
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr., 18 August 2020
Sec. Pediatric Immunology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00480
This article is part of the Research TopicDietary Interventions and Nutritional Factors in the Prevention of Allergic Diseases in InfantsView all 16 articles
 Irene Trambusti1
Irene Trambusti1 Giulia Nuzzi1
Giulia Nuzzi1 Giorgio Costagliola1
Giorgio Costagliola1 Elvira Verduci2,3
Elvira Verduci2,3 Enza D'Auria3
Enza D'Auria3 Diego G. Peroni1*
Diego G. Peroni1* Pasquale Comberiati1
Pasquale Comberiati1Asthma is the most frequent chronic disease in children, and its pathogenesis involves genetic, epigenetic, and environmental factors. The rapid rise in the prevalence of asthma registered over the last few decades has stressed the need to identify the environmental and modifiable factors associated with the development of the disease. In particular, there is increasing interest in the role of modifiable nutritional factors specific to both the prenatal and post-natal early life as, during this time, the immune system is particularly vulnerable to exogenous interferences. Several dietary factors, including maternal diet during pregnancy, the duration of breastfeeding, the use of special milk formulas, the timing of the introduction of complementary foods, and prenatal and early life supplementation with vitamins and probiotics/prebiotics, have been addressed as potential targets for the prevention of asthma. In this review, we outline recent findings on the potential role of prenatal and perinatal dietary and nutritional interventions for the primary prevention of pediatric asthma. Moreover, we addressed unmet needs and areas for future research in the prevention of childhood-onset asthma.
Asthma is the most frequent chronic disease in children and is associated with significant morbidity and potential impairment of lung function in adulthood (1). Both genetic, epigenetic, and environmental determinants are involved in the pathogenesis of asthma. Childhood-onset asthma, as opposed to adult-onset asthma, is typically featured by a history of atopy and related markers of type-2 allergic inflammation (2). From 1960, the prevalence of allergy and asthma has progressively increased worldwide, reaching a plateau in some developed countries (3), while continuing to rise in low-income and mid-income countries (4). As a result, over the last few decades, there has been increasing interest in the identification of risk factors for allergy and asthma (5, 6). Recent research has focused on tackling both prenatal and postnatal modifiable factors for the prevention of allergic diseases, as these factors can influence the immune system in a crucial phase of its development (7). A relationship between environmental tobacco smoke, air pollution, respiratory infections, and the development of asthma has been demonstrated and extensively reviewed elsewhere (8, 9). On the contrary, the influence of in utero and early-life dietary and nutritional drivers needs to be more fully elucidated. The role of several dietary factors, including maternal diet and vitamin status, composition of the microbiome, duration of breastfeeding, the use of hydrolyzed formulas and the introduction of complementary foods, has been investigated in recent clinical trials, to identify potential targets for the prevention of childhood-onset asthma (10).
This review focuses on the latest findings on the role of prenatal and perinatal dietary factors in the development of asthma, and whether the modulation of such factors could contribute to the primary prevention of childhood-onset asthma.
Given the central role of the first 1,000 days of life for the development of the immune system, numerous studies have investigated the role of intrauterine exposures in the pathogenesis of allergic diseases (11). Recent evidence supports the hypothesis that colonization by a healthy gut microbiome during early infancy can affect the immune system development and the predisposition to immune- mediated diseases later in life, including asthma (12, 13). It has been shown that the maternal diet during pregnancy could influence the composition of the gut microbiome and the immune system development of the neonate, and therefore potentially affect the predisposition to asthma and allergies in childhood (14, 15). There is conflicting evidence on the role of prenatal maternal intake of certain foods and nutrients and the development of asthma and allergies during childhood. In 2015 Beckhaus et al. (16) reported that maternal in-utero intake of vitamin D (VD), vitamin E, and zinc had a protective effect against early life wheezing in offspring, but not on childhood-onset asthma or other atopic conditions. One recently published study, assessing the impact of pre-pregnancy diet on the risk of allergic outcomes in children (17), highlighted that the consumption of specific foods during pregnancy, such as cooked green vegetables and eggs, may protect against pediatric wheezing and asthma, while higher maternal intake of meat may increase the risk of wheezing, allergic rhinitis, and atopic dermatitis in children. Further studies are needed to confirm these results and understand the mechanisms related to maternal nutrition and the development of asthma and allergic disorders. Understanding the connection between maternal diet during pregnancy and neonatal microbiome composition may help identify effective prevention strategies, such as providing pregnant women and women desiring pregnancy with nutrition recommendations, particularly regarding products that may influence the development of allergic diseases.
The recent increase in the prevalence of asthma and allergic disorders in Westernized countries has closely paralleled a VD deficiency epidemic (18). Experimental evidence shows that VD contributes to the fetal-neonatal lung growth and modulates both innate and adaptive immune responses, inhibiting some pro-inflammatory responses associated with allergy and asthma (19). These findings have supported the hypothesis that maternal VD status may play a role in the development of pediatric asthma. Three observational studies found that the risk of asthma in school-age decreases as maternal VD levels increase (20–22), although a recent meta-analysis of observational studies showed that lower prenatal exposure to VD was associated with an increased risk of respiratory infections, but not with asthma and allergic rhinitis development (23). In an early randomized controlled trial (RCT), Goldring et al. (24) randomized 180 women at 27 weeks of gestation to receive either no VD, 800 IU/VD2 daily until delivery or single oral bolus of 200,000 IU/VD3, reporting no reduction in wheezing in offspring at 3 years of age. In the more recent Vitamin D Antenatal Asthma Reduction Trial (VDAART), 881 pregnant women with high atopy risk were randomized to receive either 4,400 IU VD3/daily (active group) or 400 IU VD3/daily (control group), during the 2nd and 3rd semesters of gestation (25). The offspring of the active group showed a clinically relevant, although not statistically significant, reduction (20% or greater) in the risk of asthma/recurrent wheezing by age 3 years, but not by age 6 years (25, 26). These results are in line with those reported by a similar 6-year follow-up trial, the COPSAC study (27, 28), in which pregnant women from an unselected cohort were randomized to receive either 2,400 IU or 400 IU VD3/daily during the 3rd trimester of gestation. These findings show that increasing VD supplementation during pregnancy may reduce the incidence of transient pre-school wheezing, but is not sufficient to prevent school-age asthma, which is usually an allergic-type of asthma (29) (Table 1). Further studies are needed to address the influence of VD maternal status on the development of asthma in children before high dose VD supplementation could be recommended during pregnancy.
