- 1Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan
- 2Center for Natural Products Lab, Chengdu Institute of Biology, Sichuan, China
- 3Department of Ethnobotany, Institute of Botany, Ilia State University, Tbilisi, Georgia
- 4Key Laboratory of Mountain Ecological Restoration, Bioresource Utilization and Ecological Restoration Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- 5University of Chinese Academy of Sciences, Beijing, China
- 6Medicinal Aromatic and Poisonous Plants Research Center, College of Pharmacy King Saud University, Riyadh, Saudi Arabia
- 7Phytochemistry Department, National Research Centre, Giza, Egypt
Hypertension is one of the most important factors responsible for cardiovascular ailments worldwide. It has been observed that herbal products and alternative herbal therapies played a significant role in decreasing hypertension. The aim of the current study is to provide significant ethnopharmacological information, both qualitative and quantitative on medicinal plants related to hypertension from Northern Pakistan. The documented data were quantitatively analyzed for the first time in this area. A total of 250 participants were interviewed through semi-structured discussions and questionnaires. Quantitative indices including FC (Frequency citation), FIV (Family importance value), RFC (Relative frequency of citation) and DCI (Disease Consensus index) were calculated. A total of 192 plant species, belonging to 77 families were reported to be used in treatment of hypertension in Northern Pakistan. The most dominant life form reported was herbs (54%), with decoction (72 reports) and leaves (55.1%) were commonly utilized plant part. Highest FIV was recorded in Lamiaceae (327 FIV). RFC ranged from 0.08 to 1.08% while DCI varied from 0.233 to 0.000. In this study original data was compared with thirty one previous national and international published papers from neighboring region to compare the medicinal uses and obtain some novel plant species. About 42% of the medicinal plant species were reported for the first time in treatment of hypertension in comparison to these 31 published papers. Different phytochemical activities of antihypertensive plants were also reported from literature. This research work documents the traditional knowledge of medicinal plants usage and provides baseline in designing clinical trials and pharmacological analysis for treatment of hypertension.
Introduction
Hypertension is one of the most common cardiovascular diseases that become major health concern in various parts of the world. Arterial hypertension is a chronic medical condition in which the pressure in the arteries raised above 140/90 mmHg (Osamor and Owumi, 2010). There are two types of hypertension, systolic and diastolic hypertension. Based on detected blood pressure (BP) rates there are different groups of hypertension, mild or grade I (BP 140–159/90–99 mm Hg), moderate or grade II (BP 160–179/100–109 mm Hg), and severe or grade III and IV (BP>180–210/110–120 mm Hg) (Salud, 2001).
Family history, extensive use of alcohol, high sodium intake and high sugar intake might be one of the cause hypertension (Eddouks et al., 2002). Smoking and coffee consumption is also reported to be cause of hypertension. Ecological aspects such as lead polluted drinking water and cadmium contamination have also been shown to favor hypertension (Pirkle et al., 1985). The incidences of uncontrolled hypertension occur among individuals of above 50 years (Tee et al., 2010). Each year about half million strokes and more than a million heart attacks are caused due to hypertension (Jaffer and Weissleder, 2005; Sarafidis et al., 2008; Grassi et al., 2013). Many other conditions such as insulin resistance, obesity, kidney failure, nervous system, concomitance, atherosclerosis and cardiovascular diseases have been found to be related with high blood pressure (Ghosh and Bandyopadhyay, 2007; Heisler et al., 2008). Hypertension is estimated to cause 4.5% of the disease burden globally (World Health Organization, 2002; Cardoso and Salles, 2016).
Hypertension has become a worldwide concern with important scale of morbidity and mortality. It has been observed that 1 billion persons all over the World suffer from hypertension triggering up to 7.1 million casualties per year (Hajjar and Kotchen, 2003; Tahraoui et al., 2007). In Africa particularly in Gabon, it is reported (World Health Organization, 2001) that 26.4% of the inhabitants (26.1% female and 26.6% male) suffered from hypertension (Opie and Seedat, 2005). Similarly the prevalence of hypertension in the Asia-Pacific areas ranges from 7 to 38% in women and from 5 to 47% in men (Lawes et al., 2004). Around the globe, the lowest percentage of people with hypertensions (less than 5.2%) live in rural North India (Kearney et al., 2005).
In Pakistan a health survey (NHSP) between 1990 and 1994 emphasized on the extent of the burden of hypertension in Pakistan. Hypertension affected 18% of adults above 15 years and 33% of adults above 45 years, with 3% suffering from low hypertension at 140/90 mm Hg or below. In Pakistan it is reported that every third person over the above 40 years of ageis affected by hypertension (Saleem et al., 2010). In Ayurvedic and Greek traditional medicinal systems, hypertension was diagnosed by its apparent symptoms. In the current study local healers gave information about symptoms and signs of hypertension and indicated also which other sources of information about hypertension they might have had. The local healers spend much time listening to the patients suffering from any illness, and discussing the possible causes of the disease and the course of treatment with the patients. The perceived causes of hypertension by traditional healers include diet and hereditary causes, as found in other studies (Meli et al., 2009). The traditional healers also described other symptoms such as severe headache, fatigue, chest pain, irregular heart beat among others for a diagnosis of hypertension.
In homeopathy and traditional Chinese herbal medicinal system there are numerous treatments for hypertension (Gress et al., 2000). However, there is still a need for supplementary means for treatment, and also for non-pharmacological management (Gallagher et al., 2006). Such on-pharmacological methods include biofeedback, relaxation, weight reduce, drug treatment and dietary modifications, e.g., reduced salt intake, avoidance of excessive alcohol use and exercise (Farpour-Lambert et al., 2009), and non-smoking (Puddey and Beilin, 2006) for controlling mild hypertension.
The traditional use of plants as medicines started with the evolution of societies (Bandaranayake, 2006). Plants and animals have promising possibilities for drugs discovery (Newman and Cragg, 2007; Sahraie-Rad et al., 2015; Sharifi-Rad et al., 2017; Salehi et al., 2018a). Pure compounds might be helpful for treatment of various ailments (Bandaranayake, 2006; Sharifi-Rad et al., 2017, 2018; Salehi et al., 2018b). Alternative herbal medicines are often preferred over modern medicines (Nuwaha and Muganzi, 2008). According to the WHO about 80% of people rely on traditional medicines (Calixto, 2005). A recent study showed that 25% of modern drug and 75% of new medicines against virulent diseases are obtained from natural plant resources (Bedoya et al., 2009). Hypertension, has become one of the most common non-communicable diseases internationally and it is affecting up to 20% of the world's adult population (Osamor and Owumi, 2010). Educated people have more awareness about the side effects of allopathic drugs so they are more expected to take herbal extracts for treatment of hypertension (Gulla and Singer, 2000).
The aim of this study was to document indigenous knowledge related to herbal remedies for the treatment of hypertension by indigenous communities of Northern Pakistan. It is hoped that these results may help the conservation of traditional medicinal knowledge for future generations.
Materials and Methods
Study Area
Northern Pakistan includes Swat valley, Hazara division, Manshera, Naran, Dir and other tribal areas (Figure 1). It shares a border with Gilgit Baltistan in the north east, FATA (Federal Administered Tribal Areas) in the West-South, Punjab in the South east, and Azad Jammu and Kashmir in the North. The northern regions of Pakistan are home of its largest mountain ranges, and these cover 72,496 km2. This region includes the foothills of Himalayas, Hindukush and Karakorum ranges (Hamayun, 2003), and harbor particular plant species used as edible plants, medicinal and aromatic plants (Ali and Qaiser, 1986). The temperature varies from 3.4°C to 34.3°C, with winter snowfall in hilly areas (Chevallier et al., 2011). The area has old cultural traditions, festival, dresses and ceremonies. The majority of people speak Pushto, other local languages are Potohari, Gujrati, and Hindko.
Ethics Approval and Consent to Participate
Before conducting interviews, the individual prior informed consent was obtained from all participants. No further ethics approval was required. All work conducted was carried out under the stipulations of the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity. The right to use and authorship of any traditional knowledge of all participants is maintained, and any use of this information, other than for scientific publication, does require additional prior consent of the traditional owners, as well as a consensus on access to benefits resulting from subsequent use.
Field Survey and Demographic Data
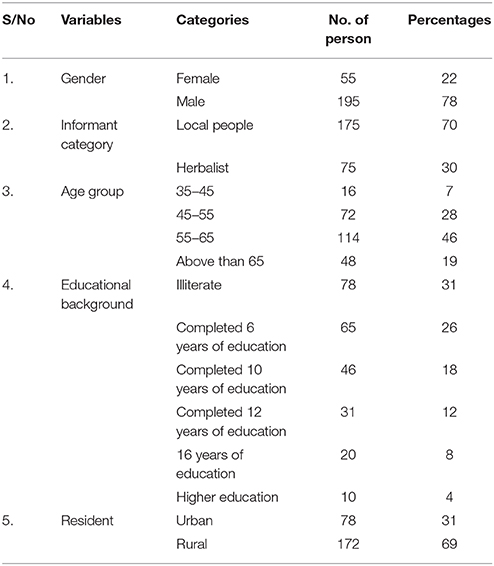
Field surveys were conducted during July 2015 to March 2016 in order to document traditional knowledge in different areas of Northern Pakistan. During the field surveys, the indigenous uses of plants for the treatment of hypertension were recorded through interviews with experienced Traditional Health Practitioners (THPs) and local people. The objectives of the study were clearly explained, prior informed oral consent was obtained, and interviews were conducted in local languages to obtain the knowledge of medicinal plants. Semi-structured questionnaires were used (Martin, 1995). Data documentation and field surveys were assessed by using quantitative indices. Questionnaire (Table 1) was mainly focused on the ethnobotanical knowledge of native communities. In these interviews, the first part of the questionnaire was concerned with demographic information of the participants including gender, age, informant category, educational background and residence while second part contain informative questions about the local names of plant, identification, habit, mode of preparation, plant part used, medicinal use and method of administration. Research articles, relevant web pages and books were also studied with the aim to collect data of phytochemical compounds and toxicity present in the plants. Data documentation and field surveys were assessed by using quantitative ethnobotanical indices.
In this, total of 250 traditional healers (175 local people and 75 herbalists) were interviewed. The local healers diagnosed hypertension by watching for the following symptoms: severe headache, fatigue, chest pain, vision problems, breathing problems, irregular heartbeat, blood in the urine, pounding in the chest, cardiovascular problems and dizziness. The majority of informant falls in the age category of 35–45 years (7%) followed by 45–55 years (28%), 55–65 years (46%) and above than 65 years of age (19%). The participants were divided into different categories on the basis of educational background i-e illiterate (31%), completed 6 years of education (26%), 10 years of education (18%), 12 years of education (12%), and 16 years of education (8%) while (4%) were of higher education (Table 1). Large number of herbal plants were used for treatment of diseases by illiterate villagers showing that in under developed areas people still depend on ethno medicinal plants. Other demographic data was collected with 31% of the participants living in urban and 69% in rural communities (Table 1).
Plant Collection and Identification
The plants were collected from different areas of northern Pakistan in different seasons during the year. In present study medicinal plants reported by the local informants were identified by vernacular names and collected in the field. These specimens were later reconfirmed for correct identification using the services of senior Plant Taxonomist Professor Dr. Mir Ajab Khan (PhD in Plant Taxonomy), from department of Plant Sciences Quaid-i- Azam University, Islamabad. The collected plant specimens were dried and preserved by following standard herbarium techniques recommended by Jain and Rao (1977). The plant names were verified by using databases such as KEW medicinal plant name services (mpns: http://www.kew.org/mpns). Voucher specimens were deposited in the herbarium, of Department of Plant Sciences, Quaid-i-Azam University (ISL), Islamabad.
Quantitative Analysis
Results were analyzed using quantitative indices like Disease Consensus Index (DCI), Frequency of Citation (FC), Relative Frequency Citation (RFC) and Family Importance Value (FIV).
Diseases Consensus Index (DCI)
It is used to evaluate the plant knowledge to cure specific ailment and the degree of consensus that how people recommend plant to treat specific disease. Diseases Consensus Index (DCI) is calculated by using formula followed by Andrade-Cetto et al. (2006).
Where∑(Vxi) = Total sum of the ideal uses for a species. Vx is calculated by using formula Vx = No of questions answered for a species/Total questions asked. The value of Vx ranges from 0 to 1. mVx represents statistical mean of total ideal values (Vxi); CC is correlation coefficient, Pm−0.1 is the compensation factor that analyzes the dispersion of indigenous knowledge considering the mode of preparation and parts used.
Relative Frequency of Citation (RFC)
The RFC is calculated to determine the level of traditional knowledge about the use of medicinal plants in the study areas. Relative Frequency of Citation (RFC) was calculated by using the formula.
Where the number of informants who mentioned the use of the species is “Fc” and “N” is the total number of informants (Tardío and Pardo-De-Santayana, 2008).
Family Importance Value (FIV)
FIV values show the knowledge of informant about the families of plant species used. FIV of the medicinal plant being calculated by using formula as under (Molares and Ladio, 2009).
High FIV value reveals that there is plenty of knowledge and several authors are more known while less FIV values indicates that there is less awareness about the use of family.
