- 1Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, China
- 2Department of General Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Triple-negative breast cancer (TNBC) is a subtype of aggressive breast cancer and characterized by a lack of the expression of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2. BRCA genes are tumor-suppressor genes that are involved in DNA damage repair and mutations of BRCA genes may increase the risk of developing breast cancer and/or ovarian cancer due to defective DNA repair mechanisms. However, the relationship between BRCA status and TNBC needs to be further investigated and validated. The aim of this meta-analysis was to evaluate the association between BRCA status and TNBC. We systematically searched the electronic databases of MEDLINE (PubMed), Embase, and Cochrane Library to identify relevant publications from April, 1959 to November, 2017. The data from the studies were examined by a meta-analysis using STATA software to calculate the odds ratio (OR) with 95% confidence interval (CI) by fixed-effect and random-effect models. We identified 16 qualified studies from 527 publications with 46,870 breast cancer patients including 868 BRCA1 mutations (BRCA1Mut) carriers, 739 BRCA2 mutations (BRCA2Mut) carriers, and 45,263 non-carriers. The results showed that breast cancer patients with BRCA1Mut carriers were more likely to have TNBC than those of BRCA2Mut carriers (OR: 3.292; 95% CI: 2.773–3.909) or non-carriers (OR: 8.889; 95% CI: 6.925–11.410). Furthermore, high expression of nuclear grade and large tumor burden (>2 cm) were significantly more common in breast cancer patients with BRCA1Mut carriers than those of BRCA2Mut carriers (OR: 2.663; 95% CI: 1.731–4.097; P = 0.211) or non-carriers (OR: 1.577; 95% CI: 1.067–2.331; P = 0.157). The data suggest that breast cancer patients with BRCA1Mut are more likely to have TNBC, high nuclear grade, and larger tumor burden.
Introduction
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer with a higher risk of both local and distant recurrence and poor overall prognosis and it accounts for about 10–20% of all cases of breast cancer (Foulkes et al., 2010; Ovcaricek et al., 2011; Boyle, 2012). TNBC is characterized by a lack of the expression of estrogen receptor (ER), progesterone receptors (PR) and human epidermal growth factor receptor two (HER2/neu), thus, offers no validated molecular targets for treatment (Onitilo et al., 2009). The BRCA1 and BRCA2 genes are tumor-suppressor genes and involved in DNA damage repair and recombination, cell-cycle checkpoint control, apoptosis and transcriptional regulation (Venkitaraman, 2014). Mutations in BRCA genes induce defective DNA repair mechanisms, which are associated with the risk of development of breast and/or ovarian cancers (Peng et al., 2016). Some studies showed that BRCA1 mutation (BRCA1Mut) carriers were more likely to have ER-negative/PR-negative breast cancer (Musolino et al., 2007; Byrski et al., 2008; Kirk, 2010). In contrast, BRCA2 mutation (BRCA2Mut) carriers seem to share the pathologic characteristics similar to those of patients with normal BRCA genes (non-carriers) (Noguchi et al., 1999). However, Comen et al. (2011) found that the association between TNBC and BRCA mutations was not only limited to BRCA1, but also a significant proportion of women with TNBC had BRCA2Mut. Currently, the relationship between the status of BRCA mutation and the statuses of ER, PR, HER2/neu and P53 have been inconsistent (Maegawa and Tang, 2010; Wu et al., 2010). With the development of targeted therapies for breast cancer patients, designation of treatment regimens has become more specific, and breast cancer patients with BRCA mutations should be treated differently from the patients without BRCA mutations. Therefore, the exact relationship between BRCA status and TNBC needs to be further investigated and validated.
We therefore performed a meta-analysis to investigate the association between the status of BRCA mutations and TNBC and the effect of BRCAMut on nuclear grade and tumor size in patients with breast cancer.
Materials and Methods
Data Sources and Search Strategy
We systematically searched the databases of MEDLINE (PubMed, http://www.ncbi.nlm.nih.gov/pubmed/), Embase (http://www.embase.com), and Cochrane Library (www.cochranelibrary.com) for relevant publications of primary studies, and used the following search algorithm: breast cancer, breast carcinoma, mammary cancer, breast tumor and BRCA1 or BRCA2, BRCA, and triple negative breast cancer, TNBC or molecular typing, type or subtype of breast cancer. The databases were searched for the studies published from April, 1959 to November, 2017.
