
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 02 September 2020
Sec. Neonatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00494
This article is part of the Research Topic Research Model Innovations in Advancing Neonatal Care View all 21 articles
 Marie-Pierre Cayer1
Marie-Pierre Cayer1 Nathalie Dussault1
Nathalie Dussault1 Marie Joëlle de Grandmont1
Marie Joëlle de Grandmont1 Marc Cloutier1,2
Marc Cloutier1,2 Antoine Lewin1,3
Antoine Lewin1,3 Danny Brouard1,4*
Danny Brouard1,4*Background: Bacteriological testing of donor human milk is mostly done both before and after pasteurization to control contamination in the end-product and meet the microbiological standards. Although the plate count method represents a reliable and sensitive technique and is considered the gold standard for bacteriological testing, it is recognized for being time-consuming and requiring qualified personnel. Recently, faster testing technologies, mostly geared toward the food industry, have been developed. Among these, the bioMérieux TEMPO® system uses the most probable number method to assess microbiological content in a semi-automated fashion.
Objective: The performances of the TEMPO® system in enumerating bacterial quality indicators in human milk were assessed and compared to the reference plate count method.
Methods: Naturally and artificially contaminated human milk samples were used to compare the analytical performances of the TEMPO® system to the plate count technique. More specifically, bacteria belonging to the genera Bacillus, Enterobacteriaceae, Staphylococcus aureus, and total aerobic flora were screened using both methods. Bacteria isolated on agar plates containing selective media were identified by supplemental testing. Bacterial testing results and method parameters were compared using linear regression analyses and Bland-Altman approaches.
Results: Naturally contaminated milk samples (n = 55) tested for total aerobic flora showed < 1 log (CFU/ml) discrepancy between the two methods in the output results for 98% of the samples. Comparative linear regression analyses demonstrate good correlations between the two methods (R2 > 0.9). At lower levels of bacterial contamination, the TEMPO® method precision (C.V. < 8%) and accuracy (> 83%) were comparable to plate counts.
Conclusions: The analytical performances of the TEMPO® system for human milk bacteriological testing are equivalent to the reference plate count method. Results from the TEMPO® system are available within a 24-h turnaround time from sample inoculation without the need for further supplemental testing, suggesting that this semi-automated method could be implemented within milk bank operations as an in-process monitoring technology to optimize end-product quality and safety.
A human milk diet has multiple, well-established benefits for all infants and reduces many risks associated with prematurity. Human milk banks provide an essential source to allow human milk diet for infants whose needs exceed what their own mothers can provide. These institutions need to follow standard safety guidelines regarding end-product bacteriological screening to ensure product safety (1–3). Since the inception of its Public Mother's Milk Bank in 2014, Héma-Québec has overseen the milk donation process and is responsible for the processing and distribution of pasteurized donor human milk (PDHM) to premature infants born at 32 weeks or earlier who require medical care and whose mother is unable to breastfeed. Recent studies have shown that breast tissue itself may contain several hundred bacterial species whose diversity may vary according to lactation stages (4–6). Although a normal milk microflora contributes to the establishment of a healthy intestinal flora in neonates, harmful pathogens in human milk can resist thermal processing and have been related to premature babies' infections (7–11).
Bacterial content analysis of pooled mother's milk prior to thermal processing helps identifying potential sources of contamination and allows educational feedback to donors when inadequate collection processes are suspected. The Human Milk Banking Association of North America (HMBANA) guidelines provide recommendations on bacteriological testing of heat-processed milk. Specifically, the guidelines states that any bacterial growth is deemed unacceptable for banked human milk (1). These recommendations on PDHM have been implemented by most milk banks. However, bacteriological screening of milk before pasteurization has not been systematically implemented in all milk banks and there is still no consensus regarding the microbiological criteria to be applied (12–14). Monitoring contaminants in “raw” milk remains relevant, since some pathogens produce toxins or spores that may resist thermal processing (15, 16). Spore-forming bacteria including Bacillus and Bacillus-derived bacterial genera species, represent the most common contaminants found in PDHM and are responsible for a significant fraction of rejected batches (14, 17). Most milk banks who apply pre-pasteurization milk screening use a total aerobic flora (TAF) threshold of 105-106 colony-forming units per milliliter (CFU/ml) (18, 19). Threshold values for maximal bacterial counts vary among milk banks and countries. For instance, the National Institute for Health and Care Excellence (NICE) in the UK recommends a maximal bacterial count of 104 CFU/ml for Staphylococcus aureus (S. aureus) and Enterobacteriaceae, and maximum of 105 for total colony count. In contrast to Australian guidelines, which do not accept any Enterobacteriaceae, Enterococci or potential pathogens capable of producing heat-stable enterotoxins (2, 20–22). Héma-Québec's Public Mother's Milk Bank adopted post-pasteurization microbiological criteria based on practices and guidelines; accordingly, unpasteurized milk containing a TAF > 105 CFU/ml or an Enterobacteriaceae content > 104 CFU/ml or any harmful pathogens including S. aureus, Bacillus, and Bacillus-like species, is discarded.
The plate count method (PCM) is currently the best practice recommended by the HMBANA for bacterial content testing of milk (1). This method can require up to 4 days for the results to be available and its application might lack standardization since procedure variations such as media composition, sample plating volume and raw milk preparations (i.e. dilutions) have been observed among milk banks (23–25). This methodology precludes a rapid intervention upon the human milk processing chain and can result in time, material and product wastage.