Jaccard Index (JI)
Jaccard Index (JI) is calculated by comparing previously published research articles from local, regional and global level by calculating the percentages of cited plants species and their medicinal utilization by using the following formula:
where “a” is the number of plants of the region A, “b” is the number of plants of the region B, and “c” is the number of plants common to A and B (Kayani et al., 2015) (Table 2).
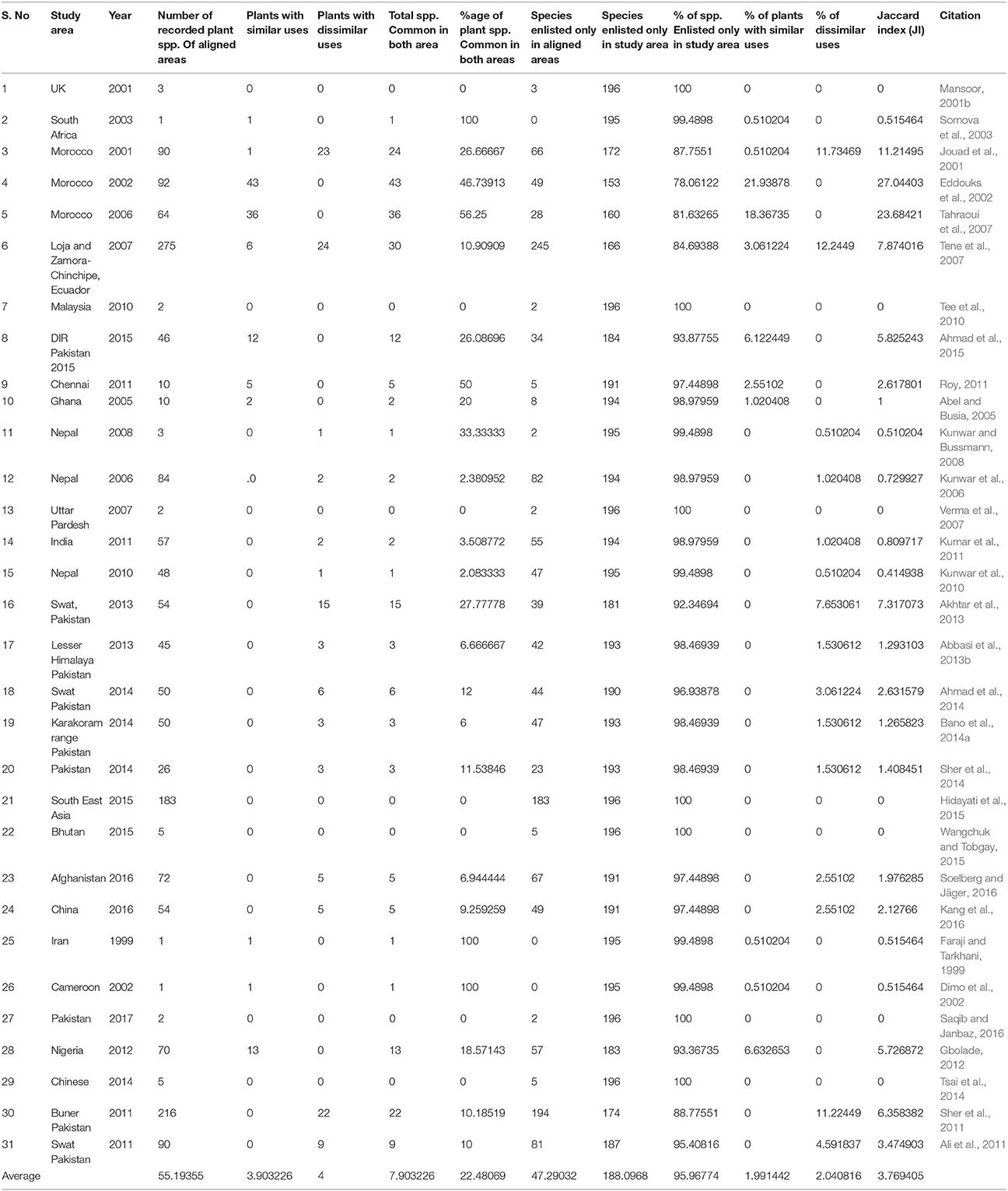
Table 2. Comparison of present study with previous studies at neighboring, regional and global level.
Results
Diversity of Medicinal Plants Hypertension
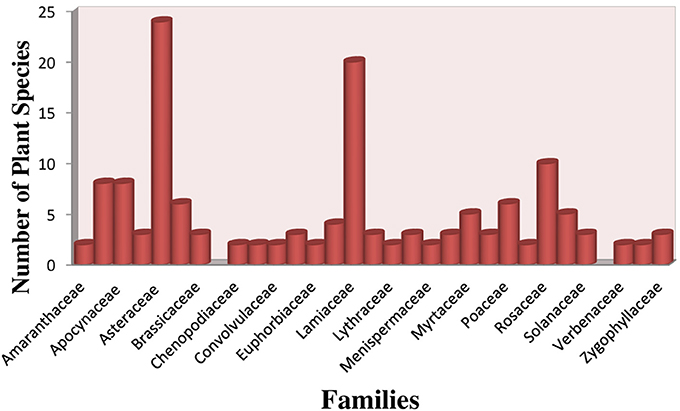
Total 192 plant species have been reported for the treatment of hypertension. Plant information with botanical, English, vernacular and family name, mode of utilization, habit, phytochemistry, toxicity, RFC, FIV, and DCI are given in Supplementary Table 1. In this study medicinal plants belonging to 77 plant families and 171 Genera were reported. Among these Asteraceae was observed to be predominant family (23 species), followed by Lamiaceae (19 species), Rosaceae (10 species), Apiaceae and Apocynaceae (8 species each), Boraginaceae and Poaceae (6 species each) Rutaceae (5 species). Fabaceae (4 species), Asclepiadaceae, Caesalpiniaceae, Brassicaceae, Cucurbitaceae, Moraceae, Zygophyllaceae and Solanaceae (3 species each), Amaranthaceae, Chenopodiaceae, Commelinaceae, Crassulaceae, Euphorbiaceae, Lythraceae, Malvaceae, Menispermaceae, Papaveraceae, Polygonaceae, Valerianaceae, Verbenaceae, Vitaceae (2 species each) and rest of families reported to have only (1 species each) used in hypertension treatment (Figure 2).
Plant Parts Used and Life Forms
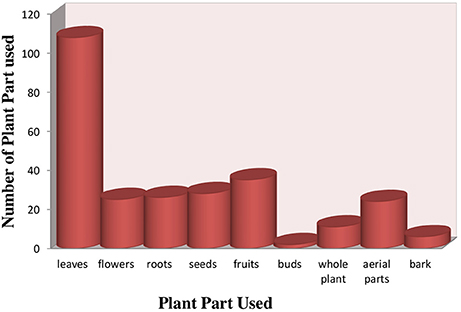
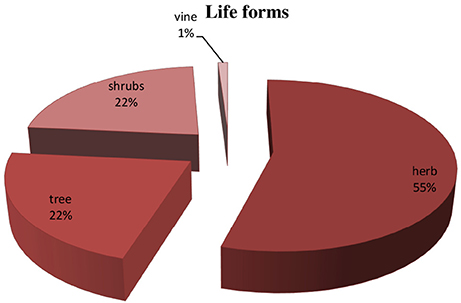
Different plant part used in preparation of herbal recipes include root, stem, leaves, flower, seed, fruit, bark, aerial parts, and whole plant are also used when small herbal plants was practiced. Leaves were most frequently part used (55.1%), followed by fruit (17.8%), seed (14.2%), roots, aerial part and flower (12.7% each), whole plant (5.6%) and bark (3.0%) (Figure 3) (Supplementary Table 1). Essential source of native medicines were as herbs (54%), shrubs (23%), trees (22%) (Figure 4).
Preparation of Herbal Medicines
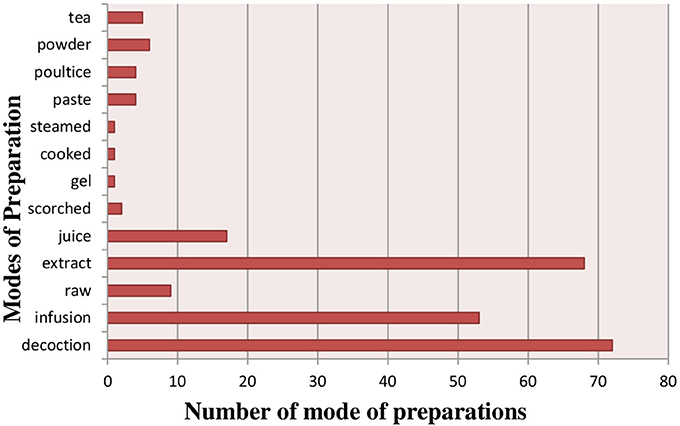
Herbal preparations were made by using different preparation modes i.e., infusion, powder, decoction, extract, juice, paste, tea, poultice, scorched, gel, cooked and steamed (Figure 5) (Supplementary Table 1). The most frequently reported mode of preparation was decoction (72 species), followed by extracts (68 species), infusions (53 species), juice (17 species) and Powder (7 species). Infusions were prepared by soaking a plant in water for more than an hour at room temperature, while decoctions were obtained from boiling the plant part in water until the water volume is reduced to half of its original volume. Juice was obtained by extracting and grinding the fresh part of plants and then mix in any liquid. Paste was made by grinding fresh plant material with water. Different dosage of herbal preparations was used by local communities for treatment of various ailments.
Quantitative Data Analysis
FIV (Family Importance Value)
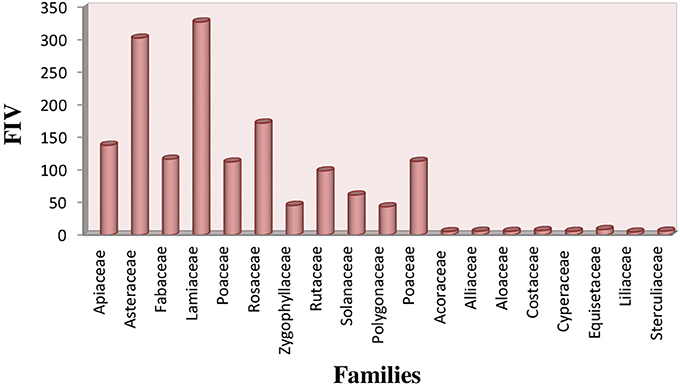
The most common families of indigenous herbal plant species as represented by its FIV were Lamiaceae (327 FIV) and Asteraceae (302 FIV), followed by Rosaceae (174 FIV), Apiaceae (138 FIV), Fabaceae (117 FIV). The least value of FIV was observed for Acoraceae, Alliaceae, Alocaceae and Cyperaceae (5 FIV) (Figure 6).
Disease-Consensus Index DCI
In this study DCI value ranges from 0.233 to 0.000. DCI values are found to be the highest in Citrus limon (0.233). Followed by Crataegus aronia (0.071), Citrus aurantifolia (0.063), Crataegus oxycantha (0.048), Coccinia grandis (0.047), Butea monosperma (0.044), Dodonaea viscosa (0.042), Carex baccans (0.034), Caralluma tuberculata (0.025), Allium cepa (0.022), Acorus calamus (0.020), Bidens pilosa (0.019), Gnaphalium uliginosum (0.018), Cissus simsiana (0.017), Amaranthus spinosus (0.015) and the lowest value observed in Syzygium aromaticum (0.000) (Supplementary Table 1).
RFC (Relative Frequency of Citation)
RFC value ranges from 0.08 to 1.08. The highest RFC value was recorded for Bidens biternata (1.08), followed by Marrubium vulgare (0.97), Stevia rebaudiana, Syzygium aromaticum, Rheum ribes, Phytolacca dioica, Heliotropium lasiocarpum, Foeniculum vulgare, Phyllanthus emblica (0.54) and lowest value was observed for Allium cepa (0.08) (Supplementary Table 1).
Systematic Reviews of Herbal Medicines Used for Hypertension Therapies
The ethnobotanical data is compared with 31 published articles on the use of medicinal plants for hypertension. The analysis showed that some medicinal herbal plants are used for same purposes globally, but some novel uses were also recorded. Out of 192 plant species for hypertension, 81 species were reported with similar uses, while 112 species were documented for hypertension for the first time. These newly reported species for hypertension should be further investigated for detailed clinical and phytochemical studies (Supplementary Table 1). Some potential species reported first time against hypertension include Abrus precatorius, Amarathus spinosus, Cissus simsiana, Citrus aurantifolia, Ficus palmata, Senna tora, Teucrium rogleanom, Valeriana officinalis, Ziziphus mauritiana and others.
Phytochemical Data of Antihypertensive Plants
The active phytochemicals of the plants have been shown in (Supplementary Table 1). These phytochemicals have variety of pharmacological activities that are used to treat hypertension. In this study documentation of plants species indicates, few species are pharmacologically assessed in the literature while other plant species needs more screening in future.
