- Division of Neonatology, Department of Pediatrics, Istanbul Medeniyet University, Istanbul, Turkey
Failure of ductus arteriosus closure after preterm birth is associated with significant morbidities. Ductal closure requires and is regulated by a complex interplay of molecular and mechanical mechanisms with underlying genetic factors. In utero patency of the ductus is maintained by low oxygen tension, high levels of prostaglandins, nitric oxide and carbon monoxide. After birth, ductal closure occurs first by functional closure, followed by anatomical remodeling. High oxygen tension and decreased prostaglandin levels mediated by numerous factors including potassium channels, endothelin-1, isoprostanes lead to the contraction of the ductus. Bradykinin and corticosteroids also induce ductal constriction by attenuating the sensitivity of the ductus to PGE2. Smooth muscle cells of the ductus can sense oxygen through a mitochondrial network by the role of Rho-kinase pathway which ends up with increased intracellular calcium levels and contraction of myosin light chains. Anatomical closure of the ductus is also complex with various mechanisms such as migration and proliferation of smooth muscle cells, extracellular matrix production, endothelial cell proliferation which mediate cushion formation with the interaction of blood cells. Regulation of vessel walls is affected by retinoic acid, TGF-β1, notch signaling, hyaluronan, fibronectin, chondroitin sulfate, elastin, and vascular endothelial cell growth factor (VEGF). Formation of the platelet plug facilitates luminal remodeling by the obstruction of the constricted ductal lumen. Vasa vasorum are more pronounced in the term ductus but are less active in the preterm ductus. More than 100 genes are effective in the prostaglandin pathway or in vascular smooth muscle development and structure may affect the patency of ductus. Hemodynamic changes after birth including fluid load and flow characteristics as well as shear forces within the ductus also stimulate closure. Current pharmacological treatment for the closure of a patent ductus is based on the blockage of the prostaglandin pathway mainly through COX or POX inhibition, albeit with some limitations and side effects. Further research for new agents aiming ductal closure should focus on a clear understanding of vascular biology of the ductus.
Fetal circulation is a unique event, functionally different from pediatric and adult circulation. Ductus arteriosus (DA) is a small, simple vessel with just a few milimeters length and width, but has a profound impact on the survival of the fetus and newborn and plays an essential role in normal postnatal adaptation after birth. It functions as a bridge between two large vessels in fetal life, but sometimes it becomes the “bridge between life and death” in preterm infants. It has baffled scientists since ancient times, and beginning from Galen and Ibn-al Nafis, Vesalius, Leonardo Botallo and William Harvey have commented on its structure and function (1).
During fetal development, cardiovascular septation occurs during days 22–28 of gestation and looping of ventricles completes. At this stage, bilateral aortic arches and bilateral DA form but over the next week, the right aortic arch and ductus involutes. Ductus arteriosus arises from the left sixth embryonic arch; leaves the pulmonary artery very close to the bifurcation point and inserts at the transition zone between the aortic arch and descending aorta, distal to the origin of the left subclavian artery. Usually it has a tubular shape but may be funnel-shaped with a narrow end on the pulmonary side and a wide end on the aortic side (2). Although they all derive from the same embryonic tissue, histological structure of the DA is completely different from that of the aorta or pulmonary arteries. The walls of both great vessels are composed of mainly elastic layers, whereas DA wall is mainly muscular. The inner layer of its wall is composed of longitudinal arranged smooth muscle cells whereas the arrangement of outer layer is mainly circular. On the luminal side, a layer of intimal endothelial cells resides on an internal elastic lamina. This unique structure of the DA sets the stage for the constriction of the vessel after birth.
Ductus arteriosus needs to be remain open in fetal life, in order to sustain the “serial” circulation of the blood. Magnetic resonance studies show that 41% of combined ventricular output passes through the DA in fetal life (3). However, after birth, as the lungs begin to function, a “parallel” circulation is maintained, rendering DA non-functional. Initially it was thought that the ductus was a flabby structure that remained passively open. However, in fetal life, ductus in actively kept dilated by continuous intramural production of prostaglandins (4). The transition from intrauterine to extrauterine life is a critical period and dysregulation of this process may lead to cardiopulmonary instability. Cardiopulmonary adaptation is completed by the closure of DA, followed by closure of ductus venosus and lastly by foramen ovale. In 88% of normal term infants, the ductus is closed by the 8th week of life (5). Preterm infants are born before complete maturation of the cardiovascular system, and they manifest most of the characteristics of fetal life. In a retrospective study of 280 preterm infants who did not receive any therapy directed at closing the ductus, the median time to closure was 71 days in infants born <26 weeks; 13 days in infants born 26–27 weeks, 8 days in infants born 28–29 weeks and 6 days in infants born >30 weeks (6). This implies that extremely preterm infants will be subjected to a period of left-to-right shunting and complications relevant to this shunting.
Ductal closure occurs in two steps: The functional step (vasoconstriction) and the anatomical step (remodeling). Interestingly, the closure of DA resembles the process after vascular injury or atherosclerosis in the adult arteries (7). Understanding different mechanisms which are involved in maintaining ductal tone is essential to comprehend why preterm infants have a high incidence of ductal patency, why some of them do not respond to treatment and where to direct novel therapeutic approaches. On the other hand, it must be stated that most of the experiments and studies toward understanding the physiology of ductal closure are done in animals such as rats or lambs and extrapolated to humans. However, some mechanisms as well as their magnitudes may not be the same in humans.
Ductal Patency in utero
Smooth muscle tone is regulated by phosphorylation/dephosphorylation of myosin light chain (MLC). MLC is phosphorylated by Calcium/calmodulin-dependent MLC-kinase (MLCK) and dephosphorylated by calcium independent MLC-phosphate (MLCP). Increased cytosolic calcium activates MLCK, which leads to MLC phosphorylation and vasoconstriction (8) (Figure 1). Patency of DA is regulated by counteractive forces causing vasodilation or vasoconstriction. In the fetus, DA needs to remain open, therefore vasodilating factors are more active than vasoconstrictive factors. Prostaglandins play a dominant role to oppose constriction. Since the pulmonary artery pressure is very high, the pressure within the DA is very high, preventing it from constriction.
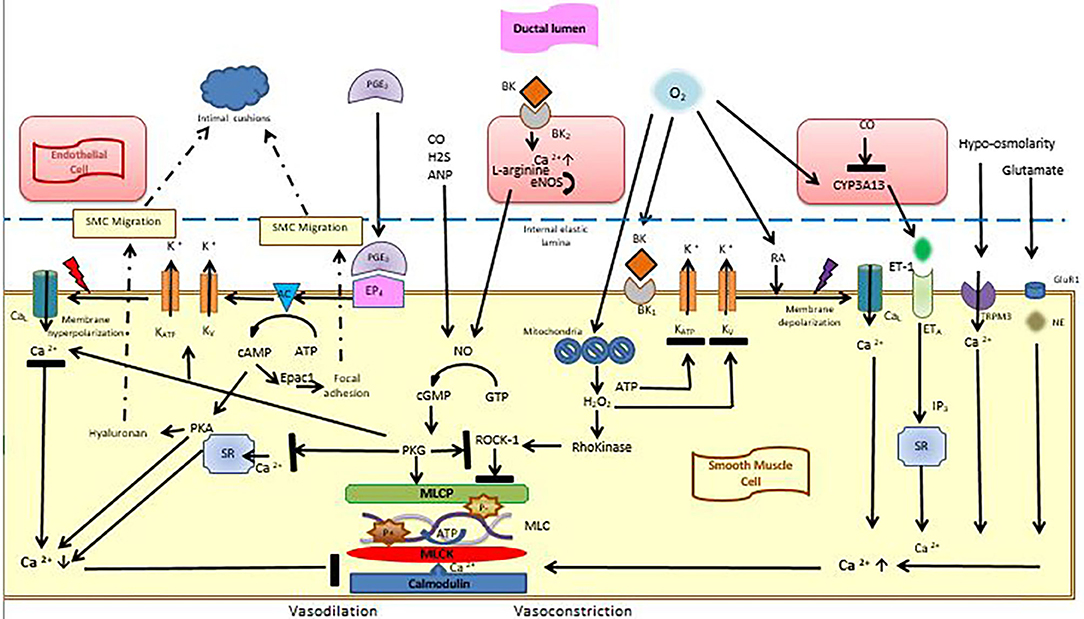
Figure 1. Vasoconstrictive and vasodilatory effects in the ductal smooth muscle cell. Smooth muscle cells are lined with an internal elastic lamina, on which endothelial cells reşide. Vasodilation is maintained by the interaction of PGE2 with its receptor EP4, which triggers potassium outflow through voltage-sensitive potassium channels, which leads to membrane hyperpolarization. Thereby, calcium entry into the cells decreases and binding of calmodulin to MLCK is inhibited. cAMP and cGMP are also effective during this process. After birth, high oxygen tension, which is sensed through the mitochondria triggers the formation of H2O2 which blocks Rho-kinase pathway as well as potassium channels leading to membrane depolarization and calcium entry into cells. Calcium entry is also stimulated by endothelin-1, hypo-osmolarity and glutamate. Calcium activates MLCK, which leads to MLC phosphorylation and vasoconstriction. cAMP is also effective in smooth muscle migration and intimal cushion formation. AC, adenylyl cyclase; ANP, Atrial Natriuretic peptide; ATP, Adenosine Tri Phosphate; BK, Bradykinin; BK1, Bradykinin receptor; CaL, calcium channels; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosin monophosphate; CO, Carbon monoxide; CYP3A13, cytochrome P450; eNOS, endogenous nitric oxide synthase; EP4, Prostaglandin receptor 4; ET-1, Endothelin-1; ETA, endothelin receptor A; epac, exchange protein activated cAMP; H2, Hydrogen sülfite; GluR1, glutamate receptor-1; KATP, KV, Voltage-dependent potassium channels; NE, Norepinephrine; MLC, Myosin Light Chain; MLCK, Myosin Light Chain Kinase; MLCP, Myosin Light Chain Phosphatase; NO, nitric oxide; PKA, cAMP-dependent protein kinase; PGE2, Prostaglandin E2; PKG, cGMP-dependent protein kinase; RA, Retinoic acid; ROCK-1, Rho-associated protein kinase-1; SR, Sarcoplasmic Reticulum; SMC, Smooth Muscle Cell; TRMP3, transient receptor potential melastatin 3 (Figure courtesy of Fahri Ovali).
Prostaglandins
Prostaglandins are synthesized from arachnidonic acid by cyclooxygenases COX1 and COX2 within the ductal muscle. Circulating prostaglandins, originated from the placenta contribute to the patency of DA (Figure 2). Prostaglandins are metabolized in the lung and low pulmonary blood flow in the fetus leads to reduced clearance of prostaglandins, increasing their concentration further.
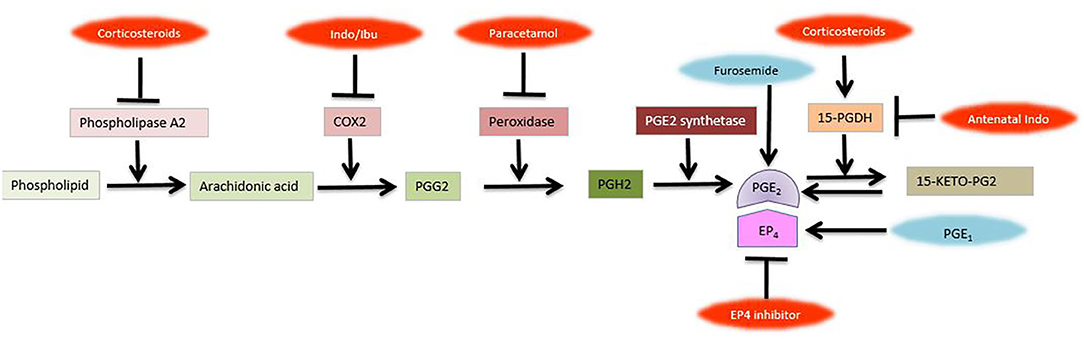
Figure 2. Prostaglandin pathway and metabolism. Inhibitors of various steps are shown in red., COX-1, cyclooxygenase-1; EP4, Prostaglandin receptor 4; Indo, Indomethacin; Ibu, Ibuprofen; PG, prostaglandin; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase; 15-KETO-PG2 (Figure courtesy of Fahri Ovali).
Prostacylin (PGI2) is the major arachnidonic acid product of the ductus but although it is more prevalent, it is less potent than prostaglandin E2 (PGE2), which is the most important prostaglandin to regulate the patency of DA (9). PGE2 interacts mainly with its receptor EP4. EP4 activates voltage-gated potassium channel (Kv) and outward K+ current increases, thereby inducing membrane hyperpolarization, which inhibits Ca2+ influx via the voltage-gated L-type calcium channels (CaL) and decreases intracellular calcium (10). PGE2also activates cyclic adenosine monophosphate (cAMP), which inturn activates protein kinase (PKA), which inhibits myosin light chain creatine kinase (MLCK). Inhibition of MLCK blocks phosphorylation of myosin light chains, which results in inhibition of vasoconstriction, hence vasodilation occurs (11–13) (Figure 1). In utero vasodilation of the ductus is maintained by dephosphorylation of MLC to MLCP. MLCP is enhanced due to the inhibitory effect of PKG on the Rho-kinase pathway. PKG also activates Kv channels, leading to hyperpolarization and inhibition of Ca2+ influx through CaL channels. Stimulation of sarcoplasmic reticulum calcium ATPase (SERCA), by PKG leads to uptake of Ca2+ in sarcoplasmic reticulum, lowering intracellular calcium (14, 15).
Nitric Oxide
Nitric oxide (NO) is produced by endothelial nitric oxide synthase in the luminal endothelium and endothelium of vasa vasorum and maintains the patency of DA by acting through cGMP. After its production, it diffuses into the SMC and binds with soluble guanylyl cyclase, producing cyclic guanosine monophosphate (cGMP). cGMP activates cGMP-dependent protein kinase (PKG), which induces vasodilation (16) (Figure 1). NO is also formed in small amounts in the SMC by inducible nitric oxide synthase (iNOS) and neuronal NOS, which contribute to ductal relaxation (17). Nitric oxide is used commonly in preterm infants for the management of pulmonary hypertension and may affect the patency of the ductus. PGE2 and NO are coupled for reciprocal compensation such that inhibition of one of them increases the concentration of the other one (18). This might explain why some preterm infants fail to close their ductus in response to COX inhibitors.
Carbon Monoxide
Hem oxygenase 1 and hem oxygenase 2 which produce carbon monoxide (CO) are found in the endothelial and smooth muscle cells of the DA. CO dilates the ductus by inhibition of O2 sensing cytochrome P450, thus interrupting endothelin-1 signaling (19, 20) (Figure 1). Carbon monoxide produced under physiological conditions seems to be negligible with regard to ductal patency but in cases of upregulation such as endotoxemia, it may have a relaxing effect on the ductus. Carbon monoxide is also formed in a molar ration during bilirubin production. Theoretically, high bilirubin levels may be associated with ductal patency. However, since high bilirubin levels are not allowed and treated accordingly in human neonates, this effect does not seem to be an important contributor to PDA. Similarly hydrogen sulfide inhibits DA tone (21).
