- 1Stem Cell Transplantation and Cellular Therapies, MSK Kids, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Princess Máxima Center for Pediatric Oncology and UMC Utrecht, Utrecht, Netherlands
Hematopoietic cell transplantation (HCT) is often a last resort, but potentially curative treatment option for children suffering from hematological malignancies and a variety of non-malignant disorders, such as bone marrow failure, inborn metabolic disease or immune deficiencies. Although efficacy and safety of the HCT procedure has increased significantly over the last decades, the majority of the patients still suffer from severe acute toxicity, viral reactivation, acute or chronic graft-versus-host disease (GvHD) and/or, in case of malignant disease, relapses. Factors influencing HCT outcomes are numerous and versatile. For example, there is variation in the selected graft sources, type of infused cell subsets, cell doses, and the protocols used for conditioning, as well as immune suppression and treatment of adverse events. Moreover, recent pharmacokinetic studies show that medications used in the conditioning regimen (e.g., busulphan, fludarabine, anti-thymocyte globulin) should be dosed patient-specific to achieve optimal exposure in every individual patient. Due to this multitude of variables and site-specific policies/preferences, harmonization between HCT centers is still difficult to achieve. Literature shows that adequate immune recovery post-HCT limits both relapse and non-relapse mortality (death due to viral reactivations and GvHD). Monitoring immune parameters post-HCT may facilitate a timely prediction of outcome. The use of standardized assays to measure immune parameters would facilitate a fast comparison between different strategies tested in different centers or between different clinical trials. We here discuss immune cell markers that may contribute to clinical decision making and may be worth to standardize in multicenter collaborations for future trials.
Introduction
The probability of long-term survival after hematopoietic cell transplantation (HCT) has steadily improved in the last decade with advances in treatments (1, 2). However, long term survival after HCT is still hampered by adverse events, such as graft-versus-host disease (GvHD), infections and relapse of the underlying disease (3–6). Delayed immune reconstitution plays a central role in most of these events (7, 8), suggesting that strategies that increase immune recovery are of great interest to increase survival chances post-HCT (9).
Among the graft-related variables that can influence immune reconstitution are the graft source and composition, degree of HLA-match and the cell dose (10–12). Transplantation with matched related bone marrow (BM) or peripheral blood (PB) (stem) cells is considered the standard for allo-HCT, but also matched unrelated donors (MUD) (mis)matched unrelated cord blood (CB), a haplo-identical or a mismatched family donor can be considered as alternative graft sources. The HCT-source and its manipulation, such as CD34+ positive selection (13) or other ex vivo T-cell depletion (14) and in vivo T cell depletion with serotherapy [e.g., anti-thymocyte globulin (ATG) or Alemtuzumab] may be important factors defining the probability of T-cell immune reconstitution (IR). Moreover, grafts are selected based on HLA-matching criteria, where the degree of HLA-match is, in general, stricter for BM/PB than for CB grafts, and may depend on the indication. Also, the use of grafts from younger donors is preferred by centers because it associates with better survival chances, due to lower non-relapse mortality (15–17). The graft's cell dose is considered, particularly in CB transplants, because units with low numbers of CD34+ cells were associated with inferior outcomes (18). A retrospective European Society for Blood and Marrow Transplantation (EBMT) analysis by Czerw et al. showed that T cell numbers in the HCT graft are highly variable (range; 50–885 × 10e6/kg) and positively correlate with an increased incidence of grade III-IV acute (a) GvHD (19). Others found that higher numbers of γδT cells (20) in the graft associate with favorable immune reconstitution and superior clinical outcome (21). However, whether the combination of graft source, match grade, cell dose and graft composition will result in optimal immune recovery post-HCT is impossible to deduce from these data. To get more insight into the contribution of different parameters to outcome, we need to identify the best combination of markers that associate with clinical outcome. A reliable predictor for outcomes, across the variety of transplant platforms (e.g., T replete and T deplete), seems to be CD4+ T cell counts above 50/μL within 100 days after transplant (22–24).