Discussion
In communities of Northern Pakistan people rely mostly on herbal medicines for treatment of various diseases. Mostly the herbal remedies are practiced in the rural communities (Ibrar et al., 2007). Most of the local participants who showed the interest in traditional medicinal knowledge belong to the older age group (Ahmad et al., 2015). Local healers used a large number of different herbs for the treatment of hypertension, and seemed to play a relevant role in the management of hypertension in rural communities, which has important implications for health care workers. Majority of the informants stated that the transmission knowledge about usage of medicinal plants was not efficient due to lack of interest shown by the younger people. It was also observed that some people avoid to used traditional medicines due to availability and convenience of allopathic drugs (Kayani et al., 2015).
The most dominant family reported in terms of medicinal plants was Asteraceae. Of about 350,000 species of identified flowering plants, nearly 10% belong to Asteraceae, and almost every environment in different regions (Barker et al., 2008). This fact could possibly describe the usual occurrence of plant family Asteraceae. Alongside the Lamiaceae has maximum proportion in ethno medicine (Amira and Okubadejo, 2007). However, in study area, more species belonging to Apiaceae and Fabaceae were used as reported in previous literature by Asase et al. (2010).
Participants recognize diverse kinds of plant species with a variability of medicinal properties present in a single family (Abbasi et al., 2013a). Another reason of high citation of Lamiaceae and Asteraceae may be their higher occurrence in mountainous areas as reported in earlier studies (Pieroni, 2008; Mustafa et al., 2011).
Among the medicinal plant species herbs were most commonly used, because of the large number of species naturally abundant in these geographical regions (Abbasi et al., 2013a; Butt et al., 2015) and easily accessible to communities residing in these areas (Tabuti et al., 2003; Uniyal et al., 2006; Sanz-Biset et al., 2009). Herbs are easily accessible having high healing potential and yield secondary metabolites having therapeutical properties against diseases (Bano et al., 2014a; Yaseen et al., 2015a). It was noted that herbalists utilized herbs commonly for formulations due to ease in collection and availability (Uniyal et al., 2006).
In this study leaves were the major plant parts used. It was found that leaves preferably used in treatment of ailments due to significant amount of bioactive compounds present in the leaves (Chaudhary et al., 2010; Rashid et al., 2015). Similar results were reported from studies carried out the in previous literature (Mahishi et al., 2005; Abo et al., 2008; Shil et al., 2014) The flower, stem and leaves of different medicinal plants are used for curing various ailments like hypertension, digestive disorders, fever and others (Lev, 2000; Leporatti and Ivancheva, 2003).
Decoction was the most common method of utilization in present study, this result is in accord with the previous studies (Nadembega et al., 2011; Rehecho et al., 2011). In Pakistan most people prefer to use decoctions of herbal medicines (Mahmood et al., 2011; Khan et al., 2015). In our study Mentha longifolia leaf extract made from the leaves are used for treating hypertension this result is similar with previous study of (Hwang et al., 1987) in which aqueous-methanolic extract of this plant, showed a significant decrease in the blood pressure and heart rate. Tinospora malabarica, Sonchus asper, Cestrum racemosum, Callisia gracilis, and Cymbopogon citratus were also used against hypertension in other areas (Poffenberger and Singh, 1992; Steyn et al., 2001; Dharmananda, 2012). In high altitudes areas in winter season, dried powder is favored for curing different diseases (Ahmad et al., 2014; Bano et al., 2014b; Kayani et al., 2014). The most common method of administration of herbal drugs was oral (70%), like in other areas (Hammond et al., 1998; De-La-Cruz et al., 2007). Herbal medicines are mostly bitter in taste and prepared by mixing with sugar and honey (Sadeghi et al., 2014). In study area, the dosage of herbal remedies varied, like found in others studies (Ahmed et al., 2015). Many plants have been reported in previous literature for treating hypertension, blood pressure diseases such as Allium sativum, Berberis vulgaris, Coriandrum sativum and various other similar plants have been identified in present study are reported for curing hypertension (Sher et al., 2016).
DCI was evaluated in present study to analyze the disease consensus of informants for traditional remedies of hypertension (Choudhury et al., 2015; Yaseen et al., 2015b). Species having higher DCI value can be preferred for further future studies (Andrade-Cetto and Wiedenfeld, 2011). Pharmacological studies show that Allium sativum, Lepidium sativum, and Ocimum basilicum, Mentha sp., Trigonella foenum-graecum, Urtica dioica, Olea species, and Eucalyptus globulus are effective species in treating hypertension (Jouad et al., 2001; El-Hilaly et al., 2003; Gurib-Fakim, 2006). The root infusion of Acorus calamus is used as plant for anti-hypertension properties in this study which is in accord with the study of (Patel et al., 2012) who reported antihypertensive effect of rhizome part of Acorus calamus on renal artery occlusion induced hypertension in rats.
RFC refers the local importance of each plant species with reference to informant who cited this specie (Vitalini et al., 2013). The reason for high RFC value may be the easy availability of species, wide distribution and high medicinal properties for treating various ailments (Mukherjee and Wahile, 2006). Foeniculum vulgare, Nerium oleander, Olea europaea, Allium sativum, Mentha sp., Eucalyptus globulus, Nigella sativa, and Lepidium sativum were documented for the first time for treating hypertension (Eddouks et al., 2002; Zaoui et al., 2002) (Supplementary Table 1).
The comparison of our results shows broad variations with previous studies regarding JI when the findings of these studies were compared with the present work (Leonti et al., 2003; Ladio et al., 2007). In our study JI varies from 0 to 27.04%. The highest degree of similarity was recorded with (Eddouks et al., 2002; Tahraoui et al., 2007), while lowest similarity was found with (Mansoor, 2001a; Somova et al., 2003; Abel and Busia, 2005; Ahmad et al., 2005; Tee et al., 2010). The phytochemicals have variety of pharmacological activities that are used to treat hypertension. The study indicates many important medicinal plant species which are pharmacologically active but still unexplored and need to be explored further.
Conclusion
The present study gave an overview about traditional medicinal knowledge of plants as anti-hypertensive drug. 192 medicinal plant species belonging to 77 families were reported to be in present study to treat hypertension. It was first attempt to document ethno-botanical information using quantitative approaches on hypertension in the study area. The leaves were reported to be most used plant part (55.1%) while herbs were the most used life form (54%) and decoction being the most common mode of administration (72 reports). The quantitative approaches such as Family importance value (FIV), Relative Frequency of Citation (RFC) and Disease Consensus index (DCI) were used to assess the importance of traditional knowledge obtained in the study used in the present study. Highest DCI and FIV values were reported for Rutaceae and Lamiaceae. Meanwhile there are so many of these naturally occurring plant substances cover a wide range they offers a good opportunity of delivering useful medicinal complexes for the management of hypertension. To the best of our knowledge, Ficus palmata, Senna tora, Teucrium polium, Valeriana officinalis, and Ziziphus mauritiana were recorded for the first time as anti-hypertensive medicinal drugs. The study provides basic leads for future pharmacological and phytochemical investigation to explore the potential of such plants in herbal drug discovery. It is thus recommended that strategies for cultivation and conservation of important species be designed.
Author Contributions
KM carried out field surveys and data collection. MZ and NR helped in analysis of data while MA and RB revised the manuscript critically to its present form. AT, RU, AA, AS, SS, and SNS helped in the revision of the manuscript and helps in checking the consistency of data. All authors read the final manuscript and agreed to its submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors acknowledge the continuous efforts of the Research Centre, College of Pharmacy, and Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia. The authors are thankful to all key medicinal plant practitioners and informants for sharing their valuable knowledge on medicinal flora.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00789/full#supplementary-material
References
Abbasi, A. M., Khan, M. A., Khan, N., and Shah, M. H. (2013a). Ethnobotanical survey of medicinally important wild edible fruits species used by tribal communities of lesser Himalayas-Pakistan. J. Ethnopharmacol. 148, 528–536. doi: 10.1016/j.jep.2013.04.050
Abbasi, A. M., Khan, M. A., Shah, M. H., Shah, M. M., Pervez, A., and Ahmad, M. (2013b). Ethnobotanical appraisal and cultural values of medicinally important wild edible vegetables of lesser Himalayas-Pakistan. J. Ethnobiol. Ethnomed. 9:66. doi: 10.1186/1746-4269-9-66
Abdel-Sattar, E., Harraz, F. M., Al-Ansari, S. M., El-Mekkawy, S., Ichino, C., Kiyohara, H., et al. (2008). Acylated pregnane glycosides from Caralluma tuberculata and their antiparasitic activity. Phytochemistry 69, 2180–2186. doi: 10.1016/j.phytochem.2008.05.017
Abel, C., and Busia, K. (2005). An exploratory ethnobotanicastudy of the practice of herbal medicine by the Akan peoples of Ghana. Alter. Med. Rev. J. clin. Ther. 10, 112–122.
Abo, K. A., Fred-Jaiyesimi, A. A., and Jaiyesimi, A. E. (2008). Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J. Ethnopharmacol. 115, 67–71. doi: 10.1016/j.jep.2007.09.005
Abubacker, M., and Ramanathan, R. (2012). Antibacterial activities of argemone mexicana L. (Papavaraceae) leaf extract on pathogenic bacterial strains. Drug Invent. Today 4, 385–387. doi: 10.1016/S2221-1691(12)60316-5
Abukakar, M., Ukwuani, A., and Shehu, R. (2008). Phytochemical screening and antibacterial activity of Tamarindus indica pulp extract. Asian. J. Biochem. 3, 134–138. doi: 10.3923/ajb.2008.134.138
Adebayo, M., Adeboye, J., and Ajaiyeoba, E. (2004). Preliminary phytochemical investigation and diuretic studies of Alstonia boonei stem bark in male Wistar rats. J. Nat. Remedies 4, 179–182. doi: 10.18311/jnr/2004/184
Adeniyi, S., Orjiekwe, C., Ehiagbonare, J., and Arimah, B. (2010). Preliminary phytochemical analysis and insecticidal activity of ethanolic extracts of four tropical plants (Vernonia amygdalina, Sida acuta, Ocimum gratissimum and Telfaria occidentalis) against beans weevil (Acanthscelides obtectus). Int. J. Phy. Sci. 5, 753–762.
Ahameethunisa, A. R., and Hopper, W. (2012). In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann. Clin. Microbiol. Antimicrob. 11:30. doi: 10.1186/1476-0711-11-30
Ahmad, A., and Misra, L. (1997). Isolation of herniarin and other constituents from Matricaria chamomilla flowers. Int. J. Pharmacognosy 35, 121–125. doi: 10.1076/phbi.35.2.121.13280
Ahmad, F., and Tawan, C. (2002). Phytochemical Studies on Piper Umbellatum L. Sarawak: ASEAN Review of Biodiversity and Environmental Conservation (ARBEC).
Ahmad, I., and Beg, A. Z. (2001). Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 74, 113–123. doi: 10.1016/S0378-8741(00)00335-4
Ahmad, L., Semotiuk, A., Zafar, M., Ahmad, M., Sultana, S., Liu, Q. R., et al. (2015). Ethnopharmacological documentation of medicinal plants used for hypertension among the local communities of DIR Lower, Pakistan. J. Ethnopharmacol. 175, 138–146. doi: 10.1016/j.jep.2015.09.014
Ahmad, M., Sultana, S., Fazl-I-Hadi, S., Ben Hadda, T., Rashid, S., Zafar, M., et al. (2014). An Ethnobotanical study of medicinal plants in high mountainous region of Chail valley (District Swat-Pakistan). J. Ethnobiol. Ethnomed. 10, 4269–4210. doi: 10.1186/1746-4269-10-36
Ahmad, S., Latif, A., Qasmi, I. A., and Amin, K. M. (2005). An experimental study of sexual function improving effect of Myristica fragrans Houtt.(nutmeg). BMC Complement. Altern. Med. 5:16. doi: 10.1186/1472-6882-5-16
Ahmadiani, A., Javan, M., Semnanian, S., Barat, E., and Kamalinejad, M. (2001). Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J. Ethnopharmacol. 75, 283–286. doi: 10.1016/S0378-8741(01)00187-8
Ahmed, M. F., Rao, A. S., Ahemad, S. R., and Ibrahim, M. (2012). Phytochemical studies and antioxidant activity of Melia azedarach Linn leaves by DPPH scavenging assay. Int. J. Pharm. Appl. 3, 271–276.
Ahmed, N., Mahmood, A., Ashraf, A., Bano, A., Tahir, S. S., and Mahmood, A. (2015). Ethnopharmacological relevance of indigenous medicinal plants from district Bahawalnagar, Punjab, Pakistan. J. Ethnopharmacol. 175, 109–123. doi: 10.1016/j.jep.2015.08.011
Akhtar, N., Rashid, A., Murad, W., and Bergmeier, E. (2013). Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. J. Ethnobiol. Ethnomed. 9:25. doi: 10.1186/1746-4269-9-25
Akubugwo, I., Obasi, N., Chinyere, G., and Ugbogu, A. (2008). Mineral and phytochemical contents in leaves of Amaranthus hybridus L and Solanum nigrum L. subjected to different processing methods. African. J. Biochem. Res. 2, 040–044.