New bacteriological testing technologies with higher throughput have been recently introduced, among others, by the food and cosmetic industries as a means of intervening faster on their production chain (26, 27). The TEMPO® system (bioMérieux, Marcy-l'Étoile, France) is the first semi-automated bacteriological testing technology able to identify contaminants typically found in human milk. This system uses testing cards with specific culture media allowing rapid bacterial growth. After distribution of culture media-sample mixtures in the wells of the card, bacteria multiply during incubation and metabolize culture media containing a fluorescent indicator. The TEMPO® system relies upon the detection of the number and volume of positive wells (fluorescent or non-fluorescent) and statistical methods that are based on the most probable number (MPN) approach to perform bacterial enumeration (28, 29). The TEMPO® system is increasingly used in the food industry, which takes advantage of its semi-automated process, built-in sample scanner for traceability purposes and its speed in achieving bacteriological count and identification without additional identification tests (30, 31). Human milk and dairy products have similar matrices in terms of fat and inhibitory substances, which suggest that the TEMPO® system might be an asset for milk banks. This technology could potentially be used as a faster means to perform in-process bacteriological monitoring of raw mother's milk and PDHM (32, 33).
The objective of this study was to conduct a performance evaluation of the TEMPO® system for the enumeration of four typical bacterial quality indicators observed in mother's milk (TAF, Enterobacteriaceae, S. aureus, and Bacillus) and to compare the results with standard PCM. The impacts of the milk matrix on the reliability of the TEMPO® method were assessed along with the precision and accuracy. To the best of our knowledge, this study represents the first assessment of the TEMPO® system performances for human milk bacterial testing.
Before collection, donors were asked to fill a milk donation qualification form providing relevant personal and medical information and to sign an informed donation consent form, in accordance with Héma-Québec's (HQ) Research Ethics Committee guidelines. Qualified donors were subjected to the same serological screening performed for regular blood donations at HQ's blood bank (i.e., hepatitis B and C, syphilis, HIV, CMV, and HTLV-I/II). Expressed milk was self-collected and frozen in a household refrigerator by mothers at their home. Periodically, milk was sent to the Public Mothers' Milk Bank using validated HQ shipping containers. All milk donations were stored at −20°C until analysis. On the day of the analysis, milk samples were rapidly thawed in a water bath at 37°C. Naturally Contaminated Milk Samples (NCMS), and uncontaminated milk samples sterilized by pasteurization were spiked with known concentrations of specific bacterial strains and labeled as Artificially Contaminated Milk Samples (ACMS). Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 27217) and Bacillus cereus spores (isolated from a milk donation) were used to prepare ACMS. Mother's milk bacteriological contents were confirmed by PCM and diluted to final target concentrations in order to generate ACMS. For NCMS (n = 55) and ACMS (n = 3), 10-fold serial dilutions (10−1, 10−2, or 10−3) were prepared in Buffered Peptone Water (BPW; bioMérieux) for bacterial enumeration, as described in Table 1.
The PCM, performed by the spread plate technique, was used to enumerate all four bacterial quality indicators in milk samples. The method's performances had been previously validated following controlled quality assurance protocols. Briefly, 100 μl of milk sample were seeded on different media in duplicate. Plates were inverted and incubated at 35°C for 48 h. For the enumeration of TAF and Bacillus species, sheep blood agar medium was used (Oxoid; Thermo Fisher Scientific, Waltham, MA, USA). Colonies characterized by a clear zone halo, which can be attributed to hemolytic activity, were ascribed to Bacillus. After isolation on blood sheep agar, gram staining and inoculation of sporulation agar medium were performed to confirm the Bacillus identification. Enterobacteriaceae and S. aureus were enumerated on MacConkey and mannitol medium (Laboratoire de santé publique du Québec, Québec), respectively. For NCMS, characterized by a competitive flora, only presumptive pink to purple colonies fermenting lactose were counted as Enterobacteriaceae. Triple-Sugar-Iron agar (TSI) medium plates were seeded with bacteria to confirm the presence of Enterobacteriaceae. S. aureus in NCMS could not be quantified using the previous PCM because coagulase-negative Staphylococcus (CONS) and coagulase-positive Staphylococci (CPS; S. aureus) could both grow in mannitol medium. CPS identification was confirmed by the observation of presumptive bright yellow colonies on sheep blood agar after a 24-h incubation period at 35°C. CPS was differentiated from CONS using a coagulase test, following the manufacturer's instructions (BD BBL™ Rabbit Coagulase Plasma). For all samples, average counts from duplicates are reported as original concentrations, considering the applied dilution factor.
The bioMérieux TEMPO® system consists of two independent work stations, the semi-automated sample preparation module and the reading/recording module. In this study, the TEMPO® system was used according to the manufacturer's recommendations. Briefly, each individual bacterial analysis was performed using its own specific testing card, which contains a growth-triggered fluorescent transducer scattered in a selective culture medium and a predefined sample dilution pattern for determination of the MPN. Each card contains 48 analysis wells of three different volumes (225, 22.5, and 2.25 μl), from which a fluorescence signal can be recorded and interpreted to deduce the initial bacterial content reported in CFU/ml.
Before each bacterial analysis, a 1 mL milk sample was transferred into a previously rehydrated disposable vial containing 3 mL of sterile water. The seeded medium was subsequently transferred to its attached testing card and sealed using the TEMPO® Filler. Each testing card was incubated for a specific time and temperature, following the manufacturer's recommendations (see Table 1). In this study, the TEMPO® AC testing card was used to characterize TAF and was incubated for 24–28 h at 35°C before reading. Enterobacteriaceae were enumerated using the TEMPO® EB testing card and was incubated at 35°C for 22–27 h. The TEMPO® STA testing card was used to determine the S. aureus concentration at 35°C for 24–27 h. Finally, a 22–27 h incubation period at 30°C was used with the TEMPO® BC testing card to assay Bacillus species belonging to the Bacillus cereus group (i.e., B. cereus, B. anthracis, B. thuringiensis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, and B. cytotoxicus). After their respective incubation period, all cards were analyzed sequentially in an automated fashion using the TEMPO® reader station. Results are reported in CFU/ml for the original sample after manually entering the initial dilution factor (34).