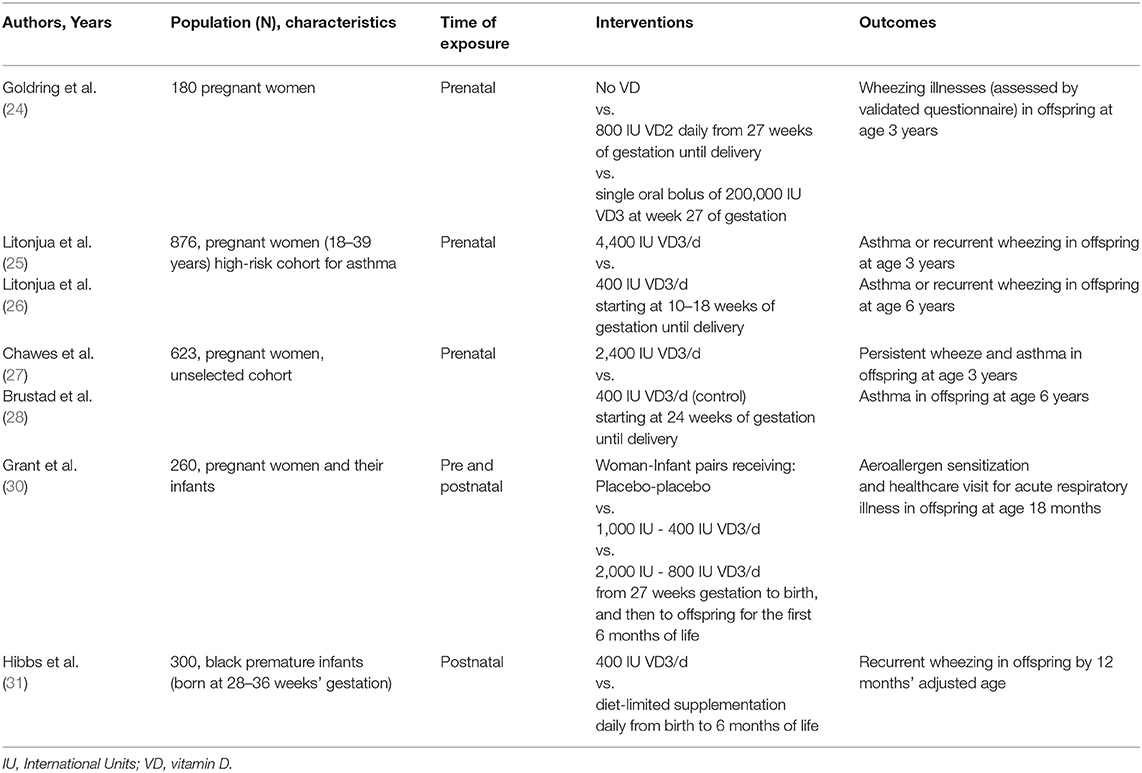
Table 1. Randomized controlled trials on vitamin D supplementation for the prevention of childhood wheezing/asthma.
Regarding other vitamins, Stone et al. (32) showed that higher levels of vitamin E, particularly in its alpha-tocopherol isoform, in postpartum maternal plasma concentration were associated with a decreased likelihood of asthma and wheezing over 2 years.
Breastfeeding is the most relevant postnatal factor that supports microbial colonization and drives the immune system development of the newborn (33, 34). Compared with formula feeding, breastfeeding has been related to lower morbidity and mortality in infants and decreased incidence of allergic diseases, including asthma (35). Increasing evidence shows that breast milk plays a central role in the development of tolerogenic immune responses during the first years of life, due to its content in immunoglobulins, vitamin A and transforming growth-factors, which promote the gut mucosal barrier integrity and homeostasis (35, 36). Significantly, it has been observed that certain aeroallergens such as dust mite allergens, when found in breast milk, can increase the risk for developing food allergy, as they could disrupt intestinal immune homeostasis through their protease activity and prevent the induction of oral tolerance to food allergens (37). Regarding asthma, breast milk appears to have a protective, dose-dependent impact on respiratory health, especially for preschool wheezing, although the mechanisms of how breastfeeding reduces the risk of childhood wheezing/asthma is not fully elucidated (38). A systematic review by Dogaru et al. (39) showed that children who were breastfed longer had a lower risk of developing childhood wheezing/asthma. This finding was more significant at ages 0–2 years, and diminished over time, although it remained evident at school age (39).
Other systematic reviews and meta-analyses reported similar findings, suggesting that breastfeeding is protective against pre-school wheezing, which is commonly triggered by viral respiratory infections, whereas this protection tended to wane in older children when heterogeneous factors can influence respiratory morbidity (38, 40). Although there is a growing body of evidence on the role of breast milk in the development of immune tolerance during the first months of life, the impact of breastfeeding on asthma pathogenesis remains controversial.
