para o Sistema Único de Saúde (PPSUS) grant supported by Departamento de Ciência e Tecnologia da Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Ministério da Saúde (Decit/SCTIE/MS, Brazil) intermediated by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) with support of Fundação Araucária and Secretaria Estadual de Saúde, Paraná (SESA-PR, Brazil); São Paulo Research Foundation under grant agreements 2011/19670-0 (Thematic Project) and 2013/08216-2 (Center for Research in Inflammatory Disease); Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil); Financiadora de Estudos e Projetos and Secretaria de Estado da Ciência, Tecnologia e Ensino Superior do Paraná under grant agreements 01.12.0294.00 (0476/11) (FINEP/SETI-PR, Brazil); and Universidade Estadual de Londrina, PROPPG, Escritório de Apoio ao Pesquisador (Edital Internacionalize 2018). SB and LS-F received a CNPq Post-doc fellowship. We also thank the support of Central Multiusuário de Laboratórios de Pesquisa da Universidade Estadual de Londrina (CMLP-UEL).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GO and handling Editor declared their shared affiliation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.01123/full#supplementary-materia
References
Ahmed, S., Mundhe, N., Borgohain, M., Chowdhury, L., Kwatra, M., Bolshette, N., et al. (2016). Diosmin modulates the NF-kB signal transduction pathways and downregulation of various oxidative stress markers in alloxan-induced diabetic nephropathy. Inflammation 39, 1783–1797. doi: 10.1007/s10753-016-0413-4
Al-Rejaie, S. S., Aleisa, A. M., Abuohashish, H. M., Parmar, M. Y., Ola, M. S., Al-Hosaini, A. A., et al. (2015). Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol. Res. 37, 924–933. doi: 10.1179/1743132815Y.0000000079
Amaral, F. A., Bastos, L. F., Oliveira, T. H., Dias, A. C., Oliveira, V. L., Tavares, L. D., et al. (2016). Transmembrane TNF-alpha is sufficient for articular inflammation and hypernociception in a mouse model of gout. Eur. J. Immunol. 46, 204–211. doi: 10.1002/eji.201545798
Amaral, F. A., Costa, V. V., Tavares, L. D., Sachs, D., Coelho, F. M., Fagundes, C. T., et al. (2012). NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 64, 474–484. doi: 10.1002/art.33355
Anjaneyulu, M., and Chopra, K. (2003). Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1001–1005. doi: 10.1016/S0278-5846(03)00160-X
Binshtok, A. M., Wang, H., Zimmermann, K., Amaya, F., Vardeh, D., Shi, L., et al. (2008). Nociceptors are interleukin-1beta sensors. J. Neurosci. 28, 14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008
Carcole, M., Castany, S., Leanez, S., and Pol, O. (2014). Treatment with a heme oxygenase 1 inducer enhances the antinociceptive effects of micro-opioid, delta-opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharmacol. Exp. Ther. 351, 224–232. doi: 10.1124/jpet.114.215681
Chen, L., Hsieh, M. S., Ho, H. C., Liu, Y. H., Chou, D. T., and Tsai, S. H. (2004). Stimulation of inducible nitric oxide synthase by monosodium urate crystals in macrophages and expression of iNOS in gouty arthritis. Nitric Oxide 11, 228–236. doi: 10.1016/j.niox.2004.09.003
Cunha, T. M., Verri, W. A. Jr., Schivo, I. R., Napimoga, M. H., Parada, C. A., Poole, S., et al. (2008). Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 83, 824–832. doi: 10.1189/jlb.0907654
Dalbeth, N., Merriman, T. R., and Stamp, L. K. (2016). Gout. Lancet 388, 2039–2052. doi: 10.1016/S0140-6736(16)00346-9
Desaulniers, P., Fernandes, M., Gilbert, C., Bourgoin, S. G., and Naccache, P. H. (2001). Crystal-induced neutrophil activation. VII. Involvement of Syk in the responses to monosodium urate crystals. J. Leukoc. Biol. 70, 659–668.