A total of 55 NCMS were tested for TAF and Enterobacteriaceae by the TEMPO® MPN and PCM methods, and results were directly compared. Since the TEMPO® BC testing card is specific to the Bacillus cereus group, comparison of enumeration results with PCM, which uses blood sheep agar allowing growth of other Bacillus species, could not be done. Since CONS and CPS could both grow in mannitol medium, the measured concentration of S. aureus could also not be determined by PCM. Hence, the comparison of methods' performances for S. aureus and Bacillus detection was made from the number of positive samples (% of the population of n = 55) analyzed by both methods. Regarding the PCM method for TAF, Enterobacteriaceae and Bacillus, plate counts were obtained by seeding the undiluted sample along with 10−1, 10−2, and 10−3 dilutions. Detection of S. aureus was performed by spreading the original undiluted milk sample. Every plate was prepared in duplicate, and results are reported as the average count from both plates.
For TEMPO® measurements, two TEMPO® AC testing cards seeded, respectively, with 100 and 10−2 dilutions of the undiluted milk sample were used to cover the TAF enumeration range (1-490 000 CFU/ml, or 0–5.7 log CFU/ml). TEMPO® EB testing cards were seeded with 10−1 dilutions giving a detection range of 10–49 000 CFU/ml, or 0–4.7 log CFU/ml. Finally, the TEMPO® STA and BC testing cards were also seeded with the original milk sample (i.e., 100 dilution), and their enumeration range is 1–4,900 CFU/ml, or 0–3.7 log CFU/ml.
The accuracy of the TEMPO® method for each bacterial quality indicator and the milk matrix effect were assessed using the corresponding PCM results as reference. A high concentration of NCMS was used for the determination of the TEMPO® accuracy and precision for the enumeration of TAF (i.e. 5 log CFU/ml). ACMS were prepared by spiking Escherichia coli for Enterobacteriaceae enumeration, Bacillus cereus spores for Bacillus testing and S. aureus for the detection of CPS. PDHM or BPW was used as diluent to decrease the bacterial concentration. To cover an enumeration ranging from 1 to 5 log CFU/ml, 100-10−2 sample dilutions were seeded on plates or TEMPO® cards.
To assess the impacts of the milk matrix on the reliability of the TEMPO® method, milk samples were diluted in PDHM or BPW to obtain standard concentrations ranging between 1 and 5 log CFU/ml for TAF and Enterobacteriaceae, and between 1 and 4 log CFU/ml for S. aureus and Bacillus cereus. From each standard, four 10-fold serial dilutions were prepared for TAF, and five 10-fold serial dilutions were needed for Enterobacteriaceae, S. aureus and Bacillus cereus enumeration tests. Negative control samples previously determined to contain 0 CFU/ml were also prepared and analyzed. The TEMPO® and PCM enumeration results for each bacterial quality indicators diluted in either pasteurized milk or BPW were compared by regression analysis. All experiments were performed in triplicate.
The precision of each method was assessed from six individual measurements of six test samples prepared from the same mother's milk sample. The accuracy of the analytical method for each bacterial quality indicator was determined using three individual test samples prepared from mother's milk samples, and final target concentrations were 5 log CFU/ml for TAF, 4 log CFU/ml for Enterobacteriaceae, 3 log CFU/ml for S. aureus and 1 log CFU/ml for Bacillus cereus. All test samples were analyzed using both the PCM and TEMPO® method. Seeding on media or TEMPO® cards was performed using sample dilutions of 10−2 for TAF, 10−1 for Enterobactericeae and S. aureus, and no dilution (100) for Bacillus cereus. The results of the precision tests are expressed by the coefficient of variation (CV), which is the relative standard error compared to the mean. The method's accuracy (%) for each quality indicator was calculated by reporting the experimentally measured concentrations to the expected values.
Bacterial counts were converted to logarithmic values. The latter were analyzed with standard statistical methods for means and standard deviations (SD). The correlation between the PCM and TEMPO® results was assessed by linear regression analysis and the coefficient of determination (R2) was used to estimate the model accuracy. The agreement between methods was evaluated by performing a Bland-Altman analysis (OriginPro8.0, OriginLab Corporation, Northampton, MA, USA; SAS 9.4, SAS Institute, Cary, NC, USA).
Samples (n = 55) were tested for all four bacterial quality indicators by both methods. Comparing bacterial counts obtained for each sample tested by the PCM and TEMPO® methods, there was no more than a 1 log CFU/ml difference in the results, irrespective of the bacterial target.
When comparing enumeration results obtained from both methods for TAF using a linear regression analysis, the coefficient of determination obtained was 0.90 (Figure 1A). The concentration of one milk sample was out of the quantification range for both methods, and was therefore excluded from the analysis. According to the Bland-Altman analysis (Figure 1C), there was a good agreement between both methods, with a mean difference of < 0.5 log CFU/ml across the entire range of values. Only a few samples (n = 4) were found to be outside the agreement limits of the Bland-Altman analysis set at 2 × SD. The degree of correlation and agreement between measurements is highlighted by the narrow 95% CI (−0.8 to 0.7 log CFU/ml) and an analytical bias of only 0.1 log CFU/ml for the TEMPO® method over the PCM.