Study Selection
The inclusion criteria were as follows: (a) comparative studies of breast cancer patients with BRCA1Mut, BRCA2Mut, and non-carriers; (b) studies were published as a full paper in English; (c) the statuses of ER, PR and HER2 were measured by immunohistochemistry; and (d) high-quality case-control studies (Newcastle-Ottawa Scale [NOS] score ≥ 7 points). The exclusion criteria were as follows: (a) review articles; (b) study was based on preclinical setting such as cell culture and/or animal models of feline mammary cancer; (c) study did not discuss BRCA1 and BRCA2 mutations separately; and (d) study had no inclusion, or duplicated data from other studies.
Data Extraction
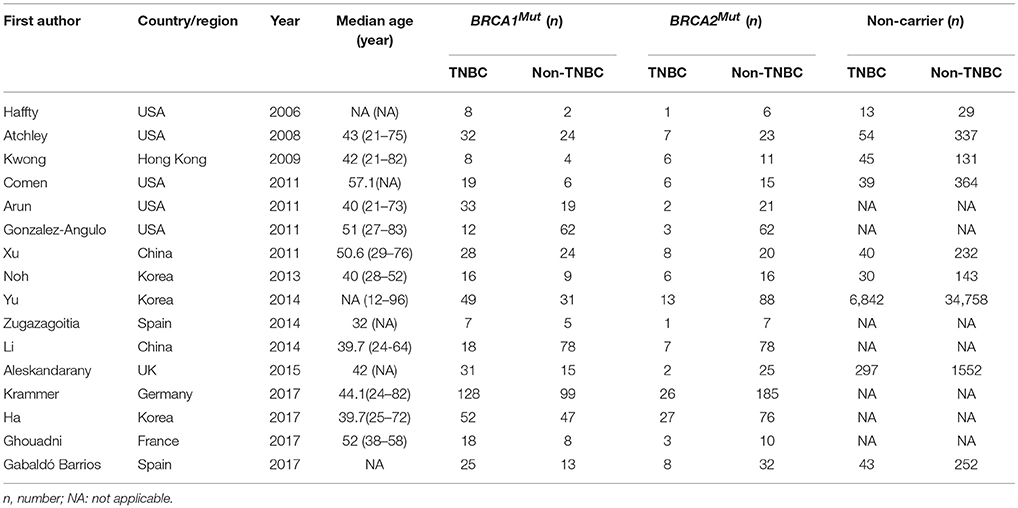
Two investigators independently extracted the date from each study including the first author; year of publication; country of study; numbers of subjects with (a) non-carrier with TNBC, (b) non-carriers without TNBC, (c) BRCA1Mut carrier with TNBC, (d) BRCA1Mut carrier without TNBC, (e) BRCA2Mut with TNBC and (f) BRCA2Mut carrier without TNBC; tumor size and nuclear grade with a standardized form. Additional investigators were consulted when discrepancies were present.
Population, Interventions, Comparators, Outcomes and Study Designs (PICOS)
The population from the study is patients with breast cancer. Genetic testing of BRCA mutations was performed in these patients. BRCA status (BRCA1 mutations carriers, BRCA2 mutations carriers, and non-carriers) was compared and the outcomes of incidence of TNBC, expression of nuclear grade and tumor burden (>2 cm) were evaluated in these patients. The study designs were to evaluate the association between BRCA status and TNBC as well as the relationship of BRCA mutations and the expression of nuclear grade and tumor burden.
Quality Assessment
The quality of each study was independently evaluated by at least two examiners who read each study and scored it according to the NOS criteria (Deeks et al., 2003). The average NOS score was 7.4 points.
Statistical Analysis
The STATA software version 12.0 (Stata Corp, College Station, TX, USA) was used to perform this meta-analysis. Dichotomous outcomes were analyzed using the OR with 95% CI as the summary statistics, as previously described in the Mantel–Haenszel method (Mantel and Haenszel, 1959; Greenland and Robins, 1985). Statistical heterogeneity was evaluated by a X2 test (Higgins et al., 2003). The Higgins I2 test measured inconsistency between studies; values of <25, 25–50, and >50% were defined as low, moderate and high, respectively (DerSimonian and Laird, 1986). Data were analyzed with the fixed-effect model for low or moderate consistency and with the random-effect model for high heterogeneity. We also performed sensitivity analysis by omitting specific studies to find potential outliers.
