- 1Key Laboratory of Anti-inflammatory and Immune Medicine, Ministry of Education, Anti-inflammatory Immune Drugs Collaborative Innovation Center, Institute of Clinical Pharmacology, Anhui Medical University, Hefei, China
- 2Menzies Institute for Medical Research, Hobart, TAS, Australia
The interaction between T cell and dendritic cells (DCs) that leads to T cell activation affects the progression of the immune response including autoimmune diseases. Antigen presentation on immune cell surface, formation of an immunological synapse (IS), and specific identification of complex by T cells including two activating signals are necessary steps that lead to T cell activation. The formation of stimulatory IS involves the inclusion of costimulatory molecules, such as ICAM-1/LFA-1 and CD28/B7-1, and so on. Some fusion proteins and monoclonal antibodies targeting costimulatory molecules have been developed and approved to treat autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), type I diabetes (T1D), inflammatory bowel disease (IBD), and psoriasis. These biological agents, including CTLA-4- and LFA-3-Ig, anti-CD3 monoclonal antibody, could prevent the successful engagement of DCs by T cell with significant efficacy and safety profile. In this article, we reviewed the molecular mechanisms of T cell activation during the interaction between T cells and DCs, and summarized some biological agents that target costimulatory molecules involved in the regulation of T cell activation.
Introduction
Various organ specific autoimmune diseases are mediated by an imbalance of T cell subsets, e.g., an absence of regulatory T cells, or tissue injury driven by pathological T helper cell responses. Examples are rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), type I diabetes (T1D), and inflammatory bowel disease (IBD) (Fletcher et al., 2010; Burmester et al., 2014; Geem et al., 2015; Suarez-Fueyo et al., 2016; Pugliese, 2017). In an inflammatory environment, autoreactive T cells are activated initially by dendritic cells (DCs). Like macrophages and B cells, DCs are professional antigen-presenting cells (APCs). However, DCs have the unique property of inducing the differentiation of naïve CD4+ T cells into helper and effector T cells with a unique combination of abilities, which allows DCs to effectively process antigen, strongly express costimulatory molecules, secrete cytokines, and migrate to tissues or lymphoid organs to prime T cells (Steinman, 2007). Therefore, DCs emerged as critical players in the initiation and development of immune response.
In identifying pathogen-associated cues, DCs undergo a series of functional changes known as maturation. Mature DCs present antigenic peptides in the context of major histocompatibility complex (MHC) class II to the T cell receptor and express co-stimulatory molecules CD40 and B7. Mature DCs are characterized by the production of cytokines, such as IL-12, and by the expression of homing receptors, such as CCR7, which directs the migration of DCs into the T-cell regions of secondary lymphoid organs. Together these changes enable DCs to effectively activate naïve T cells. At the same time, DCs induce the expression of the corresponding costimulatory molecules of CD40L, CD28, on T cells. Mature DCs promote naïve T cells differentiate into Th1, Th2, Th17, or Treg cells in a stimulus-dependent manner by secreting cytokines. In a mouse model of EAE in vivo and in vitro, DCs which express aberrant P38 can promote the differentiation of Th17 cells by inducing the secretion of IL-17 and the expression of IL-23 receptors (IL-23R) (Huang et al., 2012). Detection of mature DCs producing large quantities of IL-12 and IL-23 strongly supports the notion that DCs play a key role in autoimmune diseases by promoting a deleterious imbalance between Th1, Th2, and Th17 cells (Lebre et al., 2008; Tournadre et al., 2012; Segura et al., 2013). The majority of DCs exist in the inflammatory synovia fluid of RA patients, expressing CD1a and secreting IL-23 (Segura et al., 2013). Furthermore, DCs with a gene deletion of interleukin 1 receptor related kinase M (IRAK-M) show strong antigen presenting ability, resulting in the abnormal activity of diabetogenic T cells and autoimmune reaction in vitro and the rapid onset of T1D in vivo in immunodeficient NOD mice when cotransferred with diabetogenic T cells (Tan Q. et al., 2014).
DCs include immunogenicity DCs and tolerogenic DCs according to function. Interactions between tolerogenic DCs and CD4+Foxp3+ regulatory T cells (Tregs) play a critical role in maintaining peripheral tolerance and preventing activation of T cells (Audiger et al., 2017). Peripheral tolerance is associated with a high activity of Tregs and a reduced inflammatory profile of Th cells (Min et al., 2006; Li et al., 2008). CD4+ Treg in both the spleen and lymph node help to maintain tolerogenic status of DCs through the expression of CTLA-4 in mice (Wing et al., 2008).
DCs from different location exert different functions. Plasmacytoid DCs secrete large amounts of type I interferons (such as IFN-alpha and IFN-beta) after identification of the viruses through TLR7 and TLR9, which are located in intracellular compartments (Gilliet et al., 2008). The central role of plasmacytoid DC in autoimmune diseases is emphasized by its association with type I-interferon signal. Type I interferons produced by plasmacytoid DC from human PBMCs also supports IL-17 secretion and Th17 responses (Lombardi et al., 2009). Furthermore, human plasmacytoid DCs enhance thymic Treg expansion and generation of peripheral Treg through the production of indoleamine 2, 3-dioxygenase (IDO) and the expression of programmed death-ligand 1 (PD-L1) in vivo and in vitro (Chen et al., 2008; Amarnath et al., 2011; Creusot et al., 2014). Lymphoid-resident DCs rapidly extracts antigens from lymph and blood for presentation to T cells (Sixt et al., 2005). In particular, CD205+ DCs in the spleen of mice are able to induce the tolerance of CD4+ T cell under suboptimal activation conditions (Yamazaki et al., 2008).
The interaction between T cells and DC leads to the formation of immunological synapse (IS) and is maintained by highly expressing adhesion molecules (LFA-1, LFA-3, ICAM-1, ICAM-2), cytokines and chemokines (Lee et al., 2002; Tseng et al., 2008). In this article, we reviewed the molecular mechanism of T cells activation by DCs and immunotherapy targeting T cell activation in autoimmune diseases.
Molecular Mechanisms of T Cell Activation by DCs
There are three stages during T cells activation by DCs, namely antigen presenting, antigen recognition of T cells and two signals formation. In addition, IS formation between T cells and DCs plays an important role in T cell activation.
Antigen Presenting
Germline encoded pattern recognition receptors (PPR) specific for pathogen-associated patterns (PAM) are present on immature DCs. An engagement of these membrane-bound receptors trigger a maturation of DCs and lead to an up-regulation of costimulatory molecules (Kabelitz and Medzhitov, 2007). Mature DCs in mice express chemokine receptor 7 (CCR7) and begin to migrate into regional lymph nodes after an encounter with antigen (Ritter et al., 2004).
For a presentation with MHC class II, antigen is degraded by DCs to a suitable length (approximately 12 amino acids) utilizing proteasomes in the endogenous pathway. These antigenic peptides bind to specific grooves in the MHC class II molecules (Jones et al., 2006). Peptide-MHC II complexes are formed in the rough endoplasmatic reticulum and transported to the cell surface for presentation (Vyas et al., 2008; Neefjes et al., 2011). At the local draining lymph node, DC present complexes of processed peptides together with MHC class II to naïve CD4+ T cells which bind to this combination with their TCR and initiate signaling. The peptide binding to MHC class I and the subsequent presentation to CD8+ T cells is similar in many aspects and will not be discussed in detail. Overall, antigen presentation with MHC class II and MHC class I are mainly two modes for DCs.