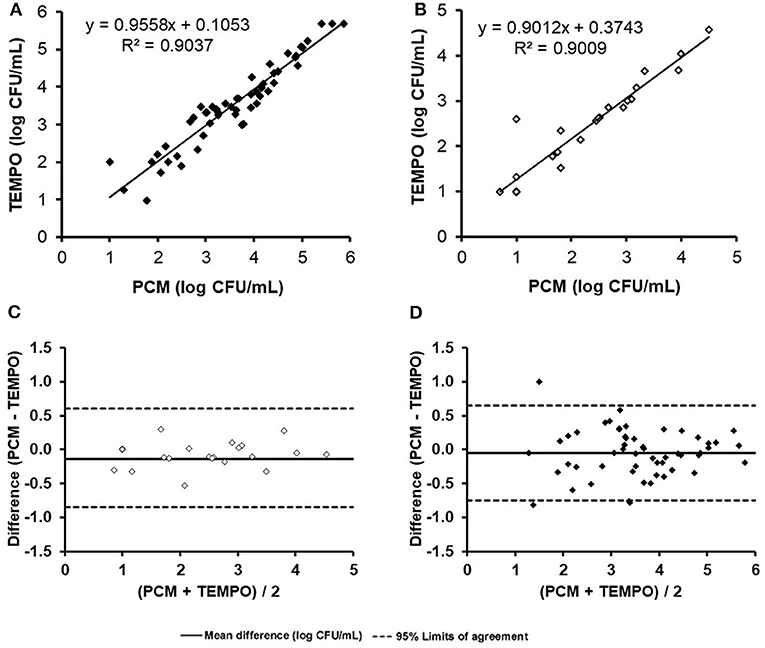
Figure 1. Comparison of total aerobic flora and Enterobacteriaceae enumerations (log count per mL) for the 55 naturally contaminated samples by the TEMPO® and plate count methods. Linear regression curve (A) and Bland-Altman analysis (C) for total aerobic flora. Linear regression curve (B) and Bland-Altman analysis (D).
Only 44% (24/55) of milk samples were found positive for Enterobacteriaceae after analysis by both methods. At low concentrations (< 1.3 log CFU/ml), two samples were positive according to the TEMPO® EB test, but only one showed growth by PCM using MacConkey agar medium (Table 2). A statistical analysis was performed on the 24 positive samples, and a linear regression coefficient of 0.90 was obtained (Figure 1B). The Bland-Altman plot (Figure 1D) shows a good agreement between the two methods, as all results are within the 95% C.I. limits (−0.9 to 0.6 log CFU/ml), with an analytical bias of only 0.1 log CFU/ml and a calculated ICC of 0.94, suggesting an excellent reliability for the TEMPO® method.
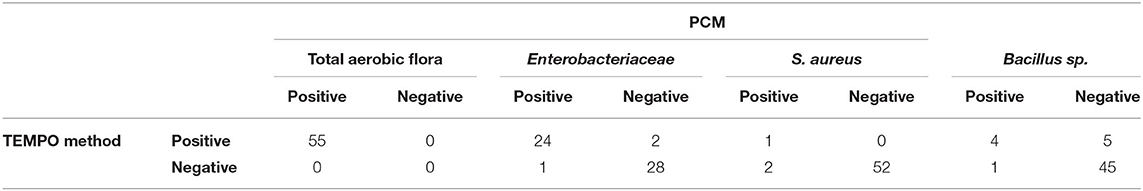
Table 2. Comparative detection of four bacterial quality indicators in 55 NCMS using TEMPO® and PCM methods.
S. aureus was detected by the PCM and the strain identity was confirmed by the coagulase test (Table 2) for three of the 55 milk samples (5.4%). The TEMPO® STA test gave a positive result for only one of them associated with an error (code 19). The code 19 is associated to an inconsistent result generated by the TEMPO® reader system. When an incoherent result is obtained, fluorescence is present in the upper wells of the card, confirming the presence of bacteria in the sample, but without a concentration estimate. Despite the fact that it was not possible to obtain a bacterial count for this sample due to a code 19, but considering that the error is associated with growth, they were identified as being contaminated. Consequently, there were two human milk samples unconfirmed positive for the presence of S. aureus by the TEMPO® system out of the 55 analyzed.
Bacillus was detected in nine of the 55 samples by TEMPO®, compared to only five by the reference PCM. Only one of the five positive samples identified by PCM was detected exclusively by the gold standard method. On the other hand, five of the nine positive samples by TEMPO were considered negative by PCM. In the end, four samples were tested positive for bacillus by both methods (Table 2). Interestingly, the single colony of a presumptive bacillus that grew on blood agar did not generate beta hemolysis, thereby questioning the identity as a cereus species. However, analysis by mass spectrometry (MALDI-TOF) confirmed that the bacterium belongs to the cereus group, and therefore should have been detected by the TEMPO® BC test; however the concentration was only 5 CFU/ml. Overall, both methods demonstrated their capacities to detect low levels of bacillus i.e., from 0 to 3.7 log CFU/ml (1–4,900 CFU/ml).
The TEMPO® system enables the quantification of bacterial quality indicators over a concentration range of four to five orders of magnitude. To assess the impacts of the complex milk matrix on the TEMPO® method's analytical performances, enumeration results for all bacterial target were compared in ACMS vs. BPW, with the exception of TAF, which was performed in NCMS only (Table 3). The statistical analysis shows a good overall agreement between the PCM and TEMPO® methods for all tested bacterial strains and sample matrices. More specifically, the linearity coefficient between the PCM and TEMPO® results for the enumeration of the TAF in milk is R2 = 0.976. Furthermore, bacterial counts obtained for Enterobacteriaceae by the PCM using MacConkey medium correlated just as well in a matrix composed of either milk (R2 = 0.995) or BPW (R2 = 0.996). The linear correlation coefficients observed when plotting S. aureus counting results by TEMPO® vs. PCM are R2 = 0.990 and R2 = 0.948 for BPW and milk matrices, respectively. Finally, there were no significant differences in the results obtained by the two methods for the enumeration of B. cereus using BPW (R2 = 0.994) or milk (R2 = 0.990) samples artificially contaminated with spores. Note that an R2 value of 1.0 indicates a perfect fit between variables and indicating that the model explains all the relationship, between the two factors.