Al-Ghaithi, F., El-Ridi, M. R., Adeghate, E., and Amiri, M. H. (2004). Biochemical effects of Citrullus colocynthis in normal and diabetic rats. Mol. Cell. Biochem. 261, 143–149. doi: 10.1023/B:MCBI.0000028749.63101.cc
Al-Tameme, H. J., Hameed, I. H., Idan, S. A., and Hadi, M. Y. (2015). Biochemical analysis of Origanum vulgare seeds by fourier-transform infrared (FT-IR) spectroscopy and gas chromatography-mass spectrometry (GC-MS). J. Pharmacognosy Phytother. 7, 221–237. doi: 10.5897/JPP2015.0362
Ali, B. H., Blunden, G., Tanira, M. O., and Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food. Chem. Toxicol. 46, 409–420. doi: 10.1016/j.fct.2007.09.085
Ali, B. H., Wabel, N., and Blunden, G. (2005). Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariff a L.: a review. Phytother. Res. 19, 369–375. doi: 10.1002/ptr.1628
Ali, H., Sannai, J., Sher, H., and Rashid, A. (2011). Ethnobotanical profile of some plant resources in Malam Jabba valley of Swat, Pakistan. J. Med. Plants Res. 5, 4676–4687.
Ali, S. I., and Qaiser, M. (1986). A phytogeographical analysis of the phanerogams of Pakistan and Kashmir. Proc. R. Soc. Edinb. Biolsci. 89, 89–101. doi: 10.1017/S0269727000008939
Alok, S., Jain, S. K., Verma, A., Kumar, M., Mahor, A., and Sabharwal, M. (2013). Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): A review. Asian Pac. J. Trop. Dis. 3, 242–251. doi: 10.1016/S2222-1808(13)60049-3
Alqasoumi, S. I., Basudan, O. A., Al-Rehaily, A. J., and Abdel-Kader, M. S. (2014). Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia. Saudi Pharm. J. 22, 460–471. doi: 10.1016/j.jsps.2013.12.010
Amira, O. C., and Okubadejo, N. U. (2007). Frequency of complementary and alternative medicine utilization in hypertensive patients attending an urban tertiary care centre in Nigeria. BMC Complement. Altern. Med. 7:30. doi: 10.1186/1472-6882-7-30
Andrade-Cetto, A., Becerra-Jiménez, J., Martínez-Zurita, E., Ortega-Larrocea, P., and Heinrich, M. (2006). Disease-consensus Index as a tool of selecting potential hypoglycemic plants in Chikindzonot, Yucatán, México. J. Ethnopharmacol. 107, 199–204. doi: 10.1016/j.jep.2006.03.005
Andrade-Cetto, A., and Wiedenfeld, H. (2011). Anti-hyperglycemic effect of Opuntia streptacantha Lem. J. Ethnopharmacol. 133, 940–943. doi: 10.1016/j.jep.2010.11.022
Anwar, F., Abdul Qayyum, H. M., Ijaz Hussain, A., and Iqbal, S. (2010). Antioxidant activity of 100% and 80% methanol extracts from barley seeds (Hordeum vulgare L.): stabilization of sunflower oil. Grasas y Aceites 61, 237–243. doi: 10.3989/gya.087409
Anwar, F., Latif, S., Ashraf, M., and Gilani, A. H. (2007). Moringa oleifera: a food plant with multiple medicinal uses. Phytother. Res. 21, 17–25. doi: 10.1002/ptr.2023
Arunkumar, S., and Muthuselvam, M. (2009). Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J. Agri. Sci. 5, 572–576.
Asaolu, M., Oyeyemi, O., and Olanlokun, J. (2009). Chemical compositions, phytochemical constituents and in vitro biological activity of various extracts of Cymbopogon citratus. Pak. J. Nutr. 8, 1920–1922. doi: 10.3923/pjn.2009.1920.1922
Asase, A., Akwetey, G. A., and Achel, D. G. (2010). Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J. Ethnopharmacol. 129, 367–376. doi: 10.1016/j.jep.2010.04.001
Ashafa, A. O., Sunmonu, T. O., and Afolayan, A. J. (2010). Toxicological evaluation of aqueous leaf and berry extracts of Phytolacca dioica L. in male Wistar rats. Food Chem. Toxicol. 48, 1886–1889. doi: 10.1016/j.fct.2010.04.029
Ayatollahi, S., Kobarfard, F., Asgarpanah, J., Roodsari, M. R., Fanai, G., and Choudhary, M. I. (2015). Diterpenoids of Otostegia persica (Burm.) Boiss. DARU J. Pharm. Sci. 17, 290–293.
Ayoola, P., and Adeyeye, A. (2010). Phytochemical and nutrient evaluation of Carica papaya (pawpaw) leaves. IJRRAS 5, 325–328.
Bahramsoltani, R., Rostamiasrabadi, P., Shahpiri, Z., Marques, A. M., Rahimi, R., and Farzaei, M. H. (2018). Aloysia citrodora Paláu (Lemon verbena): a review of phytochemistry and pharmacology. J. Ethnopharmacol. 22, 34–51. doi: 10.1016/j.jep.2018.04.021
Bandaranayake, W. M. (2006). Quality control, screening, toxicity, and regulation of herbal drugs. Modern phytomedicine. Turning Med. Plants Drugs 10, 25–57. doi: 10.1002/9783527609987.ch2
Bano, A., Ahmad, M., Ben Hadda, T., Saboor, A., Sultana, S., Zafar, M., et al. (2014a). Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. J. Ethnobiol. Eethnomed. 10:43. doi: 10.1186/1746-4269-10-43
Bano, A., Ahmad, M., Zafar, M., Sultana, S., Rashid, S., and Khan, M. A. (2014b). Ethnomedicinal knowledge of the most commonly used plants from Deosai Plateau, Western Himalayas, Gilgit Baltistan, Pakistan. J. Ethnopharmacol. 155, 1046–1052. doi: 10.1016/j.jep.2014.05.045
Banso, A., and Adeyemo, S. (2006). Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemistri 18, 39–44.
Barker, M. S., Kane, N. C., Matvienko, M., Kozik, A., Michelmore, R. W., Knapp, S. J., et al. (2008). Multiple paleopolyploidizations during the evolution of the compositae reveal parallel patterns of duplicate gene retention after millions of years. Mol. Biol. Evol. 25, 2445–2455. doi: 10.1093/molbev/msn187
Basma, A. A., Zakaria, Z., Latha, L. Y., and Sasidharan, S. (2011). Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac. J. Trop. Med. 4, 386–390. doi: 10.1016/S1995-7645(11)60109-0
Bedoya, L. M., Bermejo, P., and Abad, M. J. (2009). Anti-infectious activity in the cistaceae family in the Iberian Peninsula. Mini. Rev. Med. Chem. 9, 519–525. doi: 10.2174/138955709788167600
Bettaieb, I., Bourgou, S., Wannes, W. A., Hamrouni, I., Limam, F., and Marzouk, B. (2010). Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J. Agric. Food Chem. 58, 10410–10418. doi: 10.1021/jf102248j
Bhatt, I. D., Dauthal, P., Rawat, S., Gaira, K. S., Jugran, A., Rawal, R. S., et al. (2012). Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Sci. Hortic. 136, 61–68. doi: 10.1016/j.scienta.2011.12.032
Bhore, N., Pishawikar, S., and More, H. (2012). Phytochemical screening and antioxidant activity of flowers (inflorescence) of Saccharum officinarum linn. Int. J. Res. Pharm. Biomed. Sci. 3, 620–624.
Brand-Williams, W., Cuvelier, M., and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Tech. 28, 25–30. doi: 10.1016/S0023-6438(95)80008-5
Brandão, M. G., Krettli, A. U., Soares, L. S., Nery, C. G., and Marinuzzi, H. C. (1997). Antimalarial activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. J. Ethnopharmacol. 57, 131–138. doi: 10.1016/S0378-8741(97)00060-3
Britto, A., and Gracelin, H. (2011). Phytochemical analysis and antibacterial activity of Momordica charantia descourt., a know n medicinal plant. J. Basic and Appl. Biol. 5, 307–311.
Butt, M. A., Ahmad, M., Fatima, A., Sultana, S., Zafar, M., Yaseen, G., et al. (2015). Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. J. Ethnopharmacol. 168, 164–181. doi: 10.1016/j.jep.2015.03.045
Babajide, J. O., Mabusela, W. T., and Green, I. R. (2015). Some alkaloids and flavonoids from Cissampelos capensis. J. Med. Plants Res. 9, 16–29. doi: 10.5897/JMPR2014.5659
Billeter, M., Meier, B., and Sticher, O. (1991). 8-Hydroxyflavonoid glucuronides from Malva sylvestris. Phytochemistry 30, 987–990. doi: 10.1016/0031-9422(91)85292-8
Bischoff, T. A., Kelley, C. J., Karchesy, Y., Laurantos, M., Nguyen-Dinh, P., and Arefi, A. G. (2004). Antimalarial activity of Lactucin and Lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 95, 455–457. doi: 10.1016/j.jep.2004.06.031
Bukhari, N., Choi, J., Jeon, C., Park, H., Kim, W., Khan, M., et al. (2008). Phytochemical studies of the alkaloids from Peganum harmala. Appl. Chem. 12, 101–104.
Bystrom, L. M., Lewis, B. A., Brown, D. L., Rodriguez, E., and Obendorf, R. L. (2009). Phenolics, sugars, antimicrobial and free-radical-scavenging activities of Melicoccus bijugatus Jacq. fruits from the Dominican Republic and Florida. Plant Foods Human Nutr. 64, 160–166. doi: 10.1007/s11130-009-0119-y
Calişkan, O., Gündüz, K., Serçe, S., Toplu, C., Kamiloglu, O., and Sengül, M., (2012). Phytochemical characterization of several hawthorn (Crataegus spp.) species sampled from the Eastern Mediterranean region of Turkey. Pharmacogn. Mag. 8:16. doi: 10.4103/0973-1296.93305
Calixto, J. B. (2005). Twenty-five years of research on medicinal plants in Latin America: a personal view. J. Ethnopharmacol. 100, 131–134. doi: 10.1016/j.jep.2005.06.004
Cardoso, C. R., and Salles, G. F. (2016). Prognostic importance of ambulatory blood pressure monitoring in resistant hypertension: is it all that matters? Curr. Hypertens. Rep. 18:85. doi: 10.1007/s11906-016-0693-y
Chaudhary, G., Goyal, S., and Poonia, P. (2010). Lawsonia inermis Linnaeus: a phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2, 91–98.
Chen, Y.-Y., Chang, F.-R., and Wu, Y.-C. (1996). Isoquinoline alkaloids and lignans from Rollinia mucosa. J. Nat. Prod. 59, 904–906. doi: 10.1021/np960414z
Chevallier, P., Arnaud, Y., and Ahmad, B. (2011). Snow cover dynamics and hydrological regime of the Hunza River basin, Karakoram Range, Northern Pakistan. Hydrol. Earth Sys. Sci. 15, 2259–2274.
Choudhury, P. R., Choudhury, M. D., Ningthoujam, S. S., Mitra, A., Nath, D., and Talukdar, A. D. (2015). Plant utilization against digestive system disorder in Southern Assam, India. J. Ethnopharmacol. 175, 192–197. doi: 10.1016/j.jep.2015.09.020
Chung, M., Lei, B., and Li-Chan, E. (2005). Isolation and structural characterization of the major protein fraction from NorMan flaxseed (Linum usitatissimum L.). Food Chem. 90, 271–279. doi: 10.1016/j.foodchem.2003.07.038
Corsi, G., Lokar, L., and Pagni, A. M. (1984). Biological and phytochemical aspects of Valeriana officinalis. Biochem. Sys. Ecol. 12, 57–62. doi: 10.1016/0305-1978(84)90011-5
Da Silva, K. L., Biavatti, M. W., Leite, S. N., Yunes, R. A., Delle Monache, F., and Cechinel Filho, V. (2000). Phytochemical and pharmacognostic investigation of Bauhinia forficata link (Leguminosae). Zeitschrift Naturforschung C 55, 478–480. doi: 10.1515/znc-2000-5-627
Dahiru, D., William, E., and Nadro, M. (2005). Protective effect of Ziziphus mauritiana leaf extract on carbon tetrachloride-induced liver injury. Afr. J. Biotechnol. 4, 1177–1179.
Dall'acqua, S., Cervellati, R., Loi, M. C., and Innocenti, G. (2008). Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 106, 745–749.
Daniel, V., Daniang, I., and Nimyel, N. (2011). Phytochemical analysis and mineral elements composition of Ocimum basilicum obtained in JOS METROPOLIS, Plateau state Nigeria. IJET-IJENS 11, 161–165.
De-La-Cruz, H., Vilcapoma, G., and Zevallos, P. A. (2007). Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. J. Ethnopharmacol. 111, 284–294. doi: 10.1016/j.jep.2006.11.018
Dhale, D., and Mogle, U. (2011). Phytochemical screening and antibacterial activity of Phyllanthus emblica (L.). Sci. Res. Rep. 1:138–142.
Dharmananda, S. (2012). Bidens: a popular remedy escapes notice of western practitioners. Inst. Tradit. Med.
Dimo, T., Rakotonirina, S. V., Tan, P. V., Azay, J., Dongo, E., and Cros, G. (2002). Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J. Ethnopharmacol. 83, 183–191. doi: 10.1016/S0378-8741(02)00162-9
Dinda, B., Gosh, B., Arima, S., Sato, N., and Harigaya, Y. (2004). Phytochemical investigation of Gomphrena globosa aerial parts. Ind. J. Chem. Sect. B Organ. Chem. 43, 2223–2227.