Phosphodiesterase 3 (PDE3) inhibitors such as milrinone and amrinone have positive inotrope and vasodilator effects. Inhibition of PDE3 also induces dilation of the fetal and postnatal ductus arteriosus in humans. This effect is mediated by an increase in cAMP and cGMP in vascular smooth muscle (22) (Figure 1).
Transient receptor potential melastatin 3 (TRPM3) is expressed heavily in ductal SMC and acts as a calcium channel which increases intracellular Ca2+ independent of CaL channels. Progesterone is a natural inhibitor of TRPM3 and may prevent ductal closure in utero (23).
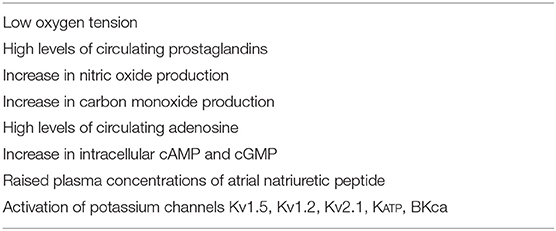
Factors that maintain ductal patency in utero are summarized in Table 1 (24).
Functional Closure
Functional closure of the ductus occurs by 8 h in 44% of normal term infants and almost 100% are closed by 72 h. (25). The rate and degree of this closure is determined by the balance of vasodilator and vasoconstrictive factors. Several changes in late gestation contribute to an increase in ductal tone.
Constriction of the ductus is maintained via3 factors:
a) Decreased concentration of prostaglandins by the loss of placental source and increased removal by the lungs, and decreased number of EP4 receptors on the ductal wall.
b) Increased arterial oxygen pressure.
c) Decreased pulmonary vascular resistance resulting in decreased blood pressure within the lumen of the DA.
Decreased Prostaglandins
After the cord is clamped, PGE2 concentrations drop rapidly due to removal of placental production and increased metabolism in the lungs (9). The metabolism of PGE2in the lung is mediated by PG transporter, which controls the uptake of PGE2into type II alveolar epithelial cells (26). In the alveolar epithelial cell, PGE2is degraded reversibly to biologically inactive metabolite 15-keto-PGE2 by 15-hydroxyprostaglandin dehydrogenase (15-PGDH) (27). The activity of 15-PGDH increases with increasing gestational age (28). 15-keto-PGE2is metabolized irreversibly to 13, 14-dihydro- 15-keto-PGE2by Δ-13-reductase. After birth, expression of EP4 decreases and increased oxygen reduces the sensitivity of the ductus to PGE2 (29). On the other hand, lungs also produce bradykinin (27). Bradykinin induces vasodilation in utero, but in higher concentrations, it induces vasoconstriction (30).
In the preterm infant, the sensitivity of DA to PGE2 is higher than that in the term infant, which is attributed to increased binding to EP2, EP3, and EP4 receptors (18). The catabolism of PGE2 is slower due to low 15-PGDH activity in early gestation. The preterm ductus is also six times more sensitive to dilation to NO than the mature DA (31). Postnatal increase in oxygen might enhance NO production, which induces vasodilation (9).
Although PGE2 is the most important agent in maintaining ductal patency in fetal life, there is some evidence that in late gestation, it may paradoxically prepare the ductus for postnatal closure. At that time, PGE2 may promote the expression of some genes involved in vasoconstriction (32, 33). In late gestation, some phosphodiesterases that degrade cAMP are upregulated, which results in limited accumulation of cAMP and attenuates the vasodilator effects of PGE2 (34).
Increased Oxygen
There are a number of oxygen sensing tissues in human body, such as fetoplacental arteries in the placenta, pulmonary artery, DA, adrenomedullary chromaffin cells, neuroepithelial body and carotid body. This oxygen-sensing and responding system is called the Homeostatic Oxygen Sensing System (HOSS) and should be considered one of the body's major systems such as cardiovascular, nervous or endocrine systems (35).
During fetal life, oxygen pressure in the ductal lumen is about 18–28 mmHg, which is suitable for maintaining the ductus open (36). After birth, arterial oxygen rises sharply to 80–100 mmHg, which leads to vasoconstriction of almost all vascular smooth muscle, with the exception of pulmonary artery (37). Oxygen-induced constriction begins within 4.6 ± 1.2 min after a rise in PaO2 (38).
Oxygen-induced vasoconstriction is a multistep process. Oxygen sensing involves a change in redox state, determined by mitochondria and nicotinamide adenine dinucleonide phosphate (NADPH) oxidase. Proximal electron transport chain is the major oxygen sensor in the ductal SMC. This is reflected in the observation that electron transport chain inhibitors (i.e., rotenone and antimycin) mimic hypoxia and constrict the ductus, as well as activating the carotid body. When PO2 rises, mitochondria respond by production of reactive O2 species (ROS) especially H2O2, which changes the cellular redox potential and alters the function of redox-sensitive genes, second messenger systems and oxygen-sensitive potassium channels, which control SMC membrane potential. Production of adenosine triphosphate (ATP) through oxidative phosphorylation increases with rising oxygen levels and inhibits KATP channels (39). These channels compose of many subunits such as Kv1.2, Kv1.5, Kv2.1, Kv31b, Kv4.3, Kv9.3, and BKca (40).
Response to oxygen is mediated through potassium channels. Although the pulmonary artery and the ductus are continuous and have similar Kv channels, their response to oxygen is reversed. Hypoxia causes pulmonary artery vasoconstriction and ductal vasodilation. Reduced expression of Kv1.5 and Kv2.1 channels results in failure of ductal closure. It may be speculated that the failed response of premature infants' ductus to normoxia may be related to “deficient” Kv channels, reduced PGE2induced gene expression of ion channels (41) and a gene transfer which restores K2.1 or Kv1.5 expression might be helpful for vasoconstriction (40).
Inhibition of potassium channels leads to membrane depolarization, influx of calcium into the cells through L type (CaL) and T type (CaT) calcium channels, and opening of inositol triposphate (IP3) sensitive sarcoplasmic reticulum calcium stores which release calcium (11, 42). This causes DA constriction without depolarization. Subsequent calcium entry through these channels promotes constriction of the DA.
L-type calcium channels are themselves oxygen-sensitive (43). T-type calcium channels also regulate calcium entry into the cells and they are upregulated with increased oxygenation (44). In response to oxygen, calcium may also enter the cell through reverse-mode function of the Na/Ca exchanger also. Maturation of the sensor, mediator and effectors goes in parallel (45).
Cytochrome P450 enzymes catalyze a number of reactions to modulate inflammation, angiogenesis and vascular tone. A member of this system also acts as a sensor for oxygen (46). Monooxygenase and lipooxygenase metabolites of arachidonic acid have the ability to respond to changes in oxygen tension (47). Stimulation of the endothelin A receptor and production of endothelin-1 (ET-1) mediates ductal constriction (48).
Isoprostanes are prostaglandin-like compounds which are produced in response to oxidative stress via non-enzymatically free radical mediated peroxidation of arachidonic acid, without the effect of COX enzymes. They increase as a response to increased arterial PO2 and increased ROS and mediate inflammation as well as constriction of the DA (49).
Retinoic acid is also active in oxygen signaling and maternally administered vitamin A accelerates the development of oxygen sensing mechanism by increasing the expression of Cav1.2 and Cav3.1 in the rat DA (50).
Rho-kinase pathway is effective in sustaining the contractile state of the ductus. Increased ROS, mainly H2O2 in the mitochondria induces RhoB and Rho-associated protein kinase-1 (ROCK-1) expression, which promotes phosphorylation of myosin phosphatase inhibiting MLCP. Activation of the Rho-kinase pathway induces calcium sensitization, which sustains ductal constriction through positive feedback mechanism (51). Rho-kinase system balances MLCK and MLCP (Figure 1). Inhibitors of Rho-kinase such as fasudil lead to pulmonary vascular relaxation, thus pulmonary hypertension (52). In the preterm infant, mitochondrial ROS system is immature, and together with failure to upregulate Rho-kinase expression to oxygen, leads to ductal vasodilation. Exogenous H2O2 mimics the effects of increased oxygen.
Oxygen stimulates the release and synthesis of endothelin-1 (ET-1), acting on type A receptors (ETA). Stimulation of these receptors induces IP3 production by phospholipase c, which leads to an increase in intracellular Ca2+ (53). Endothelin receptor antagonists are found to inhibit ductal constriction (54).
In the preterm infant smooth muscle myosin isoforms are immature and the contractile capacity of the muscle cells are weak. L-type calcium channels are immature with impaired calcium entry into the cells. Reduced expression of Rho kinase expression and activity as well as oxygen sensing KV channels contribute to the ductus patency (45). Inhibition of these potassium channels by oxygen leads to SMC depolarization, opening of the voltage-gated L-type Ca channels, influx of calcium into the SMC and vasoconstriction. The preterm ductus has increased sensitivity to the vasodilating effects of prostaglandins and nitric oxide, which prevents constriction. This sensitivity is affected by increased cAMP signaling and decreased cAMP degradation by phosphodiesterases (55). High rates of response to PG inhibitors such as indomethacin and ibuprofen may be explained by this exaggerated sensitivity of the ductus to PGs. On the contrary, elevated levels of PGs during sepsis or necrotizing enterocolitis may mediate the re-opening of ductus in preterm infants (56).
Non-oxygen Pathways
In the preterm ductus energy metabolism begins to fall after birth, with decreased amounts of glucose, oxygen and adenosine triphosphate (ATP). This is not profound enough to cause cell death but interferes with the ability of the ductus to close (57).
Glutamate promotes ductal constriction by glutamate inotropic receptor subunit 1 (GluR1)-mediated noradrenalin production in animal models (58). It may be suggested that nutritional supply of glutamate may have a therapeutic role in the closure of PDA (59).
Caffeine is an adenosine receptor blocker, but it also affects several mechanisms involved in ductal closure. It increases cAMP, releases Ca+2 from endoplasmic reticulum, and inhibits the production and activity of prostaglandins. It is used frequently in extremely low birth weight infants for the prevention and treatment of apnea and bronchopulmonary dysplasia. However, it does not affect the contractile response of the ductus at therapeutic concentrations (60).
There is a transient decline in serum osmolarity after birth, which recovers to adult levels within a few days There is some evidence that hypo-osmolarity may mediate ductal closure. In preterm infants with hemodynamically significant PDA, osmolarity is 5 mOsm/L higher compared to non-hemodynamically significant PDA group (61). Hypo-osmotic receptor transient receptor potential melastatin 3 (TRPM3) is upregulated in the ductus and regulates calcium influx into the cells, promoting vasoconstriction. Rats with transient hypo-osmolarity after birth have increased rates of ductal closure (62).
B-type Natriuretic peptide (BNP) plays an important role in regulating the body fluid volume and blood pressure. It is secreted from the ventricles in response to increased cardiac stress and increases intracellular cGMP, inducing vasodilation. In PDA, the volume of left-to-right shunting is associated with the BNP level and higher levels predict a hemodynamically significant PDA and a poor response to indomethacinin preterm infants (63, 64). Bradykinin induces ductal constriction through BK-1 receptors (29).
Progesterone
Progesterone regulates prostaglandin synthesis and prostaglandin sensitivity of smooth muscle cells. Progesterone withdrawal in late pregnancy coincides with the increasing sensitivity of ductus arteriosus to constriction by indomethacin (65). This effect is similar to that of glucocorticoids (66). However, this effect seems to be pharmacological, rather than physiological (67).
In summary, the overall mechanism of functional closure of the ductus involves a drop in PGE2 levels after birth, which impairs ductal vasodilation and a concomitant rise in oxygen which leads to vasoconstriction. Intracellular calcium increases, secondary to inhibition of Kv channels and KATP induced membrane depolarization. Increased calcium within the cell activates Ca2+–Calmodulin dependent MLCK activation, which phosphorylates MLC (8). Calmodulin expression is upregulated after birth (68). Increased ET-1 synthesis releases intracellular calcium from the sarcoplasmic reticulum (69). Rho-kinase pathway inhibits MLCP, inducing calcium sensitization by reducing the requirement for calcium influx, maintaining vasoconstriction (9).
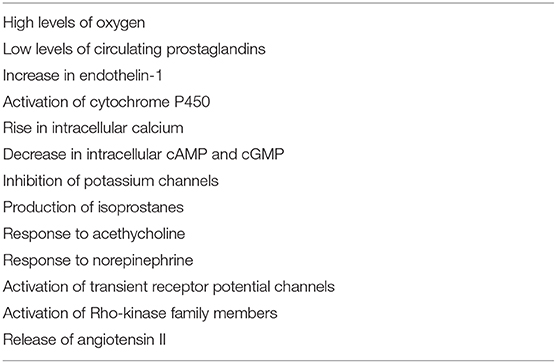
Factors that contribute to ductal constriction after birth are summarized in Table 2 (23).
Anatomic Closure and Remodeling
The initial constriction of the ductus alone is insufficient for the permanent cessation of blood flow through it. Permanent anatomic closure is essential to prevent re-opening, which occurs through a process of remodeling. This process involves intimal cushion formation, disassembly of internal elastic lamina and loss of elastic fibers in the medial layer, migration and proliferation of the SMCs, extracellular matrix production and endothelial cell proliferation and finally blood cell interaction. Each step is related to each other and occurs in a sequential way. After complete anatomic closure, the ductus undergoes apoptosis and becomes the ligamentum arteriosum.
Vasa Vasorum
A substantial amount of nutrients of the ductal wall is provided by vasa vasorum, which supply the outer portion of the wall. Vasa vasorum enters the outer wall of the ductus and grow toward the lumen. They stop growing ~400–500 μm from the lumen. The distance between the lumen and the tip of vasa vasorum is called the avascular zone of the vessel. The thickness of the avascular zone defines the furthest distance that still maintain oxygen and nutrient homeostasis in the tissue. Vasa vasorums appear in the ductal wall only after wall thickness exceeds 400 μm which is not evident before 28 weeks of gestation (70, 71). The thickness of the ductal wall in the full term fetus is 1,000 μm. When the ductus constricts after birth, it increases up to 1,250 μm, in lieu of the lumen (9). In the preterm fetal ductus, the wall thickness is about 480 μm and it increases to 670 μm after birth, when constricted (9) (Figure 3). In the full-term ductus, the increased tissue pressure occurring during ductus constriction occludes the vasa vasorum and prevents flow of nutrients to the outer wall of the vessel. This results in the effective avascular zone expanding from 500 μm to the entire thickness of the vessel wall. When this occurs, the center of the ductus wall becomes profoundly ischemic (73). However, in the 24-week-old preterm infant, the ductus is only 200 μm thick. The vasa vasorum do not penetrate the muscle media. This thin walled ductus does not need the vasa vasorum for nutrient flow. As a result, there is no vulnerable region of the wall during closure of the ductus. The preterm ductus arteriosus is less likely to develop the severe degree of hypoxia that is necessary for ductal remodeling (74).