The role of partially and extensively hydrolyzed milk formulas (pHF and eHF, respectively) in asthma prevention is still controversial. Two early RCTs showed no significant difference between pHF and eHF in the prevention of allergic diseases in children, including asthma (41, 42). In a prospective, double- blind RCT, high-risk children that could not be breastfed were randomized to receive whey-based pHF or eHF, casein-based eHF, or standard cow's milk formula (CMF) for the first 4 months of life. Hydrolysate nutrition did not have any preventive effect either on asthma or early and late wheezing (43). After 10 years of follow-up, the authors observed no effects on the development of asthma (44), while, between 11 and 15 years of age, the prevalence of asthma was lower in the casein-based eHF group than in the CMF group. No significant effect was found in the whey-based eHF group on any manifestation, nor was there any effect on sensitization with any formula (45).
In a recent birth cohort study, infants received breast milk only, pHF with or without a hypoallergenic label, or non- hydrolyzed formula. The use of the pHF-with hypoallergenic label, compared to non-hydrolyzed formula, had no protective effect on the risk of asthma up to 2 years of age and was related to a higher risk of wheezing at 1 year in high-risk infants (46). A recent Cochrane review concluded that the use of hydrolyzed formula in the early days of infancy, compared to exclusive breastfeeding, showed no significant differences in terms of infant allergy prevention. In particular, the authors found no evidence to support the use of pHF compared to CMF to prevent allergic diseases among non- exclusively breastfed infants (47).
The hypothesis that VD status in childhood might influence the susceptibility to childhood asthma and allergy is supported by the evidence on the role of VD as a key modulator of lung growth and innate and adaptive anti-inflammatory immune responses (48–50). Experimental data have shown that low VD levels are associated with increased type 2-mediated responses, interleukin-10 production, and reduced T-regulatory cells (48). In addition, recent data have shown that early postnatal colonization of the airways by pathogenic bacteria, a risk factor for the development of asthma, is influenced by VD status (50). VD may also affect airway remodeling by direct inhibition of airway smooth muscle cell growth and contractility and fibroblast proliferation (51). Observational studies have shown that VD deficiency in early life is associated with the occurrence and persistence of childhood asthma (19, 52). In a high-risk Australian cohort, VD deficiency in early childhood was associated with a higher risk for persistent asthma at 10 years of age (53). VD deficiency in infancy was also associated with increased risk of early allergic sensitization and susceptibility to respiratory infections (53), which are known risk factors for both preschool and school-age asthma (54).
Interestingly, VD supplementation during pregnancy and infancy has been related to a reduced risk of sensitization to house dust mites at age 18 months (30).
The results of the D-Wheeze trial, in which 300 black infants born prematurely were randomized to receive either a sustained supplementation with 400 IU/day of VD (active group) or a diet-limited supplementation (control group) showed a 34% reduced risk for recurrent wheezing by 12 months in the intervention group (31). There is also evidence that VD supplementation can decrease susceptibility to respiratory viral infection in older children (55). Taken together, the results from in-utero (29) and post- natal VD supplementation trials support a role for VD in reducing susceptibility to preschool viral wheezing illnesses. However, there are insufficient data to address whether postnatal VD supplementation may help in the primary prevention of persistent school-age asthma (56), and proper intervention trials with long-term follow-up are needed (Table 1). Intervention trials assessing the combination of prenatal and postnatal VD supplementation would also be needed before VD supplementation can be recommended for the primary prevention of pediatric wheezing and asthma.
There is mounting evidence showing the relationship between the composition of the early-life gut microbiome and the risk of asthma in children (57, 58), which has promoted studies on the modulation of the gut microbiome as a means of preventing asthma. However, RCTs of probiotic and prebiotic supplementation for the prevention of pediatric asthma have shown mixed efficacy outcomes (Table 2). An early systematic review and meta-analysis showed no protective role of oral probiotic supplementation during pregnancy or early life on the development of childhood wheeze and asthma (68). A study by Peldan et al. (61) in high-atopy risk children showed that prevalence of asthma at 5 and 10 years of age was similar in the group receiving oral probiotics mixture for the first 6 months of life (also including maternal supplementation starting at week 36 of gestation) and the placebo group. A recent meta-analysis of RCTs concluded that probiotic supplementation during pregnancy or early life did not influence the incidence of wheeze or asthma in infants, but seemed to reduce wheezing incidence among the subgroup of infants with atopic disease (69).