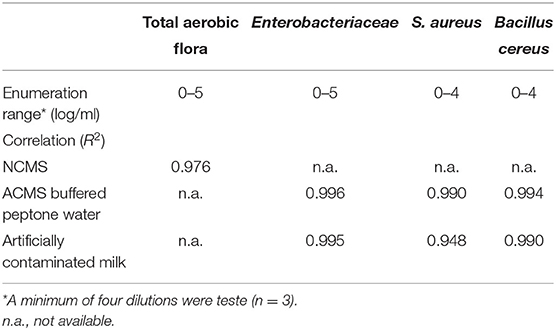
Table 3. Correlation coefficients between plate count and TEMPO® methods for the enumeration of bacterial quality indicators in ACMS and NCMS contaminated samples.
Table 4 presents the results obtained for the characterization of the precision and accuracy of both methods. For the precision expressed as the CV, a series of six measurements were taken from samples prepared so that their final concentration was strategically located within the quantification range of each bacterial target. For TAF, the expected sample concentration was 5 log CFU/ml. A CV of 3% was obtained for TEMPO, compared to 2% for PCM for paired samples. For Enterobacteriaceae, the enumeration target was set to 4 log CFU/ml and, similar to the TAF result, a slightly lower CV was obtained for PCM (1%) compared to TEMPO (3%), which was identical to the CV for TAF. A lower target concentration of 3 log CFU/ml was chosen for S. aureus, given the more stringent human milk release criteria for gram-positive bacteria. For this target species and concentration, CV of 5 and 2% were obtained for TEMPO® and PCM, respectively. A low 1 log CFU/ml concentration target was chosen for Bacillus; given this target, a CV of 9% was obtained with TEMPO®, and a much higher variability was observed in the results for PCM, with a CV of 82%.
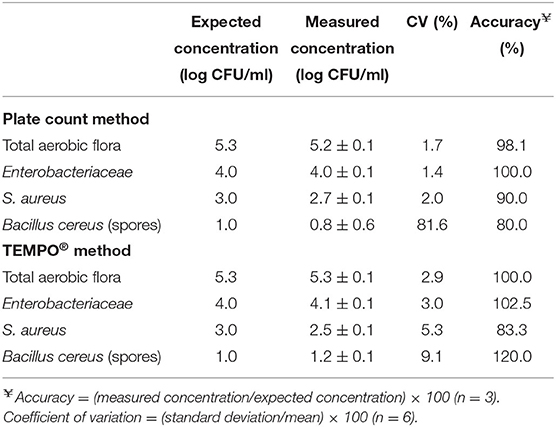
Table 4. Evaluation of the accuracy and precision of the plate count and TEMPO® methods at concentrations close to the acceptance criteria for human milk before pasteurization.
The accuracy of each method was calculated from the difference observed between the average experimental value, calculated from three separate measurements, and the expected value for each sample whose final concentration was adjusted so as to be in the quantification range. Table 4 presents the PCM and TEMPO® accuracy results observed for each target bacterium. In the case of TAF and Enterobacteriaceae, values approaching 100% were obtained with both enumeration methods at 4 and 5 log CFU/ml, respectively. For S. aureus, the target concentration to assess the accuracy of the PCM and TEMPO® methods was 3 log CFU/ml; at that concentration, the accuracy was 90 and 83% for PCM and TEMPO® method, respectively. Finally, a 1 log CFU/ml target concentration was used with Bacillus; at that target, the PCM method underestimated the milk sample contamination (80%), and the TEMPO® method overestimated it (120%).
The TEMPO® system is currently used in several countries, mainly as a quality control tool in the food industry. Indeed, all TEMPO® bacterial tests have been approved by international quality and standards organizations, such as AOAC International, AFNOR and ISO for food and environmental samples (35–38). Evaluations of the performances of the TEMPO® MPN method for the characterization of bacterial content in food products, including dairy milk, have been reported (39–45). Most of them have demonstrated a good agreement in the results between the plate count method and TEMPO® for all types of targeted bacteria. The main objective of this performance evaluation was to compare the enumeration capability of the TEMPO® system aimed at the characterization of human milk microflora and the detection of specific bacterial contaminants of interest at concentration levels as low as 1 log CFU/ml in < 24 h. The enumeration results for TAF and Enterobacteriaceae obtained from 55 NCMS demonstrated a good agreement between the PCM and TEMPO® methods and, more importantly, no significant analytical bias was observed for the TEMPO® system using plate counts as reference. Differences in the output values were not > 1 log CFU/ml for all samples, which testify to the reliability of the TEMPO® system. Interestingly, occasional discrepancies were observed between enumeration counts obtained from TEMPO® AC cards inoculated with 100 and 10−2 dilutions of the same mother's milk sample. The upper detection limit threshold of 490 000 CFU/ml was exceeded with the undiluted sample, while the 10−2 dilution generated bacterial counts within the 1–4,900 CFU/ml enumeration range. This result could be attributed to an apparent fluorescence quenching caused by diffusion or absorption of the output signal by the complex milk matrix, interpreted by the system as a sample of high bacterial content. A dilution of the sample seems to attenuate the matrix effect on the fluorescence output signal and allows obtaining results within the quantification range of the TEMPO® AC card. This in turn enables to identify milk samples with an aerobic flora content that exceeds the quality criterion of > 105 CFU/ml with a good level of confidence.