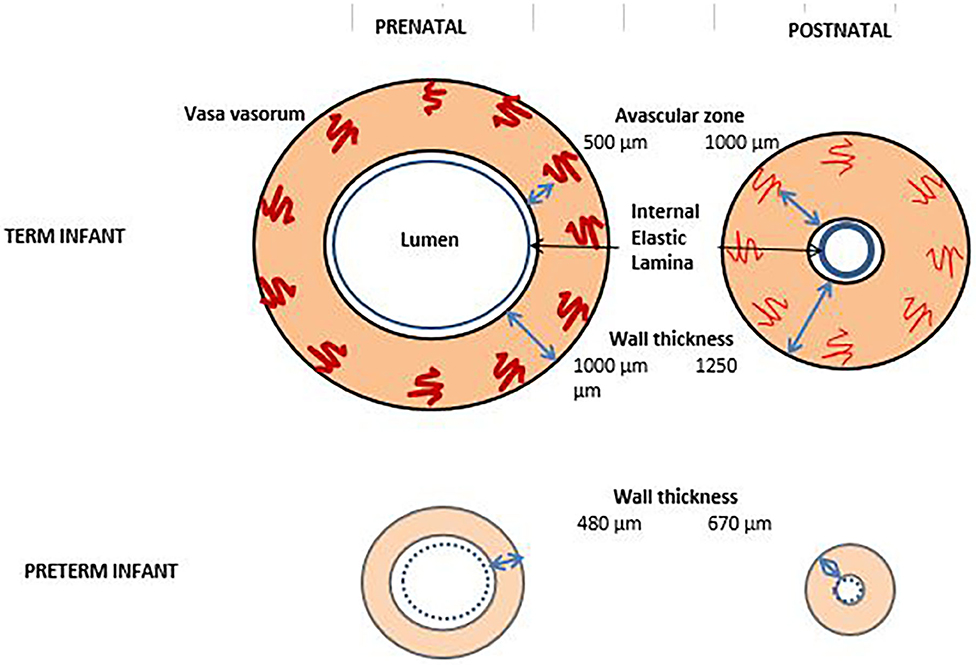
Figure 3. Vaso vasorum in term and preterm infants before and after birth. By the obliteration of vaso vasorum, the thickness of the vaso vasorum increases after birth and the lumen shrinks. In the preterm infant, vaso vasorum are absent or very scarce and the elastic lamina is thin [Figure adapted from (72)].
In the term infant, increased intramural pressure occludes vasa vasorum leading to the inhibition of provision of nutrients and oxygen to the muscular layer. Thereafter, the blood and hence the oxygen supply to the cellular layer subsides, leading to hypoxia and cell death (73). The profound hypoxia that follows induces local production of hypoxia inducible factors 1α and VEGF, inhibits the production of PGE2 and results in apoptosis of SMCs. The proliferation of endothelial cells is mediated by VEGF. Adhesion of mononuclear cells is mediated by VEGF whereas the formation of the platelet plug is mediated by platelet-derived growth factor. This induces an inflammatory response and monocytes and macrophages begin to recruit to the ductal wall, producing platelet-derived growth factor which is essential for migration and proliferation. During the initial functional vasoconstriction, loss of luminal blood flow causes hypoxia in the ductal muscle, inducing VEGF secretion (74).
Since the ductal vessel wall is very thin in the preterm infant, it does not contain vasa vasorum and the cells receive oxygen by diffusion from the lumen. This means that as long as luminal patency continues, the cells will receive oxygen and will fail to undergo anatomic remodeling. The preterm ductus requires a greater degree of constriction than that of the term infant in order to achieve a similar degree of hypoxia to trigger the cascade of events necessary for anatomic closure. At the same time, they will be responsive to prosglandins, and be susceptible to re-opening in case of prostaglandin surge as it happens in sepsis or necrotizing enterocolitis. In contrast, preterm ductus produces NO after birth, due to the growth of new vasa vasorum that synthesize NO (63). Therefore, the ductus becomes more dependent on vasodilators other than prostaglandins after the first few weeks. This is reflected in the decreased response to indomethacin with increasing postnatal age. In animal data, combined use of NO-synthase inhibitors with indomethacin produces a greater ductus constriction than indomethacin alone (75).
Elastic Fibers
The structure of the ductal wall is strikingly different from the aortic wall with regard to its elastic content. In great arteries, complex extracellular matrix and elastic fibers are essential to overcome the mechanical and hemodynamic stress of the pulsatile flow. Well-developed elastic fibers in the aorta prevents it from collapsing. However, in the ductal wall, there are very few and thin elastic fibers which do not prevent, but in fact facilitate ductal collapse. Ductal wall is composed of just a media layer which consists SMCs and a superficial thin internal elastic lamina (Figure 3). This lamina is also very prone to fragmentation. The intima layer contains the endothelial cells facing the lumen of the ductus. The structural features of the ductus prepares it for immediate closure after birth. Abnormalities of the elastic fibers and elastic lamina may be responsible for some of the PDA cases by preventing intimal cushion formation and collapse of the ductal wall (73).
In the ductal wall, there is less elastin binding protein leading to less elastin deposition. Moreover, increased chondroitin sulfate in the ductus leads to dissociation of tropoelastin from the cell surface, interfering with elastin fiber assembly further (76). Tropoelastin is a chemoattractant for SMCs. Lysl oxidase (LOX) catalyzes elastin cross-linking and it is degraded in the ductal cell when EP4 receptor is stimulated by the prostaglandins (77). EP4 is a Gs protein coupled receptor which increases intracellular cAMP by adenylyl cyclases. Signaling coupled with PGE2 and EP4 stimulates vascular dilation and intimal thickening.
Intimal Cushion Formation
After the functional phase, progressive intimal thickening and fragmentation of the internal elastic lamina occurs, ultimately forming protrusions which occlude the ductal lumen. During the remodeling process; endothelial cells and internal elastic lamina separate, leaving a subendothelial space in-between for the migration of SMCs and endothelial cells. Integrins, which are transmembrane receptors on the cell surface participate in the interaction of these cells, promoting Intimal cushion formation. In patients whose ductus is not closed, integrin expression is downregulated (78). Intimal thickening is due to the migration of smooth muscle cells from the media layer to the intima and to the proliferation of luminal endothelial cells. During this process, the first step is the migration of SMC from the media to the subendothelial layer, leading to neointimal cushion formation (79). SMC migration is mediated by PGE2, Transforming Growth Factor Beta (TGFβ-1), notch signaling, Vascular Endothelial Growth Factor (VEGF) and fibronectin coupled with hyaluronan. The proliferation of SMCs is mediated by retinoic acid and notch signaling. Mounds are formed by expansion of the neointima by hyaluronan, which creates a suitable space for migrating SMC from the muscle into the intima and proliferating endothelial cells. The process begins with the accumulation of hyaluronan beneath the endothelial cells, accompanied by loss of laminin and type IV collagen and their separation from the internal elastic lamina. Influx of water into the hyaluronan widens the subendothelial space. Hyaluronan potentiates the migration of SMCs to the subendothelial space through hyaluronan binding protein, synthesized by SMCs (80). This wide space is suitable for SMC migration. Smooth muscle migration is facilitated by impaired elastin assembly and elastin fragmentation. Accumulation of hyaluronan is associated with increased production of TGFβ and prostaglandins via activation of adenylylcyclase 6 (81). Ductal SMCs also secrete fibronectin and chondroitin sulfate which facilitate the migration of these cells (82). SMC also secrete laminin, which is a promigratory protein (83).
In addition to its effects on vasodilation, PGE2 stimulates SMC migration through exchange protein activated cAMP (epac) pathway in the ductal wall (84) (Figure 1). Cyclic AMP has 2 targets: protein kinase A (PKA) and epac. Epac is a guanine nucleotide exchange protein independent of PKA, which regulates the activity of small G proteins. It is upregulated during the perinatal period and promotes SMC migration without hyaluronan production (85). cAMP is degraded by phosphodiesterases (PDE) to 5'AMP. Activation of PDE can suppress cAMP mediated EP4 signaling and PDE inhibitors can enhance it (12). Neointimal mounds are less well-developed in the preterm infant, rendering the occlusion of the lumen difficult.
Retinoic Acid
Retinoic acid stimulates the growth of SMCs and decreases apoptosis (86). Prenatal administration of vitamin A increases the production of fibronectin and hyaluronic acid, leading to intimal tickening, as well as the intracellular calcium response and the contractile response of the ductus to oxygen (50). This response is activated by the upregulation of α1G subunit of voltage-dependent calcium channel, which is activated by oxygen-induced inhibition of potassium channel (32). In other words, retinoic acid stimulates both functional and anatomic closure of the ductus. Early trials in human neonates concluded that administration of vitamin A after birth does not result in constriction of DA, but retinoic acid receptor activation may be have a therapeutic potential for treating PDA (87, 88). However, in a recent trial on preterm infants administering 10,000 units of Vitamin A on alternate days for 28 days, rate of PDA was significantly reduced (89).
TGF-β1
TGF-β1 binds the SMC through integrins to the extracellular matrix, slowing down the migration. This is necessary during the remodeling process for maintaining the tension to sustain DA contraction (90).
Interleukin-15
(IL-15) has many pro-inflammatory effects, but also inhibits SMC proliferation and hyaluronan accumulation during the remodeling process of the ductus. The up-regulation of IL-15 in the ductus is mediated through the prostaglandin pathway. IL-15 upregulates the expression of EP4 mRNA, as well as stimulating angiogenesis through binding to endothelial cells (91). In late gestation, several factors try to promote the structural closure of the ductus in preparation for the postnatal life and IL-15 may balance this tendency by a counteractive mechanism (92).
Notch Signaling
Notch receptors are critical for cell differentiation and there are 4 Notch receptors in humans. Notch 2 and Notch3 receptors are abundant in smooth muscle and they regulate proliferation, migration and angiogenesis in the vasculature. A decrease in the number of Notch receptors in SMCs is associated with downregulation of gene expressions related to contractile elements (84). Notch signaling is required for contractile SMC differentiation in mice (91).
Extracellular Matrix Production and Proliferation
Extracellular matrix consists of hyaluronan, fibronectin, chondroitin sulfate and elastin, which are mediated by retinoic acid, TGFβ, PGE2, IL-15, and oxygen (51).
Hyaluronan is effective in SMC migration and regulated by PGE2, IL-15, and TGFβ. PGE2-mediated EP4 activation increases the cAMP production through protein kinase A (PKA), which stimulates hyaluronan production in the SMCs (93).
Fibronectin is secreted by the SMCs and promotes SMC migration in the process of intimal cushion formation. Ductal SMC produce twice more fibronectin than aortic SMCs (94). It is possible that preventing fibronectin dependent intimal thickening may be utilized for the patency of DA in congenital heart diseases (82). Other mediators which play an important role in the proliferation and migration of SMCs include versican, a hyaluronan-binding proteoglycan and tanacin, a hexameric glycoprotein (95, 96).
Chondroitin sulfate causes the release of elastin binding protein from the SMCs, impairing the elastin assembly and stabilizing the hyaluronan. Therefore, it indirectly promotes the migration of SMCs and upregulates the synthesis of fibronectin (97).
Elastin contributes to the patency of the ductus by maintaining the elasticity of the ductal wall. Its production is regulated by oxygen and PGE2. Inhibition of elastogenesis by PGE2 causes vascular collapse and ductal closure. Oxygen reduces elastin secretion in the SMCs. Stated otherwise, PGE2 and oxygen stimulates elastogenesis, leading to vasodilation and patency of the ductus (72, 98).
Blood Cell Interactions
Vascular wall ischemia induces a local inflammatory response, which activates mononuclear cells in the blood. Leukocyte interactions with P-selectin and Intercellular Adhesion Molecule-1 (ICAM-1) is necessary for recruitment when physiologic shear forces are present and this interaction is only possible when the flow is very slow. Therefore, leukocytes can adhere to the ductal wall only after vasoconstriction and loss of ductal flow has occurred (99, 100) Monocytes and phagocytes adhere to the wall of the ductus via vascular cell adhesion molecule-1 (VCAM-1), whose expression increase after birth (7, 91). VEGF is a direct chemoattractant for monocytes (101). The magnitude of this adhesion is correlated by the extent of intimal cushion formation (102). Monocytes also stimulate PDGF-B, which is a mediator of smooth muscle migration (103).
During the initial process of vasoconstriction, detachment of endothelial cells triggers the adhesion of platelets to the “injured” site, forming a platelet plug, which further blocks the lumen. Mice with disrupted platelet function or administered antibody against platelets have impaired ductus remodeling (104). The effect of platelets on ductal closure is minimal in term infants but it is more pronounced in preterm infants. Since the intimal cushion formation is mainly triggered by hypoxia and the preterm ductus is less likely to become hypoxic when it constricts after birth, platelets play a more significant role in ductal plug formation and closure in this setting (105). It is reported that platelet-derived growth factor is lower in infants with PDA (106). Low platelet count is also correlated with hemodynamically significant PDA and delayed closure of the ductus (104, 107), whereas high platelet counts promote the initial constriction of the ductus (108).
Genetic Background
Although they originate from the same neural crest lineage, there are major transcriptional differences between the DA and aorta. Several studies highlight the effect of genetic predisposition to the patency of DA. These include either the prostaglandin pathway (i.e., smooth muscle contraction), or vascular smooth muscle development or structure. Most of these information is obtained through mouse models. Overall, there are more than 100 genes are effective in the development of DA (33, 109).
Arachidonic acid is metabolized by PGH2 synthase to prostaglandin H2, which in turn is metabolized by PGE synthase to prostaglandin E2 (PGE2). Prostaglandin endoperoxide synthase 1 (Ptgs1 or COX1) and (Ptgs2 or COX2) is the rate limiting enzyme in the production of prostaglandins from arachidonic acid. Mice deficient in Ptgs1 have normal survival after birth whereas 35% of mice deficient in Ptgs2 die shortly after birth because of PDA (110). If both are lacking, 100% of mice die in the first day (111). Prostaglandin E2 binds to the cell through its receptor EP4 (Ptger4) and mediates vascular smooth muscle relaxation and intimal thickening. Mice lacking these receptors die due to PDA (11). Furthermore, Hpgd gene encodes hydroxyprostaglandin dehydrogenase 15-(NAD) which is responsible for PGE2 metabolism. The deletion of this gene leads to elevated levels of PGE, leaving the DA open and resulting in death (112). Mice deficient in prostaglandin transporter gene Slco2a1 also die within 2 days after birth (113).