Dittbrenner, A., Lohwasser, U., Mock, H.-P., and Börner, A. (2007). “Molecular and phytochemical studies of Papaver somniferum in the context of infraspecific classification,” in V International Symposium on the Taxonomy of Cultivated Plants, Vol. 799 (Gatersleben), 81–88.
Ebana, R. U., Madunagu, B. E., Ekpe, E. D., and Otung, I. N. (1991). Microbiological exploitation of cardiac glycosides and alkaloids from Garcinia kola, Borreria ocymoides, Kola nitida and Citrus aurantifolia. J. Appl. Microbiol. 71, 398–401.
Eberhardt, M. V., Lee, C. Y., and Liu, R. H. (2000). Nutrition: antioxidant activity of fresh apples. Nature 405, 903–904. doi: 10.1038/35016151
Eddouks, M., Maghrani, M., Lemhadri, A., Ouahidi, M. L., and Jouad, H. (2002). Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 82, 97–103. doi: 10.1016/S0378-8741(02)00164-2
Edeoga, H., Ikem, C., and Jäger, A. (2002). Tannins, saponins and calcium oxalate crystals from Nigerian species of Boerhavia L. (Nyctaginaceae). South Afr. J. Bot. 68, 386–388. doi: 10.1016/S0254-6299(15)30403-8
El-Hilaly, J., Hmammouchi, M., and Lyoussi, B. (2003). Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J. Ethnopharmacol. 86, 149–158. doi: 10.1016/S0378-8741(03)00012-6
Erosa-Rejón, G., Peña-Rodríguez, L. M., and Sterner, O. (2009). Secondary metabolites from Heliotropium angiospermum. J. Mex. Chem. Soc. 53, 44–47.
Faraji, M. H., and Tarkhani, A. H. (1999). The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 65, 231–236. doi: 10.1016/S0378-8741(98)00157-3
Farpour-Lambert, N. J., Aggoun, Y., Marchand, L. M., Martin, X. E., Herrmann, F. R., and Beghetti, M. (2009). Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J. Am. Coll. Cardiol. 54, 2396–2406. doi: 10.1016/j.jacc.2009.08.030
Fattouch, S., Caboni, P., Coroneo, V., Tuberoso, C. I., Angioni, A., Dessi, S., et al. (2007). Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J. Agric. Food Chem. 55, 963–969. doi: 10.1021/jf062614e
Gallagher, M., Perkovic, V., and Chalmers, J. (2006). Diuretics: a modern day treatment option?(Review Article). Nephrology 11, 419–427. doi: 10.1111/j.1440-1797.2006.00598.x
Gbolade, A. (2012). Ethnobotanical study of plants used in treating hypertension in Edo State of Nigeria. J. Ethnopharmacol. 144, 1–10. doi: 10.1016/j.jep.2012.07.018
Genig, A. Y., and Ladnaya, L. Y. (1980). Phytochemical investigation of Filipendula ulmaria Maxim. and Filipendula hexapetala Gilib. of Lvov region flora. Farmatsevtichnii Zhurnal (Kiev) 1, 50–52.
Germanó, M. P., De Pasquale, R., D'angelo, V., Catania, S., Silvari, V., and Costa, C. (2002). Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J. Agric. Food Chem. 50, 1168–1171. doi: 10.1021/jf010678d
Gharzouli, K., Khennouf, S., Amira, S., and Gharzouli, A. (1999). Effects of aqueous extracts from Quercus ilex l. root bark, Punica granatum l. fruit peel and Artemisia herba-alba Asso leaves on ethanol-induced gastric damage in rats. Phytother. Res. 13, 42–45.
Ghosal, S. (1985). Steryl glycosides and acyl steryl glycosides from Musa paradisiaca. Phytochemistry 24, 1807–1810. doi: 10.1016/S0031-9422(00)82556-X
Ghosh, J., and Bandyopadhyay, A. (2007). Comparative evaluation of obesity measures: relationship with blood pressures and hypertension. Singapore Med. J. 48, 232–235.
Gilani, S. A., Kikuchi, A., Shinwari, Z. K., Khattak, Z. I., and Watanabe, K. N. (2007). Phytochemical, pharmacological and ethnobotanical studies of Rhazya stricta Decne. Phytother. Res. 21, 301–307. doi: 10.1002/ptr.2064
Gokavi, S. S., Malleshi, N. G., and Guo, M. (2004). Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods Human Nutr. 59, 105–111. doi: 10.1007/s11130-004-4308-4
González-Molina, E., Domínguez-Perles, R., Moreno, D. A., and García-Viguera, C. (2010). Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 51, 327–345. doi: 10.1016/j.jpba.2009.07.027
Govindasamy, C., and Srinivasan, R. (2012). In vitro antibacterial activity and phytochemical analysis of Catharanthus roseus (Linn.) G. Don. Asian Pac. J. Trop. Biomed. 2, S155–S158. doi: 10.1016/S2221-1691(12)60148-8
Grace, O., Light, M., Lindsey, K., Mulholland, D., Van Staden, J., Jager, A., et al. (2002). Antibacterial activity and isolation of active compounds from fruit of the traditional African medicinal tree Kigelia africana. South Afr. J. Bot. 68, 220–222. doi: 10.1016/S0254-6299(15)30424-5
Grande, M., Torres, P., Piera, F., and Bellido, I. S. (1992). Triterpenoids from Dittrichia viscosa. Phytochemistry 31, 1826–1828. doi: 10.1016/0031-9422(92)83159-V
Grassi, G., Bombelli, M., Seravalle, G., Brambilla, G., Dell'oro, R., and Mancia, G. (2013). Role of ambulatory blood pressure monitoring in resistant hypertension. Curr. Hypertens. Rep. 15, 232–237. doi: 10.1007/s11906-013-0349-0.
Grosso, C., Vinholes, J., Silva, L. R., Pinho, P. G. D., Gonçalves, R. F., Valentão, P., et al. (2011). Chemical composition and biological screening of Capsella bursa-pastoris. Revista Brasileira de Farmacognosia 21, 635–643. doi: 10.1590/S0102-695X2011005000107
Gress, T. W., Nieto, F. J., Shahar, E., Wofford, M. R., and Brancati, F. L. (2000). Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. N. Engl. J. Med. 342, 905–912. doi: 10.1056/NEJM200003303421301
Guendez, R., Kallithraka, S., Makris, D. P., and Kefalas, P. (2005). An analytical survey of the polyphenols of seeds of varieties of grape (Vitis vinifera) cultivated in Greece: implications for exploitation as a source of value-added phytochemicals. Phytochem. Anal. 16, 17–23. doi: 10.1002/pca.804
Gülçin, I., Küfrevioglu, O. I., Oktay, M., and Büyükokuroglu, M. E. (2004). Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 90, 205–215. doi: 10.1016/j.jep.2003.09.028
Gulfraz, M., Sadiq, A., Tariq, H., Imran, M., Qureshi, R., and Zeenat, A. (2011). Phytochemical analysis and antibacterial activity of Eruca sativa seed. Pak. J. Bot. 43, 1351–1359.
Gulla, J., and Singer, A. J. (2000). Use of alternative therapies among emergency department patients. Ann. Emerg. Med. 35, 226–228. doi: 10.1016/S0196-0644(00)70072-2
Gupta, R. K., Bajracharya, G. B., and Jha, R. N. (2014). Antibacterial activity, cytotoxicity, antioxidant capacity and phytochemicals of Rheum australe rhizomes of Nepal. J. Pharmacognosy and Phytochem. 2, 125–128.
Gupta, V., and Mittal, P. (2010). Phytochemical and pharmacological potential of Nerium oleander. Pharm. Sci. Res. 1, 21–27.
Gurib-Fakim, A. (2006). Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 27, 1–93. doi: 10.1016/j.mam.2005.07.008
Hajjar, I., and Kotchen, T. A. (2003). Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA 290, 199–206. doi: 10.1001/jama.290.2.199
Hamayun, M. (2003). Ethnobotanical studies of some useful shrubs and trees of District Buner, NWFP, Pakistan. J. Ethnob. Leaflets 2003:12. Available online at: http://opensiuc.lib.siu.edu/ebl/vol2003/iss1/12
Hammond, G. B., Fernández, I. D., Villegas, L. F., and Vaisberg, A. J. (1998). A survey of traditional medicinal plants from the Callejon de Huaylas, department of Ancash, Peru. J. Ethnopharmacol. 61, 17–30. doi: 10.1016/S0378-8741(98)00009-9
Harisaranraj, R., Suresh, K., and Saravanababu, S. (2009). Evaluation of the chemical composition Rauwolfia serpentina and Ephedra vulgaris. Adv. Biol. Res. (Rennes). 3, 174–178.
Hayes, J., Allen, P., Brunton, N., O'grady, M., and Kerry, J. (2011). Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 126, 948–955. doi: 10.1016/j.foodchem.2010.11.092
Heisler, J., Glibert, P., Burkholder, J., Anderson, D., Cochlan, W., Dennison, W., et al. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. doi: 10.1016/j.hal.2008.08.006
Hajhashemi, V., Ghannadi, A., and Sharif, B. (2003). Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 89, 67–71. doi: 10.1016/S0378-8741(03)00234-4
Helal, A. M., Nakamura, N., El-Askary, H., and Hattori, M. (2000). Sesquiterpene lactone glucosides from Sonchus asper. Phytochemistry 53, 473–477. doi: 10.1016/S0031-9422(99)00516-6
Hosni, K., Hassen, I., Sebei, H., and Casabianca, H. (2013). Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: chemical composition and biological activities. Ind. Crops Prod. 44, 263–271 doi: 10.1016/j.indcrop.2012.11.033
Hidayati, S., Franco, F. M., and Bussmann, R. W. (2015). Ready for phase 5-current status of ethnobiology in Southeast Asia. J. Ethnobiol. Ethnomed. 11:17. doi: 10.1186/s13002-015-0005-7
Huang, H., Liu, Y., Meng, Q., Wei, S., Cui, H., and Zhang, C. (2010). Flavonolignans and other phenolic compounds from Sorghum halepense (L.) Pers. Biochem. Syst. Ecol. 38, 656–658. doi: 10.1016/j.bse.2010.03.013
Hussain, A. I., Anwar, F., Chatha, S. A., Latif, S., Sherazi, S. T., Ahmad, A., et al. (2013). Chemical composition and bioactivity studies of the essential oils from two thymus species from the Pakistani flora. LWT Food Sci. Tech. 50, 185–192. doi: 10.1016/j.lwt.2012.06.003
Hwang, I. S., Ho, H., Hoffman, B. B., and Reaven, G. M. (1987). Fructose-induced insulin resistance and hypertension in rats. Hypertension 10, 512–516. doi: 10.1161/01.HYP.10.5.512
Ibrar, M., Hussain, F., and Sultan, A. (2007). Ethnobotanical studies on plant resources of Ranyal hills, District Shangla, Pakistan. Pak. J. Bot. 39:329.
Idrissi, A. I., and Fkih-Tetouani, S. (2001). Phytochemical study of Mentha longifolia of Morocco. Fitoterapia 72, 596–598. doi: 10.1016/S0367-326X(01)00279-9
Ismail, M., Hussain, J., Khan, A. U., Khan, A. L., Ali, L., Khan, F. U., et al. (2012). Antibacterial, antifungal, cytotoxic, phytotoxic, insecticidal, and enzyme inhibitory activities of Geranium wallichianum. Evid. Based Compl. Alter. Med. 2012. doi: 10.1155/2012/305906
Jaffer, F. A., and Weissleder, R. (2005). Molecular imaging in the clinical arena. JAMA 293, 855–862. doi: 10.1001/jama.293.7.855
Jain, N., Alam, M. S., Kamil, M., Ilyas, M., Niwa, M., and Sakae, A. (1990). Two flavonol glycosides from Chenopodium ambrosioides. Phytochemistry 29, 3988–3991. doi: 10.1016/0031-9422(90)85389-W
Jain, S. K., and Rao, R. R. (1977). A Handbook of Field and Herbarium Methods. New Delhi: Today and Tomorrow's Printers and Publishers.
Javed, S., Shahid, A. A., Haider, M. S., Umeera, A., Ahmad, R., and Mushtaq, S. (2012). Nutritional, phytochemical potential and pharmacological evaluation of Nigella Sativa (Kalonji) and Trachyspermum Ammi (Ajwain). J. Med. Plants Res. 768–775.
Jouad, H., Haloui, M., Rhiouani, H., El Hilaly, J., and Eddouks, M. (2001). Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J. Ethnopharmacol. 77, 175–182. doi: 10.1016/S0378-8741(01)00289-6
Kamatou, G. P., Makunga, N. P., Ramogola, W. P., and Viljoen, A. M. (2008). South African Salvia species: a review of biological activities and phytochemistry. J. Ethnopharmacol. 119, 664–672. doi: 10.1016/j.jep.2008.06.030
Kamboj, A., and Saluja, A. K. (2009). Bryophyllum pinnatum (Lam.) Kurz.: phytochemical and pharmacological profile: a review. Pharmacogn. Rev. 3:364.