Testing a 10−1 sample dilution would be recommended for the enumeration of S. aureus; this dilution, corresponding to a limit of detection of < 10 CFU/ml, conforms to the quality criterion threshold for this bacterial species and corresponds to the current plate count method performed on mannitol agar in terms of limit of detection. Finally, 100 and 10−1 dilutions of the mother's milk sample would be recommended to span appropriate concentration ranges for the enumeration of Bacillus and Enterobacteriaceae with TEMPO® BC and EB cards, according to the quality criteria applicable to these bacterial species. During the evaluation of the TEMPO® analytical performances, a few samples, independently of the targeted bacteria, generated an error code (code 19), which could be attributed to incoherent results according to the TEMPO® user's manual. The error code is believed to be associated to the opacity of the human milk matrix since all events were observed with undiluted samples. Light absorption by proteins or scattering by the milk fat globules, among others, could interfere with the bacterial growth transducer excitation of its fluorescence emission resulting in a noisy optical signal. According to bioMérieux's technical staff, adherence to the incubation period recommended for each testing card should allow sufficient growth from the lower wells and solve the issue. Repeating the same sample analyses with new testing cards and using the upper recommended limit as the incubation period, the same error code was obtained. One must conclude on the possibility that some human milk constituents may interfere with the TEMPO® detection process. Proper identification of the principal interfering constituents and, extensively, of maternal factors impacting detection performances of the TEMPO® system could be investigated. Nevertheless, to maintain a detection limit of 1 CFU/ml for S. aureus and Bacillus sp. it requires performing TEMPO® analyses on undiluted samples. Consequently, it would be well-advised to conduct secondary testing in the case of positive samples for S. aureus or Bacillus sp. in order to determine the bacterial concentration. It is worth noting that some Bacillus species outside the cereus group would not be detected by the TEMPO® BC test. However, this test remains efficient at quantifying some of the most harmful contaminants with pathogenic potential related to this genus (46). Finally, TEMPO®'s precision and accuracy were comparable to the PCM and, interestingly, the semi-automated detection method performed better than the PCM at low concentration levels, which might prevent the distribution use of human milk contaminated with small numbers of bacteria belonging to the Bacillus cereus group.
Within a 24-h period, characterization of the bacterial content in mother's milk donations and pasteurized human milk bottles were performed using the TEMPO® technology from bioMérieux. More specifically, TEMPO® was used for the enumeration of the total aerobic bacterial flora, Enterobacteriaceae, B. cereus group and S. aureus from raw human milk with sample dilutions as the only sample preparation steps prior to the incubation and detection. Rapid and reliable detection of bacterial content would help screening for contaminated milk donations with heat-resistant bacteria prior to pasteurization, thereby reducing rejection rates, and improving overall end-product safety and quality. The bioMérieux TEMPO® system includes a built-in scanning device ensuring sample traceability. It is designed to be integrated within a human milk production line for in-process testing and determining pooling strategies before thermal processing. This study underlines the importance for human milk banks to keep an eye on innovative technologies, especially those dealing with the characterization of bacterial content, as these could lead to enhancements in productivity but most of all, in the safety of a precious product intended for preterm infants.
All data sets are stored on a dedicated external hard drive. They can be made available upon request to the corresponding author.
The studies involving human participants were reviewed and approved by the legal affairs department at Héma-Québec. The patients/participants provided written informed consent to participate in this study.
M-PC, MG, and DB conceived and designed the study and wrote the article. ND collected the data and contributed to the discussion of the results. AL performed the statistical analysis. MC provided suggestions concerning the content of the article and were responsible for critically revising the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank, Manon Landry for her role in supervising the activities of the human milk bank at Héma-Québec (Montreal), Dr. Gilles Delage, France Bernier, and Jean-François Leblanc for critically reading the manuscript, and bioMérieux Canada for their technical support all along the study.
1. Human Milk Banking of North America (HMBANA). Guidelines for the Establishment and Operation of a Donor Human Milk Bank. 10th ed. Raleigh, NC: Human Milk Bank Association of North America (2018).
2. National Institute for Health and Care Excellence (NICE). Donor Breast Milk Banks: The Operation of Donor Milk Bank Services. service operation. NICE Clinical Guidelines, No. 93 (2010).