A third mode of antigen presentation utilizing the conserved non-classical MHC class I molecule CD1 plays an important role in microbial infection and the immune response to lipid antigens (Shao et al., 2005; Barral and Brenner, 2007). CD1 has 30% homology with MHC class I molecules, and there are main five types of CD1 in humans, termed CD1a-e (Barral and Brenner, 2007). Probably the best studied member of the CD1 family is CD1d which presents predominantly lipids to CD1-restricted T cells that have a limited repertoire of TCR and have been termed Natural Killer (Kang et al., 2014). Although interferon (IFN)-gamma secretion by CD1-restricted T cells during infection had been shown before, the function of CD1 restricted T cells was not entirely understood for a long time (Gilleron et al., 2004). Only recently, it was demonstrated that the expression of human class I CD1 molecules in transgenic mice caused a rapid response of CD1-restricted T cells after re-exposure to antigen, suggesting a protective effect of CD1-restricted T cells (Felio et al., 2009). Natural sebum can be used as a headless antigen and presented to autoreactive T cells through CD1a (de Jong et al., 2010). In addition, researchers have found a group of highly conserved T cells in tuberculosis (TB) patients. These conserved T cells could recognize specifically mycobacterial antigens presented by CD1b (Van Rhijn et al., 2013). These findings suggest that the antigen-presenting molecule CD1 plays an important role during special antigen presentation.
Antigen Recognition of T Cells
T cell receptor (TCR) on T cells not only identify peptide-MHC complexes derived from host cells infected by pathogens, but also recognize similar structures derived from healthy host cells, so called autoantigens. The specificity of the TCR for antigen is located in the V segment, which is composed of N-terminal of two TCR polypeptides (Govers et al., 2010). Both V alpha and V beta have 3 hypervariable regions and are also known as the complementarity determining region, namely CDR1, CDR2, and CDR3. CDR3 is a largest hypervariable region and directly determines the antigen binding specificity of TCR (Feng et al., 2015). The TCR identifies simultaneously the entire complex of antigenic peptide and MHC molecule as a first step in T cell activation (Reiser et al., 2000). The comparison between the TCR conformation and the conformation of TCR bound to the peptide-MHC complex indicates that CDR3 region undergoes a large conformational transition in order to obtain a diagonal position that allows the binding to peptide-MHC complex (Garcia, 2012). When the V segment of the TCR identifies an antigen/MHC complex, the TCR heterodimer deliver the activation signal to the cell nucleus through the constant transmembrane components of the CD3 complex. (Schamel et al., 2005). Therefore, TCR signaling has a key role in determining T cell fate. TCR-peptide-MHC complexes appear to support a model of physical specificity between TCR germline V regions and MHC.
Two Signals Are Necessary for Activation of Naïve T Cells
Naïve T cell activation by DCs requires two signals, termed signal-1 and signal-2 (Anderson and Siahaan, 2003). Signal-1 is equivalent to the binding of TCR to peptide-MHC complex (Garg et al., 2010; Vesely et al., 2011; Manikwar et al., 2012). Signal-2 requires the interaction of costimulatory molecules at the interface between DCs and T cells (B7/CD28, LFA-1/ICAM-1 and ICAM2, CD2/LFA-3) (Figure 1). The combination of TCR and peptide-MHC complex (signal 1) will lead to phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) on CD3, which is closely adjacent to TCR by Lck kinase (Rossy et al., 2013). T cells receive signal-1 when the activation cascade leads to signaling through multiple TCR for several hours (Frauwirth and Thompson, 2004). This sustained signaling is necessary for the effective activation of signal transduction pathways that lead to the activation of nuclear transcription factors. The clustering of TCR in IS at the interface between T cells and DC is necessary for continuous signal transduction and will be discussed in more detail later. Signal-2 was initialized by the interaction of costimulatory molecules expressed DCs and T cells. Positive signals, such as CD28/B7-1 (CD80) and CD28/B7-2 (CD86), and negative signals, such as CTLA-4/CD80 and CTLA-4/CD86 have been identified (Huang et al., 2012; Manikwar et al., 2012). As mentioned above pairs of costimulatory molecules (CD80/CD28, LFA-1/ICAM-1, or ICAM2, CD2/LFA-3) provide signal 2. The activation signal of these costimulatory molecules is delivered to T cells via the ITAM of the cytoplasmic domain, which enhances the TCR response to antigen (Acuto et al., 2008). It was two signals model for T-cell activation. T cells could be activated in simultaneously receiving signal-1 from T-cell recognition of antigen and signal-2 from costimulatory molecular. However, it was an off signal to T cells in only receiving signal-1, and T cells would translate into tolerance, clone incompetent or deletion.
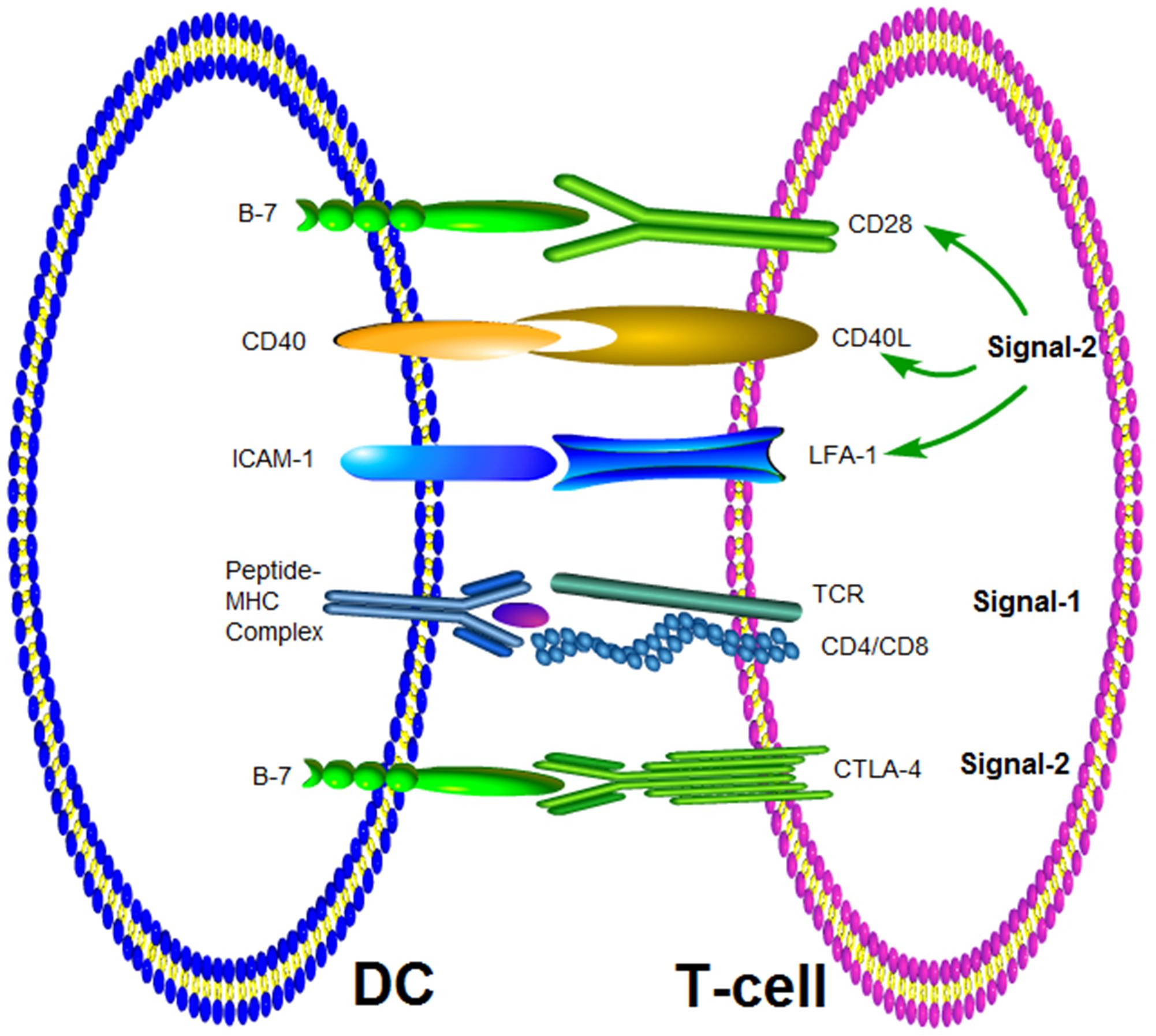
FIGURE 1. Molecular interactions at the interface of T-cell and APC. Signal-1 is provided by the interaction between TCR and the MHC-peptide complex. The co-stimulatory signal (Signal-2) can be delivered by different pairs of molecules.