Results
Study Selection and Patient Characteristics
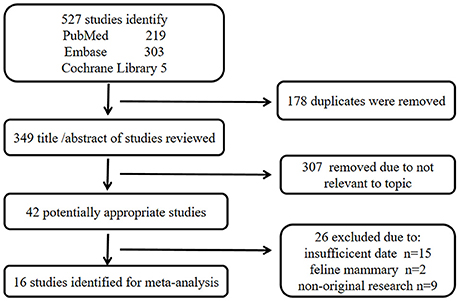
A total of 527 publications were identified from the three databases, 219 from PubMed, 303 from Embase, and five from Cochrane Library. The titles and abstracts of all remaining publications (n = 349) were reviewed after removing the duplicate publications (n = 178) and 307 more publications were excluded as irrelevant to the topic. Next, 26 publications were further excluded for insufficient data (n = 15), feline mammary focus (n = 2), and non-original research (n = 9) after carefully examining the full texts of the remaining 42 publications. Finally, 16 eligible publications were included in the study of meta-analysis (Haffty et al., 2006; Atchley et al., 2008; Kwong et al., 2009; Arun et al., 2011; Comen et al., 2011; Gonzalez-Angulo et al., 2011; Xu et al., 2012; Noh et al., 2013; Li et al., 2014; Yu et al., 2014; Zugazagoitia et al., 2014; Aleskandarany et al., 2015; Gabaldó Barrios et al., 2017; Ghouadni et al., 2017; Ha et al., 2017; Krammer et al., 2017). The screening method and results of the relevant studies are shown in Figure 1 and the main characteristics of participated patients are summarized in Table 1.
The included studies were conducted in eight countries or regions as USA 5, Korea 3, China 2, Hong Kong 1, UK 1, Germany 1, France 1, and Spain 2, the published date was between 2006 and 2017. 45,870 patients were included in the studies, with the median age ranged from 32.0 to 57.1 years, 868 BRCA1Mut carriers, 739 BRCA2Mut carriers, and 45,263 non-carriers (Table 1).
Association of BRCA Status and TNBC
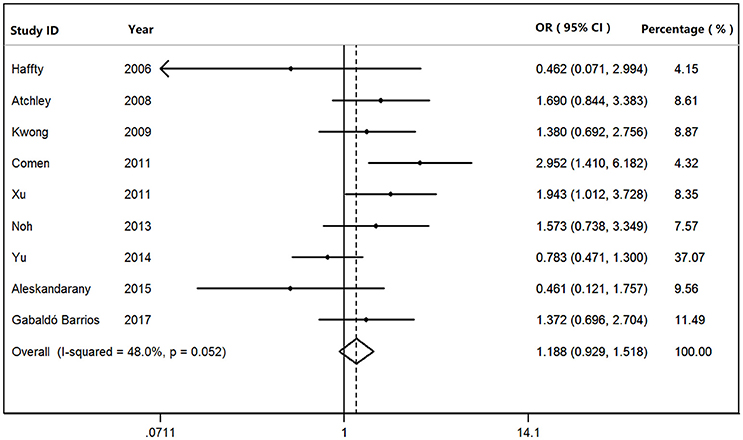
We found that BRCA1Mut carriers were more likely to have TNBC than those of BRCA2Mut carriers (OR: 3.292; 95% CI: 2.773–3.909) or non-carriers (OR: 8.889; 95% CI: 6.925–11.410) among the patients with breast cancer (Figure 2). Because heterogeneity was found across the studies (I2 = 35.2%, heterogeneity X2 = 23.16; d.f. = 15; P = 0.081), the pooled OR was calculated as 3.292 (95% CI: 2.773–3.909) by a fixed-effect model. Furthermore, BRCA1Mut carriers were significantly more likely to have TNBC than those of non-carriers (Figure 3). There was significant heterogeneity in the studies (I2 = 59.9%, heterogeneity X2 = 19.94; d.f. = 8; P = 0.011), the pooled OR was calculated as 4.011 (95% CI: 3.362–4.786) by a fixed-effect model. Interestingly, the incidence of TNBC was not significantly different between BRCA2Mut carries and non-carriers (Figure 4). Because the studies were significantly heterogeneous (I2 = 48.0%, heterogeneity X2 = 15.39; d.f. = 8; P = 0.052), the pooled OR was calculated as 1.188 (95% CI: 0.929–1.518) by a random-effects model.
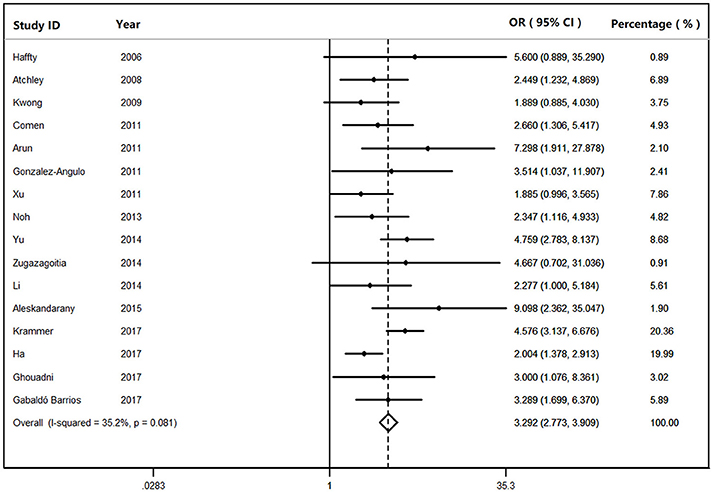
Figure 2. The odds ratio (OR) of BRCA1 mutations vs. BRCA2 mutations in patients with TNBC by Forest Plot.