3. Moro GE, Billeaud C, Rachel B, Calvo J, Cavallarin L, Christen L, et al. Processing of donor human milk: update and recommendations from the European milk bank association (EMBA). Front Pediatr. (2019) 7:49. doi: 10.3389/fped.2019.00049
4. Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. (2016) 82:5039–48. doi: 10.1128/AEM.01235-16
5. Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother's milk: a purposeful contribution to the development of the infant microbiota and immunity. Front Immunol. (2018) 9:361. doi: 10.3389/fimmu.2018.00361
6. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. (2012) 96:544–51. doi: 10.3945/ajcn.112.037382
7. Lombard F, Marchandin H, Jacquot A, Cambonie G, Rodiere M, Filleron A. Streptococcus agalactiae late-onset neonatal infections: should breast milk be more systematically tested for bacterial contamination? Acta Paediatr. (2012) 101:e529–30. doi: 10.1111/apa.12006
8. Hilliard NJ, Schelonka RL, Waites KB. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol. (2003) 41:3441–4. doi: 10.1128/JCM.41.7.3441-3444.2003
9. Decousser JW, Ramarao N, Duport C, Dorval M, Bourgeois-Nicolaos N, Guinebretiere MH, et al. Bacillus cereus and severe intestinal infections in preterm neonates: putative role of pooled breast milk. Am J Infect Control. (2013) 41:918–21. doi: 10.1016/j.ajic.2013.01.043
10. Nakamura K, Kaneko M, Abe Y, Yamamoto N, Mori H, Yoshida A, et al. Outbreak of extended-spectrum beta-lactamase-producing Escherichia coli transmitted through breast milk sharing in a neonatal intensive care unit. J Hosp Infect. (2016) 92:42–6. doi: 10.1016/j.jhin.2015.05.002
11. Byrne PA, Miller C, Justus K. Neonatal group B streptococcal infection related to breast milk. Breastfeed Med. (2006) 1:263–70. doi: 10.1089/bfm.2006.1.263
12. Picaud JC. VIII. Human milk banks: How to organize the collection of human milk to feed preterm infants. J Pediatr Gastroenterol Nutr. (2015) 61(Suppl. 1):S10–2. doi: 10.1097/01.mpg.0000471456.78296.a6
13. Weaver G, Bertino E, Gebauer C, Grovslien A, Mileusnic-Milenovic R, Arslanoglu S, et al. Recommendations for the establishment and operation of human milk banks in Europe: a consensus statement from the European milk bank association (EMBA). Front Pediatr. (2019) 7:53. doi: 10.3389/fped.2019.00053
14. Froh EB, Vanderpool J, Spatz DL. Best practices to limit contamination of donor milk in a milk bank. J Obstet Gynecol Neonatal Nurs. (2018) 47:547–55. doi: 10.1016/j.jogn.2017.12.002
15. Necidova L, Bogdanovicova K, Harustiakova D, Bartova K. Short communication: pasteurization as a means of inactivating staphylococcal enterotoxins A, B, and C in milk. J Dairy Sci. (2016) 99:8638–43. doi: 10.3168/jds.2016-11252
16. Alles AA, Wiedmann M, Martin NH. Rapid detection and characterization of postpasteurization contaminants in pasteurized fluid milk. J Dairy Sci. (2018) 101:7746–56. doi: 10.3168/jds.2017-14216
17. Jang HL, Cho JY, Kim MJ, Kim EJ, Park EY, Park SA, et al. The experience of human milk banking for 8 years: Korean perspective. J Korean Med Sci. (2016) 31:1775–83. doi: 10.3346/jkms.2016.31.11.1775
18. Arslanoglu S, Bertino E, Tonetto P, De Nisi G, Ambruzzi AM, Biasini A, et al. Guidelines for the establishment and operation of a donor human milk bank. J Matern Fetal Neonatal Med. (2010) 23:1–20. doi: 10.3109/14767058.2010.512414
19. Ministère de la Santé de la Jeunesse et des Sports. Décrets, Arrêtés, Circulaires. Textes Généraux. Décision du 3 Décembre 2007 Définissant les Règles de Bonnes Pratiques Prévues à L'alinéa 3 L'article L. 2323-1 du Code de la Santé Publique. JORF; texte 22 sur 165 (2008).
20. Hartmann BT, Pang WW, Keil AD, Hartmann PE, Simmer K. Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Hum Dev. (2007) 83:667–73. doi: 10.1016/j.earlhumdev.2007.07.012
21. Omarsdottir S, Casper C, Akerman A, Polberger S, Vanpee M. Breastmilk handling routines for preterm infants in Sweden: a national cross-sectional study. Breastfeed Med. (2008) 3:165–70. doi: 10.1089/bfm.2007.0033
22. Chang FY, Cheng SW, Wu TZ, Fang LJ. Characteristics of the first human milk bank in Taiwan. Pediatr Neonatol. (2013) 54:28–33. doi: 10.1016/j.pedneo.2012.11.004
23. Mullie C, Obin O, Outurquin G, Grognet S, Leke A, Adjide C. Breastmilk donations: bacteriological assessment, analysis of causes of non-compliance and suggestions for improvement. Arch Pediatr. (2018) 25:263–8. doi: 10.1016/j.arcped.2018.02.006
24. Owen M, Willis C, Lamph D. Evaluation of the TEMPO((R)) most probable number technique for the enumeration of enterobacteriaceae in food and dairy products. J Appl Microbiol. (2010) 109:1810–6. doi: 10.1111/j.1365-2672.2010.04810.x
25. Lee DY, Kim HY, Cho YS. Comparison of TEMPO BC and MYP plate methods for the enumeration of bacillus cereus in various foods. J Food Hyg Saf. (2017) 32:249–53. doi: 10.13103/JFHS.2017.32.4.249
26. Yossa N, Smiley J, Huang MJ, Yin L, Bell R, Tallent S, et al. Comparison of TEMPO((R)) BC with spiral plating methods for the enumeration of bacillus cereus in cosmetic products either naturally preserved or preserved with phenoxyethanol. J AOAC Int. (2019) 102:1080–90. doi: 10.5740/jaoacint.18-0375
27. Yörük NG. Most probable number technique in Escherichia coli count using ISO 16649-3, ISO 7251, and rapid test enumeration device (TEMPO EC) methods in milk and dairy products. J Food Saf. (2018) 38:e12502. doi: 10.1111/jfs.12502
28. Cochran WG. Estimation of bacterial densities by means of the “most probable number”. Biometrics. (1950) 6:105–16. doi: 10.2307/3001491
29. Woodward RL. How probable is the most probable number? J Am Water Works Assoc. (1957) 49:1060–8. doi: 10.1002/j.1551-8833.1957.tb16906.x
30. Tallent SM, Hait JM, Ferguson M. Comparative study of tempo BC automated MPN for the enumeration of bacillus cereus group in food. J Food Saf. (2018) 38:e12472. doi: 10.1111/jfs.12472
31. Lobacz A, Kowalik J, Zulewska J. Determination of the survival kinetics of Salmonella spp. on the surface of ripened raw milk cheese during storage at different temperatures. Int J Food Sci Technol. (2020) 55:610–8. doi: 10.1111/ijfs.14315
32. Crowley ES, Bird PM, Torontali MK, Agin JR, Goins DG, Johnson R. TEMPO TVC for the enumeration of aerobic mesophilic flora in foods: collaborative study. J AOAC Int. (2009) 92:165–74. doi: 10.1093/jaoac/92.1.165
33. Lindemann S, Kmet M, Reddy R, Uhlig S. Matrix-specific method validation of an automated most-probable-number system for use in measuring bacteriological quality of grade “A” milk products. J Food Prot. (2016) 79:1911–8. doi: 10.4315/0362-028X.JFP-16-141
34. U. S. Food and Drug Administration. Bacteriological Analytical Manual. Appendix 2. (2010). Available online at: https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions (accessed February 18, 2020).