The effects of prostaglandins are mediated through cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP). Phosphodiesterases degrade these compounds and several genes have been proposed for phosphodiesterases. Pde5a mediates vasoconstriction in SMC of DA and its expression decreases in 6 h after birth (114).
The genes upregulated during ductal closure are phosphodiesterase 4B (PDE4B), desmin pyruvate dehydrogenase kinase isozyme 4 (PDK4), and insulin-like growth factor binding protein 3 (IGFBP3). Downregulated genes include cyclin-dependent kinase inhibitor 1C (CDKN1C) and alpha-2-glycoprotein1 (AZGP1). PDE4B limits the accumulation of cAMP, minimizing the vasodilator effect of endogenous prostaglandins. Therefore, increasing PDE4B would facilitate ductal constriction. Desmin is a component of the cytoskeleton and its increase indicates the maturation of SMCs. PDK4 and IGFBP3 regulate cell proliferation and apoptosis, which are operative in ductal closure. Increased transforming growth factor β-receptor II regulates PDK4 also (90, 115, 116). Wingless integrin pathway (Wnt), which is a part of IGFBP3 system plays a major role in ductal closure through upregulation of cyclooxygenase 2 and apoptotic processes mediated by macrophages (117).
Smooth muscle of the DA contracts in response to oxygen. Mice deficient in myosin heavy chain gene (Myh11) have delayed closure of DA (118). Myocardin regulates expression of genes for contractile proteins in smooth muscle cells (SMC) and selective deletion or ablation of Mycocd results in death (119). Tcfap2b is the gene which encodes the transcription factor in neural crest cells, from which the DA originates. Mice deficient in Tcfap2b die within the first day of life with PDA and pulmonary overload (120). The DA of these mice lack markers of differentiated SMCs such as calponin and hypoxia-inducible factor 2α (HIF2α) which are also involved in oxygen sensing, suggesting that Tcfap2b plays an important role in the development of SMCs (121). Some genes act together resulting in PDA. Whereas, the deletion of Notch2 gene results in PDA in only 40% of cases, when combined with the deletion of Notch3, PDA is observed in 100% of cases (122). Deletion of the Notch ligand, jag1 also results in lethal PDA on day 1 because of significant defects in SMC differentiation (123). On the other hand, deletion of the Brahma-related gene (Brg1-a) which is responsible for chromatin remodeling results in cardiovascular anomalies, including PDA (124). Genes involved in platelet production or adhesion such as Nfe2 or Itga2b affect the complete occlusion and remodeling of the DA (104).
Changes in the expression of structural genes also affect the patency of DA. Vascular SMC contraction is mediated by myosin II. Myosin II has two components, namely heavy chains and light chains. The genes encoding these proteins such as Myh11, Myl2, Myl5, Myl7, and Myl9 may be effective in the regulation of ductal patency, but more studies are needed to reach a definite conclusion (68, 125). Contraction of the myosin light chains requires phosphorylation and this step is regulated by the Rho-Rho kinase system. The gene encoding the RhoBGTPase (Rhob) and its effector Rock2 is upregulated in the newborn DA and inhibition of this system may result in the patency of DA (38).
L-type calcium channels are essential for ductal closure and the genes encoding the Na/K ATPase (Akq1b1) and a subunit of L-type calcium channels (Cacna2d1) are highly expressed in the DA (126). Potassium channels control the resting membrane potential of SMCs and the genes encoding these channels (Kcnk3 and Abcc9) are also upregulated in the DA (127). Large conductance calcium-activated potassium channels (BKca) are abundant in vascular smooth muscle and they are expressed significantly higher in DA (109). These ion channels may be promising targets for novel PDA therapies.
Vasoconstrictive effect of oxygen in the DA is mediated by endothelin-1 (ET-1). There are 2 membrane receptors for ET-1, ETA receptor is encoded by the Ednra gene and ETB receptor is encoded by the Ednrb gene. ETB receptor functions as a scavenger for ET-1 and inhibits endothelin dependent vasoconstriction. Downregulation of this receptor would result in increased levels of ET-1, leading to vasoconstriction of the ductus, which takes place in late preterm infants (68, 126).
In humans, there are a number of syndromes associated with PDA, as well as non-syndromic PDA which are associated with single nucleotide polymorphisms (128). In twin studies, a familial component has been suggested for PDA in preterm infants (129). Consanguineous marriages provide insights to specific chromosome regions associated with increased susceptibility to PDA (130). A polymorphism in the estrogen receptor alpha gene ESR1 rs2234693, prostaglandin I2 synthase gene (rs493694) and another polymorphism in the interferon gamma gene rs2430561 is associated with a decreased risk of PDA and bronchopulmonary dysplasia (131, 132). On the other hand, polymorphisms in the TNF receptor associated factor 1 TRAF1 (rs1056567) gene, angiotensin II type 1 receptor AGTR1 gene and TFAP2β (rs987237) gene, are associated with increased risk of PDA (133, 134). TFAP2β is also associated with altered levels of calcium and potassium channels in SMCs, which are essential for proper contraction (135). Angiotensin II is vasoconstrictive for the ductus and its receptor (Agtr2) is upregulated in late preterm infants also (128).
Although their significance remain to be elucidated, there are other genes involved in the development of matrix molecules of the DA, such as Tcfab2b, lysyl oxidase (Lox), and fibronectin (Fn1) genes which may draw attention in the future (128). Lysyl oxidase is a cross-linking enzyme for elastic fibers and stabilizes the extracellular matrix. Reduction of LOX activity is demonstrated in the pathogenesis of many vascular diseases (136). Activation of PGE2-EP4 pathway promotes the degradation of this enzyme, leading to poor elastogenesis, suggesting that control of elastogenesis is clinically important (72). Paradoxically, there are some data that indomethacin treatment of preterm infants may have an adverse effect on the inhibition of elastic fiber formation (72). Regulation of the LOX protein through PGE2-EP4 pathway may be a basis for further therapeutic strategies that target vascular elastogenesis (72).
Mechanical and Hemodynamical Factors
Hemodynamic Changes After Birth
In fetal life, high pulmonary arterial pressure, coupled with low systemic resistance in the placenta results in right to left shunting of blood through the ductus so that a large proportion of blood coming from the umbilical circulation is directed to the systemic circulation without passing through the pulmonary vascular bed. During fetal life, 10–20% of total biventricular cardiac output enters the lungs but in late gestation, this increases to 30% (137). After birth, umbilical cord clamping increases the systemic resistance whereas ventilation of the lungs decreases the pulmonary vascular resistance and increases the pulmonary blood flow; the shunt through the ductus reverses to left to right. This sudden reversal of shunting across the DA rises the pulmonary blood flow even further. In term infant, dominant left to right shunting is achieved by 10 min after birth and it is entirely left to right by 24 h of age (138). In critical cyanotic congenital heart disease, patency of ductus is life-saving. In persistent pulmonary hypertension, high resistance in pulmonary arteries results in persistance of right to left shunting through the ductus. In the preterm neonate, if the ductus remains open, left to right shunting will occur both during systole and diastole and large amounts of blood would flood the lungs. This will expose the endothelium to increased shearing forces, which will induce the production of vasodilatory mediators such as nitric oxide, bradykinin, prostacyclin, and inactivate the production of vasoconstrictor mediators such as thromboxane, endothelin and leukotriens. Arachnidonic acid may be released from pulmonary tissue damaged by shear stress, secondary to mechanical ventilation. Prolonged mechanical ventilation can also induce the release of prostacyclin, often associated with increased ductal shunting (139). This would lead to alveolar edema, and increased need for respiratory support. Positive pressure ventilation during resuscitation, oxygen, and exogenous surfactant treatment induce a rapid fall in PVR. Especially in preterm infants <32 weeks, surfactant administration is associated with an increase in the right ventricular output and absolute ductal diameter (140, 141). Acute rise in pulmonary blood low causes a significant increase in left heart preload and rise in left atrial pressure which results in increased pressure in the left atrium and contraction of foramen ovale (142). The myocardium of the preterm infant is less contractile due to fewer contractile units, increased water content and immature sarcoplasmic reticulum (143). Thus, hemodynamic stability of the preterm infant is highly dependent on myocardial function, and a sudden increase in the workload of myocardium after birth may result in failure of the myocardium resulting in pulmonary overload and hypertension. In preterm infants, delayed cord clamping does not seem to affect the incidence of PDA (144). Early adrenal insufficiency may also have a role in prolonged ductal patency (145).
Fluid dynamics state that the shunt volume across a tubular structure is directly proportional to the 4th power of the radius of the tube (i.e., ductus) and the pressure gradient between both ends (i.e., aorta and pulmonary artery), and inversely proportional to the blood viscosity and length of the tube (i.e., ductus). Moreover, increased venous return from the lungs to the left ventricle will cause an increase in the left ventricular end-diastolic pressure, which will increase atrial natriuretic peptide (ANP), with evolution of pulmonary venous hypertension and pulmonary hemorrhage. On the other hand, left to right shunting at the ductus will decrease the systemic perfusion (i.e., ductal steal) leading to systemic hypoxia and organ dysfunction. Shorter diastolic time and increased myocardial oxygen demand may result in endocardial ischemia (142).
There is scarce information on the effects of dilation mediated by the mechanical force of the flow, or through shear forces and shear stress. Although there is no clear evidence, it may be speculated that there are some mechanosensors in the ductal wall, which can sense the biomechanical changes in the lumen and regulate ductal closure. The effect of laminar flow vs. turbulent flow across the ductus is also understudied. In case of laminar flow, endothelial cells are less likely to proliferate and nitric oxide synthase and prostacyclin are upregulated (146, 147). However, if turbulent flow develops, endothelial cells begin to upregulate vasoconstrictors such as endothelin-1 and adhesion molecules such as VCAM-1 and ICAM-1 (148, 149). Matrix modeling is an essential component of intimal cushion formation. Stretch of the ductal wall induces the release of angiotensin II from the endothelial cell, leading to smooth muscle proliferation and production of matrix metalloproteinases which trigger extracellular matrix remodeling and vasoconstriction (149, 150). Circumferential stretch also triggers phenotypic switching of SMCs from contractile to synthetic cells, which means more proliferation and migration of SMCs (150).
Fluid Load
A sharp decrease in pulmonary artery pressure, as it happens after surfactant replacement therapy may increase the left-to-right shunt through the ductus, leading to pulmonary overflow and hemorrhage. In preterm infants, increased pulmonary fluid and protein load is eliminated by an increased lung lymph flow, named as “edema safety factor.” Therefore, ductal closure within the first 24 h of birth do not have any significant effect on the course of respiratory distress syndrome. However, if ductus remains open for several days, this compensation cannot be maintained and alteration in the pulmonary mechanics and pulmonary edema ensue. The impact of patent ductus arteriosus on patient outcomes seems to be a function of magnitude of shunt, rather than its mere presence. Closure of the ductus improves lung mechanics in these infants (151). Early ductal closure is associated with less pulmonary hemorrhages in preterm infants (152). Ibuprofen leads to increased expression of sodium channels and increased removal of fluid from the alveolar compartment (153). In some studies, fluid restriction is recommended as an alternative to treatment; such an approach may reduce pulmonary circulation but also compromises end organ flow in patients already suffering from ductal steal phenomenon (154). However, high (liberal) fluid intake is associated with an increased risk of PDA and BPD (155). Therefore, close monitoring of fluid intake and individualized treatment of infants are of utmost importance in preterm infants, especially in the first days of life.
Early Adrenal Function
Cortisol is central to the ability of the infant to attenuate its response to inflammation and is potent inhibitor of inflammatory edema. Animal and human studies have shown that adrenal insufficiency can lead to increased inflammatory response to injury and that glucocorticoids can affect patency of the ductus (156, 157). Glucocorticoids induce production of lipocortin which inhibits phospholipase A2. Phospholipase A2 stimulates the release of arachnidonic acid precursors needed for prostaglandin synthesis; thus in response to steroids, prostaglandin production is inhibited (158). Animal studies have shown that glucocorticoid administration decreases the sensitivity of ductal tissue to the dilating influence of PGE2 (156). Sick infants do not have elevated serum corticol levels compared to well infants. These infants are also prone to developing bronchopulmonary dysplasia (159). Therefore, it is not surprising that patent ductus arteriosus is one of the most prominent risk factors in the development of bronchopulmonary dysplasia (160).
Effects of Surgical Ligation
Surgical ligation of the ductal immediately after birth increases the expression of genes involved in lung inflammation and decreases the expression of genes involved in epithelial sodium channels (161). Therefore, the pulmonary mechanics do not improve as much as expected. Furthermore, early ligation may slow down lung growth (162). On the other hand, in the presence of atelectasis, the persistence of left to right shunt through the PDA maintains an elevated arterial oxygen pressure in the lungs and after PDA ligation, a decrease in the systemic arterial pressure may be observed (163).
Effects of Pharmacological Agents
Oxygen is the most powerful agent for closure of the ductus. In numerous studies investigating the effect of different levels of oxygen on preterm morbidities, ductal closure was associated with high levels of oxygen but oxygen-related co-morbidities in these infants raised significant concerns (164). These trials including Surfactant, Positive Pressure, Pulse Oxymetry Randomized Trial (SUPPORT), Canadian Oxygen Trial (COT), and Benefits of Oxygen Saturation Targeting (BOOST) II trials have reported neurodevelopmental outcomes after various oxygen targeting, as well as other complications; however the discussion of these trials are out of the context of this article (165–167).
All available pharmacological treatments aiming for changing the ductal tone use the prostaglandin pathway by either the cyclooxygenase pathway (indomethacin and ibuprofen) or the peroxidase pathway (acetaminophen). The infant's gestational age, as well as the infant's race, ethnicity, growth, and exposure to antenatal corticosteroids modify the effects of prostaglandins and determine the rate of drug-induced closure (168, 169).
In the term infant PGE2 receptors are down-regulated after birth. However, in the preterm infant, the production of receptors increases, rendering the ductus susceptible to vasodilation and resistant to indomethacin or ibuprofen. COX1 and COX2 expression increases with advancing maturity in the fetus (170, 171). COX inhibitors decrease vaso vasorum flow, leading to hypoperfusion and ischemic injury in the vessel wall (85). Preterm ductus also synthesizes other vasodilators such as NO, TNF-α, and IL-6 in response to wall hypoxia. Decreased ATP concentrations also prevent the ductus from contracting (172). Therefore, the preterm ductus becomes more resistant to treatment with constrictor agents (173).