CD28/CD80 was a pair of co-stimulatory molecules that mediated and enhanced immune responses, but these molecules were not directly involved in memory immune responses (Kopf et al., 2000). Furthermore, the co-stimulatory signal of CD28 was related mainly to initiating interaction between DCs and T cells. The CD28/CD80 signal activate T cells to express multiple other costimulatory molecules, these costimulatory molecules control the balance of immune response and the stability of internal environment. The interaction between cytotoxic T lymphocytes (CTL) and Th or Th and B cells rests on other costimulatory molecules, such as OX40 (CD134), inducible T-cell costimulator (ICOS) (Bansal-Pakala et al., 2004). The costimulatory molecule 4-1BB and its ligand 4-1BBL can control adaptive immunity (Lee et al., 2008). Treg cells up-regulate the expression of 4-1BB in response to IL-2 and suppressed T cell proliferation. At the same time, the synergy of 4-1BB and CD28 signal can affect cell polarization, and promote Th0 cells differentiation into Th1 cells which are characterized by the production of IFN-gamma (Elpek et al., 2007).
CTLA-4 (CD152) is homologous to CD28 and also expressed on activated T cells, but the cytoplasmic domain of CTLA-4 has an immunoreceptor tyrosine-based inhibitory motif (ITIM) (Topalian et al., 2015). Therefore, CTLA-4 binds to CD80 in competition with CD28 with an affinity that is 20 times higher than CD28/CD80 and can send an inhibitory signal to activated T cells through its ITIM motif to restore the balance of immune response (Pentcheva-Hoang et al., 2004; Vogel et al., 2015). CTLA-4 activates protein tyrosine phosphatase (PTP) through the ITIM structure and inhibits T cell activation signal transduction, leading to a negative regulation of T cell activation (Chemnitz et al., 2004). Additionally, CTLA-4 inhibits the expression of IL-2 receptor alpha chain, IL-2 secretion and IL-2 mRNA accumulation, also resulting in an inhibition of the activation of T cells in preclinical mouse models (Hannani et al., 2015). Hence, costimulatory signals mediated by costimulatory molecules, including positive signals and negative signals, play important role in regulating interaction between T cell and DCs and maintaining the balance of immune response.
IS Formation Plays an Important Role in T Cell Activation
IS play an important role in T cells activation, and IS formation involves a variety of costimulatory molecules, such as ICAM-1/LFA-1, CD28/B7-1, and so on (Schwartz et al., 2002).
Formation Mechanisms of IS
In the process of T cell and DC interaction, a variety of transmembrane molecules accumulate in a “raft” structure that is rich in sphingomyelin and cholesterol, and are clustered at the interface of T cell and DC contact. This special “raft” structure has been termed IS. Before the formation of IS, T cells form pseudopods in search of peptide MHC complexes on APCs. After the initial contact the formation of IS is a dynamic process that has been described to depend on a planar lipid bilayer. IS formation includes three phases: (i) The first stage is the connection of TCR and peptide MHC complex. The adhesion molecules such as LFA-1/ICAM-1 and CD2/LFA-3 are recruited to the nascent rafts (Barreiro et al., 2007); (ii) The second stage has been termed the peptide MHC complex transfer stage. In the early stages of IS formation, TCR- peptide MHC complex is transported to the central region of IS, while LFA-1/ICAM-1 is transferred to the peripheral region to form mature IS; (iii) The third stage is the formation of a stable contact region at the interface of T cell and antigen presenting cell. In this stage, the super molecular structure of a mature IS can be maintained for 1 h, while PKC theta, Bcl10, IKK beta are recruited to IS by cytoskeleton changes (Dustin, 2005).
Molecular Structure of IS
The molecular structure of IS include three areas, namely central supermolecular activation cluster (cSMAC), peripheral SMAC (pSMAC), and distal SMAC (dSMAC). The molecules in cSMAC area mainly includes TCR- peptide MHC complex, CD3, CD28, and signal transduction molecules such as PKC theta and Lck (Valitutti, 2008). Adhesion molecules such as LFA-1/ICAM-1 or DC-SIGN surround pSMAC area (Dustin, 2005). CD2/LFA-3 is located between cSMAC and pSMAC, and CD43, CD45, and PSGL-1 are located at dSMAC. The number of TCRs in cSMAC is only double that of in pSMAC, but the number of LFA in pSMAC is almost 6 times that of in cSMAC. In fact, cSMAC and pSMAC do not show obvious boundaries. Although cSMAC and pSMAC can be maintained for several hours, the numbers of receptors and molecules in IS are changed dynamically (Dustin, 2005). CD45 is a unique molecule that is transferred to dSMAC from cSMAC, which may be related to the activation of Lck at the stage of early IS formation (Grigorian et al., 2009). cSMAC take part in the reuse and degradation of TCRs, which down-regulate the TCR and attenuate signals (Figure 2). Thus it can be seen that the molecular structure of IS was very complex involving in many molecules and signals, which take part in IS formation through interaction and dynamically balance.