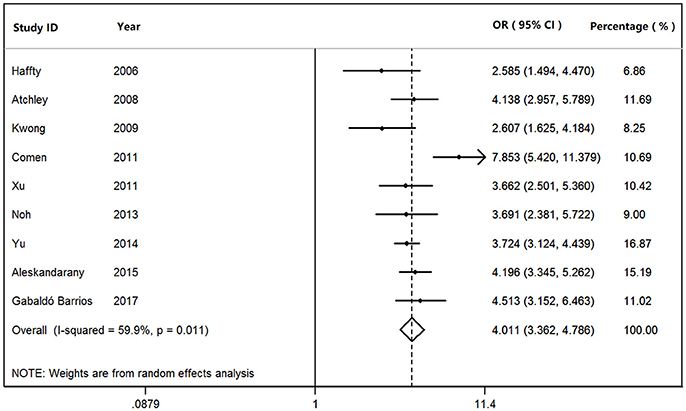
Figure 3. The odds ratio (OR) of BRCA1 mutations vs. non-carriers in patients with TNBC by Forest Plot.
Association of BRCA Status and Nuclear Grade or Tumor Burden
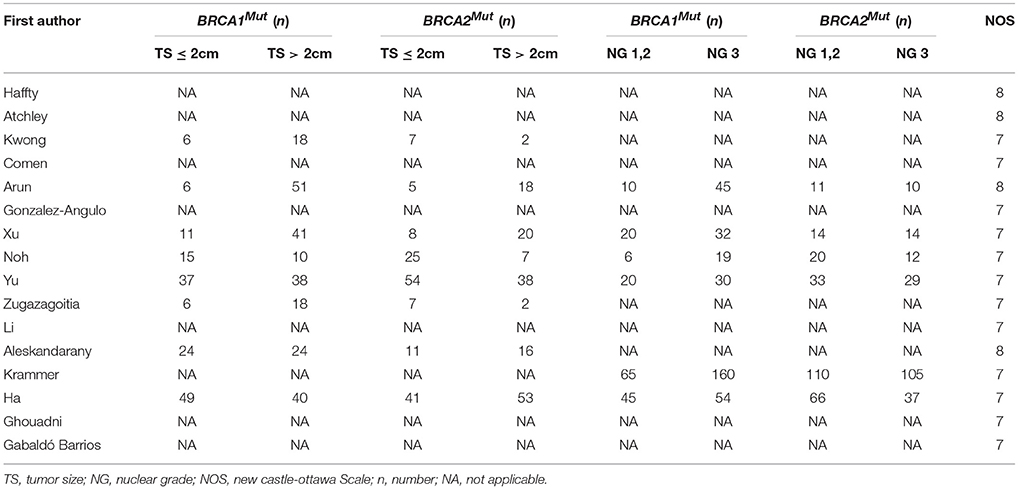
As shown in Table 2, high expression of nuclear grade was more common in the breast cancer patients with BRCA1Mut carriers than those of patients with BRCA2Mut carriers (OR: 2.663; 95% CI: 1.731–4.097; P = 0.211). Moreover, Tumors were more likely to exceed 2 cm in the breast cancer patients with BRCA1Mut carriers than those of patients with BRCA2Mut carriers (OR: 1.577; 95% CI: 1.067–2.331; P = 0.157).
Sensitivity Analyses and Publication Bias
Sensitivity analyses showed that two publications from Li et al. (2014) and Xu et al. (2012) accounted for all the observed heterogeneity. The I2 was 34.5% when all studies were included in the analysis. However, the I2 was reduced to 20.5% when the study of Li et al. (2014) was excluded and it was further dropped to 18.1% when the study of Yu et al. (2014) was also removed from the analysis. The results suggest that those two papers significantly influenced the overall analysis. Begg's tests indicated that no publication bias was observed in this meta-analysis for association between BRCA1Mut and BRCA2Mut (P = 0.499; Figure 5), or between BRCA1Mut and non-carriers (P = 0.348; Figure 6).