35. Association française de normalisation (AFNOR). Validation Certificate for Alternative Analytical Method According to Standard EN ISO 16140: 2003, Certificate no. BIO 12/13-02/05: TEMPO EC- Method Validated for the Enumeration of Escherichia coli in Food Products. (2005). Available online at: http://www.afnor-validation.com/afnor-validation-validated-methods/e-coli.html (accessed February 18, 2020).
36. Association française de normalisation (AFNOR). Validation Certificate for Alternative Analytical Method According to Standard EN ISO 16140: 2003, Certificate no. BIO 12/17-12/05: TEMPO TC- Method Validated for the Enumeration of Total Coliforms in Food Products. (2005). Available online at: http://www.afnor-validation.com/afnor-validation-validated-methods/coliforms.html (accessed February 18, 2020).
37. Association française de normalisation (AFNOR). Validation Certificate for Alternative Analytical Method According to Standard NF EN ISO 16140-2: 2016, Certificate no. BIO 12/35-05/13: TEMPO AC- Method Validated for the Enumeration of Aerobic Mesophilic Flora in Human Food, Pet Food and Environmental Samples. (2016). Available online at: https://nf-validation.afnor.org/wp-content/uploads/2014/03/Synt-BIO-12-35-05-13 (accessed February 18, 2020).
38. Association française de normalisation (AFNOR). ISO 16140 – Part 2: Validation Study of a TEMPO Method for Bacillus Cereus Enumeration in Food Samples and Environmental Samples. (2016). Available online at: https://assets.bettyblocks.com/2e826c3765f4401c84671a06de961fc7_e1e4b8ec2b22479080d36038777c5403/17066/2014LR47_-_TEMPO_BC_-_SUMMARY_REPORT___MicroVal_-_Version_1_.pdf (accessed February 18, 2020).
39. Katase M, Tsumura K. Enumeration of micro-organisms in processed soy products with an automated most probable number method compared with standard plate method. Lett Appl Microbiol. (2011) 53:539–45. doi: 10.1111/j.1472-765X.2011.03143.x
40. Torlak E, Akan IM. Evaluation of TEMPO STA for the enumeration of coagulase-positive staphylococci in cheese. Food Sci Technol. (2012) 18:645–50. doi: 10.3136/fstr.18.645
41. Paulsen P, Schopf E, Smulders FJ. Enumeration of total aerobic bacteria and Escherichia coli in minced meat and on carcass surface samples with an automated most-probable-number method compared with colony count protocols. J Food Prot. (2006) 69:2500–3. doi: 10.4315/0362-028X-69.10.2500
42. Paulsen P, Borgetti C, Schopf E, Smulders FJ. Enumeration of Enterobacteriaceae in various foods with a new automated most-probable-number method compared with petrifilm and international organization for standardization procedures. J Food Prot. (2008) 71:376–9. doi: 10.4315/0362-028X-71.2.376
43. Line JE, Stern NJ, Oakley BB, Seal BS. Comparison of an automated most-probable-number technique with traditional plating methods for estimating populations of total aerobes, coliforms, and Escherichia coli associated with freshly processed broiler chickens. J Food Prot. (2011) 74:1558–63. doi: 10.4315/0362-028X.JFP-11-024
44. Kunicka A. Evaluation of the TEMPO system: an automated method for food microbiological quality control. J Biotechnol. (2007) 131:69–72. doi: 10.1016/j.jbiotec.2007.07.119
45. Zitz U, Domig KJ, Hoehl A, Weiss H, Wilrich PT, Kneifel W. Evaluation of three applications of a semi-automated most-probable-number for the assessment of microbiological parameters in dairy products. Accred Qual Assur. (2011) 16:299–309. doi: 10.1007/s00769-011-0772-3
Keywords: donor human milk, human milk bank, bacteriology testing, donor screening, Bacillus, quality control, in-process monitoring
Citation: Cayer M-P, Dussault N, de Grandmont MJ, Cloutier M, Lewin A and Brouard D (2020) Evaluation of the Tempo® System: Improving the Microbiological Quality Monitoring of Human Milk. Front. Pediatr. 8:494. doi: 10.3389/fped.2020.00494
Received: 25 March 2020; Accepted: 14 July 2020;
Published: 02 September 2020.
Edited by:
Yuan Shi, Children's Hospital of Chongqing Medical University, ChinaReviewed by:
Sanjeet K. Panda, Texas Tech University Health Sciences Center El Paso, United StatesCopyright © 2020 Cayer, Dussault, de Grandmont, Cloutier, Lewin and Brouard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danny Brouard, ZGFubnkuYnJvdWFyZEBoZW1hLXF1ZWJlYy5xYy5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.