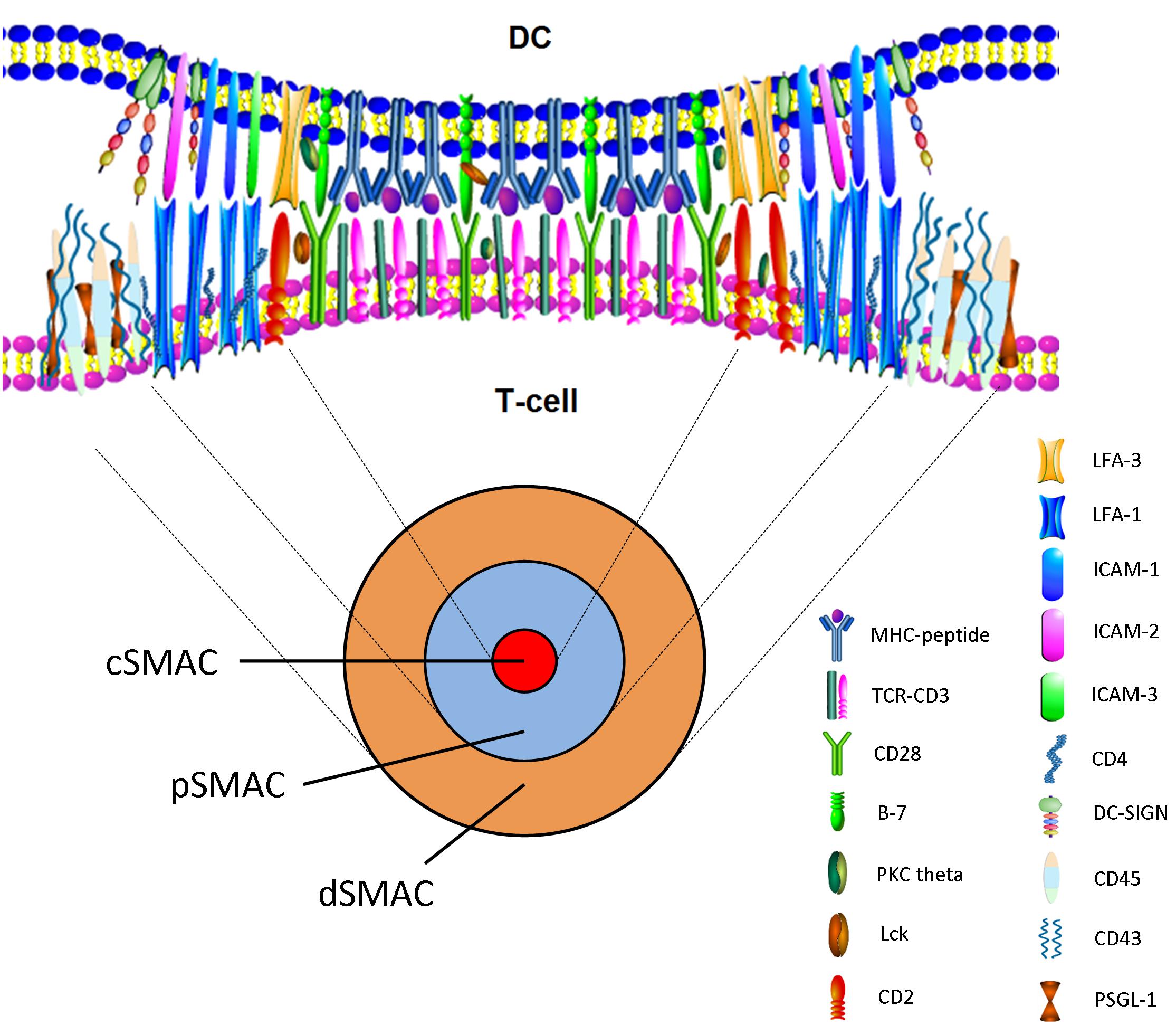
FIGURE 2. En face view of the IS with cSMAC, pSMAC, and dSMAC. Active reorganization via cytoskeleton-directed movements gives rise to the cSMAC. The mature synapse with the cSMAC, the pSMAC, and the dSMAC is observed after T-cell–APC contact.
Factors That Influence IS Formation
TCR signaling is necessary for the maintenance of IS. TCR-microclusters (TCR-MCs) are formed immediately after the TCR on T cells adheres to peptide MHC complex including many signal molecules such as CD3, SLP-76, TCR, and ZAP-70 (Campi et al., 2005; Saito and Yokosuka, 2006). TCR-MCs are the activation site of initial signal. TCR stimulation, calcium influx, tyrosine activation occur before the formation of cSMAC. TCR-MCs are continuously produced, even after IS formation (Barda-Saad et al., 2005). TCR is rapidly internalized about 5 min after the exposure to DC, but TCR stimulation will continue for several hours, which results in the activation signal in the peripheral MCs rather than cSMAC. Blocking TCR-peptide–MHC complex within 10 hours results in IS dissolution, decreasing the level of calcium influx and causes cell separation. These findings indicate that the maintenance of TCR signaling is necessary for the maintenance of IS and the full activation of T cells.
CD4 molecule could promote IS formation. CD4 can improve the sensitivity of T cells to antigens and can accumulate Lck to the center region of IS after the initial exposure of DCs to T cells. CD4-deficient T cells have a reduced proliferative response to antigen stimulation and the effect of IS formation is also significantly reduced. The cells expressing CD4 or displaying a peptide MHC complex have a strong binding, suggesting that CD4 is not only an auxiliary receptor but also contributes to cell adhesion. The actual lipid raft is the key components of a functional IS. The accumulation of lipid rafts was observed at cSMAC, indicating that the formation of IS was accompanied by the movement of lipid rafts to cSMAC and gathered on the contact interface of cells (Henel et al., 2006). Lck and LAT are linked to lipid rafts by deacylation. Other signaling molecules such as PLC gamma, SLP76, and Vav are transferred to IS by binding to phosphorylated tyrosine on LAT (Phee et al., 2005; Braiman et al., 2006; Soares et al., 2013). CTLA-4 limits the accumulation of lipid rafts, thereby inhibiting T cell proliferation and cytokine secretion (Teft et al., 2006). Actin movement is associated with the transportation of cytoskeleton and can be blocked by the myosin inhibitor butanedione monoxime (van der Honing et al., 2010).
Overall, TCR signaling, CD4 molecule, lipid raft, PLC gamma and CTLA-4, and so on not only involve in IS information but also modulate IS information. Any abnormal signals or the imbalance among molecules would lead to abnormal T cell activation in autoimmune diseases. These molecules might be new drug targets, and it would offer new therapy strategies through developing new drugs targeting above molecules.
Immunotherapy Targeting T Cells Activating in Autoimmune Diseases
Detailed insights into the molecular mechanisms of the interaction between T cells and DCs are helpful to design immunotherapy strategies that target T cell activation in autoimmune diseases. At present, some biological agents, such as CTLA-4Ig (Abatacept), Anti CD3 monoclonal antibody, LFA-3 Ig fusion protein (Alefacept) that target co-stimulation molecules on T cell have been developed and approved to treat autoimmune diseases.
CTLA-4Ig Modulates Co-stimulatory Signals and Inhibits T Cell Activation
The recombinant fusion protein, CTLA-4Ig (Abatacept) that comprises the extracellular domain of human CTLA4 and a fragment of Fc domain of human IgG1 belongs to a new type of selective co-stimulatory modulators. Abatacept prevents complement fixation and modulates the necessary co-stimulatory signal for T cell activation. Furthermore, it binds to CD80 and CD86, thus competing with CD28 and reducing T cell activation (Cutolo and Nadler, 2013; Keating, 2013). The fusion protein affects multiple downstream pathways through modulation of upstream events of T cells activation. Additionally, Abatacept inhibits T-cell proliferation and the secretion of IFN-gamma, IL-1, IL-6, and TNF-alpha (Koenders et al., 2012; Whitfield et al., 2017).