A decisive factor that may even overrule the graft-related effects, is the type and timing of the conditioning regimen used. Pharmaco-kinetic and -dynamic studies show strong correlations between post-transplant recovery of immune cells and the timing and dose of conditioning agents [e.g., fludarabine (Flu), busulfan (Bu), and ATG] (25, 26). These data point toward personalized dosing strategies to achieve an optimal exposure in every individual patient (27–29).
Due to the multitude of variables described above and the site-specific policies/preferences, a precise understanding of how these variables can be influenced to optimize outcomes of HCT is difficult to achieve. While donor-selection in cell transplantation has been increasingly standardized over the last decades (30, 31), harmonization of the conditioning regimen is still lacking and the highly variable pharmacokinetics of drugs used in the conditioning regimen and their dramatic effects on outcome are still not widely considered. In the first part of this review, we will provide an overview of the association between different conditioning regimens, outcomes and IR post-HCT. Given the strong relationship between immune recovery and outcome (32, 33), monitoring immune parameters post-HCT may serve as a relatively fast predictor for outcomes (including survival, relapse, and non-relapse mortality) and accelerate comparisons between different strategies in different centers and between different clinical trials. The use of standardized assays across laboratories will be imperative for this purpose. In the second part, we discuss a rationale for selection of a minimal parameter set to monitor immune recovery that could be considered for standardization.
Conditioning Regimens, Immune Reconstitution, and Outcomes: Toward Individualized Dosing
We recently showed that the pharmacokinetics of rabbit ATG (rATG) is highly dependent on absolute lymphocyte count (ALC) as a representation for the receptor-load (26). In adults, the ALC before rATG-dosing was the only predictor for rATG clearance, while in pediatric patients a weight of <40 kg also influenced clearance. In Table 1 the suggested rATG (Thymoglobulin) exposure targets for pediatric and adult patients are listed. It is important to realize that the ligands recognized by rATG and rATLG (anti-T-lymphocyte globulin; Neovii Biotech, Rapperswil, Switzerland) are not identical, so the target exposures for rATG do not apply for rATLG (26, 35, 36). A nomogram for ATLG should still be made, as its effects are likely similar to those of rATG. Soiffer et al. showed that ATLG was associated with inferior chronic-GvHD-free, leukemia-free survival in a post-hoc analysis of a randomized controlled trial with three different regimens (25). This study nicely illustrates that it is essential to understand the effects of all agents on immune reconstitution and the interrelationships between the agents that may significantly alter the outcomes. In this trial, patients receiving total body irradiation (TBI) + cyclophosphamide (Cy) had lower “absolute lymphocyte counts” on the day of rATLG dosing compared to patients receiving a chemo (Bu-Cy or Bu-Flu)-based regimen or patients that received Cy first, before receiving TBI (usually because of practical issues). This resulted in a slower clearance of rATLG, delayed immune reconstitution and subsequently higher transplantation related mortality. Other forms of serotherapy, such as Alemtuzumab, as well as equine-ATG (less frequently used), can have highly variable PK and a significant influence on IR and survival chances.
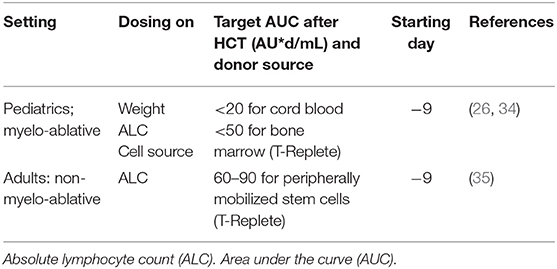
Table 1. Suggested novel ATG (thymoglobulin) dosing nomograms based on PKPD modeling for (non-)myelo-ablative settings in pediatrics and adults [For pediatrics based in PARACHUTE clinical trial; (34)].