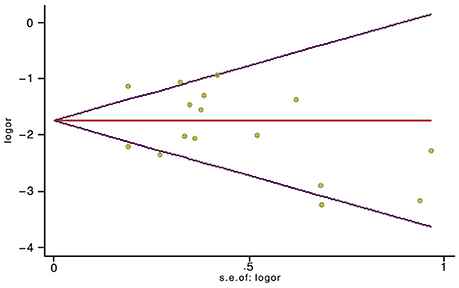
Figure 5. Indication of publication bias for the association between BRCA1 mutations and BRCA2 mutations by Begg's Funnel Plot with pseudo 95% confidence limits. The data indicate that there was no obvious indication of publication bias.
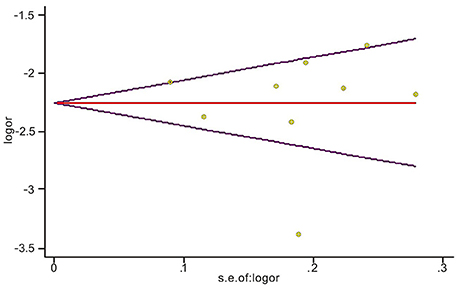
Figure 6. Indication of publication bias for the association between BRCA1 mutations and non-carriers by Begg's Funnel Plot with pseudo 95% confidence limits. The data indicate that there was no obvious indication of publication bias.
Discussion
We investigated the association between BRCA status and TNBC (a subtype of breast cancer) and the characteristics of breast cancer patients with BRCA1Mut and BRCA2Mut using a meta-analysis. The currently specific criteria of guidelines from the National Comprehensive Cancer Network (NCCN) for test of BRCA1Mut and BRCA2Mut include patients' ages at diagnosis and their family members; family histories of breast, ovarian, pancreatic and prostate cancers, and diagnosed TNBC (National Comprehensive Cancer Network, 2017). Up to date, approximately 300 mutations within the BRCA1Mut gene have been identified, including small insertions, deletions and non-sense mutations, most of them lead to functionally inactive proteins (Miki et al., 1994; Simard et al., 1994). BRCA2 is a tumor suppressor gene that mediates the repair of chromosomal damage (Yoshida and Miki, 2004). In the present study of meta-analysis, we found that TNBC was more common among the breast cancer patients with BRCA1Mut than those of patients with BRCA2Mut (OR: 3.292; 95% CI: 2.773–3.909) or non-carriers (OR: 8.889; 95% CI: 6.925–11.410). In an unselected cohort study in 77 patients with TNBC, it was found that 15 (19.5%) had BRCA mutations including 12 (15.6%) in BRCA1 (one somatic) and 3 (3.9%) in BRCA2 (Gonzalez-Angulo et al., 2011). In addition, a significantly lower risk of relapse was found in TNBC patients with BRCA mutations (Gonzalez-Angulo et al., 2011).
The underlying mechanism that links BRCA1Mut to ER negativity has been the focus of ongoing investigations. Hosey et al. (2007) discovered that BRCA1Mut tumors fail to express ER due to the loss of BRCA1-mediated transcriptional activation of estrogen receptor 1 (ESR1). Reduction or absence of BRCA1 in breast cancer occurs through several mechanisms including hypermethylation of the BRCA1 promoter, loss of heterozygosity, and transcriptional regulation of BRCA1 (Catteau et al., 1999; Baldassarre et al., 2003). However, the exact mechanism for the transcription of BRCA1 is highly complex and remains unknown. Further studies are needed to gain insight into the interaction between BRCA1 and ER, and its potential effects on the expressions of PR and HER2.
BRCA2Mut breast cancer has the pathologic features similar to those of sporadic breast cancers (Lee et al., 2010). The incidence of TNBC was not significantly different between patients with BRCA2Mut and non-carriers (OR: 1.203; 95% CI: 0.871–1.660). Hosey et al. (2007) suggested that breast cancer patients with BRCA2Mut were unlikely to be ER-deficient because of the ability of estrogen metabolites to induce loss of the second BRCA1 allele, thus, estrogen may somehow facilitate the survival of BRCA1-deficient cells in hormonally responsive tissues.
Interestingly, in the present study, we found that a high nuclear grade was also more common in the tumors from BRCA1Mut patients than in those of patients with BRCA2Mut carriers (OR 2.663; 95% CI: 1.731–4.097; P = 0.211) and the tumors with BRCA1Mut were more likely to have nuclear grade three than those of tumors with BRCA2Mut. This finding is consistent with the earlier studies in the literature (Musolino et al., 2007; Li et al., 2008; Xu et al., 2012). We also found that the tumors with BRCA1Mut were more likely to exceed 2 cm than those of tumors with BRCA2Mut ($OR 1.577; 95% CI: 1.067–2.331), although several studies have reported that the sizes of tumors were not significantly different between the tumors with BRCA1Mut and the tumors with BRCA2Mut (Xu et al., 2012; Noh et al., 2013; Yu et al., 2014). The observed difference of tumor size may be due to different clinical and pathological characteristics from the tumors with BRCA1Mut and BRCA2Mut leading to different prognosis in the patients with BRCA mutations. The findings may be significant with valuable information for oncologists to better understand the role of BRCA mutations in breast cancer patients and optimal treatment of TNBC.