Kang, J., Kang, Y., Ji, X., Guo, Q., Jacques, G., Pietras, M., et al. (2016). Wild food plants and fungi used in the mycophilous Tibetan community of Zhagana (Tewo County, Gansu, China). J. Ethnobiol. Ethnomed. 12: 21. doi: 10.1186/s13002-016-0094-y
Karimi, E., Oskoueian, E., Hendra, R., Oskoueian, A., and Jaafar, H. Z. (2012). Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 17, 1203–1218. doi: 10.3390/molecules17021203
Kaur, G. J., and Arora, D. S. (2009). Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 9:30. doi: 10.1186/1472-6882-9-30
Kayani, S., Ahmad, M., Sultana, S., Khan Shinwari, Z., Zafar, M., Yaseen, G., et al. (2015). Ethnobotany of medicinal plants among the communities of Alpine and Sub-alpine regions of Pakistan. J. Ethnopharmacol. 164, 186–202. doi: 10.1016/j.jep.2015.02.004
Kayani, S., Ahmad, M., Zafar, M., Sultana, S., Khan, M. P., Ashraf, M. A., et al. (2014). Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies–Abbottabad, Northern Pakistan. J. Ethnopharmacol. 156, 47–60. doi: 10.1016/j.jep.2014.08.005
Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., and He, J. (2005). Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223. doi: 10.1016/S0140-6736(05)70151-3
Khan, S. S., Khan, A., Ahmed, A., Ahmad, V. U., Farooq, U., Arshad, S., et al. (2010). Two new disulfated triterpenoids from Zygophyllum fabago. Helv. Chim. Acta. 93, 2070–2074. doi: 10.1002/hlca.200900477
Khan, M. P., Ahmad, M., Zafar, M., Sultana, S., Ali, M. I., and Sun, H. (2015). Ethnomedicinal uses of edible wild fruits (EWFs) in Swat Valley, Northern Pakistan. J. Ethnopharmacol. 173, 191–203. doi: 10.1016/j.jep.2015.07.029
Kim, Y., Goodner, K. L., Park, J.-D., Choi, J., and Talcott, S. T. (2011). Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 129, 1331–1342. doi: 10.1016/j.foodchem.2011.05.012
Krishnadhas, L., Santhi, R., and Annapurani, S. (2016). Isolation and identification of flavonoid fractions from the leaves of Volkameria inermis and its in-vitro cytotoxic study. Int. J. Pharm. Clin. Res. 8, 1648–1653.
Krishnaiah, D., Devi, T., Bono, A., and Sarbatly, R. (2009). Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 3, 067–072.
Krolikiewicz-Renimel, I., Michel, T., Destandau, E., Reddy, M., Andr,é, P., Elfakir, C., et al. (2013). Protective effect of a Butea monosperma (Lam.) Taub. flowers extract against skin inflammation: antioxidant, anti-inflammatory and matrix metalloproteinases inhibitory activities. J. Ethnopharmacol. 148, 537–543. doi: 10.1016/j.jep.2013.05.001
Kumar, D., Gupta, N., Ghosh, R., Gaonkar, R. H., and Pal, B. C. (2013). α-Glucosidase and α-amylase inhibitory constituent of Carex baccans: bio-assay guided isolation and quantification by validated RP-HPLC–DAD. J. Funct. Foods 5, 211–218. doi: 10.1016/j.jff.2012.10.007
Kumar, M., Sheikh, M. A., and Bussmann, R. W. (2011). Ethnomedicinal and ecological status of plants in Garhwal Himalaya, India. J. Ethnobiol. Ethnomed. 7:32. doi: 10.1186/1746-4269-7-32
Kunwar, R. M., and Bussmann, R. W. (2008). Ethnobotany in the nepal himalaya. J. Ethnobiol. Ethnomed. 4:24. doi: 10.1186/1746-4269-4-24
Kunwar, R. M., Nepal, B. K., Kshhetri, H. B., Rai, S. K., and Bussmann, R. W. (2006). Ethnomedicine in Himalaya: a case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J. Ethnobiol. Ethnomed. 2:27. doi: 10.1186/1746-4269-2-27
Kunwar, R. M., Shrestha, K. P., and Bussmann, R. W. (2010). Traditional herbal medicine in Far-west Nepal: a pharmacological appraisal. J. Ethnobiol. Ethnomed. 6:35. doi: 10.1186/1746-4269-6-35
Lacaze, B. (1996). Stationary clock changes on stationary processes. Signal Process. 55, 191–205. doi: 10.1016/S0165-1684(96)00130-2
Ladio, A., Lozada, M., and Weigandt, M. (2007). Comparison of traditional wild plant knowledge between aboriginal communities inhabiting arid and forest environments in Patagonia, Argentina. J. Arid Environ. 69, 695–715. doi: 10.1016/j.jaridenv.2006.11.008
Li, J., Huang, D., Chen, W., Xi, Z., Chen, C., Huang, G., et al. (2013). Two new phenolic glycosides from Gnaphalium affine D. Don and their anti-complementary activity. Molecules 18, 7751–7760. doi: 10.3390/molecules18077751
Lakshmi, T., Anitha, R., Durgha, K., and Manjusha, V. (2011). Coping with hypertension using safer herbal medicine–A therapeutic review. Int. J. Drug Dev. Res. 3, 1–8.
Lamchouri, F., Benali, T., Bennani, B., Toufik, H., Hassani, L. I. M., Bouachrine, B., et al. (2012). Preliminary phytochemical and antimicrobial investigations of extracts of Haloxylon scoparium. J Mater Enviro. Sci. 3, 754–759.
Lawes, C. M., Bennett, D. A., Feigin, V. L., and Rodgers, A. (2004). Blood pressure and stroke an overview of published reviews. Stroke 35, 776–785. doi: 10.1161/01.STR.0000116869.64771.5A
Leonti, M., Sticher, O., and Heinrich, M. (2003). Antiquity of medicinal plant usage in two Macro-Mayan ethnic groups (Mexico). J. Ethnopharmacol. 88, 119–124. doi: 10.1016/S0378-8741(03)00188-0
Leporatti, M. L., and Ivancheva, S. (2003). Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 87, 123–142. doi: 10.1016/S0378-8741(03)00047-3
Lev, B. (2000). Intangibles: Management, Measurement, and Reporting. Washington, DC: Brookings Institution Press.
Löffler, C., Sahm, A., Wray, V., Czygan, F.-C., and Proksch, P. (1995). Soluble phenolic constituents from Cuscuta reflexa and Cuscuta platyloba. Biochem. Syst. Ecol. 23, 121-128.
Lozoya-Saldana, H., and García, G. D. L. R. (1996). Electrotherapy and shoot tip culture eliminate potato virus X in potatoes. Am. Potato J. 73, 149–154. doi: 10.1007/BF02853073
Lu, R., Zhang, H., Tan, X., and Li, B. (2011). Studies on chemical constituents from Bud of Trachycarpus fortunei. Chinese J. Exper. Trad. Med. Formulae 7:34.
Lu, Y., and Foo, L. Y. (2000). Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 55, 263–267. doi: 10.1016/S0031-9422(00)00309-5
Macías, F. A., Molinillo, J. M., Torres, A., Varela, R. M., and Castellano, D. (1997). Bioactive flavonoids from Helianthus annuus cultivars. Phytochemistry 45, 683–687. doi: 10.1016/S0031-9422(97)00011-3
Machado, D. G., Cunha, M. P., Neis, V. B., Balen, G. O., Colla, A., Bettio, L. E., et al. (2013). Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 136, 999–1005. doi: 10.1016/j.foodchem.2012.09.028
Mahishi, P., Srinivasa, B. H., and Shivanna, M. B. (2005). Medicinal plant wealth of local communities in some villages in Shimoga District of Karnataka, India. J. Ethnopharmacol. 98, 307–312. doi: 10.1016/j.jep.2005.01.035
Mahmood, A., Riffat, N., Zabta, K., and Aqeel, M. (2011). Ethnobotanical survey of plants from Neelum, Azad Jammu and Kashmir, Pakistan. Pak. J. Bot. 43, 105–110.
Mahmoud, Z., Kassem, F., Abdel-Salam, N., and Zdero, C. (1986). Sesquiterpene lactones from Lactuca sativa. Phytochemistry 25, 747–748. doi: 10.1016/0031-9422(86)88039-6
Mandal, M., Paul, S., Uddin, M. R., Mondal, M. A., Mandal, S., and Mandal, V. (2016). In vitro antibacterial potential of Hydrocotyle javanica Thunb. Asian Pac. J. Trop. Dis. 6, 54–62. doi: 10.1016/S2222-1808(15)60985-9
Mangas, S., Moyano, E., Osuna, L., Cusido, R. M., Bonfill, M., and Palazón, J. (2008). Triterpenoid saponin content and the expression level of some related genes in calli of Centella asiatica. Biotechnol. Lett. 30: 1853. doi: 10.1007/s10529-008-9766-6
Mansoor, G. A. (2001a). Herbs and alternative therapies in the hypertension clinic. Am. J. Hypertens. 14, 971–975. doi: 10.1016/S0895-7061(01)02172-0
Mansoor, G. A. (2001b). Herbs and Alternative Therapies in the Hypertension Clinic. Oxford, UK: Oxford University Press.
Mansouri, A., Embarek, G., Kokkalou, E., and Kefalas, P. (2005). Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 89, 411–420.
Mary Helen, P., Vargheese, T. A., Jj, J. K., Abiramy, M., Sajina, N., and Jaya Sree, S. (2012). Phytochemical analysis and anticancer activity of essential oil from Myristica fragrans. Int. J. Curr. Pharm. Rev. Res. 2, 188.
Masinde, W. R. G. (2014). Phytochemical investigation of zanthoxylum gilletii (rutaceae) for antiplasmodial biomolecules. Nairobi: University of Nairobi.
Matias, E. F. F., Alves, E. F., Do Nascimento Silva, M. K., De Alencar Carvalho, V. R., Coutinho, H. D. M., and Da Costa, J. G. M. (2015). The genus Cordia: botanists, ethno, chemical and pharmacological aspects. Rev. Bras. de Farmacognosia 25, 542–552. doi: 10.1016/j.bjp.2015.05.012
Marco, J. A., Sanz, J. F., and Albiach, R. (1992). A sesquiterpene ester from Lactuca serriola. Phytochemistry 31, 2539–2540. doi: 10.1016/0031-9422(92)83321-O
Markham, K. R., Wallace, J. W., Babut, Y. N., Murty, V. K., and Rao, M. G. (1989). 8-C-glucosylscutellarein 6, 7-dimethyl ether and its 2 ″-O-apioside from Abrus precatorius. Phytochemistry 28, 299–301. doi: 10.1016/0031-9422(89)85069-1
Merfort, I., Buddrus, J., Nawwar, M., and Lambert, J. (1992). A triterpene from the bark of Tamarix aphylla. Phytochemistry 31, 4031–4032. doi: 10.1016/S0031-9422(00)97580-0
Mena, P., García-Viguera, C., Navarro-Rico, J., Moreno, D. A., Bartual, J., Saura, D., et al. (2011). Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 91, 1893–1906. doi: 10.1002/jsfa.4411
Meli, J., Benedicta, N., Chungag, T., Mope, J. G. D., and Samuel Kingue, J. S. (2009). Perceptions of the etiology and treatment of hypertension among some traditional healers in cameroon. Open Health J. 2, 39–43. doi: 10.2174/1874944500902010039
Menichini, F., Conforti, F., Rigano, D., Formisano, C., Piozzi, F., and Senatore, F. (2009). Phytochemical composition, anti-inflammatory and antitumour activities of four Teucrium essential oils from Greece. Food Chem. 115, 679–686. doi: 10.1016/j.foodchem.2008.12.067
Menshikov, G., and Kuzovkov, A. (1949). Research on the alkaloids of Heliotropium lasiocarpum. J. Gen. Chem. USSR. 19:137.
Metwally, A. M., Omar, A. A., Harraz, F. M., and El Sohafy, S. M. (2010). Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 6:212. doi: 10.4103/0973-1296.66939
Michel, T., Destandau, E., Le Floch, G., Lucchesi, M. E., and Elfakir, C. (2012). Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 131, 754–760. doi: 10.1016/j.foodchem.2011.09.029
Mikail, H. (2010). Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbits. J. Med. Plant Res. 4, 322–326.
Mohan, R., and Middha, A. (2017). Pharmacognostical and pharmacological profile of Crataegus songrica. Int. J. Interdiscipl. Res. Centre 3, 54–79.
Mojab, F., Kamalinejad, M., Ghaderi, N., and Vahidipour, H. R. (2010). Phytochemical screening of some species of Iranian plants. Iran. J. Pharmaceut. Res. 2, 77–82.
