
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr., 15 October 2020
Sec. Neonatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.553519
 Hai-Bo Huang1
Hai-Bo Huang1 Yi-Hua Chen1
Yi-Hua Chen1 Jing Wu2
Jing Wu2 Matt Hicks3
Matt Hicks3 Yan-Zhi Yi1
Yan-Zhi Yi1 Qian-Shen Zhang1
Qian-Shen Zhang1 Chun-Bong Chow1
Chun-Bong Chow1 Po-Yin Cheung1,3*
Po-Yin Cheung1,3*Objective: To investigate the incidence and risk factors of retinopathy of prematurity (ROP) in very and extremely preterm (28+0- <32+0, and <28+0 weeks gestation, respectively) neonates, and the predictive factors for ROP in the early hours after birth and during hospitalization.
Methods: Using a prospective database supplemented with a retrospective chart review, we identified preterm neonates born at gestation <32 weeks at the University of Hong Kong-Shenzhen Hospital between January 2015 and August 2018. Demographic and clinical variables were studied including indicators of disease acuity in the first 24 h after birth. We also compared the difference in risk factors between survivors with ROP and survivors without ROP.
Results: During the study period, there were 529 preterm neonates admitted to our neonatal intensive care unit with 120 (23%) born at <32 weeks' gestation. Thirteen (11%) neonates died. Among the 107 survivors, 23 (21%) had ROP, of whom five (22%) received laser and/or medical therapy for severe ROP. Compared with survivors without ROP, infants with ROP had lower mean blood pressure in the first 12 and 24 h after birth, respectively. Using multivariate regression, gestation age, mean blood pressure in the first 12 h after birth, hospital length of stay, and total days of blood gases pH <7.2 were independent risk factors for ROP.
Conclusions: In this small cohort of Chinese neonates born <32+0 weeks' gestation, survivors with ROP had a lower blood pressure in the early hours after birth, younger gestation, longer hospital stay, and duration of acidosis when compared to those without ROP.
Improved obstetrical and neonatal care has increased the survival of very preterm (28+0- <32+0 weeks gestation) and extremely preterm (<28+0 weeks gestation) neonates, who may subsequently develop complications including retinopathy of prematurity (ROP). Retinopathy of prematurity is a developmental, proliferative, vascular disorder that occurs in the retina of preterm infants who have incomplete retinal vascularization (1). Globally, it was estimated that 184,700 of 14.9 million preterm neonates (1.2%) developed any stage of ROP, with an incidence of 16% in the very and extremely preterm neonates (2). In high-income countries, ~20% of infants with ROP would subsequently require treatment (2). However, in China, the incidence of ROP was 6.6–28.1% with up to 40% (13.2–39.9%) with severe type I ROP requiring laser and/or medical therapy (3–6).
The most important risk factor for developing ROP is prematurity. In multivariate analyses, other independent risk factors for ROP include low birth weight (BW), low gestational age (GA), assisted ventilation for longer than 1 week, surfactant therapy, high blood transfusion volume, cumulative illness severity, low caloric intake, hyperglycemia, and insulin therapy (7–14). However, there is little information regarding predictive factors for ROP in the early hours after birth.
We aimed to investigate the incidence and risk factors of ROP in the very and extremely preterm neonates. We hypothesized that the indicators of disease acuity including blood pressure (BP) during the early hours after birth would be associated with the subsequent development of ROP.
This study was performed using a prospective database supplemented with retrospective chart reviews. The study received the approval of institutional ethics board [#(2018)88r]. Informed consent was obtained from the parents before the initial screening for ROP as per institutional practice.