As therapy, Abatacept is mainly used in RA treatment (Genovese et al., 2005; Dorner et al., 2010). It has been proven to be efficient, safe, and tolerable in combination with methotrexate (MTX) in clinical trials with RA patients when the response to MTX was inadequate (Kremer et al., 2005). In Europe, Abatacept is approved for the treatment of patients with highly active and progressive RA, who have never received MTX treatment. It is also approved for the treatment of patients with moderate to severe active RA, who have shown inadequate responses in previous therapies with at least one conventional disease-modifying antirheumatic drug (cDMARDs) such as MTX or a TNF inhibitor. In phase III clinical trials, intravenous or subcutaneous injection regimens of Abatacept were beneficial for RA symptoms, disease activity, structural damage progression and physical function of the joint. In a long-term follow-up, the efficacy could be shown to be maintained. The drug-free remission rates following discontinuation of all RA treatment were significantly higher after treatment of Abatacept plus methotrexate than of methotrexate alone. Intravenous and subcutaneous injections of Abatacept were generally well tolerated and showed low immunogenicity (Blair and Deeks, 2017). Previous studies using synovial tissue from RA patients treated with Abatacept found the inhibition of B-cell proliferation and down regulation of the expression of B-cell markers (Buch et al., 2009).
Abatacept was also used to treat lupus nephritis by inhibiting CD28 engagement on T cells and plasma cells (Bahlis et al., 2007). This mechanistic rationale is strongly supported by the studies in SLE murine models, in which treatment with Abatacept or other forms of CTLA4-Ig have been shown to arrest and even reverse established lupus nephritis. Treatment with Abatacept induced remission by binding to CD80 on renal podocytes in patients with focal segmental glomerulosclerosis (Yu et al., 2013; Group, 2014).
Anti-CD3 mAbs by Induce Anergy and Apoptosis of Activated T Cells
Anti CD3 monoclonal antibodies are an immunosuppressant. Muromonab-CD3 is a murine IgG2, which specifically binds to CD3 on T cells and blocks proliferation and function of T cells. Tolerance induction by anti-CD3 mAbs is related to the induction of Tregs that control pathogenic autoimmune responses preferentially by inducing anergy or apoptosis in activated T cells while ignoring Tregs (You et al., 2008; Penaranda et al., 2011). Consequently, anti-CD3 mAb therapy is associated with an increase in number and function of Treg and regulatory cytokines such as TGF-beta and IL-10. The heterogeneity of TCR expression by different T-cell subsets might explain the differential effect of anti-CD3 mAb on effector, regulatory or naïve T cells (Valle et al., 2015). At the same time anti-CD3 mAb-induced signaling through the CD3/TCR complex can render the T cell anergic or trigger apoptosis. Various studies have shown that anti-CD3 mAbs effectively treat chronic inflammatory and autoimmune diseases, such as IBD, T1D and MS. Intravenous administration of anti-CD3 mAb has been successfully tested in numerous animal models of autoimmunity, including the experimental autoimmune encephalomyelitis (EAE) model of MS, diabetic NOD mice, TNP-KLH induced colitis (a model of IBD) and collagen-induced arthritis (Kohm et al., 2005; Chatenoud and Bluestone, 2007; Notley et al., 2010; Wu et al., 2010).
Biological agents targeting CD3 include teplizumab, otelixizumab, and visilizumab. It has been observed that administration of otelixizumab, teplizumab, or visilizumab result in positive clinical responses (Keymeulen et al., 2005, 2010; Plevy et al., 2007). Otelixizumab and teplizumab were foremost tested in T1D patients, while visilizumab and foralumab were mainly studied in IBD (Yu et al., 2008; Daifotis et al., 2013). In clinical trials, the tolerogenic activity of humanized anti-CD3 mAb (visilizumab) in T1D was found to be excellent. In a second Phase I/II trial, teplizumab improved insulin production and metabolic control in patients with recent onset T1D. A phase II trial that assessed the safety and efficacy of visilizumab in patients with severe corticosteroid-refractory ulcerative colitis had promising results (Plevy et al., 2007). In general, non-FcR binding anti-CD3 mAb are promising models for treatment of autoimmune and inflammatory diseases (Herold et al., 2003).
LFA-3 Ig (Alefacept) and Anti-LFA-1 Antibody (Efalizumab) Inhibit CD2 Signaling in T Cells
It had been demonstrated that an LFA-3 Ig fusion protein (Alefacept) could reduce psoriasis lesions (Nickoloff and Nestle, 2004). Alefacept competes with LFA-3 for binding to CD2 on T cells and efficiently interferes with LFA-3/CD2 binding, consequently stopping T cell activation. Furthermore, the Ig part of Alefacept binds to immunoglobulin receptor Fc-gamma-RIII on the surface of natural killer cells and some T cell subpopulations inducing apoptosis of memory T cell subgroups (da Silva et al., 2002; Rigby et al., 2015). Finally, administered intramuscularly or intravenously Alefacept inhibits T cell activation and proliferation, and induces the apoptosis of memory-effector (CD45RO+) T cells (Konig et al., 2016).
In psoriasis the presence of LFA-1 in IS is very important. A separate anti-LFA-1 antibody (Efalizumab) has been shown to block adhesive interaction in the treatment of psoriasis. The antibody reduced skin lesions by blocking the adhesion molecule on T cells and was well tolerated and resulted in significant improvement in patients with moderate to severe plaque psoriasis (Papp et al., 2006).
Conclusion
In summary, the interaction between T cells and DCs involves in the pathogenesis of autoimmune disease. Autoreactive T cells are activated by autoantigens presented by DCs during the interaction between T and DC (Tan T. et al., 2014). The underlying molecular mechanisms of T cell activation by DCs have been well understood. Three stages during T cells activation by DCs, including antigen presenting, antigen recognition of T cells, and two signals formation have been investigated in great detail. T cells could be activated in two signals model by simultaneously receiving signal-1 from T-cell recognition of antigen and signal-2 from costimulatory molecular. In addition, IS formation between T cells and DCs plays an important role in T cell activation. cSMAC, pSMAC, and dSMAC form the molecular structure of IS. IS molecular structure is very complex involving in a variety of molecules and signals, which take part in IS formation through interaction and dynamically balance.
Understanding the molecular mechanisms of the interaction between T cells and DCs is helpful to discover new drug targets and design immunotherapy strategies that target T cell activation in autoimmune diseases. At present, some recombinant fusion protein and monoclonal antibodies targeting costimulatory molecules, such as CTLA-4- and LFA-3-Ig, anti-CD3 monoclonal antibody, and so on have been developed and approved to treat autoimmune diseases, such as RA, SLE, IBD, MS, and psoriasis. These biological drugs show a significant efficacy and have a high safety profile. More biological agents that modulate T cell activation will be developed based on a better understanding of the molecular mechanisms of T cell activation in the near future.
Author Contributions
YT and QW collected data and wrote this paper. HK and LZ revised the article. WW designed the work.