A prospective validation of a new dosing nomogram for rATG in pediatric patients (PARACHUTE trial) proved to be effective to expedite immune reconstitution (primary endpoint) and survival (secondary endpoint) (34). This study confirms our previous results showing that a timely recovery of CD4 T cells (>50 CD4+ T cells/μL at two consecutive measurements within 100 days post-HCT) is the strongest predictor of leukemia-free survival, non-relapse mortality, as well as overall survival (23, 35, 37, 38). We previously showed that adequate CD4 recovery is associated with a lower chance for relapse in acute myeloid leukemia (AML) (22) and with higher survival chances for patients with adenoviral reactivation (24). Recently this association was also found to be a significant predictor in a predominantly T cell depleted cohort of pediatric and adult patients (23, 26, 39). Moreover, a recent multicenter trial also showed the association of CD4 T cell recovery with survival chances in patients developing GvHD (23). Interestingly, the CD4 IR is preceded by increasing cell numbers of the myeloid lineage and could even be retraced to the proliferative potential of myeloid cells in BM and CB grafts (38). This makes CD4+ T >50/μL a reliable biomarker for clinical decision making, e.g., patients who fail to reconstitute CD4 T cells may, for instance, be eligible for prophylactic therapy or for pre-emptive treatment upon the first positive virus measurement. Alternatively, patients with adequate CD4 T cell numbers should be monitored carefully, but would not receive treatment, as there is a reasonable chance that the reconstituting immune system will clear the pathogen by itself. Of course, these observations should be further studied in the context of a clinical trial, and follow-up studies and validations are needed to confirm whether predictions can be further improved.
In a recent retrospective cohort analysis (including > 650 pediatric and young adult patients), the cumulative exposure to Bu significantly influenced clinical outcomes (40). The optimal Bu-exposure (80–100 mg*h/L) was associated with the highest survival chances and lowest toxicity and was independent of the indication, chemo-combination (Bu + Flu, Cy + Bu, or Bu + Cy and Melphalan), age and donor source. The method of Bu-exposure estimation may differ between centers and could be responsible for a large variation in the reported estimated exposures (40). This emphasizes the value of standardizing the entire process of sample logistics up to data reporting, in order to be able to compare different treatment strategies.
More recently, Flu-exposure (given prior to transplant) was also found to influence survival by negatively affecting IR in patients who were over-exposed (41, 42). This is an interesting observation, given that the exposure in blood was only before transplant, and it is important to note that the PK in the organs in experimental models can be different, as discussed by the authors. These studies show that the pharmacokinetic variation between individuals is high and that these differences in exposure can have significant impact on outcomes, including survival. It is still daily practice that a variety of conditioning regimens are used, which can complicate comparisons of HCT outcomes across different centers and even within trials as illustrated by Soiffer et al. (25). Also, post-transplant Cy is a frequently used (and a simple and cheap) transplant platform to cross the HLA barrier in allogeneic HCT and induce a state of immunologic tolerance (43, 44). While its simplicity makes it an attractive approach, there is not much data available about immune reconstitution in this transplant setting, which would be of interest to study in more detail to identify predictors for failure or success.
Together, these data suggest that the recovery of (CD4+) T cells may be an easy-to-obtain biological marker and a potential biomarker/predictor to monitor treatment success in population studies. Additional work is needed to identify further biomarkers with clinical predictive value. A biological predictor for clinical outcomes, even before clinical signs will be visible, may be valuable to anticipate graft-versus host responses. It may also facilitate stratification of patient subgroups for treatment interventions after HCT, e.g., prophylactic antiviral therapy, or the use of checkpoint inhibition, to ensure adequate treatment for responsive patients, while predicting non-responders or patients with a high probability of developing life-threatening side effects who may be directly eligible for other treatments (45). As different institutions have their own policies on sample collection and monitoring, it is of crucial importance to set up multicenter validation trials to establish standardized protocols for sampling, handling logistics, measurements and data processing, to reduce result variability and allow for more accurate data comparison.