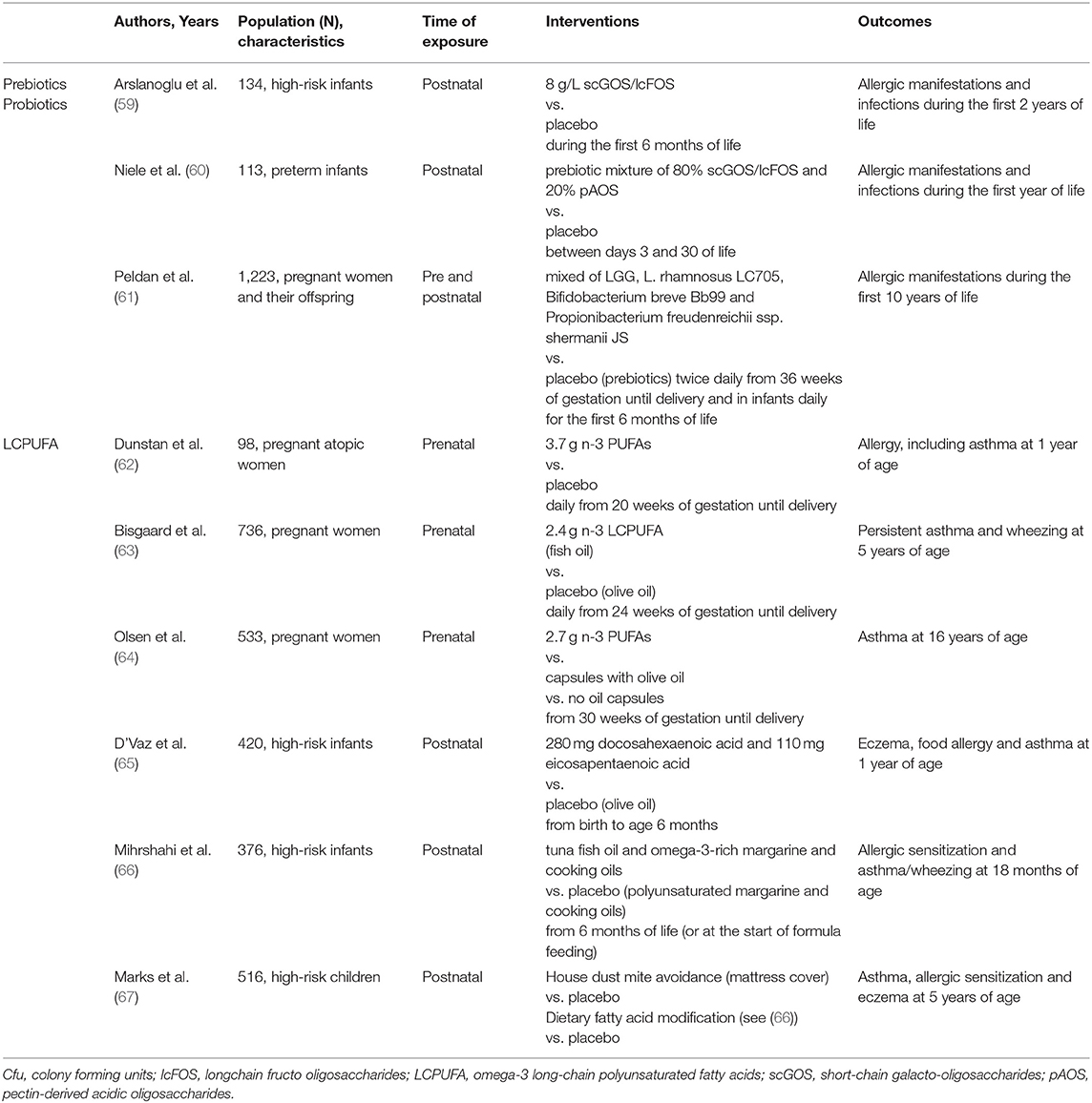
Table 2. Randomized controlled trials on prebiotics/probiotics and LCPUFA supplementation for the prevention of childhood wheezing/asthma.
In a 2-year follow-up RCT involving 132 infants at risk of atopy, infants that were fed with a formula containing a mixture of prebiotic oligosaccharides reported a lower incidence for recurrent wheezing compared to the placebo group (59). In a 1-year follow-up RCT involving 113 preterm infants, supplementation of non-human neutral and acidic oligosaccharides during the neonatal period did not reduce the incidence of allergies, bronchial hyper-reactivity, and respiratory infections (60). In 2013, a Cochrane review reported no significant effect of oral prebiotics for the prevention of childhood- onset asthma (70). A more recent meta-analysis of RCTs concluded that the role of prebiotics in the prevention of allergies is still uncertain (71).
Taken together, the evidence on the effects of oral probiotics and prebiotics for the prevention of pediatric asthma is so controversial that no definitive recommendation can be made. Differences in the probiotic strain specificity, the population treated, the timing of administration, and the duration of the intervention all contribute to the heterogeneity of the meta-analysis and of RCT outcomes.
The supplementation with omega-3 long-chain polyunsaturated fatty acids (LCPUFA) during pregnancy and early life, through the administration of fish oil, has been proposed for the prevention of allergic sensitization and atopic disease, including asthma (72, 73). LCPUFA influence the membrane structure and function, potentially modulating the function of the cells involved in the immune and inflammatory response (74). Most of the studies on maternal fish oil supplementation during pregnancy showed a reduced risk of allergic sensitization to both foods and aeroallergens in children (62, 75–78). In some of these studies, also a lower incidence of eczema was reported (77, 78), while other authors reported no differences in the incidence of allergic diseases (76, 79). Two meta-analyses reported a beneficial effect of maternal supplementation with fish oil on the reduction of allergic sensitization and eczema (80), and on the sensitization to egg and peanut (81), respectively. However, a recent Cochrane review concluded that there is “limited evidence” to support that supplementation with LCPUFA during pregnancy and lactation could reduce the incidence of allergies in children (82). Regarding the prevention of wheeze/asthma, the literature data available show mixed efficacy results for the supplementation of LCPUFA during pregnancy (62–64) (Table 2). Indeed, a recent meta-analysis concluded that LCPUFA supplementation during pregnancy is not associated with a significant protective effect on wheeze/asthma in offspring (81).
Conflicting results have been found in studies investigating fish oil supplementation in infants and children for the prevention of allergic sensitization and asthma (65–67) (Table 2). A meta-analysis of RCTs found no evidence of a protective role of LCPUFA supplementation in infants and children in the prevention of asthma (83). Overall, the available studies show methodological heterogeneity and risk for suboptimal adherence bias. Therefore, further trials are needed to clarify the role of LCPUFA supplementation during pregnancy and early life in the prevention of pediatric asthma.