Domiciano, T. P., Wakita, D., Jones, H. D., Crother, T. R., Verri, W. A. Jr., Arditi, M., et al. (2017). Quercetin inhibits inflammasome activation by interfering with ASC oligomerization and prevents interleukin-1 mediated mouse Vasculitis. Sci. Rep. 7:41539. doi: 10.1038/srep41539
Fattori, V., Amaral, F. A., and Verri, W. A. Jr. (2016). Neutrophils and arthritis: role in disease and pharmacological perspectives. Pharmacol. Res. 112, 84–98. doi: 10.1016/j.phrs.2016.01.027
Fattori, V., Borghi, S. M., Guazelli, C. F., Giroldo, A. C., Crespigio, J., Bussmann, A. J., et al. (2017a). Vinpocetine reduces diclofenac-induced acute kidney injury through inhibition of oxidative stress, apoptosis, cytokine production, and NF-kappaB activation in mice. Pharmacol. Res. 120, 10–22. doi: 10.1016/j.phrs.2016.12.039
Fattori, V., Serafim, K. G., Zarpelon, A. C., Borghi, S. M., Pinho-Ribeiro, F. A., Alves-Filho, J. C., et al. (2017b). Differential regulation of oxidative stress and cytokine production by endothelin ETA and ETB receptors in superoxide anion-induced inflammation and pain in mice. J. Drug Target. 25, 264–274. doi: 10.1080/1061186X.2016.1245308
Fattori, V., Pinho-Ribeiro, F. A., Borghi, S. M., Alves-Filho, J. C., Cunha, T. M., Cunha, F. Q., et al. (2015). Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-kappaB activation. Inflamm. Res. 64, 993–1003. doi: 10.1007/s00011-015-0885-y
Gloire, G., and Piette, J. (2009). Redox regulation of nuclear post-translational modifications during NF-kappaB activation. Antioxid. Redox Signal. 11, 2209–2222. doi: 10.1089/ARS.2009.2463
Grace, P. M., Gaudet, A. D., Staikopoulos, V., Maier, S. F., Hutchinson, M. R., Salvemini, D., et al. (2016). Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 39, 862–879. doi: 10.1016/j.tins.2016.10.003
Guerrero, A. T., Verri, W. A. Jr., Cunha, T. M., Silva, T. A., Rocha, F. A., Ferreira, S. H., et al. (2006). Hypernociception elicited by tibio-tarsal joint flexion in mice: a novel experimental arthritis model for pharmacological screening. Pharmacol. Biochem. Behav. 84, 244–251. doi: 10.1016/j.pbb.2006.05.008
Hattori, H., Subramanian, K. K., Sakai, J., Jia, Y., Li, Y., Porter, T. F., et al. (2010). Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 107, 3546–3551. doi: 10.1073/pnas.0914351107
Hohmann, M. S., Cardoso, R. D., Fattori, V., Arakawa, N. S., Tomaz, J. C., Lopes, N. P., et al. (2015). Hypericum perforatum reduces paracetamol-induced hepatotoxicity and lethality in mice by modulating inflammation and oxidative stress. Phytother. Res. 29, 1097–1101. doi: 10.1002/ptr.5350
Jalalvand, F., Amoli, M. M., Yaghmaei, P., Kimiagar, M., and Ebrahim-Habibi, A. (2015). Acarbose versus trans-chalcone: comparing the effect of two glycosidase inhibitors on obese mice. Arch. Endocrinol. Metab. 59, 202–209. doi: 10.1590/2359-3997000000038
Jin, X., and Gereau, R. W. (2006). Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 26, 246–255. doi: 10.1523/JNEUROSCI.3858-05.2006
Juliana, C., Fernandes-Alnemri, T., Wu, J., Datta, P., Solorzano, L., Yu, J. W., et al. (2010). Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802. doi: 10.1074/jbc.M109.082305
Karimi-Sales, E., Mohaddes, G., and Alipour, M. R. (2018). Chalcones as putative hepatoprotective agents: preclinical evidence and molecular mechanisms. Pharmacol. Res. 129, 177–187. doi: 10.1016/j.phrs.2017.11.022
Karkhaneh, L., Yaghmaei, P., Parivar, K., Sadeghizadeh, M., and Ebrahim-Habibi, A. (2016). Effect of trans-chalcone on atheroma plaque formation, liver fibrosis and adiponectin gene expression in cholesterol-fed NMRI mice. Pharmacol. Rep. 68, 720–727. doi: 10.1016/j.pharep.2016.03.004
Kobayashi, A., Kang, M. I., Watai, Y., Tong, K. I., Shibata, T., Uchida, K., et al. (2006). Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 26, 221–229. doi: 10.1128/MCB.26.1.221-229.2006
Lamoke, F., Labazi, M., Montemari, A., Parisi, G., Varano, M., and Bartoli, M. (2011). Trans-Chalcone prevents VEGF expression and retinal neovascularization in the ischemic retina. Exp. Eye Res. 93, 350–354. doi: 10.1016/j.exer.2011.02.007
Latz, E., Xiao, T. S., and Stutz, A. (2013). Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411. doi: 10.1038/nri3452
Lima-Junior, D. S., Costa, D. L., Carregaro, V., Cunha, L. D., Silva, A. L., Mineo, T. W., et al. (2013). Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 19, 909–915. doi: 10.1038/nm.3221
Marim, F. M., Silveira, T. N., Lima, D. S. Jr., and Zamboni, D. S. (2010). A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One 5:e15263. doi: 10.1371/journal.pone.0015263
Martin, W. J., and Harper, J. L. (2010). Innate inflammation and resolution in acute gout. Immunol. Cell Biol. 88, 15–19. doi: 10.1038/icb.2009.89