Some limitations of the study should be acknowledged in this meta-analysis. The methods of assessing ER/PR-negative status were varied among the studies. Most studies defined ER/PR-negative specimens as having <10% immunoreactive cells, whereas newer immunohistochemistry guidelines have used a threshold of <1%. BRCA mutation tests also lack uniformity, which may affect the outcomes. Therefore, selection bias was inevitable.
Conclusion
The present study suggests that TNBC was more common among the breast cancer patients with BRCA1Mut tumors than those of patients with BRCA2Mut tumors or non-carriers. Furthermore, a high expression of nuclear grade and large tumor burden (> 2cm) were significantly more common in BRCA1Mut patients than that of BRCA2Mut patients. The study provides valuable information for clinicians to better understand the role of BRCA mutations in breast cancer patients for providing optimal treatment and improving outcome clinically.
Author Contributions
HC, JW, SC, and XZL designed the study and analyzed the data; HC, JW, ZZ, and XZL collected and assembled the data; HC, JW, YT, XXL, SL, SC, and XZL reviewed, analyzed, and interpreted the data. HC, JW, SC, and XZL assessed the risk of bias; HC, JW, and XZL wrote the first draft and SC wrote the final version of the manuscript. All authors discussed the results and contributed to the manuscript.
Funding
The work was supported by the Grants from National Natural Science Foundation of China (Grant No. 81774013), Novel Drug Development Program of China (Grant No. 2014X09102-043001), the Science and Technology Planning Project of Sichuan Province, China (Grant No. 14JC0798), Educational Commission of Sichuan Province, China (Grant No. 16ZA0187), the Collaborative Fund of Luzhou Government and Southwest Medical University (Grant No. 2016LZXNYD-T03) and the Distinguished Professor Research Startup Funding (SC) from Southwest Medical University (2015-RCYJ0002), Southwest Medical University (Grant No. 2017-ZRQN-010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aleskandarany, M., Caracappa, D., Nolan, C. C., Macmillan, R. D., Ellis, I. O., Rakha, E. A., et al. (2015). DNA damage response markers are differentially expressed in BRCA-mutated breast cancers. Breast Cancer Res. Treat. 150, 81–90. doi: 10.1007/s10549-015-3306-6
Arun, B., Bayraktar, S., Liu, D. D., Gutierrez Barrera, A. M., Atchley, D., Pusztai, L., et al. (2011). Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J. Clin. Oncol. 29, 3739–3746. doi: 10.1200/JCO.2011.35.2682
Atchley, D. P., Albarracin, C. T., Lopez, A., Valero, V., Amos, C. I., Gonzalez-Angulo, A. M., et al. (2008). Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J. Clin. Oncol. 26, 4282–4288. doi: 10.1200/JCO.2008.16.6231
Baldassarre, G., Battista, S., Belletti, B., Thakur, S., Pentimalli, F., Trapasso, F., et al. (2003). Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma. Mol. Cell Biol. 23, 2225–2238. doi: 10.1128/MCB.23.7.2225-2238.2003
Boyle, B. (2012). Triple-negative breast cancer: epidemiological considerations and recommendations. Ann. Oncol. 6, 7–12. doi: 10.1093/annonc/mds187
Byrski, T., Gronwald, J., Huzarski, T., Grzybowska, E., Budryk, M., Stawicka, M., et al. (2008). Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res. Treat. 108, 289–296. doi: 10.1007/s10549-007-9600-1
Catteau, A., Harris, W. H., Xu, C. F., and Solomon, E. (1999). Methylation of the BRCA1 promoter region in sporadic breast and ovarian cancer: correlation with disease characteristics. Oncogene 18, 1957–1965. doi: 10.1038/sj.onc.1202509
Comen, E., Davids, M., Kirchhoff, T., Hudis, C., Offit, K., and Robson, M. (2011). Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in ashkenazi women. Breast Cancer Res. Treat. 129, 185–190. doi: 10.1007/s10549-011-1433-2
Deeks, J. J., Dinnes, J., D'Amico, R., Sowden, A. J., Sakarovitch, C., Song, F., et al. (2003). Evaluating non-randomised intervention studies. Health Technol. Assess. 7, 1–173. doi: 10.3310/hta7270
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control Clin. Trials. 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Foulkes, W. D., Smith, I. E., and Reis-Filho, J. S. (2010). Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948. doi: 10.1056/NEJMra1001389