Monitoring Immune Cell Reconstitution
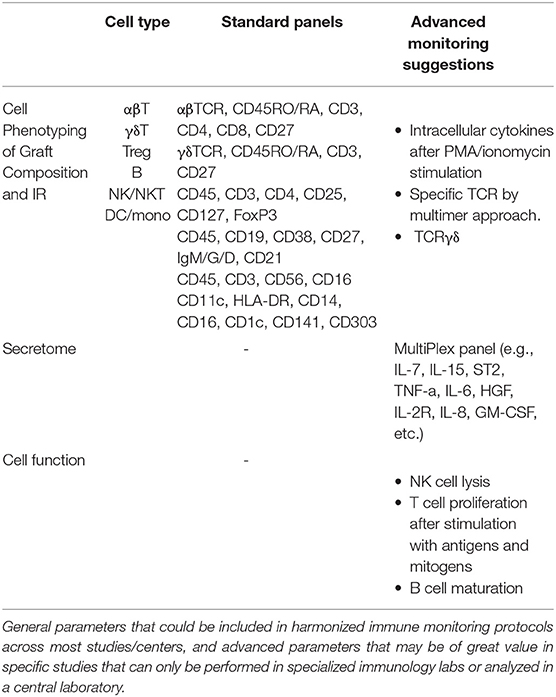
Multicolor flow cytometry is the technology by default in many accredited transplant laboratories for immune cell reconstitution monitoring. It enables the analysis of a large number of parameters simultaneously, in a short time and for a reasonable cost. Many centers monitor the recovery of the common leukocyte subsets based on CD45 (leukocytes), CD3, CD4, CD8 (T cells), CD19 (B cells), αβTCR, γδTCR and CD16/CD56 (NK cells), and may analyze the maturation of T cells from naïve to effector/memory cells to discriminate lymphopenic proliferation and the recovery of thymic output. There are, however, no stringent guidelines for clinical decisions based on quantification of cell subsets post-HCT.
Many effector and regulatory cell types have been linked to the anti-cancer immune responses (46). Different subsets of T cells play crucial roles in controlling disease progression, and effectors like CD8+ and CD4+ T cells have been associated with direct anti-tumor activity and a favorable prognosis, particularly when those T cells express memory and activation markers (47–50). NK cells were shown to mediate tumor regression in AML patients, eliminate graft rejection and protect patients against GvHD (51, 52). The presence of mature antigen-presenting dendritic cells (DCs) has been correlated with improved survival (53–56). T regulatory (Treg) cells down-modulate T-cell activation through the production of immunosuppressive cytokines (TGFβ, IL-10), as well as through surface receptors (CTLA4), and can drastically impact both the anti-tumor immune response as well as the control of GvHD (57–59). Myeloid suppressive cells (60–62) and regulatory B cells (63–65) also play a potential role in attenuating the immune response and controlling effector cells and signaling mechanisms. Moreover, the presence of certain biomarkers may be predictive for the functioning of effector mechanisms; e.g., sorafenib-related IL-15 production causes an increase in CD8+CD107a+IFN-γ+ T cells with features of longevity and eliminates leukemia in secondary recipients, indicating that sorafenib after HCT might be more effective through induction of IL-15-mediated metabolic reprogramming of leukemia-reactive T cells (66). Others showed that the presence of peripheral blood DCs in high frequencies relates to clinical response to high-dose IL-2 (67). This data suggests that DCs may be instrumental for endogenous- and immunotherapy-induced immunity against cancer.
In summary, data from multiple studies suggest that monitoring immune cell subsets may hold predictive value for outcome, development of adverse effects, or may be used for patient stratification for certain treatment modalities. Multicenter validation studies are required using standardized protocols for sampling, handling logistics, measurements and data processing. Diagnostic laboratories in transplant centers have standardized protocols to diagnose and measure minimal residual disease burden in patients with hematological malignancies. Most centers provide validated assays for CD34 cells and immune monitoring of leukocyte subsets post-HCT in a standardized way. It thus seems a small step to collect and collectively report these data in international databases (e.g., EBMT), and to relate biological markers, such as CD4 T cell reconstitution, to outcome parameters and to the treatment procedures.