Cyclooxygenase (COX) Inhibitors
Indomethacin and Ibuprofen non-selectively inhibit cyclooxygenase enzymes; thus preventing arachidonic acid conversion to prostaglandins (Figure 2). Both drugs are associated with increased amiloride-sensitive alveolar epithelial sodium channels, suggesting improved lung water clearance and lung compliance (174). Ductal sensitivity to indomethacin increases with gestational age. Response rate to indomethacin is about 5–10% in infants <27 weeks of gestation, whereas it is almost 100% above 34 weeks (175). Half-life of indomethacin is 4–5 h longer (i.e., 17 h) in preterm infants <32 weeks of gestation. It can be given by intravenous, oral, rectal or intraarterial route. Closure rates vary from 48 to 98.5%, depending upon the dose, method and duration of administration (176, 177). Early use of indomethacin within the first few days of life decreases the risk of pulmonary hemorrhage, intraventricular hemorrhage and the need for surgical ligation, although does not affect the development of BPD (178). If needed, a second course may be given, with a success rate of 40–50% (179). Since more courses are associated with periventricular leukomalacia, repeat doses are not recommended (180). Efficacy of indomethacin declines with decreasing gestational age and becomes doubtful at 22–23 weeks of gestation (176). Indomethacin have several side effects including renal failure, oliguria, proteinuria, hyperkalemia, cerebral white matter damage, necrotizing enterocolitis, intestinal perforation and platelet dysfunction (181).
Ibuprofen is a non-selective COX inhibitor with less side effects than those of indomethacin. It can be given orally or intravenously. Elimination is slower after oral administration and the rate of closure is also higher (182). Its efficacy for ductal closure is the same as indomethacin. Major side effects include gastrointestinal hemorrhage, oliguria, high bilirubin levels, and pulmonary hypertension (183, 184). These effects may vary depending on the relevant genetic polymorphisms (185).
Peroxidase (POX) Inhibitors
Paracetamol (Acetaminophen) inhibits POX enzyme which catalyzes the conversion of PG2 to PGH2, without any peripheral vasoconstriction (Figure 2). Although it is commonly discussed under the family of non-steroidal anti-inflammatory drugs, it does not have any anti-inflammatory effect (186). Its gastrointestinal tolerance is better than other NSAIDs and it lacks antiplatelet activity. Paracetamol induced POX inhibition is competitive and independent of COX activity (187). Paracetamol induced reduction in PGH2 production can be counteracted by lipid peroxides and PGG2 itself (188). The maturational difference in lipid hydroperoxide production in preterm newborns may contribute to paracetamol-induced ductal closure (188). Since the mechanism of action is different than that of indomethacin or ibuprofen, the efficacy of paracetamol in extremely low birth weight infants may be less than other NSAIDs, although solid evidence is lacking. On the other hand, its pharmacokinetics has not been well-studied in ductal closure. Inhibition of the POX dilators may lead to an increase in the pulmonary vascular resistance via peroxynitrite production (189). Furthermore, there are some data that paracetamol may synergistically enhance the inhibitory effects of other anti-inflammatory drugs on platelets (190). In preterm infants, very few cases of toxicity have been reported, which may be attributed to the low activity of cytochrome P450 in the immediate postnatal period, minimizing the formation of toxic metabolites (191). Meta-analysis comparing oral paracetamol vs. oral ibuprofen concluded that paracetamol confers comparable efficacy with fewer adverse effects (192). Paracetamol may be used as a rescue treatment for infants with persistent PDA (193). Due to the small number of studies, it is difficult do decide whether paracetamol is superior or inferior to other NSAIDs. Although there are no randomized controlled trials, there are some observational studies which suggest that paracetamol use in pregnancy or in early infancy might be associated with attention deficit/ hyperactivity disorder, autism spectrum disorder or hyperactivity symptoms. Although the exact mechanism is unknown and a cause-effect relation is difficult to establish, a small but significant odds ratio is observed in many studies (194–196). However, another study suggests that there is not an increased risk of autism in these infants at 2-year follow-up (197). There is no data on the possible long-term neurodevelopmental effects of paracetamol use in preterm infants. Therefore, paracetamol should be used cautiously until further safety data exists.
Some authors have suggested to use a combination therapy of ibuprofen and paracetamol as a first line therapy for ductal closure. However, preliminary reports suggest that the effectiveness of combination therapy does not differ compared to monotherapy of either drug (198). In another study, in infants who failed two previous cycles of monotherapy, combination therapy with oral ibuprofen and oral paracetamol was effective in 9 infants out of 12 (199). Results of an ongoing randomized controlled trial are pending (https://clinicaltrials.gov/ct2/show/NCT03103022).
Other non-steroidal anti-inflammatory drugs such as aspirin, metamizol, nimesulid, and diclophenac have constrictor effects on the ductus (200–203).
Glucocorticoids have also vasoconstrictive effects on the ductus, as well as synergistic effects with non-steroidal anti-infective drugs (NSAIDs), as a result of decreasing the sensitivity of the ductal SMCs to prostaglandins (204) (Figure 2). In the preterm fetus, elevated levels of corticosteroids is associated with decreased sensitivity of the ductus to the vasodilating effects of prostaglandins and prenatal administration of corticosteroids, mainly for the prevention of respiratory distress syndrome have also decreased the incidence of PDA in these infants (205). Similarly, postnatal corticosteroid administration may constrict the ductus (206). Angiotensin II does not have a role in ductal closure after birth (207).
There are many substances and foods with anti-inflammatory and anti-oxidant properties, such as those rich in polyphenols or flavonoids. For example, 30–40% of solid extract of green tea composes of polyphenols, mainly catechins. They exert their anti-inflammatory effects through the inhibition of endogenous prostaglandins. Similar effects have been reported with black tea, resveratrol, mate tea, orange juice and dark chocolate (207). Excessive consumption of such foods by the pregnant woman may influence the dynamics of the DA, by inhibiting COX and PG synthesis pathway. The amount of flavonoids necessary to trigger clinically significant ductal closure remains to be determined (208).
Genetic therapies may include endothelin-signaling genes or blockage of potassium channels. Channel isoforms have distinct pharmacological and physiological characteristics, making them ideal targets for ductal-selective therapies (209). Glibenclamide is a non-specific KATP channel inhibitor, used for treating diabetes. It is shown that DA constricts in response to glibenclamide, and constriction is inhibited by diazoxide, a KATP channel activator (210). Children with Cantu syndrome, which is caused by mutations in the subgroup of KATP channel genes, have PDA, often resistant to medical treatment, requiring surgical closure most of the time (16). Agonist or antagonist molecules targeting KATP channels may modulate DA tone.
Novel treatment strategies for PDA include combined use of ibuprofen and paracetamol (211), EP4 inhibition (212), NOS inhibition (213), enhancing ion channel expression (50), glutamate supplementation (59) especially in situations where COX inhibitors have proven to be ineffective.
Prostaglandin E1
PGE1 is the main drug used for vasodilatatory effects of the ductus. PGE1 binds to the EP4receptor which increases intracellular cAMP. Milrinon is a phosphodiesterase 3 inhibitor, which can also increase cAMP levels and dilate the ductus (12).
Non-selective endothelin receptor antagonist TAK-044 and inhibition of Notch pathway may have vasodilating effects on the ductus (51, 91). Natriuretic peptide (NP) is an activator of PKG-cGMP and can prevent ductal closure also (51).
Antenatal corticosteroids are used for the maturation of preterm lung, but their effects on the development of patent ductus arteriosus, intracranial hemorrhage, and necrotizing enterocolitis are also well-studied (214). However, some reports conclude that the incidence of PDA is not effected significantly by antenatal corticosteroids (215). Antenatal steroids increase 15-PGDH activity, thereby promoting PGE2 breakdown (216).
Antenatal MgSO4 which is a calcium channel blocker may result in delayed ductal closure in infants (217). However, recent evidence in extremely low birth weight infants suggests that antenatal MgSO4 exposure is not associated with an increased risk of hemodynamically significant PDA; in fact, it may be associated with a decreased likelihood of hemodynamically significant PDA (218). Diazoxide, which is a KATP channel activator may induce ductal opening (173). Furosemide increases renal PGE2 production, thus dilating the ductus in animals but its effect is limited in humans (219).
Conclusion
Ductus arteriosus is an intriguing and complex vessel with many distinct features. Understanding the precise mechanisms of regulation of the ductus is essential in order to improve individual pharmacological treatments. The debate about PDA should not be “treat all” or “treat none,” but rather, “who to treat.” This approach necessitates individualized treatment. Treatments targeting endogenous ductus regulation pathways other than prostaglandins, such as cell cycle regulators, transcription factors, and epigenomic regulators should be developed. Since vascular constriction and ductal wall remodeling are required for complete closure, treatments that favor vasoconstriction and remodeling would also be ideal for infants with persistent PDA. Novel therapies for ductal closure in preterm infants should also focus on a clear understanding of genes which are operative on ductal patency, as new genome-wide studies uncover genes associated with PDA. Evolving pediatric clinical pharmacology heralds next generation solutions for unresolved issues of today, such as drugs with selective DA effects, without any side effects.
Author Contributions
FO is responsible for the design, research, writing, and editing of the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raju TN. From Galen to Gross and beyond: a brief history of the enigmatic ductus arteriosus. J Perinatol. (2019) 39:1442–8. doi: 10.1038/s41372-019-0517-4
2. Reese J. Towards a greater understanding of the ductus arteriosus. Sem Perinatol. (2018) 42:199–202. doi: 10.1053/j.semperi.2018.05.001
3. Prsa M, Sun L, Van Amerom J, Yoo SJ, Wortmann LG, Jaeggi E, et al. Reference ranges of blood flow in the majör vessels on the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging. (2014) 7:663–70. doi: 10.1161/CIRCIMAGING.113.001859
4. Hammenman C. Patent ductus arteriosus. Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clin Perinatol. (1995) 22:457–79. doi: 10.1016/S0095-5108(18)30293-8
5. Matsui H, McCarthy KP, Ho SY. Morphology of the patent arterial duct: features relevant to treatment. Images Paediatr Cardiol. (2008) 10:27–38.
6. Semberova J, Sirc J, Miletin J, Kucera J, Berka I, Sebkova S, et al. Spontaneous closure of patent ductus arteriosus in infants ≤ 1500 g. Pediatrics. (2017) 140:e20164258. doi: 10.1542/peds.2016-4258
7. Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. (2000) 190:300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I
8. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II modulated by G proteins, kinases and myosin phosphatase. Physiol Rev. (2003) 83:135–8. doi: 10.1152/physrev.00023.2003
9. Hundscheid T, vanden Boek M, van der Lee R, de Boode W. Understanding the pathobiology in patent ductus arteriosus in prematurity-beyond prostaglandins and oxygen. Pediatr Res. (2019) 86:28–38. doi: 10.1038/s41390-019-0387-7
10. Fan F, Ma A, Guan Y, Huo J, Hu Z, Tian H, et al. Effect of PGE2 on DA tone by EP4 modulating Kv channels with different oxygen tension between preterm and term. Int J Cardiol. (2011) 147:58–65. doi: 10.1016/j.ijcard.2009.07.045
11. Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, et al. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. (1997) 390:78–81. doi: 10.1038/36342
12. Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharm Rev. (2013) 65:1010–52. doi: 10.1124/pr.112.007195
13. Crichton CA, Smith GC, Smith GL. alpha-Toxin-permeabilised rabbit fetal ductus arteriosus is more sensitive to Ca2+ than aorta or main pulmonary artery. Cardiovasc Res. (1997) 33:223–9. doi: 10.1016/S0008-6363(96)00171-X
14. Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. (2011) 3:a003970. doi: 10.1101/cshperspect.a003970
15. Clyman RI, Waleh N, Kajino H, Roman C, Mauray F. Calcium-dependent and calcium-sensitizing pathways in the mature and immature ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. (2007) 293:R1650–6. doi: 10.1152/ajpregu.00300.2007
16. Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharm Rev. (2010) 62:525–63. doi: 10.1124/pr.110.002907
17. Baragatti B, Brizzi F, Barogi S, Laubach VE, Sodini D, Shesely EG, et al. Interactions between NO, CO and an endothelium-derived hyperpolarizing factor (EDHF) in maintaining patency of the ductus arteriosus in the mouse. Br J Pharm. (2007) 151:54–62. doi: 10.1038/sj.bjp.0707211
18. Sodini D, Baragatti B, Barogi S, Laubach VE, Coceani F. Indomethacin promotes nitric oxide function in the DA in the mouse. Br J Pharmacol. (2008) 153:1631–40. doi: 10.1038/bjp.2008.36
19. van der Sterren S, Kleikers P, Zimmermann LJ, Villamor E. Vasoactivity of the gasotransmitters hydrogen sulfide and carbon monoxide in the chicken ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. (2011) 301:R1186–98. doi: 10.1152/ajpregu.00729.2010
20. Coceani F, Kelsey L, Seidliz E, Mars GS, McLaughlin BE, Vreman HJ, et al. Carbon monoxide formation in the ductus arteriosus in the lamb: implications for regulation of muscle tone. Br J Pharmacol. (1997) 120:599–608. doi: 10.1038/sj.bjp.0700947
21. Majeed Y, Tumova S, Green BL, Seymour VAL, Woods DM, Agarwal AK, et al. Pregnenolone sulphate-independent inhibition of TRPM3 channels by progesterone. Cell Calcium. (2012) 51:1–11. doi: 10.1016/j.ceca.2011.09.005
22. Yasuda K, Koyama N, Nomura T, Makino Y, Murata M, Takenaka M, et al. Effects of phosphodiesterase 3 inhibitors to premature PDA (abstract). J Japan Soc Premature Newborn Med. (2004) 16:395.
23. Crockett SL, Berger CD, Shelton EL, Reese J. Molecular and mechanical factors contributing to ductus arteriosus patency and closure. Congenital Heart Dis. (2019) 14:15–20. doi: 10.1111/chd.12714
24. Park SM, Ahn CM, Hong SJ, Kim YH, Park JH, Shim WJ, et al. Acute changes of left ventricular hemodynamics and function during percutaneous coronary intervention in patients with unprotected left main coronary artery disease. Heart Vessels. (2015) 30:432–40. doi: 10.1007/s00380-014-0495-6
25. Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharm. (2004) 65:973–8. doi: 10.1124/mol.65.4.973
26. Printz MP, Skidgel RA, Friedman WF. Studies of pulmonary prostaglandin biosynthetic and catabolic enzymes as fctors in ductus arteriosus patency and closure. Evidence for a shift in products with gestational age. Pediatr Res. (1984) 18:19–24.