Funding
This work was financially supported through the National Natural Science Foundation of China (Nos. 81330081, 81202541, 81473223, and 81673444), the Anhui Provincial Natural Science Foundation for Distinguished Young Scholars (1808085J28), the Key Projects of Natural Science Research of Anhui Colleges and Universities (KJ2017A176), Anhui University Excellent Youth Talent Support Program (gxyqZD2017025), Innovation and Entrepreneurship Support Program for Returnees of Anhui Province.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acuto, O., Di Bartolo, V., and Michel, F. (2008). Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 8, 699–712. doi: 10.1038/nri2397
Amarnath, S., Mangus, C. W., Wang, J. C., Wei, F., He, A., Kapoor, V., et al. (2011). The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl. Med. 3:111ra120. doi: 10.1126/scitranslmed.3003130
Anderson, M. E., and Siahaan, T. J. (2003). Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides 24, 487–501. doi: 10.1016/S0196-9781(03)00083-4
Audiger, C., Rahman, M. J., Yun, T. J., Tarbell, K. V., and Lesage, S. (2017). The importance of dendritic cells in maintaining immune tolerance. J. Immunol. 198, 2223–2231. doi: 10.4049/jimmunol.1601629
Bahlis, N. J., King, A. M., Kolonias, D., Carlson, L. M., Liu, H. Y., Hussein, M. A., et al. (2007). CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood 109, 5002–5010. doi: 10.1182/blood-2006-03-012542
Bansal-Pakala, P., Halteman, B. S., Cheng, M. H., and Croft, M. (2004). Costimulation of CD8 T cell responses by OX40. J. Immunol. 172, 4821–4825. doi: 10.4049/jimmunol.172.8.4821
Barda-Saad, M., Braiman, A., Titerence, R., Bunnell, S. C., Barr, V. A., and Samelson, L. E. (2005). Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6, 80–89. doi: 10.1038/ni1143
Barral, D. C., and Brenner, M. B. (2007). CD1 antigen presentation: how it works. Nat. Rev. Immunol. 7, 929–941. doi: 10.1038/nri2191
Barreiro, O., De La Fuente, H., Mittelbrunn, M., and Sanchez-Madrid, F. (2007). Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol. Rev. 218, 147–164. doi: 10.1111/j.1600-065X.2007.00529.x
Blair, H. A., and Deeks, E. D. (2017). Abatacept: a review in rheumatoid arthritis. Drugs 77, 1221–1233. doi: 10.1007/s40265-017-0775-4
Braiman, A., Barda-Saad, M., Sommers, C. L., and Samelson, L. E. (2006). Recruitment and activation of PLCgamma1 in T cells: a new insight into old domains. EMBO J. 25, 774–784. doi: 10.1038/sj.emboj.7600978
Buch, M. H., Boyle, D. L., Rosengren, S., Saleem, B., Reece, R. J., Rhodes, L. A., et al. (2009). Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann. Rheum. Dis. 68, 1220–1227. doi: 10.1136/ard.2008.091876
Burmester, G. R., Feist, E., and Dorner, T. (2014). Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 77–88. doi: 10.1038/nrrheum.2013.168
Campi, G., Varma, R., and Dustin, M. L. (2005). Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202, 1031–1036. doi: 10.1084/jem.20051182
Chatenoud, L., and Bluestone, J. A. (2007). CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 7, 622–632. doi: 10.1038/nri2134
Chemnitz, J. M., Parry, R. V., Nichols, K. E., June, C. H., and Riley, J. L. (2004). SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 173, 945–954. doi: 10.4049/jimmunol.173.2.945
Chen, W., Liang, X., Peterson, A. J., Munn, D. H., and Blazar, B. R. (2008). The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 181, 5396–5404. doi: 10.4049/jimmunol.181.8.5396
Creusot, R. J., Giannoukakis, N., Trucco, M., Clare-Salzler, M. J., and Fathman, C. G. (2014). It’s time to bring dendritic cell therapy to type 1 diabetes. Diabetes Metab. Res. Rev. 63, 20–30. doi: 10.2337/db13-0886
Cutolo, M., and Nadler, S. G. (2013). Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun. Rev. 12, 758–767. doi: 10.1016/j.autrev.2013.01.001
da Silva, A. J., Brickelmaier, M., Majeau, G. R., Li, Z., Su, L., Hsu, Y. M., et al. (2002). Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2(+) cells. J. Immunol. 168, 4462–4471. doi: 10.4049/jimmunol.168.9.4462
Daifotis, A. G., Koenig, S., Chatenoud, L., and Herold, K. C. (2013). Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin. Immunol. 149, 268–278. doi: 10.1016/j.clim.2013.05.001
de Jong, A., Pena-Cruz, V., Cheng, T. Y., Clark, R. A., Van Rhijn, I., and Moody, D. B. (2010). CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat. Immunol. 11, 1102–1109. doi: 10.1038/ni.1956
Dorner, T., Kinnman, N., and Tak, P. P. (2010). Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol. Ther. 125, 464–475. doi: 10.1016/j.pharmthera.2010.01.001
Dustin, M. L. (2005). A dynamic view of the immunological synapse. Semin. Immunol. 17, 400–410. doi: 10.1016/j.smim.2005.09.002
Elpek, K. G., Yolcu, E. S., Franke, D. D., Lacelle, C., Schabowsky, R. H., and Shirwan, H. (2007). Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J. Immunol. 179, 7295–7304. doi: 10.4049/jimmunol.179.11.7295
Felio, K., Nguyen, H., Dascher, C. C., Choi, H. J., Li, S., Zimmer, M. I., et al. (2009). CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J. Exp. Med. 206, 2497–2509. doi: 10.1084/jem.20090898
Feng, Y., Van Der Veeken, J., Shugay, M., Putintseva, E. V., Osmanbeyoglu, H. U., Dikiy, S., et al. (2015). A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature 528, 132–136. doi: 10.1038/nature16141
Fletcher, J. M., Lalor, S. J., Sweeney, C. M., Tubridy, N., and Mills, K. H. (2010). T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11. doi: 10.1111/j.1365-2249.2010.04143.x
Frauwirth, K. A., and Thompson, C. B. (2004). Regulation of T lymphocyte metabolism. J. Immunol. 172, 4661–4665. doi: 10.4049/jimmunol.172.8.4661
Garcia, K. C. (2012). Reconciling views on T cell receptor germline bias for MHC. Trends Immunol. 33, 429–436. doi: 10.1016/j.it.2012.05.005
Garg, A. D., Nowis, D., Golab, J., Vandenabeele, P., Krysko, D. V., and Agostinis, P. (2010). Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim. Biophys. Acta 1805, 53–71. doi: 10.1016/j.bbcan.2009.08.003
Geem, D., Harusato, A., Flannigan, K., and Denning, T. L. (2015). Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1409–1418. doi: 10.1097/MIB.0000000000000343
Genovese, M. C., Becker, J. C., Schiff, M., Luggen, M., Sherrer, Y., Kremer, J., et al. (2005). Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 353, 1114–1123. doi: 10.1056/NEJMoa050524
Gilleron, M., Stenger, S., Mazorra, Z., Wittke, F., Mariotti, S., Bohmer, G., et al. (2004). Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 199, 649–659. doi: 10.1084/jem.20031097
Gilliet, M., Cao, W., and Liu, Y. J. (2008). Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606. doi: 10.1038/nri2358
Govers, C., Sebestyen, Z., Coccoris, M., Willemsen, R. A., and Debets, R. (2010). T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends Mol. Med. 16, 77–87. doi: 10.1016/j.molmed.2009.12.004