All neonates born before 32 weeks' gestation between January 2015 and August 2018 were included in this study. Infants were excluded from the study if they had congenital retinal abnormalities or if a follow-up eye examination was not completed or non-survival before initial hospitalization. All infants who were admitted into the Neonatal Intensive Care Unit of the University of Hong Kong-Shenzhen Hospital had demographic and clinical information collected in a prospective database by clinical assistants supervised by a senior doctor (Y-H C). The demographic and clinical information included GA, BW, gender, delivery mode, single or multiple births, Apgar score at 1 and 5 min, mean BP, lowest body temperature, urine output in the 1st and 2nd 12 h periods of life, vasoactive medications in the 1st 24 h, hospital length of stay, duration of total parenteral nutrition (TPN) and ventilation (non-invasive and invasive), intubation in the delivery room, surfactant therapy, weight gain, respiratory distress syndrome, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage, necrotizing enterocolitis, neonatal sepsis, total number of days with blood gases pH <7.2, pH > 7.5, PCO2 <30 mmHg, PCO2 > 80 mmHg, PO2 <50 mmHg, PO2 > 150 mmHg, highest C-reactive protein level, lowest leukocyte and platelet counts, and number of red blood cells transfusion.
Eye examinations were performed under indirect ophthalmoscopy by JW, who has extensive experience in neonatal retinal examination, on all very and extremely preterm infants according to the unit policy. The International Classification of ROP guidelines were used to record the stage of ROP, location by zone, and signs of plus disease (15). All infants were scheduled to have their first examination between 4 and 6 weeks of life. If ROP was detected, the examinations were repeated at intervals according to the severity of findings. Ophthalmic examinations were continued until full retinal vascularization and the maximum stage of ROP for each infant was reported. The data were analyzed based on the most advanced stage of ROP in the eye with the most severe disease. Severe ROP was defined as ROP that required treatment for laser photocoagulation and/or intravitreal bevacizumab. Criteria for treatment of ROP were based on the Early Treatment for ROP (16).
Among demographic and clinical information in the clinical database, BPD was defined as oxygen dependency beyond 28 days after birth and required ventilatory support and/or supplemental oxygen at 36 weeks of post-menstrual age (17). Sepsis was defined by the presence of a positive blood culture with clinical symptoms and signs. In our unit, we transfused infants with 15 mL/kg of red blood cells each time under the discretion of the clinical team.
Statistical analyses were conducted using IBM Statistical Product and Service Solutions software Version 24 (SPSS Inc., Chicago, IL). Continuous data were examined as mean ± standard deviation and median and interquartile ranges for parametric and non-parametric variables, respectively. Comparisons of categorical variables were performed using the Fisher's exact test. Variables with p-value < 0.2 in the univariate analysis were entered into a backward stepwise multivariate logistic regression model to identify the association with ROP. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Model fitness was assessed with the Hosmer–Lemeshow goodness-of-fit test. Area under the curve (AUC) of the receiver operating curves (ROC) was analyzed to assess the prognostic performance. Sensitivity, specificity, and positive and negative predictive values were also determined. All probability values were two-sided and a p-value < 0.05 was considered statistically significant.
From January 2015 to August 2018, 529 preterm neonates were admitted to our NICU with 120/529 (23%) born at <32 weeks' gestation. Of these 120 neonates, there were 36 (30%) extremely preterm neonates of <28+0 weeks and 84 (70%) very preterm neonates of 28+0 to 31+6 weeks' gestation. Thirteen (11%) neonates died. The 107 survivors (24 extremely preterm and 83 very preterm infants) met criteria for ROP screening and were included with complete retinal follow-up examinations. Retinopathy of prematurity was diagnosed in 23 (21%) of 107 infants. Five (4.7% of 107; 22% of 23) infants had severe type I ROP that required laser and/or medical therapy. Fifteen (63%) and 3 (12.5%; 20% of 15) of 24 surviving extremely preterm infants had ROP and severe type I ROP, respectively.
The surviving infants with ROP had younger GA and lower BW than that of survivors without ROP [27.6 (26.4–28.3) vs. 30.3 (28.9–31.3) weeks and 988 ± 243 g vs. 1,383 ± 320 g, respectively, both p <0.001] (Table 1). In univariate analyses, risk factors for ROP were categorized into those within the first 24 h of life (early hours) and during the hospital stay. Risk factors for ROP included Apgar score at 1 and 5 min, mean BP, lowest body temperature, urine output, vasoactive medications, intubation in the delivery room, respiratory distress syndrome, and surfactant therapy in the first 24 h (Table 1). Sex, delivery mode, multiple birth, lowest body temperature, and urine output during the first 24 h after birth were not different between infants with and without ROP.