Recent advances in the field of allergy prevention showed that early 2000s recommendation to delay the introduction of solid allergenic foods to the infant's diet is not an effective approach to reduce the risk of allergic sensitization and atopic diseases in children (84–88). More recently, high-quality clinical trials showed that the early introduction of some food allergens, such as peanut and egg, is associated with a reduced risk of food allergy to those foods (84). There is conflicting evidence regarding early complementary feeding and the prevention of pediatric asthma, with some observational studies reporting that the early introduction of some solid foods (before 1 year of age), such as oat, fruit, vegetables, and fish, was associated with a reduced prevalence of wheezing and asthma in childhood (89–91), while others reporting no such association (92).
The timing of the introduction of fish is of particular interest to the purpose of primary prevention of asthma, given its high content in LCPUFA. Despite the heterogeneity in the methods of analysis and outcome measures, the early introduction of fish has been associated, in many observational studies, with a reduced risk of allergic sensitization (93, 94). However, the protective effect of early introduction of fish on the development of infant wheezing and asthma seems more controversial, with some observational studies reporting such association (93, 95–98), while others did not confirm this protective effect (90, 99). Indeed, two recent meta-analyses conclude that introducing fish before the age of 9 months is associated with low-to-very low evidence of reduced allergic sensitization and rhinitis (94), but there is limited evidence that early introduction of fish could reduce the risk of developing wheezing and asthma in childhood (100).
The significant increase in the prevalence of asthma and allergic diseases registered in recent years has promoted research on the identification of modifiable risk factors for the prevention of such disorders. It is well-acknowledged that respiratory health is determined by a complex interaction between genetic factors and environmental drivers that occur during prenatal and early postnatal life, including dietary factors. However, it remains difficult to define the contribution of specific dietary supplements and nutritional food sources to the risk of developing pediatric asthma, due to the heterogeneous pathogenesis of this disease and the limitations of the currently available evidence, in terms of study design, type and duration of interventions and outcomes measures.
Further research is needed to accurately identify dietary and nutritional modifiable risk factors for asthma and to address whether the modulation of such factors, either alone or in combination, could contribute to the primary prevention strategies of pediatric asthma.
DP, GN, IT, GC, and PC made substantial contributions to conception, design, and acquisition of data. GC, IT, GN, and PC drafted the initial manuscript. DP, EV, ED'A and PC critically reviewed it for important intellectual content. All authors approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bui DS, Walters HE, Burgess JA, Perret JL, Bui MQ, Bowatte G, et al. Childhood respiratory risk factor profiles and middle-age lung function: a prospective cohort study from the first to sixth decade. Ann Am Thorac Soc. (2018) 15:1057–66. doi: 10.1513/AnnalsATS.201806-374OC
2. Oksel C, Custovic A. Development of allergic sensitization and its relevance to paediatric asthma. Curr Opin Allergy Clin Immunol. (2018) 18:109–16. doi: 10.1097/ACI.0000000000000430
3. Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. (2016) 137:1–7. doi: 10.1542/peds.2015-2354
4. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
5. Polk BI, Bacharier LB. Potential strategies and targets for the prevention of pediatric asthma. Immunol Allergy Clin North Am. (2019) 39:151–62. doi: 10.1016/j.iac.2018.12.010
6. Peroni DG, Bonomo B, Casarotto S, Boner AL, Piacentini GL. How changes in nutrition have influenced the development of allergic diseases in childhood. Ital J Pediatr. (2012) 38:22 doi: 10.1186/1824-7288-38-22
7. Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol. (2017) 17:495–507. doi: 10.1038/nri.2017.54
8. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedón JC. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. (2016) 4:1111–22. doi: 10.1016/j.jaip.2016.05.003
9. Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. (2015) 386:1075–85. doi: 10.1016/S0140-6736(15)00156-7
10. Maciag MC, Phipatanakul W. Prevention of asthma: targets for intervention. Chest. (2020). doi: 10.1016/j.chest.2020.04.011. [Epub ahead of print].
11. Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. (2003) 3:125–32. doi: 10.1097/00130832-200304000-00006
12. Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome composition and its impact on the development of allergic diseases. Front Immunol. (2020) 11:700. doi: 10.3389/fimmu.2020.00700
13. Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. (2015) 12:S150–6. doi: 10.1513/AnnalsATS.201503-133AW
14. Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. (2015) 15:308–22. doi: 10.1038/nri3830
15. Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O'Mahony L. Recent developments and highlights in mechanisms of allergic diseases: Microbiome. Allergy. (2018) 73:2314–27. doi: 10.1111/all.13634