Gabaldó Barrios, X., Sarabia Meseguer, M. D., Marín Vera, M., Sánchez Bermúdez, A. I., Macías Cerrolaza, J. A., Sánchez Henarejos, P., et al. (2017). Molecular characterization and clinical interpretation of BRCA1/BRCA2 variants in families from murcia (South-eastern Spain) with hereditary breast and ovarian cancer: clinical- pathological features in BRCA carriers and non-carriers. Fam Cancer. 16, 477–489. doi: 10.1007/s10689-017-9985-x
Ghouadni, A., Delaloge, S., Lardelli, P., Kahatt, C., Byrski, T., Blum, J. L., et al. (2017). Higher antitumor activity of trabectedin in germline BRCA2 carriers with advanced breast cancer as compared to BRCA1 carriers: a subset analysis of a dedicated phase II trial. Breast 34, 18–23. doi: 10.1016/j.breast.2017.04.006
Gonzalez-Angulo, A. M., Timms, K. M., Liu, S., Chen, H., Litton, J. K., Potter, J., et al. (2011). Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin. Cancer Res. 17, 1082–1089. doi: 10.1158/1078-0432.CCR-10-2560
Greenland, S., and Robins, J. M. (1985). Estimation of a common effect parameter from sparse follow-up data. Biometrics 41, 55–68. doi: 10.2307/2530643
Ha, S. M., Chae, E. Y., Cha, J. H., Kim, H. H., Shin, H. J., and Choi, W. J. (2017). Association of BRCA mutation types, imaging features, and pathologic findings in patients with breast cancer with BRCA1 and BRCA2 mutations. Am. J. Roentgenol. 209, 920–928. doi: 10.2214/AJR.16.16957
Haffty, B. G., Yang, Q., Reiss, M., Kearney, T., Higgins, S. A., Weidhaas, J., et al. (2006). Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 24, 5652–5657. doi: 10.1200/JCO.2006.06.5664
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hosey, A. M., Gorski, J. J., Murray, M. M., Quinn, J. E., Chung, W. Y., Stewart, G. E., et al. (2007). Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J. Natl. Cancer. Inst. 99, 683–694. doi: 10.1093/jnci/djm207
Kirk, R. (2010). Surgical oncology: cancer risk reduction in BRCA mutation carriers. Nat. Rev. Clin. Oncol. 7:609. doi: 10.1038/nrclinonc.2010.157
Krammer, J., Pinker-Domenig, K., Robson, M. E., Gönen, M., Bernard-Davila, B., Morris, E. A., et al. (2017). Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 163, 565–571. doi: 10.1007/s10549-017-4198-4
Kwong, A., Wong, L. P., Wong, H. N., Law, F. B., Ng, E. K., Tang, Y. H., et al. (2009). Clinical and pathological characteristics of Chinese patients with BRCA related breast cancer. Hugo. J. 3, 63–76. doi: 10.1007/s11568-010-9136-z
Lee, E. H., Park, S. K., Park, B., Kim, S. W., Lee, M. H., Ahn, S. H., et al. (2010). Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res. Treat. 122, 11–25. doi: 10.1007/s10549-010-0859-2
Li, W. F., Hu, Z., Rao, N. Y., Song, C. G., Zhang, B., Cao, M. Z., et al. (2008). The prevalence of BRCA1 and BRCA2 germline mutations in high-risk breast cancer patients of Chinese Han nationality: two recurrent mutations were identified. Breast Cancer Res. Treat. 110, 99–109. doi: 10.1007/s10549-007-9708-3
Li, Y. T., Ni, D., Yang, L., Zhao, Q., and Ou, J. H. (2014). The prevalence of BRCA1/2 mutations of triple-negative breast cancer patients in Xinjiang Multiple ethnic region of China. Eur. J. Med. Res. 19:35. doi: 10.1186/2047-783X-19-35
Maegawa, R. O., and Tang, S. C. (2010). Triple-negative breast cancer: unique biology and its management. Cancer Invest. 28, 878–883. doi: 10.3109/07357907.2010.483507
Mantel, N., and Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748.
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S., et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71. doi: 10.1126/science.7545954
Musolino, A., Bella, M. A., Bortesi, B., Michiara, M., Naldi, N., Zanelli, P., et al. (2007). BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: A population-based study. Breast 16, 280–292. doi: 10.1016/j.breast.2006.12.003
National Comprehensive Cancer Network (2017). Clinical Practice Guidelines in Oncology: Genetic/Familial High Risk Assessment: Breast and Ovarian (2017). Available online at: https://www.genomeweb.com/sites/default/files/nccn_2017.pdf (Accessed March 2, 2018).