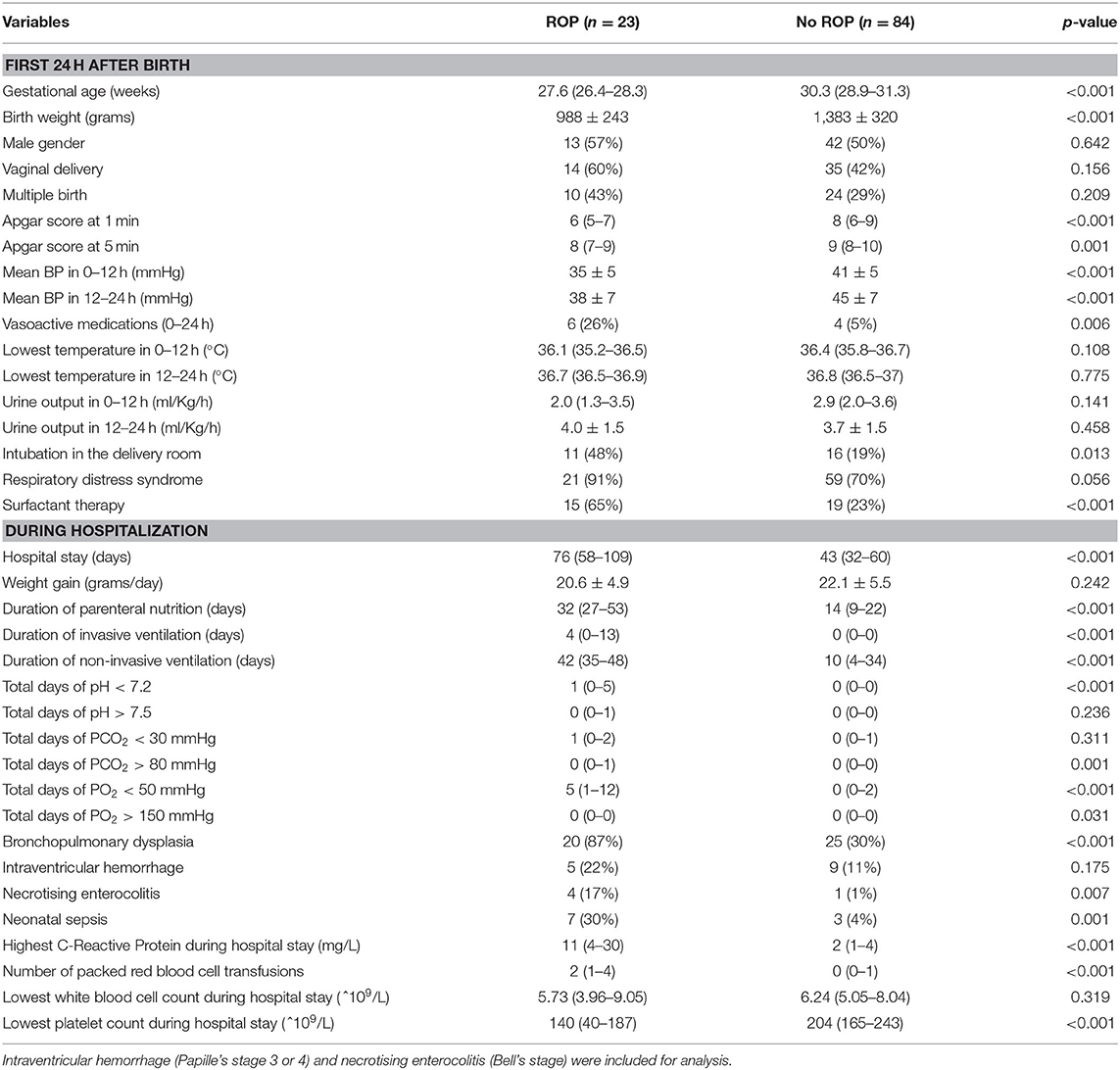
Table 1. Univariate analysis of risk factors for retinopathy of prematurity in the first 24 h after birth and during hospitalization (mean ± SD) [median (IQR)].