16. Beckhaus AA, Garcia-Marcos L, Forno E, Pacheco-Gonzalez RM, Celedón JC, Castro-Rodriguez JA. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta-analysis. Allergy. (2015) 70:1588–604. doi: 10.1111/all.12729
17. Baiz N, Just J, Chastang J, Forhan A, de Lauzon-Guillain B, Magnier AM, et al. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin Immunol. (2019) 15:40. doi: 10.1186/s13223-019-0353-2
18. Comberiati P, Tsabouri S, Piacentini GL, Moser S, Minniti F, Peroni DG. Is vitamin D deficiency correlated with childhood wheezing and asthma? Front Biosci. (2014) 6:31–9. doi: 10.2741/E687
19. Pfeffer PE, Hawrylowicz CM. Vitamin D in asthma: mechanisms of action and considerations for clinical trials. Chest. (2018) 153:1229–3919. doi: 10.1016/j.chest.2017.09.005
20. Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. (2007) 85:853–9. doi: 10.1093/ajcn/85.3.853
21. Camargo CA, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. (2007) 85:788–95. doi: 10.1093/ajcn/85.3.788
22. Allan KM, Prabhu N, Craig LC, McNeill G, Kirby B, McLay J, et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. (2015) 45:1027–36. doi: 10.1183/09031936.00102214
23. Pacheco-González RM, García-Marcos L, Morales E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatr Allergy Immunol. (2018) 29:243–53. doi: 10.1111/pai.12876
24. Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial. PLoS ONE. (2013) 8:e66627. doi: 10.1371/journal.pone.0066627
25. Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. (2016). 315:362–70. doi: 10.1001/jama.2015.18589
26. Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, O'Connor GT, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. (2020) 382:525–33. doi: 10.1056/NEJMoa1906137
27. Chawes BL, Bønnelykke K, Stokholm J, Vissing NH, Bjarnadóttir E, Schoos AM, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. (2016) 315:353–61. doi: 10.1001/jama.2015.18318
28. Brustad N, Eliasen AU, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. High-dose vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA. (2019) 321:1003–5 doi: 10.1001/jama.2019.0052
29. Comberiati P, Peroni DG. Vitamin D supplementation in pregnancy does not prevent school-age asthma. Allergy. (2020) 75:1839–54. doi: 10.1111/all.14337
30. Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. (2016) 71:1325–34. doi: 10.1111/all.12909
31. Hibbs AM, Ross K, Kerns LA, Wagner C, Fuloria M, Groh-Wargo S, et al. Effect of vitamin D supplementation on recurrent wheezing in black infants who were born preterm: the d-wheeze randomized clinical trial. JAMA. (2018) 319:2086–94. doi: 10.1001/jama.2018.5729
32. Stone CA, Cook-Mills J, Gebretsadik T, Rosas-Salazar C, Turi K, Brunwasser SM, et al. Delineation of the individual effects of vitamin E isoforms on early life incident wheezing. J Pediatr Mar. (2019) 206:156–63.e3. doi: 10.1016/j.jpeds.2018.10.045
33. Munblit D, Treneva M, Peroni DG, Colicino S, Chow LY, Dissanayeke S, et al. Immune components in human milk are associated with early infant immunological health outcomes: a prospective three-country analysis. Nutrients. (2017) 9:532. doi: 10.3390/nu9060532
34. Munblit D, Treneva M, Peroni DG, Colicino S, Chow L, Dissanayeke S, et al. Colostrum and mature human milk of women from London, Moscow, and verona: determinants of immune composition. Nutrients. (2016) 8:695. doi: 10.3390/nu8110695
35. Minniti F, Comberiati P, Munblit D, Piacentini GL, Antoniazzi E, Zanoni L, et al. Breast-milk characteristics protecting against allergy. Endocr Metab Immune Disord Drug Targets. (2014) 14:9–15. doi: 10.2174/1871530314666140121145045
36. Verhasselt V, Genuneit J, Metcalfe JR, Tulic MK, Rekima A, Palmer DJ, et al. Ovalbumin in breastmilk is associated with a decreased risk of IgE-mediated egg allergy in children. Allergy. (2020) 75:1463–6. doi: 10.1111/all.14142
37. Rekima A, Bonnart C, Macchiaverni P, Metcalfe J, Tulic MK, Halloin N, et al. A role for early oral exposure to house dust mite allergens through breast milk in IgE-mediated food allergy susceptibility. J Allergy Clin Immunol. (2020) 145:1416–29. doi: 10.1016/j.jaci.2019.12.912
38. Guibas GV, Xepapadaki P, Moschonis G, Douladiris N, Filippou A, Tsirigoti L, et al. Breastfeeding and wheeze prevalence in pre-schoolers and pre-adolescents: the genesis and healthy growth studies. Pediatr Allergy Immunol. (2013) 24:772–81. doi: 10.1111/pai.12169
39. Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. (2014) 179:1153–67. doi: 10.1093/aje/kwu072
40. Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. (2015) 104:38–53. doi: 10.1111/apa.13132
41. Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol. (2000) 11:149–61. doi: 10.1034/j.1399-3038.2000.00081.x
42. Nentwich I, Michkova E, Nevoral J, Urbanek R, Szepfalusi Z. Cow's milk-specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy. (2001) 56:1144–56. doi: 10.1111/j.1398-9995.2001x.00926.x
43. von Berg A, Filipiak-Pittroff B, Krämer U, Link E, Heinrich J, Koletzko S, et al. The German Infant Nutritional Intervention Study (GINI) for the preventive effect of hydrolyzed infant formulas in infants at high risk for allergic diseases. design and selected results. Allergol Select. (2017) 1:28–38. doi: 10.5414/ALX01462E
44. von Berg A, Filipiak-Pittroff B, Krämer U, Hoffmann B, Link E, Beckmann C, et al. Allergies in high-risk schoolchildren after early intervention with cow's milk protein hydrolysates: 10-year results from the German Infant Nutritional Intervention (GINI) study. J Allergy Clin Immunol. (2013) 131:1565–73. doi: 10.1016/j.jaci.2013.01.006