Grigorian, A., Torossian, S., and Demetriou, M. (2009). T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol. Rev. 230, 232–246. doi: 10.1111/j.1600-065X.2009.00796.x
Group, A. T. (2014). Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study. Arthritis Rheumatol. 66, 3096–3104. doi: 10.1002/art.38790
Hannani, D., Vetizou, M., Enot, D., Rusakiewicz, S., Chaput, N., Klatzmann, D., et al. (2015). Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 25, 208–224. doi: 10.1038/cr.2015.3
Henel, G., Singh, K., Cui, D., Pryshchep, S., Lee, W. W., Weyand, C. M., et al. (2006). Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood 107, 4449–4457. doi: 10.1182/blood-2005-06-2519
Herold, K. C., Burton, J. B., Francois, F., Poumian-Ruiz, E., Glandt, M., and Bluestone, J. A. (2003). Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala). J. Clin. Invest. 111, 409–418. doi: 10.1172/JCI16090
Huang, G., Wang, Y., Vogel, P., Kanneganti, T. D., Otsu, K., and Chi, H. (2012). Signaling via the kinase p38alpha programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nat. Immunol. 13, 152–161. doi: 10.1038/ni.2207
Jones, E. Y., Fugger, L., Strominger, J. L., and Siebold, C. (2006). MHC class II proteins and disease: a structural perspective. Nat. Rev. Immunol. 6, 271–282. doi: 10.1038/nri1805
Kabelitz, D., and Medzhitov, R. (2007). Innate immunity–cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr. Opin. Immunol. 19, 1–3. doi: 10.1016/j.coi.2006.11.018
Kang, T. B., Yang, S. H., Toth, B., Kovalenko, A., and Wallach, D. (2014). Activation of the NLRP3 inflammasome by proteins that signal for necroptosis. Methods Enzymol. 545, 67–81. doi: 10.1016/B978-0-12-801430-1.00003-2
Keating, G. M. (2013). Abatacept: a review of its use in the management of rheumatoid arthritis. Drugs 73, 1095–1119. doi: 10.1007/s40265-013-0080-9
Keymeulen, B., Vandemeulebroucke, E., Ziegler, A. G., Mathieu, C., Kaufman, L., Hale, G., et al. (2005). Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608. doi: 10.1056/NEJMoa043980
Keymeulen, B., Walter, M., Mathieu, C., Kaufman, L., Gorus, F., Hilbrands, R., et al. (2010). Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 53, 614–623. doi: 10.1007/s00125-009-1644-9
Koenders, M. I., Marijnissen, R. J., Joosten, L. A., Abdollahi-Roodsaz, S., Di Padova, F. E., Van De Loo, F. A., et al. (2012). T cell lessons from the rheumatoid arthritis synovium SCID mouse model: CD3-rich synovium lacks response to CTLA-4Ig but is successfully treated by interleukin-17 neutralization. Arthritis Rheum. 64, 1762–1770. doi: 10.1002/art.34352
Kohm, A. P., Williams, J. S., Bickford, A. L., Mcmahon, J. S., Chatenoud, L., Bach, J. F., et al. (2005). Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J. Immunol. 174, 4525–4534. doi: 10.4049/jimmunol.174.8.4525
Konig, M., Rharbaoui, F., Aigner, S., Dalken, B., and Schuttrumpf, J. (2016). Tregalizumab - a monoclonal antibody to target regulatory T cells. Front. Immunol. 7:11. doi: 10.3389/fimmu.2016.00011
Kopf, M., Coyle, A. J., Schmitz, N., Barner, M., Oxenius, A., Gallimore, A., et al. (2000). Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192, 53–61. doi: 10.1084/jem.192.1.53
Kremer, J. M., Dougados, M., Emery, P., Durez, P., Sibilia, J., Shergy, W., et al. (2005). Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 52, 2263–2271. doi: 10.1002/art.21201
Lebre, M. C., Jongbloed, S. L., Tas, S. W., Smeets, T. J., Mcinnes, I. B., and Tak, P. P. (2008). Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am. J. Pathol. 172, 940–950. doi: 10.2353/ajpath.2008.070703
Lee, K. H., Holdorf, A. D., Dustin, M. L., Chan, A. C., Allen, P. M., and Shaw, A. S. (2002). T cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542. doi: 10.1126/science.1067710
Lee, S. W., Park, Y., So, T., Kwon, B. S., Cheroutre, H., Mittler, R. S., et al. (2008). Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat. Immunol. 9, 917–926. doi: 10.1038/ni.1632
Li, H., Zhang, G. X., Chen, Y., Xu, H., Fitzgerald, D. C., Zhao, Z., et al. (2008). CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J. Immunol. 181, 2483–2493. doi: 10.4049/jimmunol.181.4.2483
Lombardi, V., Van Overtvelt, L., Horiot, S., and Moingeon, P. (2009). Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J. Immunol. 182, 3372–3379. doi: 10.4049/jimmunol.0801969
Manikwar, P., Kiptoo, P., Badawi, A. H., Buyuktimkin, B., and Siahaan, T. J. (2012). Antigen-specific blocking of CD4-specific immunological synapse formation using BPI and current therapies for autoimmune diseases. Med. Res. Rev. 32, 727–764. doi: 10.1002/med.20243
Min, S. Y., Park, K. S., Cho, M. L., Kang, J. W., Cho, Y. G., Hwang, S. Y., et al. (2006). Antigen-induced, tolerogenic CD11c+,CD11b+ dendritic cells are abundant in Peyer’s patches during the induction of oral tolerance to type II collagen and suppress experimental collagen-induced arthritis. Arthritis Rheum. 54, 887–898. doi: 10.1002/art.21647
Neefjes, J., Jongsma, M. L., Paul, P., and Bakke, O. (2011). Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836. doi: 10.1038/nri3084
Nickoloff, B. J., and Nestle, F. O. (2004). Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113, 1664–1675. doi: 10.1172/JCI200422147
Notley, C. A., Mccann, F. E., Inglis, J. J., and Williams, R. O. (2010). ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ Treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis Rheum. 62, 171–178. doi: 10.1002/art.25058
Papp, K. A., Miller, B., Gordon, K. B., Caro, I., Kwon, P., Compton, P. G., et al. (2006). Efalizumab retreatment in patients with moderate to severe chronic plaque psoriasis. J. Am. Acad. Dermatol. 54, S164–S170. doi: 10.1016/j.jaad.2005.10.032
Penaranda, C., Tang, Q., and Bluestone, J. A. (2011). Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J. Immunol. 187, 2015–2022. doi: 10.4049/jimmunol.1100713
Pentcheva-Hoang, T., Egen, J. G., Wojnoonski, K., and Allison, J. P. (2004). B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 21, 401–413. doi: 10.1016/j.immuni.2004.06.017
Phee, H., Abraham, R. T., and Weiss, A. (2005). Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat. Immunol. 6, 608–617. doi: 10.1038/ni1199
Plevy, S., Salzberg, B., Van Assche, G., Regueiro, M., Hommes, D., Sandborn, W., et al. (2007). A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis. Gastroenterology 133, 1414–1422. doi: 10.1053/j.gastro.2007.08.035
Pugliese, A. (2017). Autoreactive T cells in type 1 diabetes. J. Clin. Invest. 127, 2881–2891. doi: 10.1172/JCI94549
Reiser, J. B., Darnault, C., Guimezanes, A., Gregoire, C., Mosser, T., Schmitt-Verhulst, A. M., et al. (2000). Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat. Immunol. 1, 291–297. doi: 10.1038/79728
Rigby, M. R., Harris, K. M., Pinckney, A., Dimeglio, L. A., Rendell, M. S., Felner, E. I., et al. (2015). Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J. Clin. Invest. 125, 3285–3296. doi: 10.1172/JCI81722