Factors during hospital stay which were different between surviving infants with and without ROP included total number of days of PO2 <50 mmHg, PO2 > 150 mmHg, PCO2 > 80 mmHg, blood gas pH <7.2, days of non-invasive and invasive ventilation support, hospital length of stay, duration of parenteral nutrition, diagnosis of BPD, necrotizing enterocolitis, or sepsis, highest C-reactive protein levels, lowest platelet count, and number of red blood cell transfusions (Table 1). Weight gain during the hospitalization, occurrence of intraventricular hemorrhage, and lowest leukocyte count were not different between the two groups.
Based on a backward stepwise multivariate logistic regression model to predict ROP using risk factors with p-value < 0.2 in univariate analyses, GA was the only statistically significant independent risk factor (p < 0.001) in the first 24 h of life whereas mean BP in the first 12 h was modest (p = 0.057) (Table 2). Other statistically significant independent risk factors included hospital stay and total days of acidosis (pH <7.2) (Table 2). Duration of non-invasive ventilation, necrotizing enterocolitis, neonatal sepsis, and blood transfusion were not statistically significant in the regression analysis of ROP (p = 0.061–0.087). In this model, odds ratios of Apgar scores, BP at 12–24 h, lowest temperature in 0–12 h, vasoactive medications used in 0–24 h, intubation in the delivery room, respiratory distress syndrome, surfactant therapy, duration of parenteral nutrition, total days of PCO2 > 80 mmHg, PO2 <50 mmHg and >150 mmHg, BPD, and lowest platelet count had p-values > 0.1 (data not shown).
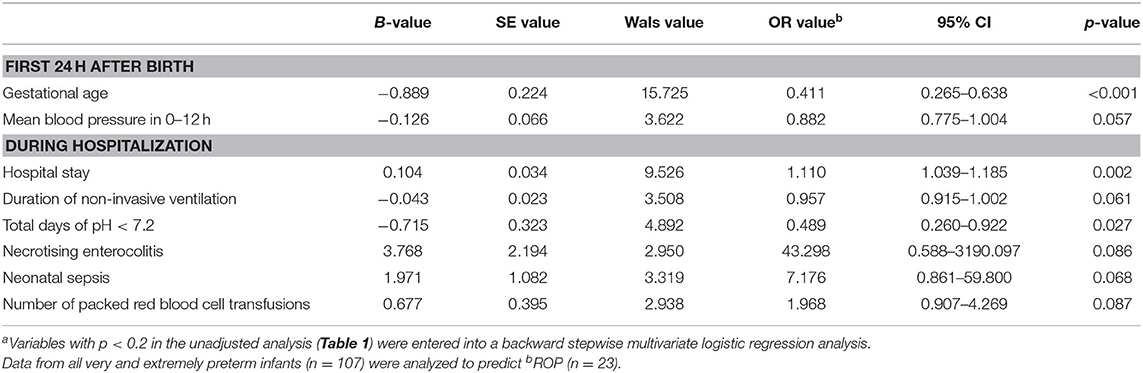
Table 2. Independent risk factors of retinopathy of prematurity in the first 24 h after birth and during hospitalizationa in infants born at <32 weeks gestation.