Molares, S., and Ladio, A. (2009). Chemosensory perception and medicinal plants for digestive ailments in a Mapuche community in NW Patagonia, Argentina. J. Ethnopharmacol. 123, 397–406. doi: 10.1016/j.jep.2009.03.033
Moustafa, A. M., Ahmed, S. H., Nabil, Z. I., Hussein, A. A., and Omran, M. A. (2010). Extraction and phytochemical investigation of Calotropis procera: effect of plant extracts on the activity of diverse muscles. Pharm. Biol. 48, 1080–1190. doi: 10.3109/13880200903490513
Mudnic, I., Modun, D., Brizic, I., Vukovic, J., Generalic, I., Katalinic, V., et al. (2009). Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomedicine 16, 462–469. doi: 10.1016/j.phymed.2008.11.004
Mukherjee, P. K., and Wahile, A. (2006). Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J. Ethnopharmacol. 103, 25–35. doi: 10.1016/j.jep.2005.09.024
Mustafa, A. K., Sikka, G., Gazi, S. K., Steppan, J., Jung, S. M., Bhunia, A. K., et al. (2011). Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 109, 1259–1268. doi: 10.1161/CIRCRESAHA.111.240242
Nadembega, P., Boussim, J. I., Nikiema, J. B., Poli, F., and Antognoni, F. (2011). Medicinal plants in baskoure, kourittenga province, Burkina Faso: an ethnobotanical study. J. Ethnopharmacol. 133, 378–395. doi: 10.1016/j.jep.2010.10.010
Naranjo, T., and Maestra, B. (1995). The effect of ph mutations on homoeolgous pairing in hybrids of wheat with Triticum longissimum. Theor. Appl. Genet. 91, 1265–1270. doi: 10.1007/BF00220939
Nascimento, G. G., Locatelli, J., Freitas, P. C., and Silva, G. L. (2000). Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 31, 247–256. doi: 10.1590/S1517-83822000000400003
Nasser, A. M. (1983). Leaf alkaloids of Rauwolfia caffra. Phytochemistry 22, 2297–2300. doi: 10.1016/S0031-9422(00)80165-X
Nawamaki, K., and Kuroyanagi, M. (1996). Sesquiterpenoids from Acorus calamus as germination inhibitors. Phytochemistry 43, 1175–1182. doi: 10.1016/S0031-9422(96)00401-3
Newman, D. J., and Cragg, G. M. (2007). Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 70, 461–477. doi: 10.1021/np068054v
Nuwaha, F., and Muganzi, E. (2008). Predictors of use of traditional medicine by patients with sexually transmitted infections in Southwest Uganda. J. Altern. Complement. Med. 14, 733–739. doi: 10.1089/acm.2007.7160
Ogunkunle, A., and Ladejobi, T. A. (2006). Ethnobotanical and phytochemical studies on some species of Senna in Nigeria. Afr. J. Biotechnol. 5, 2020–2023.
Oluwatoyin, S. M., Illeogbulam, N. G., and Joseph, A. (2011). Phytochemical and antimicrobial studies on the aerial parts of Heliotropium indicum Linn.
Opie, L. H., and Seedat, Y. K. (2005). Hypertension in sub-Saharan African populations. Circulation 112, 3562–3568. doi: 10.1161/CIRCULATIONAHA.105.539569
Oliveira, I., Sousa, A., Ferreira, I. C., Bento, A., Estevinho, L., and Pereira, J. A. (2008). Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 46, 2326–2331. doi: 10.1016/j.fct.2008.03.017
Orav, A., Arak, E., and Raal, A. (2006). Phytochemical analysis of the essential oil of Achillea millefolium L. from various European Countries. Nat. Prod. Res. 20, 1082–1088. doi: 10.1080/14786410500510849
Osamor, P. E., and Owumi, B. E. (2010). Complementary and alternative medicine in the management of hypertension in an urban Nigerian community. BMC Complement. Altern. Med. 10:36. doi: 10.1186/1472-6882-10-36
Paes, L., Mendonça, M., and Casas, L. (2013). Structural and phytochemical aspect from vegetative part of Costus spicatus (Jacq.) Sw (Costaceae). Revista Brasileira de Plantas Medicinais 15, 380–390. doi: 10.1590/S1516-05722013000300011
Pandino, G., Lombardo, S., Mauromicale, G., and Williamson, G. (2011). Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Composit. Anal. 24, 148–153. doi: 10.1016/j.jfca.2010.04.010
Park, S.-H., Ryu, S.-N., Bu, Y., Kim, H., Simon, J. E., and Kim, K.-S. (2010). Antioxidant components as potential neuroprotective agents in sesame (Sesamum indicum L.). Food Rev. Int. 26, 103–121. doi: 10.1080/87559120903564464
Parveen, A., Ali, Z., Fantoukh, O., and Khan, I. (2016). Phytochemical Constituents Of Tinospora Sinensis. Planta Med. 82:PC61. doi: 10.1055/s-0036-1578763
Patel, P., Vaghasiya, J., Thakor, A., and Jariwala, J. (2012). Antihypertensive effect of rhizome part of Acorus calamus on renal artery occlusion induced hypertension in rats. Asian Pacific J. Trop. Dis. 2, S6–S10. doi: 10.1016/S2222-1808(12)60114-5
Pereira, S. I., Freire, C. S., Pascoal Neto, C., Silvestre, A. J., and Silva, A. M. (2005). Chemical composition of the epicuticular wax from the fruits of Eucalyptus globulus. Phytochem. Anal. 16, 364–369. doi: 10.1002/pca.859
Pieroni, A. (2008). Local plant resources in the ethnobotany of Theth, a village in the Northern Albanian Alps. Genet. Resour. Crop Evol. 55, 1197–1214. doi: 10.1007/s10722-008-9320-3
Pirkle, J. L., Schwartz, J., Landis, J. R., and Harlan, W. R. (1985). The relationship between blood lead levels and blood pressure and its cardiovascular risk implications. Am. J. Epidemiol. 121, 246–258. doi: 10.1093/oxfordjournals.aje.a113995
Poffenberger, M., and Singh, S. (1992). Forest management partnerships: regenerating India's forests. Unasylva 43:46.
Puddey, I. B., and Beilin, L. J. (2006). Alcohol is bad for blood pressure. Clin. Exp. Pharmacol. Physiol. 33, 847–852. doi: 10.1111/j.1440-1681.2006.04452.x
Pundir, R. K., Jain, P., and Sharma, C. (2010). Antimicrobial activity of ethanolic extracts of Syzygium aromaticum and Allium sativum against food associated bacteria and fungi. Ethnobotanical Leaflets 2010:11.
Rajan, S., Thirunalasundari, T., and Jeeva, S. (2011). Anti—enteric bacterial activity and phytochemical analysis of the seed kernel extract of Mangifera indica Linnaeus against Shigella dysenteriae (Shiga, corrig.) Castellani and Chalmers. Asian Pac. J. Trop. Med. 4, 294–300. doi: 10.1016/S1995-7645(11)60089-8
Rasool, R., Ganai, B. A., Akbar, S., Kamili, A. N., and Masood, A. (2010). Phytochemical screening of Prunella vulgaris L.: an important medicinal plant of Kashmir. Pak. J. Phar. Sci. 23, 399–402.
Rashid, S., Ahmad, M., Zafar, M., Sultana, S., Ayub, M., Khan, M. A., et al. (2015). Ethnobotanical survey of medicinally important shrubs and trees of Himalayan region of Azad Jammu and Kashmir, Pakistan. J. Ethnopharmacol. 166, 340–351. doi: 10.1016/j.jep.2015.03.042
Rehecho, S., Uriarte-Pueyo, I., Calvo, J., Vivas, L. A., and Calvo, M. I. (2011). Ethnopharmacological survey of medicinal plants in Nor-Yauyos, a part of the Landscape Reserve Nor-Yauyos-Cochas, Peru. J. Ethnopharmacol. 133, 75–85. doi: 10.1016/j.jep.2010.09.006
Riaz, T., Abbasi, A. M., Shahzadi, T., Ajaib, M., and Khan, M. K. (2012). Phytochemical screening, free radical scavenging, antioxidant activity and phenolic content of Dodonaea viscosa. J. Serb. Chem. Soc. 77, 423–435. doi: 10.2298/JSC110621183R
Rogers, W. J., Michaux, S., Bastin, M., and Bucheli, P. (1999). Changes to the content of sugars, sugar alcohols, myo-inositol, carboxylic acids and inorganic anions in developing grains from different varieties of Robusta (Coffea canephora) and Arabica (C. arabica) coffees. Plant Sci. 149, 115–123. doi: 10.1016/S0168-9452(99)00147-8
Roussos, P. A. (2011). Phytochemicals and antioxidant capacity of orange (Citrus sinensis (l.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Horticult. 129, 253–258. doi: 10.1016/j.scienta.2011.03.040
Rovesti, P. (1936). Therapeutic and dietetic properties of Karkade (Hibiscus sabdariffa) a new colonialpink tea. Farm. Ital. 3, 13–15.
Roy, A. (2011). Coping with hypertension using safer herbal medicine-A therapeutic review. Int. J. Drug Dev. Res. 3, 1–8.
Sadeghi, Z., Kuhestani, K., Abdollahi, V., and Mahmood, A. (2014). Ethnopharmacological studies of indigenous medicinal plants of Saravan region, Baluchistan, Iran. J. Ethnopharmacol. 153, 111–118. doi: 10.1016/j.jep.2014.01.007
Sahpaz, S., Garbacki, N., Tits, M., and Bailleul, F. (2002). Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 79, 389–392. doi: 10.1016/S0378-8741(01)00415-9
Saied, S., and Begum, S. (2004). Phytochemical studies of Berberis vulgaris. Chem. Nat. Comp. 40, 137–140. doi: 10.1023/B:CONC.0000033929.60336.bb
Sahreen, S., Khan, M. R., and Khan, R. A. (2011). Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. Leaves. J. Med. Plants Res. 5, 2755–2765.
Sahraie-Rad, M., Izadyari, A., Rakizadeh, S., and Sharifi-Rad, J. (2015). Preparation of strong antidandruff shampoo using medicinal plant extracts: a clinical trial and chronic dandruff treatment. Jundishapur J. Nat. Pharmaceut. Products 10, 1–17. doi: 10.17795/jjnpp-21517
Sahu, M. C., and Padhy, R. N. (2013). In vitro antibacterial potency of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asian Pacific J. Trop. Dis. 3, 217–226. doi: 10.1016/S2222-1808(13)60044-4
Saklani, S., Chandra, S., Badoni, P., and Dogra, S. (2012). Antimicrobial activity, nutritional profile and phytochemical screening of wild edible fruit of Rubus ellipticus. Int. J. Med. Aromatic Plants 2, 269–274.
Saleem, M., Nazir, M., Ali, M. S., Hussain, H., Lee, Y. S., Riaz, N., et al. (2010). Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Product Rep. 27, 238–254. doi: 10.1039/B916096E
Salehi, B., Kumar, N. V. A., Sener, B., Sharifi-Rad, M., Kiliç, M., Mahady, G. B., et al. (2018a). Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 19:1459. doi: 10.3390/ijms19051459
Salehi, B., Mishra, A. P., Shukla, I., Sharifi-Rad, M., Contreras, M. D. M., Segura-Carretero, A., et al. (2018b). Thymol, thyme, and other plant sources: health and potential uses. Phytother. Res. doi: 10.1002/ptr.6109
Samuelsen, A. B. (2000). The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 71, 1–21. doi: 10.1016/S0378-8741(00)00212-9
Samuelsson, G. (1958). Phytochemical and pharmacological studies on Viscum album LI Viscotoxin, its isolation and properties. Sven. Farm. Tidskr. 62, 169–189.
Sanz-Biset, J., Campos-de-La-Cruz, J., Epiquién-Rivera, M. A., and Cañigueral, S. (2009). A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J. Ethnopharmacol. 122, 333–362. doi: 10.1016/j.jep.2008.12.009
Saqib, F., and Janbaz, K. H. (2016). Rationalizing ethnopharmacological uses of Alternanthera sessilis: a folk medicinal plant of Pakistan to manage diarrhea, asthma and hypertension. J. Ethnopharmacol. 182, 110–121. doi: 10.1016/j.jep.2016.02.017
Sarafidis, P. A., Li, S., Chen, S. C., Collins, A. J., Brown, W. W., Klag, M. J., et al. (2008). Hypertension awareness, treatment, and control in chronic kidney disease. Am. J. Med. 121, 332–340. doi: 10.1016/j.amjmed.2007.11.025
Satyal, P., Paudel, P., Raut, J., Deo, A., Dosoky, N. S., and Setzer, W. N. (2013). Volatile constituents of Pinus roxburghii from Nepal. Pharmacognosy Res. 5:43. doi: 10.4103/0974-8490.105650
Saxena, D. B. (1986). Phenyl indane from Acorus calamus. Phytochemistry 25, 553–555. doi: 10.1016/S0031-9422(00)85528-4
Schütz, K., Carle, R., and Schieber, A. (2006). Taraxacum—a review on its phytochemical and pharmacological profile. J. Eethnopharmacol. 107, 313–323. doi: 10.1016/j.jep.2006.07.021
Sengul, M., Yildiz, H., Gungor, N., Cetin, B., Eser, Z., and Ercisli, S. (2009). Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharmceut. Sci. 22, 102–106.
Sharifi-Rad, J., Hoseini-Alfatemi, S. M., Sharifi-Rad, M., and Setzer, W. N. (2015). Chemical composition, antifungal and antibacterial activities of essential oil from Lallemantia royleana (Benth. in Wall.) Benth. J. Food Safety 35, 19–25. doi: 10.1111/jfs.12139
Sharifi-Rad, M., Mnayer, D., Morais-Braga, M. F. B., Carneiro, J. N. P., Bezerra, C. F., Coutinho, H. D. M., et al. (2018). Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. doi: 10.1002/ptr.6101. [Epub ahead of print].