Martinez, R. M., Pinho-Ribeiro, F. A., Steffen, V. S., Caviglione, C. V., Fattori, V., Bussmann, A. J. C., et al. (2017a). trans-Chalcone, a flavonoid precursor, inhibits UV-induced skin inflammation and oxidative stress in mice by targeting NADPH oxidase and cytokine production. Photochem. Photobiol. Sci. 16, 1162–1173. doi: 10.1039/c6pp00442c
Martinez, R. M., Pinho-Ribeiro, F. A., Vale, D. L., Steffen, V. S., Vicentini, F., Vignoli, J. A., et al. (2017b). Trans-chalcone added in topical formulation inhibits skin inflammation and oxidative stress in a model of ultraviolet B radiation skin damage in hairless mice. J. Photochem. Photobiol. B 171, 139–146. doi: 10.1016/j.jphotobiol.2017.05.002
Martinez, R. M., Pinho-Ribeiro, F. A., Steffen, V. S., Silva, T. C., Caviglione, C. V., Bottura, C., et al. (2016). Topical formulation containing naringenin: efficacy against Ultraviolet B irradiation-induced skin inflammation and oxidative stress in mice. PLoS One 11:e0146296. doi: 10.1371/journal.pone.0146296
Martinon, F. (2010). Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 40, 616–619. doi: 10.1002/eji.200940168
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A., and Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. doi: 10.1038/nature04516
Menghini, L., Leporini, L., Vecchiotti, G., Locatelli, M., Carradori, S., Ferrante, C., et al. (2018). Crocus sativus L. stigmas and byproducts: qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 109, 91–98. doi: 10.1016/j.foodres.2018.04.028
Mitroulis, I., Kambas, K., Chrysanthopoulou, A., Skendros, P., Apostolidou, E., Kourtzelis, I., et al. (2011). Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One 6:e29318. doi: 10.1371/journal.pone.0029318
Mitroulis, I., Kambas, K., and Ritis, K. (2013). Neutrophils, IL-1beta, and gout: is there a link? Semin. Immunopathol. 35, 501–512. doi: 10.1007/s00281-013-0361-0
Pinho-Ribeiro, F. A., Zarpelon, A. C., Fattori, V., Manchope, M. F., Mizokami, S. S., Casagrande, R., et al. (2016). Naringenin reduces inflammatory pain in mice. Neuropharmacology 105, 508–519. doi: 10.1016/j.neuropharm.2016.02.019
Popa-Nita, O., Rollet-Labelle, E., Thibault, N., Gilbert, C., Bourgoin, S. G., and Naccache, P. H. (2007). Crystal-induced neutrophil activation. IX. Syk-dependent activation of class Ia phosphatidylinositol 3-kinase. J. Leukoc. Biol. 82, 763–773. doi: 10.1189/jlb.0307174
Rees, F., Hui, M., and Doherty, M. (2014). Optimizing current treatment of gout. Nat. Rev. Rheumatol. 10, 271–283. doi: 10.1038/nrrheum.2014.32
Ruiz-Miyazawa, K. W., Pinho-Ribeiro, F. A., Borghi, S. M., Staurengo-Ferrari, L., Fattori, V., Amaral, F. A., et al. (2018). Hesperidin methylchalcone suppresses experimental gout arthritis in mice by inhibiting NF-kappaB activation. J. Agric. Food Chem. 66, 6269–6280. doi: 10.1021/acs.jafc.8b00959
Ruiz-Miyazawa, K. W., Staurengo-Ferrari, L., Mizokami, S. S., Domiciano, T. P., Vicentini, F., Camilios-Neto, D., et al. (2017). Quercetin inhibits gout arthritis in mice: induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology 25, 555–570. doi: 10.1007/s10787-017-0356-x
Sakai, J., Li, J., Subramanian, K. K., Mondal, S., Bajrami, B., Hattori, H., et al. (2012). Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 37, 1037–1049. doi: 10.1016/j.immuni.2012.08.017
Scanu, A., Oliviero, F., Ramonda, R., Frallonardo, P., Dayer, J. M., and Punzi, L. (2012). Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor beta1 in the resolution phase. Ann. Rheum. Dis. 71, 621–624. doi: 10.1136/annrheumdis-2011-200711
Singh, A. K., and Vinayak, M. (2015). Curcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-alpha. IL-1beta and IL-6. Neurochem. Res. 40, 463–472. doi: 10.1007/s11064-014-1489-6
Singh, H., Sidhu, S., Chopra, K., and Khan, M. U. (2016). Hepatoprotective effect of trans-Chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharmacol. 94, 879–887. doi: 10.1139/cjpp-2016-0071
Steiner, A. A., Branco, L. G., Cunha, F. Q., and Ferreira, S. H. (2001). Role of the haeme oxygenase/carbon monoxide pathway in mechanical nociceptor hypersensitivity. Br. J. Pharmacol. 132, 1673–1682. doi: 10.1038/sj.bjp.0704014
Verri, W. A. Jr., Guerrero, A. T., Fukada, S. Y., Valerio, D. A., Cunha, T. M., Xu, D., et al. (2008). IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc. Natl. Acad. Sci. U.S.A. 105, 2723–2728. doi: 10.1073/pnas.0712116105
Verri, W. A. Jr., Souto, F. O., Vieira, S. M., Almeida, S. C., Fukada, S. Y., Xu, D., et al. (2010). IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 69, 1697–1703. doi: 10.1136/ard.2009.122655
Verri, W. A. Jr., Vicentini, F. T. M. C., Baracat, M. M., Georgetti, S. R., Cardoso, R. D., et al. (2012). “Flavonoids as anti-inflammatory and analgesic drugs: mechanisms of action and perspectives in the development of pharmaceutical forms,” in Studies in Natural Products Chemistry, 1st Edn, ed. A. U. Rahman (Amsterdam: Elsevier), 297–330.