To assess the potential to predict ROP using risk factors in the first 24 h, we examined the predictive value of mean BP and GA. Mean BP in the first 12 h, GA, and their combination appeared to be significant predictors and achieved an AUC of 0.78 (95% CI 0.66–0.89, p < 0.001), 0.89 (95% CI 0.82–0.96, p < 0.001), and 0.90 (95% CI 0.82–0.97, p < 0.001), respectively, for predicting the development of ROP (Figure 1). The optimal cutoff values for GA and mean BP were 28.3 weeks and 35 mmHg, respectively. The sensitivity, specificity, positive and negative predictive values of GA (<28.3 weeks), and mean BP (<35 mmHg) in the first 12 h for the development of ROP are shown in Table 3. Taken together, the positive predictive value was highest with the combination of GA and mean BP (0.72), whereas a GA <28.3 weeks had the highest negative predictive value of 0.97.
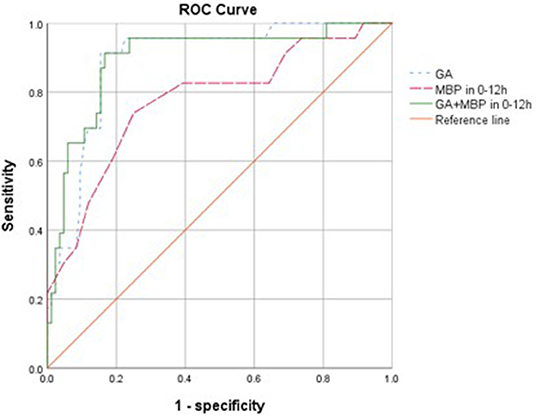
Figure 1. Receiver operating characteristic (ROC) curves for the ability of gestational age (GA), mean blood pressure (MBP) 0–12 h, and GA combined with MBP in 0–12 h in models to predict the development of retinopathy of prematurity with <32 weeks (n = 107). The reference line has an area of 0.50.
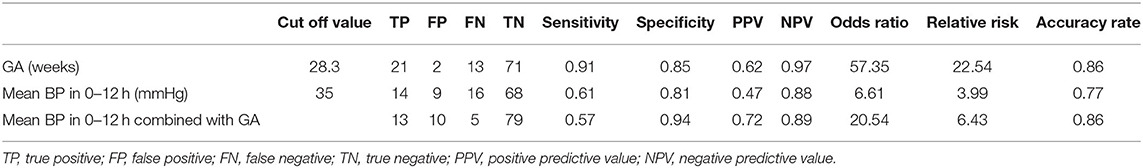
Table 3. Predictive characteristics of gestational age (GA) and mean blood pressure (BP) in the first 12 h after birth for the development of retinopathy of prematurity.
In this study, ROP was detected in 21 and 63% of the patients with GA <32+0 and <28+0 weeks, respectively. The incidence of ROP was similar to that of a national survey (2010–2012) of 6,091 preterm infants from 22 hospitals in seven administrative regions in mainland China which showed that 25% (710/2861) and 67% (112/167) preterm infants with GA <32+0 and <28+0 weeks had ROP, respectively (3). Interestingly, we found a similar incidence of severe type I ROP in preterm infants with GA <32+0 and <28+0 weeks (22 and 20%, respectively). This is in contrast to the incidence observed in the mainland China and Shenzhen reports (8% and 12% at GA <32+0 weeks vs. 21% and 37% at GA <28+0 weeks; respectively) (3, 4). We believe that the different observations could be related to variation in screening and detection algorithms, and reporting mechanisms of various centers. Further, the management of very preterm neonates may also differ between centers depending on whether or not they practice “compassionate care” for critically ill neonates of GA >28 weeks based on non-medical reasons. However, the incidence of severe type I ROP in this study (4.7%) was high when we compared with that in a recent report of two French cohorts in 2008–2013 (0.8 and 2.3% of 3,669 and 1,272 very preterm infants) (18).
Retinopathy of prematurity is a multifactorial disease. Most studies have demonstrated that prematurity and low BW are the strongest predictive factors of ROP (19–21). Other factors associated with ROP include prolonged mechanical ventilation (22–24), the need for nasal CPAP (25, 26), development of BPD (27, 28), sepsis (29), hyperoxia (30), hypoxia (31), hypercapnia, and acidosis. Higher rates of ROP were found in infants with longer length of initial hospital stay (32, 33), and prolonged parenteral nutrition, which are a proxy for cumulative illness burden. We have similar findings in the current study.