27. Tsai MY, Einzig S. Prostaglandin catabolism in fetal and maternal tissues a study of 15-hydroxyprostaglandin dehydrogenase and delta 13 reductase with specific assay methods. Prostaglandins Leukot Ess Fat Acids. (1989) 38:25–30. doi: 10.1016/0952-3278(89)90143-9
28. Smith GC, McGrath JC. Prostaglandin E2 and fetal oxygen tension synergistically inhibit response of isolated fetal rabbit ductus arteriosus to norepinephrine. J Cardiovasc Pharm. (1991) 17:861–6. doi: 10.1097/00005344-199106000-00001
29. Bateson EA, Schulz R, Olley PM. Response of fetal rabbit ductus arteriosus to bradykinin: a role of nitric oxide, prostaglandins, and bradykinin receptors. Pediatr Res. (1999) 45:568–74. doi: 10.1203/00006450-199904010-00017
30. Clyman RI, Mauray F, Roman C, Heymann MA, Payne B. Effect of gestational age on ductus arteriosus response to circulating prostaglandin E2. J Pediatr. (1983) 102:907–11. doi: 10.1016/S0022-3476(83)80023-7
31. Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, Chen YQ. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension and vasa vasorum. Pediatr Res. (1998) 43:633–44. doi: 10.1203/00006450-199805000-00012
32. Yokoyama U, Minamisawa S, Adachi-Akahane S, Akaike T, Naguro I, Funokoshi K, et al. Multiple transcripts of Ca2+channel alpha1-subunits and a novel spliced variant of the alpha1C-subunit in rat ductus arteriosus. Am J Physiol Heart Circ Physiol. (2006) 290:H1660–70. doi: 10.1152/ajpheart.00100.2004
33. Goyal R, Goyal D, Longo LD, Clyman RI. Microarray gene expression analysis in ovine ductus arteriosus during fetal development and birth transition. Pediatr Res. (2016) 80:610–8. doi: 10.1038/pr.2016.123
34. Ichikawa Y, Yokoyama U, Iwamoto M, Oshikawa J, Okumura S, Sato M, et al. Inhibition of phosphodiesterase type 3 dilates the rat ductus arteriosus without inducing intimal thickening. Circ J. (2012) 76:2456–64. doi: 10.1253/circj.CJ-12-0215
35. Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. (2005) 353:2042–55. doi: 10.1056/NEJMra050002
36. Heymann MA, Rudolph AM. Control of the ductus arteriosus. Physiology. (1975) 55:62–78. doi: 10.1152/physrev.1975.55.1.62
37. Smith GC, McGrath JC. Indomethacin but not oxygen tension affects the sensitivity of isolated neonatal rabit ductus arteriosus, but not aorta to noradrenaline. Cardiovas Res. (1988) 22:910–5. doi: 10.1093/cvr/22.12.910
38. Kajimoto H, Hashimoto K, Bonnet SN, Heromy A, Harry G, Moudgil R, et al. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species. Circulation. (2008) 115:1777–88. doi: 10.1161/CIRCULATIONAHA.106.649566
39. Nakanishi T, Gu H, Hagiwara N, Momma K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res. (1993) 72:1218–28. doi: 10.1161/01.RES.72.6.1218
40. Michelakis ED, Rebeyka I, Wu X, Nsair A, Tehabud B, Hashimoto K, et al. O2 sensing in the human ductus arteriosus regulation of voltage gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. (2002) 91:478–86. doi: 10.1161/01.RES.0000035057.63303.D1
41. Thebaud B, Michelakis ED, Wu XC, Moudgil R, Kuzyk M, Dyck JR, et al. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: implications for infants with patent ductus arteriosus. Circulation. (2004) 110:1372–9. doi: 10.1161/01.CIR.0000141292.28616.65
42. Michealakis E, Rebeyka I, Bateson J, Olley P, Puttagunta L, Archer S. Voltage gated potassium channels in human ductus arteriosus. Lancet. (2000) 356:134–7. doi: 10.1016/S0140-6736(00)02452-1
43. Thebaud B, Wu XC, Kajimoto H, Bonnet S, Hashimoto K, Michelakis ED, et al. Developmental absence of the O2 sensitivity of L type calcium channels in preterm ductus arteriosus smooth muscle cells impairs O2 constriction contributing to patent ductus arteriosus. Pediatr Res. (2008) 63:176–81. doi: 10.1203/PDR.0b013e31815ed059
44. Akaike T, Jin MH, Yokoyama U, Izumi-Nakaseko H, Jiao Q, Iwasaki S, et al. T-type Ca2+ channels promote oxygenation induced closure of the rat ductus arteriosus not only by vasoconstriction but also by neointima formation. J Biol Chem. (2009) 284:24025–34. doi: 10.1074/jbc.M109.017061
45. Cogolludo AL, Moral-Sanz J, van der Sterren S, Frazziano G, van Cleef AN, Menéndez C, et al. Maturation of O2 sensing and signaling in the chicken ductus arterious. Am J Physiol Lung Cell Mol Physiol. (2009) 297:L619–30. doi: 10.1152/ajplung.00092.2009
46. Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med. (2008) 18:20–5. doi: 10.1016/j.tcm.2007.11.002
47. Baragatti B, Coceani F. Arachidonic acid epoxygenase and 12(S) lipoxygenase: evidence of their concerted involvement in ductus arteriosus constriction to oxygen. Can J Physiol Pharmacol. (2011) 89:329–34. doi: 10.1139/y11-025
48. Coceani F, Kelsey L, Seidliz E. Evidence for an effector role of endothelin in closure of the ductus arteriosus at birth. Can J Physiol Pharmacol. (1992) 70:1061–4. doi: 10.1139/y92-146
49. Chen JX, O'Mara PW, Poole SD, Brown N, Ehinger NJ, Slaughter JC, et al. Isoprostanes as physiological mediators of transition to newborn life: novel mechanism regulating patency of the term and preterm ductus arteriosus. Pediatr Res. (2012) 72:122. doi: 10.1038/pr.2012.58
50. Wu GR, Jing S, Momma K, Nakanishi T. The effect of vitamin A on contraction of the ductus arteriosus in fetal rat. Pediatr Res. (2001) 49:747–54. doi: 10.1203/00006450-200106000-00006
51. Hung YC, Yeh JL, Hsu JH. Molecular mechanism for regulation postnatal ductus arteriosus closure. Int J Mol Sci. (2018) 19:1861. doi: 10.3390/ijms19071861
52. Hong Z, Hong F, Olschewski A, Cabrera JA, Varghese A, Nelson DP, et al. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arterisosus. Circulation. (2006) 114:1372–9. doi: 10.1161/CIRCULATIONAHA.106.641126
53. Akaike T, Minamisawa S. Role of ion channels in ductus arteriosus closure. Hum Genet Embryo. (2013) 3:116. doi: 10.4172/2161-0436.1000116
54. Takizawa T, Horikoshi E, Shen MH, Masaoka T, Takagi H, Yamamoto M, et al. Effects of TAK-044, a nonselective endothelin receptor antagonist, on the spontaneous and indomethacin- or methylene blue-induced constriction of the ductus arteriosus in rats. J Vet Med Sci. (2000) 62:505–9. doi: 10.1292/jvms.62.505
55. Waleh N, Kajino H, Marrache AM, Ginzinger D, Roman C, Seidner SR, et al. Prostaglandin E2–mediated relaxation of the ductus arteriosus: effects of gestational age on g protein-coupled receptor expression, signaling, and vasomotor control. Circulation. (2004) 110:2326–32. doi: 10.1161/01.CIR.0000145159.16637.5D
56. altered ductus Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. (1996) 128:470–8. doi: 10.1016/S0022-3476(96)70356-6
57. Levin M, McCurnin D, Seidner SR, Yoder B, Waleh N, Goldberg S, et al. Postnatal constriction, ATP depletion, and cell death in the mature and immature ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. (2006) 290:R359–64. doi: 10.1152/ajpregu.00629.2005
58. Clyman RI, Teitel D, Padbury J, Roman C, Mauray F. The role of beta-adrenoreceptor stimulation and contractile state in the preterm lamb's response to arteriosus patency. Pediatr Res. (1988) 23:316–22. doi: 10.1203/00006450-198803000-00017
59. Fujita S, Yokoyama U, Ishiwata R, Aoki R, Nagao K, Masukawa D, et al. Glutamate promotes contraction of the rat ductus arteriosus. Circ J. (2016) 80:2388–96. doi: 10.1253/circj.CJ-16-0649
60. Clyman RI, Roman C. The effects of caffeine on the preterm sheep ductus arteriosus. Pediatr Res. (2007) 62:167–9. doi: 10.1203/PDR.0b013e3180a725b1
61. Çakir U, Tayman C. A mystery of patent ductus arteriosus and serum osmolality in preterm infants. Am J Perinatol. (2018) 36:641–6. doi: 10.1055/s-0038-1673397
62. Aoki R, Yokoyama U, Ichikawa Y, Taguri M, Kumagaya S, Ishiwata R, et al. Decreased serum osmolality promotes ductus arteriosus constriction. Cardiovasc Res. (2014) 104:326–36. doi: 10.1093/cvr/cvu199
63. Hu Y, Jin H, Jiang Y, Du J. Prediction of therapeutic response to cyclooxygenase inhibitors in preterm infants with patent ductus arteriosus. Pediatr Cardiol. (2018) 39:647–52. doi: 10.1007/s00246-018-1831-x
64. Mine K, Ohashi A, Tsuji S, Nakashima JI, Hirabayashi M, Kaneko K. B-type antriuretic peptide for assessment of haemodynamically significant patent ductus arteriosus in premature infants. Acta Pediatr. (2013) 102:e347–52. doi: 10.1111/apa.12273
65. Sharpe GL, Larsson KS, Thalme MB. Studies on closure of the ductus arteriosus. XII. In vitro effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins. (1975) 9:585–95. doi: 10.1016/0090-6980(75)90064-7
66. Clyman RI, Mayray F, Roman C, Rudolph AM, Heyman M. Glucorticoids alter the sensitivity of the lamb ductus arteriosus to prostaglandin E2. J Pediatr. (1981) 98:126–8. doi: 10.1016/S0022-3476(81)80558-6
67. Pulkkinen MO, Momma K, Pulkkinen J. Constriction of fetal ductus arteriosus by progesterone. Biol Neonate. (1986) 50:270–3. doi: 10.1159/000242608
68. Costa M, Barogi S, Socci ND, Angeloni D, Maffei M, Baragatti B, et al. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genom. (2006) 25:250–62. doi: 10.1152/physiolgenomics.00231.2005
69. Coceani F, Liu YA, Seidlitz E, Kelsey L, Kuwaki T, Ackerley C, et al. Deletion of the endothelin-A-receptor suppresses oxygen induced constriction but not postnatal closure of the ductus arteriosus. J Cardiovasc Pharm. (2000) 36:S75–7. doi: 10.1097/00005344-200036001-00025
70. Clyman RI, Seidner SR, Kajino H, Roman C, Koch CJ, Ferrara N, et al. VEGF regulates remodeling during permanent anatomic closure of the ductus arteriosus. Am J Physiol. (2002) 282:R199–206. doi: 10.1152/ajpregu.00298.2001
71. Kajino H, Goldberg S, Roman C, Liu BM, Mauray F, Qi Y, et al. Vasa vasorum hypoperfusion is responsible for medial hypoxia and anatomic remodeling in the newborn lamb ductus arteriosus. Pediatr Res. (2002) 51:228-35. doi: 10.1203/00006450-200202000-00017
72. Yokoyama U, Minamismawa S, Shioda A, Ishiwata R, Jin MH, Masuda M, et al. Prostaglandin E 2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation. (2014) 129:487–96. doi: 10.1161/CIRCULATIONAHA.113.004726
73. Gitterberger-de Groot AC. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. (1977) 39:610–8. doi: 10.1136/hrt.39.6.610
74. Clyman RI, Chan CY, Mauray F, Chen YQ, Cox W, Seidner SR, et al. Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia and gestation. Pediatr Res. (1999) 45:19–29. doi: 10.1203/00006450-199901000-00005
75. Seidner SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, et al. Combined prostaglandin and nitric oxide inhibition produces anatomic remadoling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. (2001) 50:365–73. doi: 10.1203/00006450-200109000-00012
76. Hinek A, Mecham RP, Keeley F, Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb DA and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest. (1991) 88:2083–94. doi: 10.1172/JCI115538
77. Hsieh YT, Liu NM, Ohmori E, Yokota T, Kajimura I, Akaike T, et al. Transcription profiles of the DA in Brown-Norway rats with irregular elastic fiber formation. Circ J. (2014) 78:1224–33. doi: 10.1253/circj.CJ-13-1029
78. Clyman RI, Goetzman BW, Chen YQ, Mauray F, Kramer RH, Pytela R, et al. Changes in endotehial cell and smooth muscle cell integrin expression during closure of the ductus arteriosus: an immunohistochemical comparison of the fetal, preterm newborn and full term newborn rhesus monkey ductus. Pediatr Res. (1996) 40:198–208. doi: 10.1203/00006450-199608000-00004
79. Francalanci P, Camassei FD, Orzalesi M, Danhaive O, Callea F. CD44-v6 expression in smooth muscle cells in the postnatal remodeling process of ductus arteriosus. Am J Cardiol. (2006) 97:1056–9. doi: 10.1016/j.amjcard.2005.10.046
80. Boudreau N, Turley E, Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased DA smooth muscle cell migration. Dev Biol. (1991) 143:235–47. doi: 10.1016/0012-1606(91)90074-D
81. Yokoyama U, Minamisawa S, Katayama A, Tang T, Suzuki S, Iwatsubo K, et al. Differential regulation of vascular tone and remodeling via stimulation of type 2 and type 6 adenylyl cyclases in the ductus arteriosus. Circ Res. (2010) 106:1882–92. doi: 10.1161/CIRCRESAHA.109.214924
82. Mason CA, Bigras JL, O'Blenes SB, Zhou B, McIntyre B, Nakamura N, et al. Gene transfer in utero biologically engineers a patent DA in lambs by arresting fibronectin-dependent neointimal formation. Nat Med. (1999) 5:176–82. doi: 10.1038/5538
83. Clyman RI. Mechanisms regulating closure of the ductus arteriosus. In: Polin R, Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and Neonatal Physiology. New York, NY: Elsevier (2019). p. 57:592–9.