Sharifi-Rad, J., Salehi, B., Stojanović-Radić, Z. Z., Fokou, P. V. T., Sharifi-Rad, M., Mahady, G. B., et al. (2017). Medicinal plants used in the treatment of tuberculosis-Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 8, 1–18. doi: 10.1016/j.biotechadv.2017.07.001
Shenoy, C., Patil, M., Kumar, R., and Patil, S. (2009). Preliminary phytochemical investigation and wound healing activity of Allium cepa Linn (Liliaceae). Int. J. Pharm. Pharm. Sci. 2, 167–175.
Sher, H., Aldosari, A., Ali, A., and de Boer, H. J. (2014). Economic benefits of high value medicinal plants to Pakistani communities: an analysis of current practice and potential. J. Ethnobiol. Ethnomed. 10:71. doi: 10.1186/1746-4269-10-71
Sher, H., Bussmann, R. W., Hart, R., and de Boer, H. J. (2016). Traditional use of medicinal plants among Kalasha, Ismaeli and Sunni groups in Chitral district, Khyber Pakhtunkhwa province, Pakistan. J. Ethnopharmacol. 188, 57–69. doi: 10.1016/j.jep.2016.04.059
Sher, Z., Khan, Z., and Hussain, F. (2011). Ethnobotanical studies of some plants of Chagharzai valley, district Buner, Pakistan. Pak. J. Bot. 43, 1445–1452.
Shil, S., Choudhury, M., and Das, S. (2014). Indigenous knowledge of medicinal plants used by the Reang tribe of Tripura state of India. J. Ethnopharmacol. 152, 135–141. doi: 10.1016/j.jep.2013.12.037
Shikov, A. N., Kundracikova, M., Palama, T. L., Pozharitskaya, O. N., Kosman, V. M., Makarov, V. G., et al. (2010). Phenolic constituents of Gnaphalium uliginosum L. Phytochem. Lett. 3, 45–47. doi: 10.1016/j.phytol.2009.11.002
Siddiqui, B. S., Gulzar, T., Mahmood, A., Begum, S., Khan, B., Rasheed, M., et al. (2005). Phytochemical studies on the seed extract of Piper nigrum Linn. Nat. Prod. Res. 19, 703–712. doi: 10.1080/14786410512331330657
Siddiqui, B. S., Usmani, S. B., Begum, S., and Siddiqui, S. (1993). Steroidal alkaloids and an androstane derivative from the bark of Holarrhena pubescens. Phytochemistry 33, 925–928. doi: 10.1016/0031-9422(93)85306-C
Singh, A. (2008). Phytochemicals of Gentianaceae: a review of pharmacological properties. Int. J. Pharmaceut. Sci. Nanotechnol. 1, 33–36.
Singh, S. (2012). Phytochemical analysis of different parts of Prosopis juliflora. Int. J. Curr. Pharm. Res. 4, 59–61.
Soelberg, J., and Jäger, A. K. (2016). Comparative ethnobotany of the Wakhi agropastoralist and the Kyrgyz nomads of Afghanistan. J. Ethnobiol. Ethnomed. 12:2. doi: 10.1186/s13002-015-0063-x
Solihah, M., Rosli, W. W., and Nurhanan, A. (2012). Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int. Food Res. J. 19, 1533–1538.
Somova, L. I., Shode, F. O., Ramnanan, P., and Nadar, A. (2003). Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 84, 299–305. doi: 10.1016/S0378-8741(02)00332-X
Souza, J. S. N., Machado, L. L., Pessoa, O. D., Braz-Filho, R., Overk, C. R., Yao, P., et al. (2005). Pyrrolizidine alkaloids from Heliotropium indicum. J. Braz. Chem. Soc. 16, 1410–1414. doi: 10.1590/S0103-50532005000800019
Sonibare, M. A., Soladoye, M. O., Esan, O. O., and Sonibare, O. O. (2009). Phytochemical and antimicrobial studies of four species of Cola Schott and Endl. (Sterculiaceae). Afr. J. Trad. Compl. Alternat. Med. 6, 518–525.
Sowbhagya, H. (2014). Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): an overview. Crit. Rev. Food Sci. Nutr. 54, 389–398. doi: 10.1080/10408398.2011.586740
Sreelatha, S., Padma, P. R., and Umadevi, M. (2009). Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. Toxicol. 47, 702–708. doi: 10.1016/j.fct.2008.12.022
Stajner, D., Popović, B. M., Canadanović-Brunet, J., and Anackov, G. (2009). Exploring Equisetum arvense L., Equisetum ramosissimum L. and Equisetum telmateia L. as sources of natural antioxidants. Phytother. Res. 23, 546–550. doi: 10.1002/ptr.2682
Steyn, K., Gaziano, T. A., Bradshaw, D., Laubscher, R., and Fourie, J. (2001). Hypertension in South African adults: results from the demographic and health survey, 1998. J. Hypertens. 19, 1717–1725. doi: 10.1097/00004872-200110000-00004
Sukumaran, P., Nair, A. G., Chinmayee, D. M., Mini, I., and Sukumaran, S. T. (2012). Phytochemical investigation of Bidens biternata (Lour.) Merr. and Sheriff.—A nutrient-rich leafy vegetable from Western Ghats of India. Appl. Biochem. Biotechnol. 167, 1795–1801. doi: 10.1007/s12010-012-9652-5
Sumbul, S., Ahmad, M. A., Asif, M., and Akhtar, M. (2011). Myrtus communis Linn. A review. Indian J. Nat. Prod. Resour. 2, 395–402.
Tabuti, J. R., Lye, K. A., and Dhillion, S. S. (2003). Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J. Ethnopharmacol. 88, 19–44. doi: 10.1016/S0378-8741(03)00161-2
Tadhani, M., and Subhash, R. (2006). Preliminary studies on Stevia rebaudiana leaves: proximal composition, mineral analysis and phytochemical screening. J. Med. Sci. 6, 321–326. doi: 10.3923/jms.2006.321.326
Tahraoui, A., El-Hilaly, J., Israili, Z. H., and Lyoussi, B. (2007). Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 110, 105–117. doi: 10.1016/j.jep.2006.09.011
Tanaka, H., Etoh, H., Watanabe, N., Shimizu, H., Ahmad, M., and Rizwani, G. H. (2001). Erysubins C–F, four isoflavonoids from Erythrina suberosa var. glabrescences. Phytochemistry 56, 769–773. doi: 10.1016/S0031-9422(00)00441-6
Tan, R. X., Tang, H. Q., Hu, J., and Shuai, B. (1998). Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa. Phytochemistry 49, 157–161. doi: 10.1016/S0031-9422(97)00889-3
Tang, H. Q., Hu, J., Yang, L., and Tan, R. X. (2000). Terpenoids and flavonoids from Artemisia species. Planta Med. 66, 391–393. doi: 10.1055/s-2000-8538
Tardío, J., and Pardo-De-Santayana, M. (2008). Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain) 1. Econ. Bot. 62, 24–39. doi: 10.1007/s12231-007-9004-5
Tassell, M. C., Kingston, R., Gilroy, D., Lehane, M., and Furey, A. (2010). Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 4:32. doi: 10.4103/0973-7847.65324
Tatiya, A., Surana, S., Khope, S., Gokhale, S., and Sutar, M. (2007). Phytochemical investigation and Immunomodulator activity of Amaranthus spinosus Linn. Indian J. Pharm. Educ. Res. 41, 337–341.
Tee, S. R., Teoh, X. Y., Aiman, W., Aiful, A., Har, C. S. Y., Tan, Z. F., et al. (2010). The prevalence of hypertension and its associated risk factors in two rural communities in Penang, Malaysia. IeJSME 2, 27–40.
Tene, V., Malagón, O., Finzi, P. V., Vidari, G., Armijos, C., and Zaragoza, T. (2007). An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 111, 63–81. doi: 10.1016/j.jep.2006.10.032
Torrance, S. J., Wiedhopf, R. M., Cole, J. R., Arora, S. K., Bates, R. B., Beavers, W. A., et al. (1976). Antitumor agents from Jatropha macrorhiza (Euphorbiaceae). II. Isolation and characterization of jatrophatrione. J. Org. Chem. 41, 1855–1857. doi: 10.1021/jo00872a038
Tsai, D. S., Chang, Y. S., Li, T. C., and Peng, W. H. (2014). Prescription pattern of Chinese herbal products for hypertension in Taiwan: a population-based study. J. Ethnopharmacol. 155, 1534–1540. doi: 10.1016/j.jep.2014.07.047
Umamaheswari, M., and Chatterjee, T. (2008). In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr. J. Trad. Compl. Alternat. Med. 5, 61–73. doi: 10.4314/ajtcam.v5i1.31258
Uniyal, S. K., Singh, K. N., Jamwal, P., and Lal, B. (2006). Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2:14. doi: 10.1186/1746-4269-2-14
Usman, H., Abdulrahman, F., and Ladan, A. (2007). Phytochemical and antimicrobial evaluation of Tribulus terrestris L.(Zygophylaceae). Growing in Nigeria. Res. J. Bio. Sci. 2, 244–247.
Varsha, S., Agrawal, R., and Sonam, P. (2013). Phytochemical screening and determination of anti-bacterial and anti-oxidant potential of Glycyrrhiza glabra root extracts. J. Environ. Res. Dev. 7, 1552–1558.
Verma, A. K., Kumar, M., and Bussmann, R. W. (2007). Medicinal plants in an urban environment: the medicinal flora of Banares Hindu University, Varanasi, Uttar Pradesh. J. Ethnobiol. Ethnomed. 3:35. doi: 10.1186/1746-4269-3-35
Verma, R. K., and Verma, S. K. (2006). Phytochemical and termiticidal study of Lantana camara var. aculeata leaves. Fitoterapia 77, 466–468. doi: 10.1016/j.fitote.2006.05.014
Vitalini, S., Iriti, M., Puricelli, C., Ciuchi, D., Segale, A., and Fico, G. (2013). Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 145, 517–529. doi: 10.1016/j.jep.2012.11.024
Voirin, B., Bayet, C., Faure, O., and Jullien, F. (1999). Free flavonoid aglycones as markers of parentage in Mentha aquatica, M. citrata, M. spicata and M. x piperita. Phytochemistry 50, 1189–1193. doi: 10.1016/S0031-9422(98)00672-4
Wang, Y., Xiang, L., Wang, C., Tang, C., and He, X. (2013). Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 8:e71144. doi: 10.1371/journal.pone.0071144
Wangchuk, P., and Tobgay, T. (2015). Contributions of medicinal plants to the gross national happiness and biodiscovery in Bhutan. J. Ethnobiol. Ethnomed. 11:48. doi: 10.1186/s13002-015-0035-1
World Health Organization (2001). The World Health Report 2001: Mental Health: New Understanding, New Hope. Geneva: World Health Organization.
World Health Organization (2002). WHO Monographs on Selected Medicinal Plants. Geneva: World Health Organization.
Yaseen, G., Ahmad, M., Sultana, S., Suleiman Alharrasi, A., Hussain, J., and Zafar, M. (2015a). Ethnobotany of medicinal plants in the Thar Desert (Sindh) of Pakistan. J. Ethnopharmacol. 163, 43–59. doi: 10.1016/j.jep.2014.12.053
Yaseen, G., Ahmad, M., Zafar, M., Sultana, S., Kayani, S., Cetto, A. A., et al. (2015b). Traditional management of d iabetes in Pakistan: ethnobotanical investigation from traditional health Practitioners. J. Ethnopharmacol. 174, 91–117. doi: 10.1016/j.jep.2015.07.041
Keywords: hypertension, Northern Pakistan, medicinal plant, disease consensus index, ethnobotany
Citation: Malik K, Ahmad M, Bussmann RW, Tariq A, Ullah R, Alqahtani AS, Shahat AA, Rashid N, Zafar M, Sultana S and Shah SN (2018) Ethnobotany of Anti-hypertensive Plants Used in Northern Pakistan. Front. Pharmacol. 9:789. doi: 10.3389/fphar.2018.00789
Received: 09 January 2018; Accepted: 28 June 2018;
Published: 24 July 2018.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Marinella De Leo, Università degli Studi di Pisa, ItalyJavad Sharifi-Rad, Shahid Beheshti University of Medical Sciences, Iran
Andy Wai Kan Yeung, University of Hong Kong, Hong Kong
Copyright © 2018 Malik, Ahmad, Bussmann, Tariq, Ullah, Alqahtani, Shahat, Rashid, Zafar, Sultana and Shah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khafsa Malik, a2hhZnNhbWFsaWs3ODZAZ21haWwuY29t
 Khafsa Malik
Khafsa Malik Mushtaq Ahmad1,2
Mushtaq Ahmad1,2 Rainer W. Bussmann
Rainer W. Bussmann Akash Tariq
Akash Tariq Riaz Ullah
Riaz Ullah Ali S. Alqahtani
Ali S. Alqahtani Neelam Rashid
Neelam Rashid Syed N. Shah
Syed N. Shah