The major finding in this study was that lower mean BP in the first 12 h after birth and other risk factors including those previously reported were significantly associated with development of ROP in univariate analyses (Table 1). While low BP could be a marker of disease acuity, hypotension and fluctuation of oxygen saturation might affect retinal perfusion and lead to retinal ischemia (34). Both mean BP and GA were predictors of ROP. However, the predictive accuracy of mean BP (AUC of 0.78) was not better than that of GA alone (AUC of 0.89), and the addition of mean BP did not improve ROP prediction further (AUC of 0.90). Nevertheless, this may suggest that mean BP was not a confounder here but was probably an effect modifier. Gestational age displayed sensitivity of 0.91 and specificity of 0.85 at the optimal cutoff value of 28.3 weeks for predicting the development of ROP, with positive and negative predictive values of 0.62 and 0.97, respectively. Mean BP in 0–12 h displayed sensitivity of 0.61 and specificity of 0.81 to predict ROP at the optimal cutoff value of 35 mmHg, and positive and negative predictive values were 0.47 and 0.88, respectively. When combining the variables of mean BP (0–12 h) and GA, sensitivity and specificity were 0.57 and 0.94, with positive and negative predictive values of 0.72 and 0.89, respectively. Regarding the accuracy rate, it was 0.86 of GA, 0.77 of mean BP (0–12 h), and 0.86 GA and mean BP (0–12 h) (Table 3). Interestingly, our data suggests that mean BP in the early hours after birth may have a modest effect and add positive predictive value to GA as perinatal risk factors for ROP. Studies using multiple cohorts of large sample size are required to examine the predictive value of BP in the early hours after birth.
There are several limitations of this study. First, this is a retrospective study of a small cohort of patients in a single unit which precluded us to examine effects of confounding variables in details and variables at birth including complications of delivery, apnea at birth, and resuscitation interventions (e.g., oxygen supplementation, oxygen saturation, and respiratory support). We also could not identify risk factors of severe ROP. Second, there was a wide confidence interval for odds ratio of BPD and sepsis in the prediction model, which might be in part attributed to a low incidence related to the small sample size. Third, we did not have digital fundus retinal photography for screening, which can improve objectivity and comparability of retinal findings. Last but not least, for total days of blood gas abnormalities during the hospital stay, the duration was the best estimate because we did not check blood gases daily.
In conclusion, in this small cohort of very and extremely preterm Chinese neonates, those survivors with ROP had lower blood pressure in the early hours after birth, in addition to younger gestation, longer hospital stay, and duration of acidosis, when compared to survivors without ROP. The high incidence of infants with ROP in our study emphasized the need for aggressive measures for prevention and control of the disease. We did not investigate but do believe that the safe implementation of oxygen and blood pressure therapy with appropriate monitoring, better antenatal and neonatal care, meticulous attention to hygienic procedures, and control of sepsis may reduce the incidence of ROP. Therefore, monitoring standards of neonatal care and conducting quality improvement projects across the country are essential for improving neonatal outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Hong Kong-Shenzhen Hospital Institutional Ethics Committee. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
H-BH designed the study, collected, analyzed and interpreted data, and drafted the initial manuscript. Y-HC designed the data collection instruments, collected data, and carried out the initial analyses. JW performed eye examinations, ROP diagnosis, clinical type, and treatment. MH, Q-SZ, Y-ZY, and C-BC conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed important intellectual content. P-YC conceptualized and designed the study, designed the data collection instruments, interpreted the data, and critically reviewed important intellectual content. H-BH, Y-HC, JW, MH, Q-SZ, Y-ZY, C-BC, and P-YC reviewed, revised, and approved the manuscript. All authors contributed to the article and approved the submitted version.
This study was in part funded by ShenZhen SanMing Project (SZSM 201911016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Dr. Denggao Peng for statistical assistance, and the health care professionals and families of the NICU, University of Hong Kong-Shenzhen Hospital, for their assistance with this study.