45. von Berg A, Filipiak-Pittroff B, Schulz H, Hoffmann U, Link E, Sußmann M, et al. Allergic manifestation 15 years after early intervention with hydrolyzed formulas–the GINI Study. Allergy. (2016) 71:210–9. doi: 10.1111/all.12790
46. Davisse-Paturet C, Raherison C, Adel-Patient K, Divaret-Chauveau A, Bois C, Dufourg MN, et al. Use of partially hydrolysed formula in infancy and incidence of eczema, respiratory symptoms or food allergies in toddlers from the ELFE cohort. Pediatr Allergy Immunol. (2019) 30:614–23. doi: 10.1111/pai.13094
47. Osborn DA, Sinn JK, Jones LJ. Infant formulas containing hydrolysed protein for prevention of allergic disease. Cochrane Database Syst Rev. (2018) 10:Cd003664. doi: 10.1002/14651858.CD003664.pub6
48. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. (2010) 88:441–50. doi: 10.1007/s00109-010-0590-9
49. Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation. a systematic review. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L587–602. doi: 10.1152/ajplung.00117.2014
50. Foong RE, Bosco A, Jones AC, Gout A, Gorman S, Hart PH, et al. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am J Respir Cell Mol Biol. (2015) 53:664–75. doi: 10.1165/rcmb.2014-0356OC
51. Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. (2011) 184:1342–9. doi: 10.1164/rccm.201107-1239OC
52. van Oeffelen AA, Bekkers MB, Smit HA, Kerkhof M, Koppelman GH, Haveman-Nies A, et al. Serum micronutrient concentrations and childhood asthma: the PIAMA birth cohort study. Pediatr Allergy Immunol. (2011) 22:784–93. doi: 10.1111/j.1399-3038.2011.01190.x
53. Hollams EM, Teo SM, Kusel M, Holt BJ, Holt KE, Inouye M, et al. Vitamin D over the first decade and susceptibility to childhood allergy and asthma. J Allergy Clin Immunol. (2017) 139:472–81.e79. doi: 10.1016/j.jaci.2016.07.032
54. Comberiati P, Di Cicco ME, D'Elios S, Peroni DG. How much asthma is atopic in children? Front Pediatr. (2017) 5:122. doi: 10.3389/fped.2017.00122
55. Loeb M, Dang AD, Thiem VD, Thanabalan V, Wang B, Nguyen NB, et al. Effect of vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: a randomized controlled trial. Influenza Other Respir Viruses. (2019) 13:176–83. doi: 10.1111/irv.12615
56. Yepes-Nuñez JJ, Brozek JL, Fiocchi A, Pawankar R, Cuello-García C, Zhang Y, et al. Vitamin D supplementation in primary allergy prevention: systematic review of randomized and non-randomized studies. Allergy. (2018) 73:37–49. doi: 10.1111/all.13241
57. Pettersen VK, Arrieta MC. Host-microbiome intestinal interactions during early life: considerations for atopy and asthma development. Curr Opin Allergy Clin Immunol. (2020) 20:138–48 doi: 10.1097/ACI.0000000000000629
58. Mennini M, Dahdah L, Artesani MC, Fiocchi A, Martelli A. Probiotics in asthma and allergy prevention. Fronti Pediatr. (2017) 5:165. doi: 10.3389/fped.2017.00165
59. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. (2008) 138:1091–5. doi: 10.1093/jn/138.6.1091
60. Niele N, van Zwol A, Westerbeek EA, Lafeber HN, van Elburg RM. Effect of non-human neutral and acidic oligosaccharides on allergic and infectious diseases in preterm infants. Eur J Pediatr. (2013) 172:317–23. doi: 10.1007/s00431-012-1886-2
61. Peldan P, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotics decreased eczema up to 10 years of age, but at 5-10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy. (2017) 47:975–9. doi: 10.1111/cea.12924
62. Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. (2003) 112:1178–84. doi: 10.1016/j.jaci.2003.09.009
63. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AM, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. (2016) 375:2530–9. doi: 10.1056/NEJMoa1503734
64. Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. (2008) 88:167–75. doi: 10.1093/ajcn/88.1.167
65. D'Vaz NN, Meldrum SJ, Dunstan JA, Martino D, McCarthy S, et al. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. (2012) 130:674–82. doi: 10.1542/peds.2011-3104
66. Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM, et al. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr Allergy Immunol. (2004) 15:517–22. doi: 10.1111/j.1399-3038.2004.00187.x
67. Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. (2006) 118:53–61. doi: 10.1016/j.jaci.2006.04.004
68. Azad MB, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. (2013). 347:f6471. doi: 10.1136/bmj.f6471
69. Wei X, Jiang P, Liu J, Sun R, Zhu L. Association between probiotic supplementation and asthma incidence in infants: a meta-analysis of randomized controlled trials. J Asthma. (2020) 57:167–78. doi: 10.1080/02770903.2018.1561893
70. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. (2013) 2013:Cd006474. doi: 10.1002/14651858.CD006474.pub3
71. Cuello-Garcia C, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Morgano GP, Zhang Y, et al. Prebiotics for the prevention of allergies: a systematic review and meta-analysis of randomized controlled trials. Clin Exp Allergy. (2017) 47:1468–77. doi: 10.1111/cea.13042
72. Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc. (2010) 69:373–80. doi: 10.1017/S0029665110001552
73. D'Auria E, Miraglia Del Giudice M, Barberi S, Mandelli M, Verduci E, Leonardi S, et al. Omega-3 fatty acids and asthma in children. Allergy Asthma Proc. (2014) 35:233–40. doi: 10.2500/aap.2014.35.3736
74. Miles EA, Calder PC. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients. (2017) 9:784. doi: 10.3390/nu9070784
75. Best K, Makrides M. Possible protective effect of prenatal omega-3 long-chain polyunsaturated fatty acids supplementation on persistent wheeze and asthma in early childhood. Evid Based Med. (2017) 22:104. doi: 10.1136/ebmed-2017-110696
76. Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, et al. Prenatal fish oil supplementation and allergy: 6-year follow-up of a randomized controlled trial. Pediatrics. (2016) 137:e20154443. doi: 10.1542/peds.2015-4443
77. Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Böttcher MF, Fälth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. (2009) 98:1461–7. doi: 10.1111/j.1651-2227.2009.01355.x
78. Furuhjelm C, Warstedt K, Fagerås M, Fälth-Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. (2011) 22:505–14. doi: 10.1111/j.1399-3038.2010.01096.x
79. Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. (2013) 68:1370–6. doi: 10.1111/all.12233
80. Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. (2016) 103:128–43. doi: 10.3945/ajcn.115.111104
81. Vahdaninia M, Mackenzie H, Dean T, Helps S. omega-3 LCPUFA supplementation during pregnancy and risk of allergic outcomes or sensitization in offspring: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. (2019) 122:302–13. doi: 10.1016/j.anai.2018.12.008
82. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. (2015) 2015:Cd010085. doi: 10.1002/14651858.CD010085.pub2
83. Muley P, Shah M, Muley A. Omega-3 fatty acids supplementation in children to prevent asthma: is it worthy?- a systematic review and meta-analysis. J. Allergy. (2015) 2015:312052. doi: 10.1155/2015/312052
84. Comberiati P, Costagliola G, D'Elios S, Peroni D. Prevention of food allergy: the significance of early introduction. Medicina. (2019) 55.323. doi: 10.3390/medicina55070323
85. Tromp II, Kiefte-de Jong JC, Lebon A, Renders CM, Jaddoe VW, Hofman A, et al. The introduction of allergenic foods and the development of reported wheezing and eczema in childhood: the generation R study. Arch Pediatr Adolesc Med. (2011) 165:933–8. doi: 10.1001/archpediatrics.2011.93
86. Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. (2008) 121:e44–52. doi: 10.1542/peds.2006-3553
87. Nwaru BI, Erkkola M, Ahonen S, Kaila M, Haapala AM, Kronberg- Kippilä C, et al. Age at the introduction of solid foods during the first year and allergic sensitization at age 5 years. Pediatrics. (2010) 125:50–9. doi: 10.1542/peds.2009-0813
88. Greer FR, Sicherer SH, Burks AW. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. (2019) 143:e20190281. doi: 10.1542/peds.2019-0281
89. Nwaru BI, Takkinen HM, Niemelä O, Kaila M, Erkkola M, Ahonen S, et al. Timing of infant feeding in relation to childhood asthma and allergic diseases. J Allergy Clin Immunol. (2013) 131:78–86. doi: 10.1016/j.jaci.2012.10.028
90. Virtanen SM, Kaila M, Pekkanen J, Kenward MG, Uusitalo U, Pietinen P, et al. Early introduction of oats associated with decreased risk of persistent asthma and early introduction of fish with decreased risk of allergic rhinitis. Br J Nutr. (2010) 103:266–73. doi: 10.1017/S0007114509991541
91. Nja F, Nystad W, Lodrup Carlsen KC, Hetlevik O, Carlsen KH. Effects of early intake of fruit or vegetables in relation to later asthma and allergic sensitization in school-age children. Acta Paediatr. (2005) 94:147–54. doi: 10.1080/08035250410023638
92. Nwaru BI, Craig LC, Allan K, Prabhu N, Turner SW, McNeill G, et al. Breastfeeding and introduction of complementary foods during infancy in relation to the risk of asthma and atopic diseases up to 10 years. Clin Exp Allergy. (2013) 43:1263–73. doi: 10.1111/cea.12180
93. Kull I, Bergström A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. (2006) 61:1009–15. doi: 10.1111/j.1398-9995.2006.01115.x
94. Lerodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. (2016) 316:1181–92. doi: 10.1001/jama.2016.12623
95. Nafstad P, Nystad W, Magnus P, Jaakkola JJ. Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J Asthma. (2003) 40:343–8. doi: 10.1081/JAS-120018633
96. Goksör E, Alm B, Pettersson R, Möllborg P, Erdes L, Aberg N, et al. Early fish introduction and neonatal antibiotics affect the risk of asthma into school age. Pediatr Allergy Immunol. (2013) 24:339–44. doi: 10.1111/pai.12078
97. Klingberg S, Brekke HK, Ludvigsson J. Introduction of fish and other foods during infancy and risk of asthma in the All Babies In Southeast Sweden cohort study. Eur J Pediatr. (2019) 178:395–402. doi: 10.1007/s00431-018-03312-5
98. Lumia M, Takkinen HM, Luukkainen P, Kaila M, Lehtinen-Jacks S, Nwaru BI, et al. Food consumption and risk of childhood asthma. Pediatr Allergy Immunol. (2015) 26:789–96. doi: 10.1111/pai.12352
99. Hesselmar B, Saalman R, Rudin A, Adlerberth I, Wold A. Early fish introduction is associated with less eczema, but not sensitization, in infants. Acta Paediatr. (2010) 99:1861–7. doi: 10.1111/j.1651-2227.2010.01939.x
Keywords: asthma, breastfeeding, children, complementary foods, omega-3 long-chain polyunsaturated fatty acids (LCPUFAs), primary prevention, probiotics, Vitamin D
Citation: Trambusti I, Nuzzi G, Costagliola G, Verduci E, D'Auria E, Peroni DG and Comberiati P (2020) Dietary Interventions and Nutritional Factors in the Prevention of Pediatric Asthma. Front. Pediatr. 8:480. doi: 10.3389/fped.2020.00480
Received: 13 May 2020; Accepted: 09 July 2020;
Published: 18 August 2020.
Edited by:
Marzia Duse, Sapienza University of Rome, ItalyReviewed by:
Grégory Bouchaud, INRA Centre Angers-Nantes Pays de la Loire, FranceCopyright © 2020 Trambusti, Nuzzi, Costagliola, Verduci, D'Auria, Peroni and Comberiati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diego G. Peroni, ZGllZ28ucGVyb25pQHVuaXBpLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.