Cytokine Profiling
Profiling soluble markers in blood may be of value to assess the status of the immune system before, during and after immunotherapeutic interventions for infections or GvHD, or to provide additional insight into the therapeutic mechanisms of action. Blood markers should be regarded as surrogate markers and may not always reflect local responses in affected tissues, such as skin or gut in GvHD. As this topic has been reviewed recently (9, 68), we here just describe two scores that may be close to multicenter validation studies and emphasize the standardization of techniques. Previous studies characterized biomarkers for aGvHD-related mortality post-HCT; a biomarker score using ST2, TNFR1, and Reg3a to guide risk-adapted therapy at aGvHD onset irrespective of the conditioning regimen intensity (69, 70). Another formula, including the markers lactate dehydrogenase, creatinine and thrombocytes, termed the Endothelial Activation and Stress Index (EASIX), was also reported useful for prognosis of survival chances in patients suffering from aGvHD after HCT with reduced intensity conditioning (71). These scores should still be prospectively validated in the different HCT settings, preferentially in coordinated multicenter trials before they can be implemented in patient care.
The technology to acquire the parameters for EASIX may be more standardized in and between different centers than those for analyzing proteins, such as ST-2. Many technologies are available e.g., antibody-based ELISA's or multiplex platforms, liquid chromatography—mass spectrometry (LC-MS), electro-chemiluminescence (72), but concentrations may differ depending on the methods used. The latter is problematic for implementation of mathematical scores. Standardization is needed for the procedures of sample processing and isolation, selection of tubes and duration of storage/cryopreservation. Protein levels can differ considerably between serum and plasma samples (due to release of platelet-associated molecules into serum), even between the type of anticoagulant used, and are prone to changes due to variations in time (from sampling to processing and time of storage) and/or temperature (73).
In Summary
Survival after HCT depends on many factors, including a balanced and timely immune reconstitution in the first 3 months post-HCT. Delayed immune reconstitution is associated with a lack of disease control, viral infections, and seems to increase the chances of immune dysregulation, resulting in higher non-relapse mortality rates. Currently, only a few HCT programs consider therapeutic drug monitoring of agents used in the conditioning (a main factor influencing immune recovery) or apply frequent systematic monitoring of immune cell reconstitution to predict unwanted events.
Based on current knowledge, recommendations can be made with regards to personalized drug dosing and application of a standardized minimal panel for immune monitoring to generate fast responsive surrogate markers for efficacy or the development of unwanted effects. It may be valuable to register the details of the treatment modality, i.e., drug doses and if possible, drug exposure, graft composition, and standardized immune reconstitution parameters to the Center for International Blood and Marrow Transplant Research (CIBMTR) and EBMT databases. This could also initiate the establishment of consensus guidelines in clinical trials on monitoring and reporting a minimal set of parameters, which can be extended to add-on trial-specific parameters (Table 2). Efforts to harmonize HCT protocols/platforms aiming to create the optimal “immune milieu” to exert the most optimal effector mechanism may have more impact on survival chances than many novel maintenance therapies (35, 74). Moreover, it will provide a more straightforward comparison between different treatment modalities, due to better prediction of the immune milieu.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
JB has received consulting fees from Race Oncology, Takeda, Omeros, Advances Clinical, Bluebird Bio, Bluerock, Sanofi (all not related to research described in this review).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. (2013) 19:1498–501. doi: 10.1016/j.bbmt.2013.07.020