1. Stark A, Dammann C, Nielsen HC, Volpe MV. A pathogenic relationship of bronchopulmonary dysplasia and retinopathy of prematurity? A review of angiogenic mediators in both diseases. Front Pediatr. (2018) 6:125. doi: 10.3389/fped.2018.00125
2. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. (2013) 74(Suppl. 1):35–49. doi: 10.1038/pr.2013.205
3. Collaboration Group of Retinopathy of Premature Infants. Multicenter survey on the clinical features and fundus lesions of retinopathy in premature infants in mainland China. Chin J Evid Based Pediatr. (2015) 10:161–5. doi: 10.3969/j.issn.1673-5501.2015.03.001
4. Cooperative Group of Shenzhen Retinopathy of Prematurity. The incidence of retinopathy of prematurity in Shenzhen during the past ten years. Chin J Ocul Fundus Dis. (2014) 30:12–6. doi: 10.3760/cma.j.issn.1005-1015.2014.01.004
5. Li QP, Wang ZH, Li YY, Huang QM, Huang JJ, Chen Y, et al. Retinopathy of prematurity screening in 2185 premature infants. Chin J Ophthalmol. (2012) 48:903–7. doi: 10.3760/cma.j.issn.0412-4081.2012.10.010
6. Lyu Z, Mao JB, Chen YQ, Zhu MQ, Lian HL, Wu MY, et al. Association between postnatal weight gain and severe retinopathy of prematurity in preterm babies with very low birth weight. Chin J Ocul Fundus Dis. (2016) 32:172–6. doi: 10.3760/cma.j.issn.1005-1015.2016.02.014
7. Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity. A multivariate statistical analysis. Ophthalmologica. (2000) 214:131–5. doi: 10.1159/000027482
8. Hagadorn JI, Richardson DK, Schmid CH, Cole CH. Cumulative illness severity and progression from moderate to severe retinopathy of prematurity. J Perinatol. (2007) 27:502–9. doi: 10.1038/sj.jp.7211780
9. Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol. (2009) 148:451–8. doi: 10.1016/j.ajo.2009.04.018
10. Chavez-Valdez R, McGowan J, Cannon E, Lehmann CU. Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants. J Perinatol. (2011) 31:749–56. doi: 10.1038/jp.2011.19
11. Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. (2011) 31:251–7. doi: 10.1038/jp.2010.152
12. Mohsen L, Abou-Alam M, El-Dib M, Labib M, Elsada M, Aly H. A prospective study on hyperglycemia and retinopathy of prematurity. J Perinatol. (2014) 34:453–7. doi: 10.1038/jp.2014.49
13. Ying GS, Quinn GE, Wade KC, Repka MX, Baumritter A, Daniel E, et al. Predictors for the development of referral-warranted retinopathy of prematurity in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA Ophthalmol. (2015) 133:304–11. doi: 10.1001/jamaophthalmol.2014.5185
14. Stoltz Sjöström E, Lundgren P, Öhlund I, Holmström G, Hellström A, Domellöf M. Low energy intake during the first 4 weeks of life increases the risk for severe retinopathy of prematurity in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. (2016) 101:108–13. doi: 10.1136/archdischild-2014-306816
15. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. (2005) 123:991–9. doi: 10.1001/archopht.123.7.991
16. Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. (2003) 121:1684–94. doi: 10.1001/archopht.121.12.1684
17. Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. (2011) 23:167–72. doi: 10.1097/MOP.0b013e3283423e6b
18. Godeluck A, Gérardin P, Lenclume V, Mussard C, Robillard PY, Sampériz S, et al. Mortality and severe morbidity of very preterm infants: comparison of two French cohort studies. BMC Pediatr. (2019) 19:360–8. doi: 10.1186/s12887-019-1700-7