Verri, W. A. Jr., Cunha, T. M., Parada, C. A., Poole, S., Cunha, F. Q., and Ferreira, S. H. (2006). Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther. 112, 116–138. doi: 10.1016/j.pharmthera.2006.04.001
Vieira, A. T., Galvao, I., Macia, L. M., Sernaglia, E. M., Vinolo, M. A., Garcia, C. C., et al. (2017). Dietary fiber and the short-chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J. Leukoc. Biol. 101, 275–284. doi: 10.1189/jlb.3A1015-453RRR
Wang, Z. Q., Porreca, F., Cuzzocrea, S., Galen, K., Lightfoot, R., Masini, E., et al. (2004). A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 309, 869–878. doi: 10.1124/jpet.103.064154
Wu, K. C., McDonald, P. R., Liu, J., and Klaassen, C. D. (2014). Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med. 80, 97–104. doi: 10.1055/s-0033-1351097
Zhang, X., Wang, G., Gurley, E. C., and Zhou, H. (2014). Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One 9:e107072. doi: 10.1371/journal.pone.0107072
Keywords: trans-Chalcone, joint pain, gouty arthritis, gout flare, inflammation, flavonoids, natural products, rheumatic disease
Citation: Staurengo-Ferrari L, Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Fattori V, Zaninelli TH, Badaro-Garcia S, Borghi SM, Carvalho TT, Alves-Filho JC, Cunha TM, Cunha FQ, Casagrande R and Verri WA Jr (2018) Trans-Chalcone Attenuates Pain and Inflammation in Experimental Acute Gout Arthritis in Mice. Front. Pharmacol. 9:1123. doi: 10.3389/fphar.2018.01123
Received: 16 June 2018; Accepted: 13 September 2018;
Published: 02 October 2018.
Edited by:
Annalisa Bruno, Università degli Studi “G. d’Annunzio” Chieti – Pescara, ItalyReviewed by:
Giustino Orlando, Università degli Studi “G. d’Annunzio” Chieti – Pescara, ItalyCarole L. Wilson, Medical University of South Carolina, United States
Soon Yew Tang, University of Pennsylvania, United States
Copyright © 2018 Staurengo-Ferrari, Ruiz-Miyazawa, Pinho-Ribeiro, Fattori, Zaninelli, Badaro-Garcia, Borghi, Carvalho, Alves-Filho, Cunha, Cunha, Casagrande and Verri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waldiceu A. Verri Jr., d2FsZGljZXVqckB5YWhvby5jb20uYnI=; d2F2ZXJyaUB1ZWwuYnI=
†These authors have contributed equally to this work
 Larissa Staurengo-Ferrari
Larissa Staurengo-Ferrari Kenji W. Ruiz-Miyazawa
Kenji W. Ruiz-Miyazawa Felipe A. Pinho-Ribeiro
Felipe A. Pinho-Ribeiro Victor Fattori
Victor Fattori Tiago H. Zaninelli
Tiago H. Zaninelli Stephanie Badaro-Garcia
Stephanie Badaro-Garcia Sergio M. Borghi
Sergio M. Borghi Thacyana T. Carvalho
Thacyana T. Carvalho Jose C. Alves-Filho
Jose C. Alves-Filho Thiago M. Cunha
Thiago M. Cunha Fernando Q. Cunha
Fernando Q. Cunha Rubia Casagrande
Rubia Casagrande Waldiceu A. Verri Jr.
Waldiceu A. Verri Jr.