84. Lezoulach F, Fazal L, Laudette M, Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ Res. (2016) 118:881–97. doi: 10.1161/CIRCRESAHA.115.306529
85. Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. (2003) 4:733–8. doi: 10.1038/nrm1197
86. Wu LH, Xu SJ, Teng JY, Wu W, Ye DY, Wu XZ. Differential response of human fetal smooth muscle cells from arterial dut to retinoid acid. Acta Pharmacol Sin. (2008) 29:413–20. doi: 10.1111/j.1745-7254.2008.00766.x
87. Ravishankar C, Nafday S, Green RS, Kamenir S, Lorber R, Stacewicz-Sapuntzakis M, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants. J Pediatr. (2003) 143:644–48. doi: 10.1067/S0022-3476(03)00501-8
88. Momma K, Toyono M, Miyagawa-Tomita S. Accelerated maturation of fetal ductus arteriosus by maternally administered vitamin A in rats. Pediatr Res. (1998) 43:629–32. doi: 10.1203/00006450-199805000-00011
89. Basu S, Khanna P, Srivastava R, Kumar A. Oral vitamin A supplemantation in very low birth weight neonates: a randomized controlled trial. Eur J Pediatr. (2019) 178:1255–65. doi: 10.1007/s00431-019-03412-w
90. Tannenbaum JE, Waleh NS, Mauray F, Breuss J, Pytela R, Karemer RH, Clyman RI. Transforming growth factor beta 1 inhibits fetal lab ductus arteriosus smooth muscle cell migration. Pediatr Res. (1995) 37:561–70. doi: 10.1203/00006450-199505000-00001
91. Wu JR, Yeh JL, Liou SF, Dai ZK, Wu BN, Hsu JH. Gamma-secretase inhibitor prevents proliferation and migration of ductus arteriosus smooth muscle cells through the Notch3-HES1/2/5 pathway. Int J Biol Sci. (2016) 12:1063–73. doi: 10.7150/ijbs.16430
92. Giri JG, Ahdieh M, Einsenman J, Schanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. (1994) 13:2822–30. doi: 10.1002/j.1460-2075.1994.tb06576.x
93. Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. (2006) 116:3026–34. doi: 10.1172/JCI28639
94. Rabinovich M. Cell-extracellular matrix interactions in the ductus arteriosus and perinatal pulmonary circulation. Semin Perinatol. (1996) 20:531–41. doi: 10.1016/S0146-0005(96)80067-X
95. Evanko SP, Angello JC, Wight TN. Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. ArteriosclerThromb Vasc Biol. (1999) 19:1004–13. doi: 10.1161/01.ATV.19.4.1004
96. Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin C antisense prevents progression of vascular disease. J Clin Invest. (2000) 105:21–34. doi: 10.1172/JCI6539
97. Hinek A, Boyle J, Rabinovitch M. Vascular smooth muscle cell detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate indnuced shedding of the 67 kDa cell surface elastin binding protein. Exp Cell Res. (1992) 203:344–53. doi: 10.1016/0014-4827(92)90008-V
98. Kawakami S, Minamisawa S. Oxygenation decreases elastin secretion from rat ductus arteriosus smooth muscle cells. Pediatr Int. (2015) 57:541–5. doi: 10.1111/ped.12684
99. Waleh N, Seidner S, McCurnin D, Yoder B, Liu BM, Roman C, et al. The role of monocyte-derived cells and inflammation in baboon ductus arteriosus remodeling. Pediatr Res. (2005) 57:254–26. doi: 10.1203/01.PDR.0000148278.64777.EF
100. Johnston B, Issekutz TB, Kubes P. The alpha 4-integrin supports leukocyterolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med. (1996) 183:1995. doi: 10.1084/jem.183.5.1995
101. Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. (1996) 87:3336–43. doi: 10.1182/blood.V87.8.3336.bloodjournal8783336
102. Waleh N, Seidner S, McCurnin D, Giavedoni L, Hodara V, Goelz S, et al. Anatomic closure of the premature patent ductus arteriosus: the role of CD14/CD163+ mononuclear cells and ascular endothelial growth factor (VEGF) in neointimal mound formation. Pediatr Res. (2020) 70:332–8. doi: 10.1203/PDR.0b013e3182294471
103. Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheterinjury. Arterioscler Thromb. (1993) 13:1218–122. doi: 10.1161/01.ATV.13.8.1218
104. Echtler K, Stark K, Lorenz M, Kerstan S, Walch A, Jennen L, et al. Platelets contribute to postnatal occlusion of the ductus arteriosus Nat Med. (2019) 16:75–82. doi: 10.1038/nm.2060
105. Clyman R, Cherntob S. Vessel remodeling in the newborn: platelets fill the gap. Nat Med. (2010) 16:33–4. doi: 10.1038/nm0110-33
106. Engür D, Kaynak-Türkmen M, Deveci M, Yenisey C. Platelets and platelet derived growth factor in closure of ductus arteriosus. Turk J Pediatr. (2015) 57:242–7.
107. Dani C, Poggi C, Fontanell G. Relationship between platelet count and volume and spontaneous and pharmacological closure o of ductur arteriosus in preterm infants. Am J Perinatol. (2013) 30:359–64. doi: 10.1055/s-0032-1324702
108. Shah NA, Hills NK, Waleh N, McCurnin D, Seidner S, Chemto S, et al. Relationship between circulating platelet counts and ductus arteriosus patency after indomethacin treatment. J Pediatr. (2011) 158:919–23. doi: 10.1016/j.jpeds.2010.11.018
109. Shelton EL, Ector G, Galind CL, Hooper CW, Brown N, Wilkerson I, et al. Transcriptional profiling reveals ductus arteriosus specific genes that regulate vascular tone. Physiol Genomics. (2014) 46:457–66. doi: 10.1152/physiolgenomics.00171.2013
110. Loftin CD, Trivedi DB, Tiano HF, Clark JA, Lee CA, Epstein JA, et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc Natl Acad Sci USA. (2001) 98:1059–64. doi: 10.1073/pnas.98.3.1059
111. Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA. (2000) 97:9759e64. doi: 10.1073/pnas.97.17.9759
112. Ment LR, Vohr B, Allan W, Westerveld M, Sparrow SS, Schneider KC, et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. (2000) 105:485–91. doi: 10.1542/peds.105.3.485
113. Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, et al. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med. (2002) 8:91–2. doi: 10.1038/nm0202-91
114. Nagar S, Walther S, Blanchard RL. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol. (2006) 69:2084–92. doi: 10.1124/mol.105.019240
115. Grassian AR, Metallo CM, Coloff JL, Stephanopoulos G, Brugge JS. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. (2011) 25:1716–33. doi: 10.1101/gad.16771811
116. Sun Y, Daemen A, Hatzivassiliou G, Arnott D, Wilson C, Zhuang G, et al. Metabolic and transcriptional profiling reveals pyruvate dehydrogenase kinase 4 as a mediator of epithelial-mesenchymal transition and drug resistance in tumor cells. Cancer Metab. (2014) 2:20. doi: 10.1186/2049-3002-2-20
117. Kurihara T, Kubota Y, Ozawa Y, Takubo K, Noda K, Simon MC, et al. von Hippel-Lindau protein regulates transition from the fetal to the adult circulatory system in retina. Development. (2010) 137:1563–71. doi: 10.1242/dev.049015
118. Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, et al. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol. (2000) 2:371–5. doi: 10.1038/35014065
119. Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, et al. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest. (2008) 118:515–25. doi: 10.1172/JCI33304
120. Zhao F, Bosserhoff AK, Buettner R, Moser M. A heart-hand syndrome gene: Tfap2b plays a critical role in the development and remodeling of mouse ductus arteriosus and limb patterning. PLoS ONE. (2011) 6:e22908. doi: 10.1371/journal.pone.0022908
121. Ivey KN, Sutcliffe D, Richardson J, Clyman RI, Garcia JA, Srivastava D. Transcriptional regulation during development of the ductus arteriosus. Circ Res. (2008) 103:388–95. doi: 10.1161/CIRCRESAHA.108.180661
122. Baeten JT, Jackson AR, McHugh KM, Lilly B. Loss of Notch2 and Notch3 in vascular smooth muscle causes patent ductus arteriosus. Genesis. (2015) 53:738e48. doi: 10.1002/dvg.22904
123. Feng X, Krebs LT, Gridley T. Patent ductus arteriosus in mice with smooth muscle-specific Jag1 deletion. Development. (2010) 137:4191e9. doi: 10.1242/dev.052043
124. Zhang M, Chen M, Kim JR, Zhou J, Jones RE, Tune JD, et al. SWI/SNF complexes containing Brahma or Brahma-related gene 1 play distinct roles in smooth muscle development. Mol Cell Biol. (2011) 31:2618–31. doi: 10.1128/MCB.01338-10
125. Coceani F, Scebba F, Angeloni D. Gene profiling in ductus arteriosus and aorta: a question of consistency. J Physiol Sci. (2011) 61:443–4. doi: 10.1007/s12576-011-0161-z
126. Bokenkamp R, DeRuiter MC, van Munsteren C, Gittenfactor-de Groot AC. Insights into the pathogenesis and genetic background of patency of the ductus arteriosus. Neonatology. (2010) 98:6–17. doi: 10.1159/000262481
127. Tang B, Li Y, Nagaraj C, Morty RE, Gabor S, Stacher E, et al. Endothelin-1 inhibits background two-pore domain channel TASK-1 in primary human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. (2009) 41:476–83. doi: 10.1165/rcmb.2008-0412OC
128. Lewis TR, Shelton EL, Van Driest SL, Kannankeril PJ, Reese J. Genetics of the patent ductus arteriosus (PDA) and pharmacogenetics of PDA treatment. Semin Fetal Neonatal Med. (2018) 23:232–8. doi: 10.1016/j.siny.2018.02.006
129. Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. (2008) 122:479–85. doi: 10.1542/peds.2007-2313
130. Mani A, Meraji SM, Houshyar R, Radhakrishnan J, Mani A, Ahangar M, et al. Finding genetic contributions to sporadic disease: a recessive locus at 12q24 commonly contributes to patent ductus arteriosus. Proc Natl Acad Sci USA. (2002) 99:15054e9. doi: 10.1073/pnas.192582999
131. Derzbach L, Treszl A, Balogh A, Vasarhelyi B, Tulassay T, Rigó JJ. Gender dependent association between perinatal morbidity and estrogen receptor-alpha Pvull polymorphism. J Perinat Med. (2005) 33:461–2. doi: 10.1515/JPM.2005.082
132. Bokodi G, Derzbach L, Banyasz I, Tulassay T, Vasarhelyi B. Association of interferon gamma T+874A and interleukin 12 p40 promoter CTCTAA/GC polymorphism with the need for respiratory support and perinatal complications in low birthweight neonates. Arch Dis Child Fetal Neonatal Ed. (2007) 92:F25–9. doi: 10.1136/adc.2005.086421
133. Dagle JM, Lepp NT, Cooper ME, Schaa KL, Kelsey KJP, et al. Determination of genetic predisposition to patent ductus arteriosus in preterm infants. Pediatrics. (2009) 123:1116–23. doi: 10.1542/peds.2008-0313
134. Treszi A, Szabo M, Dunai G, Nobilis A, Kocsis I, Machay T, et al. Angiotensin II type 1 receptor A1166C polymorphism and prophylactic indomethacin treatment induced ductus arteriosus closure in very low birth weight neonates. Pediatr Res. (2003) 54:753–5. doi: 10.1203/01.PDR.0000088016.67117.39
135. Waleh N, Hodnick R, Jhaveri N, McConaghy S, Dagle J, Seidner S, et al. Patterns of gene expression in the ductus arteriosus are related to environmental and genetic risk factors for persistent ductus patency. Pediatr Res. (2010) 68:292–7. doi: 10.1203/PDR.0b013e3181ed8609
136. Smit-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. (1998) 16:387–98. doi: 10.1016/S0945-053X(98)90012-9
137. Neonatal Ed. (2014) 99:F408–12. Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half ou pregnancy: a randomized study. Circulation. (1998) 97:257–62. doi: 10.1161/01.CIR.97.3.257
138. van Vonderen JJ, te Pas AB, Kolster-Bijdevaate C, van Lith JM, Blom NA, Hooper SB, et al. Non-invasive measurements of ductus arteriosus flow directly after birth. Arch Dis Child Fetal Nenatal Ed. (2014) 99:F408–12. doi: 10.1136/archdischild-2014-306033
139. Hammerman C, Aramburo MJ. Effects of hyperventilation on prostacyclin formation and on pulmonary vasodilation after GBS induced pulmonary hypertension. Pediatr Res. (1991) 29:282–7. doi: 10.1203/00006450-199103000-00012
141. Sehgal A, Mak W, Dunn M, Kelly E, Whyte H, McCrindle B, et al. Haemodynamic changes after delivery room surfactant administration to verye low birth weight infants. Arch Dis Cild Fetal Neonatal Ed. (2010) 95:F345–51. doi: 10.1136/adc.2009.173724
142. Deshpande P, Baczynski M, McNamara P, Jain M. Patent ductus arteriosus: the physiology of transition. Semin Fetal Neonatal Med. (2018) 23:225–31. doi: 10.1016/j.siny.2018.05.001
143. Rychik J. Fetal cardiovascular physiology. Pediatr Cardiol. (2004) 25:201–9. doi: 10.1007/s00246-003-0586-0
144. Kresch MJ. Management of the third stage of labor: How delayed umbilical cord clamping can affect neonatal outcome. Am J Perinatol. (2017) 34:1375–81. doi: 10.1055/s-0037-1603733
145. Chung HR. Adrenal and thyroid function in the fetus and preterm infant. Korean J Pediatr. (2014) 57:425–33. doi: 10.3345/kjp.2014.57.10.425
146. Okahara K, Sun B, Kambayashi J. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. (1998) 18:1922–26. doi: 10.1161/01.ATV.18.12.1922
147. Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. (1995) 269(Pt. 1):C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371
148. Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. (1998) 82:532–9. doi: 10.1161/01.RES.82.5.532
149. Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. (2010) 298:H367–74. doi: 10.1152/ajpheart.00565.2009
150. Mantella LE, Quan A, Verma S. Variability in vascular smooth muscle cell stretch-induced responses in 2D culture. Vasc Cell. (2015) 7:7. doi: 10.1186/s13221-015-0032-0
151. Gerhardt T, Bancalari E. Lung compliance in newborns with patent ductus arteriosus before and after surgical ligation. Biol Neonate. (1980) 38:96–105. doi: 10.1159/000241348
152. Clyman RI. Commentary: recommendations for the postnatal use of indomethacin. An analysis of four separate treatment strategies. J Pediatr. (1996) 128:601–7. doi: 10.1016/S0022-3476(96)80123-5
153. Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate. (2006) 89:330–5. doi: 10.1159/000092870
154. De Buyst J, Rakza T, Pennaforte T, Johansson AB, Storme L. Hemodynamic effects of fluid irestriction in preterm infants with significant patent ductus arteriosus. J Pediatr. (2012) 161:404–8. doi: 10.1016/j.jpeds.2012.03.012
155. Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, McKinley L, et al. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. (2008) 28:123–8. doi: 10.1038/sj.jp.7211895
156. Clyman RI, Mauray F, Roman C, Heymann MA, Ballard PL, Rudolph AM, et al. Effects of antenatal glucocorticoid administration on ductus arteriosus of preter lambs. Am J Physiol. (1981) 100:H415–20. doi: 10.1152/ajpheart.1981.241.3.H415
157. Padbury JF, Ervin MG, Polk DH. Extrapulmonary effects of antenatally administered steroids. J Pediatr. (1996) 128:167–72. doi: 10.1016/S0022-3476(96)70384-0
158. Lundgren J, Hirata F, Marom Z, Logun C, Steel L, Kaliner, et al. Dexamethasone inhibits respirotary glyconjugate secretion from feline airways in vitro by the induction of lipocortin (lipomodilin) synthesis. Am Rev Respir Dis. (1988) 106:801–5.