19. Ratra D, Akhundova L, Das MK. Retinopathy of prematurity like retinopathy in full-term infants. Oman J Ophthalmol. (2017) 10:167–72. doi: 10.4103/ojo.OJO_141_2016_2016
20. Sahin A, Sahin M, Türkcü FM, Cingü AK, Yüksel H, Cinar Y, et al. Incidence of retinopathy of prematurity in extremely premature infants. ISRN Pediatr. (2014) 2014:134347. doi: 10.1155/2014/134347
21. Yau GSK, Lee JWY, Tam VTY, Liu CCL, Yip S, Cheng E, et al. Incidence and risk factors of retinopathy of prematurity from 2 neonatal intensive care units in a Hong Kong Chinese population. Asia Pac J Ophthalmol. (2016) 5:185–91. doi: 10.1097/APO.0000000000000167
22. AI-Amro SA, AI-Kharfi TM, Thabit AA, AI-Mofada SM. Risk factors for acute retinopathy of prematurity. Compr ther. (2007) 33:73–7. doi: 10.1007/s12019-007-8008-5
23. Fortes Filho JB, Borges Fortes BG, Tartarella MB, Procianoy RS. Incidence and main risk factors for severe retinopathy of prematurity in infants weighing less than 1000 grams in Brazil. J Trop Pediatr. (2013) 59:502–6. doi: 10.1093/tropej/fmt036
24. Hwang JH, Lee EH, Kim EA. Retinopathy of prematurity among very-low-birth-weight infants in Korea: incidence, treatment, and risk factors. J Korean Med Sci. (2015) 30(Suppl. 1):S88–94. doi: 10.3346/jkms.2015.30.S1.S88
25. Hadi AM, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol. (2013) 7:831–7. doi: 10.2147/OPTH.S40136
26. Kumar P, Sankar MJ, Deorari A, Azad R, Chandra P, Agarwal R, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr. (2011) 78:812–6. doi: 10.1007/s12098-011-0363-7
27. Bas AY, Demirel N, Koc E, Isik DU, Hirfanoglu IM, Tunc T, et al. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol. (2018) 102:1711–6. doi: 10.1136/bjophthalmol-2017-311789
28. Holmström G, Broberger U, Thomassen P. Neonatal risk factors for retinopathy of prematurity- a population-based study. Acta Ophthalmol Scand. (1998) 76:204–7. doi: 10.1034/j.1600-0420.1998.760216.x
29. Lee J, Dammann O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med. (2012) 17:26–9. doi: 10.1016/j.siny.2011.08.007
30. Flynn JT, Bancalari E, Snyder ES, Goldberg RN, Feuer W, Cassady J, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. (1992) 326:1050–4. doi: 10.1056/NEJM199204163261603
31. Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. (2010) 157:69–73. doi: 10.1016/j.jpeds.2010.01.046
32. Ali AA, Gomaa NAS, Awadein AR, Al-Hayouti HH, Hegazy AI. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr. (2017) 106:1919–27. doi: 10.1111/apa.14019
33. Van Sorge AJ, Termote JUM, Kerkhoff FT, van Rijn LJ, Simonsz HJ, Peer PGM, et al. Nationwide inventory of risk factors for retinopathy of prematurity in the Netherlands. J Pediatr. (2014)164:494–8.e1. doi: 10.1016/j.jpeds.2013.11.015
Keywords: prematurity, retinopathy of prematurity, hypotension, blood gases, prediction
Citation: Huang H-B, Chen Y-H, Wu J, Hicks M, Yi Y-Z, Zhang Q-S, Chow C-B and Cheung P-Y (2020) Early Risk Factors for Retinopathy of Prematurity in Very and Extremely Preterm Chinese Neonates. Front. Pediatr. 8:553519. doi: 10.3389/fped.2020.553519
Received: 19 April 2020; Accepted: 27 August 2020;
Published: 15 October 2020.
Edited by:
Anup C. Katheria, Sharp Mary Birch Hospital for Women & Newborns, United StatesReviewed by:
Hercília Guimarães, University of Porto, PortugalCopyright © 2020 Huang, Chen, Wu, Hicks, Yi, Zhang, Chow and Cheung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Yin Cheung, cG95aW5AdWFsYmVydGEuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.