Ritter, U., Wiede, F., Mielenz, D., Kiafard, Z., Zwirner, J., and Korner, H. (2004). Analysis of the CCR7 expression on murine bone marrow-derived and spleen dendritic cells. J. Leukoc. Biol. 76, 472–476. doi: 10.1189/jlb.0104037
Rossy, J., Owen, D. M., Williamson, D. J., Yang, Z., and Gaus, K. (2013). Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat. Immunol. 14, 82–89. doi: 10.1038/ni.2488
Saito, T., and Yokosuka, T. (2006). Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr. Opin. Immunol. 18, 305–313. doi: 10.1016/j.coi.2006.03.014
Schamel, W. W., Arechaga, I., Risueno, R. M., Van Santen, H. M., Cabezas, P., Risco, C., et al. (2005). Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J. Exp. Med. 202, 493–503. doi: 10.1084/jem.20042155
Schwartz, J. C., Zhang, X., Nathenson, S. G., and Almo, S. C. (2002). Structural mechanisms of costimulation. Nat. Immunol. 3, 427–434. doi: 10.1038/ni0502-427
Segura, E., Touzot, M., Bohineust, A., Cappuccio, A., Chiocchia, G., Hosmalin, A., et al. (2013). Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38, 336–348. doi: 10.1016/j.immuni.2012.10.018
Shao, L., Kamalu, O., and Mayer, L. (2005). Non-classical MHC class I molecules on intestinal epithelial cells: mediators of mucosal crosstalk. Immunol. Rev. 206, 160–176. doi: 10.1111/j.0105-2896.2005.00295.x
Sixt, M., Kanazawa, N., Selg, M., Samson, T., Roos, G., Reinhardt, D. P., et al. (2005). The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29. doi: 10.1016/j.immuni.2004.11.013
Soares, H., Henriques, R., Sachse, M., Ventimiglia, L., Alonso, M. A., Zimmer, C., et al. (2013). Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J. Exp. Med. 210, 2415–2433. doi: 10.1084/jem.20130150
Steinman, R. M. (2007). Lasker basic medical research award. Dendritic cells: versatile controllers of the immune system. Nat. Med. 13, 1155–1159. doi: 10.1038/nm1643
Suarez-Fueyo, A., Bradley, S. J., and Tsokos, G. C. (2016). T cells in Systemic lupus erythematosus. Curr. Opin. Immunol. 43, 32–38. doi: 10.1016/j.coi.2016.09.001
Tan, Q., Majewska-Szczepanik, M., Zhang, X., Szczepanik, M., Zhou, Z., Wong, F. S., et al. (2014). IRAK-M deficiency promotes the development of type 1 diabetes in NOD mice. Diabetes Metab. Res. Rev. 63, 2761–2775. doi: 10.2337/db13-1504
Tan, T., Xiang, Y., Chang, C., and Zhou, Z. (2014). Alteration of regulatory T cells in type 1 diabetes mellitus: a comprehensive review. Clin. Rev. Allergy Immunol. 47, 234–243. doi: 10.1007/s12016-014-8440-0
Teft, W. A., Kirchhof, M. G., and Madrenas, J. (2006). A molecular perspective of CTLA-4 function. Annu. Rev. Immunol. 24, 65–97. doi: 10.1146/annurev.immunol.24.021605.090535
Topalian, S. L., Drake, C. G., and Pardoll, D. M. (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461. doi: 10.1016/j.ccell.2015.03.001
Tournadre, A., Lenief, V., Eljaafari, A., and Miossec, P. (2012). Immature muscle precursors are a source of interferon-beta in myositis: role of Toll-like receptor 3 activation and contribution to HLA class I up-regulation. Arthritis Rheum. 64, 533–541. doi: 10.1002/art.33350
Tseng, S. Y., Waite, J. C., Liu, M., Vardhana, S., and Dustin, M. L. (2008). T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C theta. J. Immunol. 181, 4852–4863. doi: 10.4049/jimmunol.181.7.4852
Valitutti, S. (2008). Immunological synapse: center of attention again. Immunity 29, 384–386. doi: 10.1016/j.immuni.2008.08.002
Valle, A., Barbagiovanni, G., Jofra, T., Stabilini, A., Perol, L., Baeyens, A., et al. (2015). Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti-CD3-mediated T cell modulation. J. Immunol. 194, 2117–2127. doi: 10.4049/jimmunol.1401551
van der Honing, H. S., De Ruijter, N. C., Emons, A. M., and Ketelaar, T. (2010). Actin and myosin regulate cytoplasm stiffness in plant cells: a study using optical tweezers. New Phytol. 185, 90–102. doi: 10.1111/j.1469-8137.2009.03017.x
Van Rhijn, I., Kasmar, A., De Jong, A., Gras, S., Bhati, M., Doorenspleet, M. E., et al. (2013). A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat. Immunol. 14, 706–713. doi: 10.1038/ni.2630
Vesely, M. D., Kershaw, M. H., Schreiber, R. D., and Smyth, M. J. (2011). Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 29, 235–271. doi: 10.1146/annurev-immunol-031210-101324
Vogel, I., Kasran, A., Cremer, J., Kim, Y. J., Boon, L., Van Gool, S. W., et al. (2015). CD28/CTLA-4/B7 costimulatory pathway blockade affects regulatory T-cell function in autoimmunity. Eur. J. Immunol. 45, 1832–1841. doi: 10.1002/eji.201445190
Vyas, J. M., Van Der Veen, A. G., and Ploegh, H. L. (2008). The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 8, 607–618. doi: 10.1038/nri2368
Whitfield, S. J. C., Taylor, C., Risdall, J. E., Griffiths, G. D., Jones, J. T. A., Williamson, E. D., et al. (2017). Interference of the T Cell and antigen-presenting cell costimulatory pathway using CTLA4-Ig (Abatacept) prevents staphylococcal enterotoxin B pathology. J. Immunol. 198, 3989–3998. doi: 10.4049/jimmunol.1601525
Wing, K., Onishi, Y., Prieto-Martin, P., Yamaguchi, T., Miyara, M., Fehervari, Z., et al. (2008). CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275. doi: 10.1126/science.1160062
Wu, H. Y., Maron, R., Tukpah, A. M., and Weiner, H. L. (2010). Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J. Immunol. 185, 3401–3407. doi: 10.4049/jimmunol.1000836
Yamazaki, S., Dudziak, D., Heidkamp, G. F., Fiorese, C., Bonito, A. J., Inaba, K., et al. (2008). CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181, 6923–6933. doi: 10.4049/jimmunol.181.10.6923
You, S., Candon, S., Kuhn, C., Bach, J. F., and Chatenoud, L. (2008). CD3 antibodies as unique tools to restore self-tolerance in established autoimmunity their mode of action and clinical application in type 1 diabetes. Adv. Immunol. 100, 13–37. doi: 10.1016/S0065-2776(08)00802-X
Yu, C. C., Fornoni, A., Weins, A., Hakroush, S., Maiguel, D., Sageshima, J., et al. (2013). Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 369, 2416–2423. doi: 10.1056/NEJMoa1304572
Keywords: T cell, dendritic cells, activation, autoimmune diseases, immunological synapse, biological agents
Citation: Tai Y, Wang Q, Korner H, Zhang L and Wei W (2018) Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Front. Pharmacol. 9:642. doi: 10.3389/fphar.2018.00642
Received: 05 February 2018; Accepted: 29 May 2018;
Published: 26 June 2018.
Edited by:
Annalisa Bruno, Università degli Studi G. d’Annunzio Chieti Pescara, ItalyReviewed by:
Barbara Rossi, University of Verona, ItalyCristina Tecchio, University of Verona, Italy
Copyright © 2018 Tai, Wang, Korner, Zhang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingling Zhang, bGwtemhhbmdAaG90bWFpbC5jb20= Wei Wei, d3dlaUBhaG11LmVkdS5jbg==
†These authors have contributed equally to this work.
 Yu Tai1†
Yu Tai1† Lingling Zhang
Lingling Zhang Wei Wei
Wei Wei