159. Watterberg KL, Scott SM, Backstrom C, Gifford KL, Cook KL. Link between early adrenal function and respiratory outcome in preterm infants: Airway infllammation and patent ductus arteriosus. Pediatrics. (2000) 105:320–4. doi: 10.1542/peds.105.2.320
160. Gürsoy T, Hayran M, Derin H, Ovali FA. Clinical scoring system to predict the development of bronchopulmonary dysplasia. Am J Perinatol. (2015). 32:659–66. doi: 10.1055/s-0034-1393935
161. Waleh N, McCurnin DC, Yoder BA, Shaul PW, Clyman RI. Patent DA ligation alters pulmonary gene expression in preterm baboons. Pediatr Res. Pediatr Res. (2011) 69:212–216. doi: 10.1203/PDR.0b013e3182084f8d
162. Chang LY, McCurnin D, Yoder B, Shaul PW, Clyman RI. Ductus arteriosus ligation alveolar growth in preterm baboons with a patent DA. Pediatr Res. (2008) 63:299–302. doi: 10.1203/PDR.0b013e318163a8e4
163. Dawes GS, Mott JC, Widdicombe JG. The patency of the ductus arteriosus in newborn lambs and its physiological consequences. J Physiol. (1955) 128:361–83. doi: 10.1113/jphysiol.1955.sp005313
164. Noori S, Patel D, Friedlich P, Siassi B, Seri I, Ramanathan R. Effects of low oxygen saturation limits on the ductus arteriosus in extremely low birth weight infants. J Perinatol. (2009) 29:553–7. doi: 10.1038/jp.2009.60
165. SUPPORT study group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Target ranges of oxygen saturation in extremely preterm infants. N Eng J Med. (2010) 362:1959–69. doi: 10.1056/NEJMoa0911781
166. BOOSTII United Kingdom Collaborative Group BOOSTII Australia Collaborative Group. BOOSTII New Zealand Collaborative Group. Stenson BJ, Tarnow Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. (2013) 368:2094–104. doi: 10.1056/NEJMoa1302298
167. Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. (2013) 309:2111–20. doi: 10.1001/jama.2013.5555
168. Shelton EL, Waleh N, Plosa EJ, Benjamin JT, Milne GL, Hooper CW, et al. Effects of antenatal betamethasone on preterm human and mouse ductus arteriosus: comparison with baboon data. Pediatr Res. (2018) 84:458–65. doi: 10.1038/s41390-018-0006-z
169. Waleh N, Barrette AM, Dagle JM, Momany A, Jin C, Hills NK, et al. Effects of advancing gestation and non-Caucasian race on ductus arteriosus gene expression. J Pediatr. (2015) 167:1033–41. doi: 10.1016/j.jpeds.2015.07.011
170. Trivedi DB, Sugimoto Y, Loftin CD. Attenuated cyclooxygenase 2 expression contributes to patent ductus arteriosus in preterm mice. Pediatr Res. (2006) 60:669–74. doi: 10.1203/01.pdr.0000246480.13170.c0
171. Rheinlander C, Weber SC, Sarioglu N, Strauss E, Obladen M. Changing expression of cyclooxygenases and prostaglandin receptor EP4 during development of the human ductus arteriosus. Peditar Res. (2006) 60:270–5. doi: 10.1203/01.pdr.0000233066.28496.7c
172. Levin M, Goldberg S, Lindqvist A, Swärd A, Roman C, Liu BM, et al. ATP depletion and cell death in the neonatal lamb ductus arteriosus. Pediatr Res. (2005) 57:801–5. doi: 10.1203/01.PDR.0000157791.95954.56
173. van der Lugt NM, Lopriore E, Bokenkamp R, Smits-Wintjens VE, Steggerda SJ, Walther FJ. Repeated courses of ibuprofen are effective in closure of a patent DA. Eur J Pediatr (2012) 171:1673–7. doi: 10.1007/s00431-012-1805-6
174. Jensen EA, Dysart KC, Gantz MG, Carper B, Higgins RD, Keszler M, et al. Association between use of prophylactic indomethacin and the risk for bronchopulmonarydysplasia in extremely preterm infants. J Pediatr. (2017) 186:34–40. doi: 10.1016/j.jpeds.2017.02.003
175. Vermillon ST, Scardo JA, Lashus AG, Wiles HB. The effecs of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational ageb. Am J Obstet Gynecol. (1997) 177:256–9. doi: 10.1016/S0002-9378(97)70184-4
176. Pacifici GM. Clinical pharmacology of indomethacin in preterm infants: implications in patent ductus arteriosus closure. Paediatr Drugs. (2013) 15:363–76. doi: 10.1007/s40272-013-0031-7
177. Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. (1983) 102:895–906. doi: 10.1016/S0022-3476(83)80022-5
178. Clyman RI. The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatal. (2013) 37:102–7. doi: 10.1053/j.semperi.2013.01.006
179. Godambe S, Newby B, Shah V, Shah PS. Effect of indomethacin on closure of ductus arteriosus in very-low-birthweight neonates. Acta Paediatr. (2006) 95:1389–93. doi: 10.1080/08035250600615150
180. Sangem M, Asthana S, Amin S. Multiple courses of indomethacin and neonatal outcomes in premature infants. Pediatr Cardiol. (2008) 29:878–84. doi: 10.1007/s00246-007-9166-z
181. Mitra S, Florez D, Tamayo ME, Aune D, Mbuagbaw L, Veroniki A, et al. Effectiveness and safety of treatments used for the management of patent ductus arteriosus (PDA) in preterminfants: A protocol for a systematic review and network meta-analysis. BMJ Open. (2016) 6:e011271. doi: 10.1136/bmjopen-2016-011271
182. Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. (2015) 2:CD003481. doi: 10.1002/14651858.CD003481.pub6
183. Hirt D, Van OB, Treluyer JM, Langhendries JP, Marguglio A, Eisinger MJ, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. (2008) 65:629–36. doi: 10.1111/j.1365-2125.2008.03118.x
184. Gournay V, Roze JC, Kuster A, Daoud P, Cambonie G, Hascoet JM, et al. Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double-blind, placebo-controlled trial. Lancet. (2004) 364:1939–44. doi: 10.1016/S0140-6736(04)17476-X
185. Bardanzellu F, Neroni P, Dessi A, Fanos V. Patacatemol in patentductus arteriosus treatment: efficacious and safe? Biomed Res Intern. (2017) 2017:1438038. doi: 10.1155/2017/1438038
186. Jozwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Poloniae Pharma Drug Res. (2014) 71:11–23.
187. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular and molecular biology. Annu Rev Biochem. (2000) 69:145–82. doi: 10.1146/annurev.biochem.69.1.145
188. Allegaert K, Anderson B, Simons S, van Overmeire B. Paracetamol to induce ductus arteriosus closure: is it valid? Arch Dis Child. (2013) 98:462–6. doi: 10.1136/archdischild-2013-303688
189. Hostovsky LT, Pan J, McNamara PJ, Belik J. Acetaminophen increases pulmonary and systemic vasomotor tone in the newborn rat. Pediatr Res. (2019) 87:1171–6. doi: 10.1038/s41390-019-0725-9
190. Munsterhjelm E, Munsterhjelm NM, Niemi TT, Ylikorkala O, Neuvonen PJ, Rosenberg PH. Dose dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology. (2005) 103:712–17. doi: 10.1097/00000542-200510000-00009
191. Beringer RM, Thompson JP, Parry S, Stoddart PA. Intravenous paracetamol overdose: two case reports and a change to national treatment guidelines. Arch Dis Child. (2011) 96:307–8. doi: 10.1136/adc.2010.192005
192. Huang X, Wang F, Wang K. paracetamol versus ibuprofen for the treatment of patent ductus arteriosus in preterm neonates: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. (2018) 31:2216–22. doi: 10.1080/14767058.2017.1338263
193. Jasani B, Weisz DE, McNamara PJ. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Semin Perinatol. (2018) 42:243–52. doi: 10.1053/j.semperi.2018.05.007
194. Masarwa R, Levine H, Görelik E, Reif S, Perlman A, Matok I. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: a systematic review, meta-analysis and meta-regression analysis of cohort studies. Am J Epidemiol. (2018) 87:1817–27. doi: 10.1093/aje/kwy086
195. Chen MH, Pan TL, Wang PW, Hsu JW, Huang KL, Su TP, et al. Prenatal exposure to acetaminophen and the risk of attention deficit hyperactivity disorder: A nationwide study in Taiwan. J Clin Psychiatry. (2019) 80:18m12612. doi: 10.4088/JCP.18m12612
196. Bittker SS, Bell KR. Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops and autism: an epidemiological study. Neuropsychiatr Dis Treat. (2018) 14:1399–414. doi: 10.2147/NDT.S158811
197. Juujarvi S, Kallankari H, Patsi P, Leskinen M, Saarela T, Hallman M, et al. Follow-up study of the early, randomized paracetamol trial to preterm infant, found no adverse reactions at the two-years corrected age. Acta Paediatr. (2019) 108:452–8. doi: 10.1111/apa.14614
198. Kimani S, Surak A, Miller M, Bhattacharya S. Use of combination therapy with acetaminophen and ibuprofen for closure of patent ductus arteriosus in preterm neonates. Pediatr Child Health. (2020). doi: 10.1093/pch/pxaa057. [Epub ahead of print].
199. Yurttutan S, Bozkaya A, Hudayioglu F, Oncel MY. The effect of combined therapy for treatment of monotherapy-resistant PDA in preterm infants. J Matern Neonatal Med. (2018) 32:1–4. doi: 10.1080/14767058.2018.1481043
200. Paladini D, Marasini M, Volpe P. Severe ductal constriction in the third trimester fetus fllowing maternal self-medication with nimesulide. Ultrasound Obstet Gynecol. (2005) 25:357–61. doi: 10.1002/uog.1873
201. Fridman E, Mangel L, Mandel D, Beer G, Kapusta L, Marom R. Effects of maternal aspirin treatment on hemodynamically significant patent ductus arteriosus in preterm infants-pilot study. J Matern Fetal Neonatal Med. (2020). doi: 10.1080/14767058.2020.1736028. [Epub ahead of print].
202. Schiessl B, Schneider KT, Zimmermann A, Kainer F, Friese K, Oberhoffer R. Peranatal constriction of the fetal ductus arteriosus-related to maternal pain medication? Z Geburtshilfe Neonatol. (2005) 209:65–8. doi: 10.1055/s-2005-864116
203. Rein AJ, Nadjari M, Elchalal U, Nir A. Contraction of fetal ductus arteriosus induced by diclofenac. Case report. Fetal Diagn Ther. (1999) 14:24–5. doi: 10.1159/000020882
204. Levey R, Matitiau A, Ben Arie A, Milman D Or Y, Hagay Z. Indomethacin and corticosteroids: an additive constrictive effect on the fetal ductus arteriosus. Am J Perinatol. (1999) 16:379–83. doi: 10.1055/s-1999-6814
205. Momma K, Mishihara S, Ota Y. Constriction of the fetal DA by glucocorticoid hormones. Pediatr Res. (1981) 15:19–21. doi: 10.1203/00006450-198101000-00005
206. Vermont Oxford Network Steroid Study Group. Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics. (2001) 108:741–8. doi: 10.1542/peds.108.3.741
207. Pagni E, Baragatti B, Scebba F, Coceani F. Functional closure of the DA at birth: evidence against an intermediary role of angiotensin II. Pharmacology. (2014) 93:120–5. doi: 10.1159/000358013
208. Zielinsky P, Piccoli AL, Langer Manica JJ, Nicoloso LHS. New insights on fetal ductal constriction: role of maternal ingestion of polyhenol-rich foods. Expert Rev Cardiovas Ther. (2010) 8:291–8. doi: 10.1586/erc.09.174
209. Kharade SV, Nichols C, Denton JS. The shifting landscape of KATP channelopathies and the need for sharper therapeutics. Future Med Chem. (2016) 8:789–802. doi: 10.4155/fmc-2016-0005
210. Toyoshima K, Momma K, Ishii T, Nakanishi T. Dilatation of the ductus arteriosus by diazoxide in fetal and neonatal rats. Pediatr Int. (2017) 59:1246–51. doi: 10.1111/ped.13424
211. Dzialowski EM. Comparative physiology of the ductus arteriosus among vertebrates. Semin Perinatol. (2018) 42:203–11. doi: 10.1053/j.semperi.2018.05.002
212. Sakuma T, Akaike T, Minamisawa S. Prostaglandin E2 receptor EP4 inhibition contracts rat ductus arteriosus. Circ J. (2018) 83:209–16. doi: 10.1253/circj.CJ-18-0761
213. Takizawa T, Kihara T, Kamata A. Increased constriction of the ductus arteriosus with combined administration of indomethacin and L-NAME in fetal rats. Biol Neonate. (2001) 80:64–7. doi: 10.1159/000047122
214. Bevilacqua E, Brunelli R, Anceschi MM. Review and meta-analysis: benefits and risks of multiple courses of antenatal corticosteroids. J Matern Fetal Neonatal Med. (2010) 23:244–60. doi: 10.3109/14767050903165222
215. Travers CP, Carlo WA, McDonald S, Das A, Bell EF, Ambalavanan N, et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. (2018) 218:130e1–13. doi: 10.1016/j.ajog.2017.11.554
216. Tsai MY, Brown DM. Effect of dexamethasone on fetal lung 15-hydroxyprostaglandin dehydrogenase: possible mechanism for the prevention of patent ductus arteriosus by maternal dexamethasone therapy. Prostaglandins Leukot Med. (1987) 27:237–45. doi: 10.1016/0262-1746(87)90074-6
217. del Moral T, Gonzalez-Quintero VH, Claure N, Vanbuskirk S, Bancalari E. Antenatal exposure to magnesium sulfate and the incidence of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. (2007) 27:154–7. doi: 10.1038/sj.jp.7211663
218. Qasim A, Jain SK, Aly AM. Antenatal magnesium sulfate exposure and hemodynamically significant patent ductus arteriosus in premature infants. AJP Rep. (2019) 9:e353–6. doi: 10.1055/s-0039-3400316
Keywords: ductus arteriosus, oxygen, vasa vasorum, hemodynamics, indomethacin, ibuprofen, acetaminophen, prostaglandins
Citation: Ovalı F (2020) Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front. Pediatr. 8:516. doi: 10.3389/fped.2020.00516
Received: 28 June 2020; Accepted: 21 July 2020;
Published: 25 August 2020.
Edited by:
Ömer Erdeve, Ankara University, TurkeyReviewed by:
Mehmet Yekta Oncel, Izmir Kâtip Çelebi University, TurkeyChristoph Bührer, Charité – Universitätsmedizin Berlin, Germany
Copyright © 2020 Ovalı. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahri Ovalı, Zm92YWxpQHlhaG9vLmNvbQ==
 Fahri Ovalı
Fahri Ovalı