Noguchi, S., Kasugai, T., Miki, Y., Fukutomi, T., Emi, M., and Nomizu, T. (1999). Clinicopathologic analysis of BRCA1- or BRCA2-associated hereditary breast carcinoma in Japanese women. Cancer 85, 2200–2205. doi: 10.1002/(SICI)1097-0142.
Noh, J. M., Han, B. K., Choi, D. H., Rhee, S. J., Cho, E. Y., Huh, S. J., et al. (2013). Association between BRCA mutation status, pathological findings, and magnetic resonance imaging features in patients with breast cancer at risk for the mutation. J. Breast Cancer 16, 308–314. doi: 10.4048/jbc.2013.16.3.308
Onitilo, A. A., Engel, J. M., Greenlee, R. T., and Mukesh, B. N. (2009). Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin. Med. Res. 7, 4–13. doi: 10.3121/cmr.2008.825
Ovcaricek, T., Frkovic, S. G., Matos, E., Mozina, B., and Borstnar, S. (2011). Triple negative breast cancer - prognostic factors and survival. Radiol. Oncol. 45, 46–52. doi: 10.2478/v10019-010-0054-4
Peng, L., Xu, T., Long, L. T., and Zuo, H. (2016). Association between BRCA status and P53 status in breast cancer: a meta-analysis. Med. Sci. Monit. 8, 1939–1945. doi: 10.12659/MSM.896260
Simard, J., Tonin, P., Durocher, F., Morgan, K., Rommens, J., Gingras, S., et al. (1994). Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat. Genet. 8, 392–398. doi: 10.1038/ng1294-392
Venkitaraman, A. R. (2014). Cancer suppression by the chromosome custodians BRCA1 and BRCA2. Science 343, 1470–1475. doi: 10.1126/science.1252230
Wu, M., Mao, C., Chen, Q., Cu, X. W., and Zhang, W. S. (2010). Protein and anti-p53 antibodies are associated with increased cancer risk: a case-control study of 569 patients and 879 healthy controls. Mol. Biol. Rep. 37, 339–343. doi: 10.1007/s11033-009-9744-7
Xu, J., Wang, B., Zhang, Y., Li, R., Wang, Y., and Zhang, S. (2012). Clinical implications for BRCA genemutation in breast cancer. Mol. Biol. Rep. 39, 3097–3102. doi: 10.1007/s11033-011-1073-y
Yoshida, K., and Miki, Y. (2004). Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 95, 866–871. doi: 10.1111/j.1349-7006.2004.tb02195.x.
Yu, J. H., Lee, J. W., Son, B. H., Kim, S. W., Park, S. K., Lee, M. H., et al. (2014). Characteristics of BRCA1/2 mutation-positive breast cancers in Korea: a comparison study based on multicenter data and the Korean breast cancer registry. J. Breast Cancer 17, 129–135. doi: 10.4048/jbc.2014.17.2.129
Zugazagoitia, J., Pérez-Segura, P., Manzano, A., Blanco, I., Vega, A., Custodio, A., et al. (2014). Limited family structure and triple-negative breast cancer (TNBC) subtype as predictors of BRCA mutations in a genetic counseling cohort of early-onset sporadic breast cancers. Breast Cancer Res. Treat. 148, 415–421. doi: 10.1007/s10549-014-3167-4
Keywords: Triple-negative breast cancer (TNBC), BRCA1, BRCA2, mutation, meta-analysis
Citation: Chen H, Wu J, Zhang Z, Tang Y, Li X, Liu S, Cao S and Li X (2018) Association Between BRCA Status and Triple-Negative Breast Cancer: A Meta-Analysis. Front. Pharmacol. 9:909. doi: 10.3389/fphar.2018.00909
Received: 09 April 2018; Accepted: 24 July 2018;
Published: 21 August 2018.
Edited by:
Suzie Chen, Rutgers, The State University of New Jersey, United StatesReviewed by:
Victor C. Kok, Asia University, TaiwanAnkita Thakkar, Burke Medical Research Institute, United States
Copyright © 2018 Chen, Wu, Zhang, Tang, Li, Liu, Cao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shousong Cao, c2hvdXNvbmdjQGdtYWlsLmNvbQ==
Xianzhu Li, ZG5hMDgwNTAwMUAxNjMuY29t
† These authors have contributed equally to this work
 Haixia Chen
Haixia Chen Jianming Wu
Jianming Wu Zhihong Zhang2
Zhihong Zhang2 Yong Tang
Yong Tang Xiaoxuan Li
Xiaoxuan Li Shousong Cao
Shousong Cao