2. Vanderwalde AM, Sun CL, Laddaran L, Francisco L, Armenian S, Berano-Teh J, et al. Conditional survival and cause-specific mortality after autologous hematopoietic cell transplantation for hematological malignancies. Leukemia. (2013) 27:1139–45. doi: 10.1038/leu.2012.311
3. Atsuta Y, Hirakawa A, Nakasone H, Kurosawa S, Oshima K, Sakai R, et al. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2016) 22:1702–9. doi: 10.1016/j.bbmt.2016.05.019
4. Holmqvist AS, Chen Y, Wu J, Battles K, Bhatia R, Francisco L, et al. Assessment of late mortality risk after allogeneic blood or marrow transplantation performed in childhood. JAMA Oncol. (2018) 4:e182453. doi: 10.1001/jamaoncol.2018.2453
5. Wilhelmsson M, Vatanen A, Borgström B, Gustafsson B, Taskinen M, Saarinen-Pihkala UM, et al. Adverse health events and late mortality after pediatric allogeneic hematopoietic SCT-two decades of longitudinal follow-up. Bone Marrow Transplant. (2015) 50:850–7. doi: 10.1038/bmt.2015.43
6. Ferry C, Gemayel G, Rocha V, Labopin M, Esperou H, Robin M, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. (2007) 40:219–24. doi: 10.1038/sj.bmt.1705710
7. Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Luo X, Curtsinger J, et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. (2018) 2:909–22. doi: 10.1182/bloodadvances.2017014464
8. Parody R, Martino R, Rovira M, Vazquez L, Vázquez MJ, de la Cámara R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. (2006) 12:734–48. doi: 10.1016/j.bbmt.2006.03.007
9. de Koning C, Plantinga M, Besseling P, Boelens JJ, Nierkens S. Immune reconstitution after allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant. (2016) 22:195–206. doi: 10.1016/j.bbmt.2015.08.028
10. Elfeky R, Lazareva A, Qasim W, Veys P. Immune reconstitution following hematopoietic stem cell transplantation using different stem cell sources. Expert Rev Clin Immunol. (2019) 15:735–51. doi: 10.1080/1744666X.2019.1612746
11. de Koning C, Nierkens S, Boelens JJ. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood. (2016) 128:2607–15. doi: 10.1182/blood-2016-06-724005
12. Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. (2016) 7:507. doi: 10.3389/fimmu.2016.00507
13. Pasquini MC, Devine S, Mendizabal A, Baden LR, Wingard JR, Lazarus HM, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. (2012) 30:3194–201. doi: 10.1200/JCO.2012.41.7071
14. Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood. (2017) 130:677–85. doi: 10.1182/blood-2017-04-779769
15. Azuma E, Hirayama M, Yamamoto H, Komada Y. The role of donor age in naive T-cell recovery following allogeneic hematopoietic stem cell transplantation: the younger the better. Leuk Lymphoma. (2002) 43:735–9. doi: 10.1080/10428190290016827
16. Hirayama M, Azuma E, Jiang Q, Kobayashi M, Iwamoto S, Kumamoto T, et al. The reconstitution of CD45RBhiCD4+ naive T cells is inversely correlated with donor age in murine allogeneic haematopoietic stem cell transplantation. Br J Haematol. (2000) 111:700–7. doi: 10.1046/j.1365-2141.2000.02391.x
17. Arai Y, Kondo T, Yamazaki H, Takenaka K, Sugita J, Kobayashi T, et al. Allogeneic unrelated bone marrow transplantation from older donors results in worse prognosis in recipients with aplastic anemia. Haematologica. (2016) 101:644–52. doi: 10.3324/haematol.2015.139469
18. Konuma T, Kato S, Oiwa-Monna M, Tanoue S, Ogawa M, Isobe M, et al. Cryopreserved CD34+ cell dose, but not total nucleated cell dose, influences hematopoietic recovery and extensive chronic graft-versus-host disease after single-unit cord blood transplantation in adult patients. Biol Blood Marrow Transplant. (2017) 23:1142–50. doi: 10.1016/j.bbmt.2017.03.036
19. Czerw T, Labopin M, Schmid C, Cornelissen JJ, Chevallier P, Blaise D, et al. High CD3+ and CD34+ peripheral blood stem cell grafts content is associated with increased risk of graft-versus-host disease without beneficial effect on disease control after reduced-intensity conditioning allogeneic transplantation from matched unrelated donors for acute myeloid leukemia - an analysis from the acute leukemia working party of the european society for blood and marrow transplantation. Oncotarget. (2016) 7:27255–66. doi: 10.18632/oncotarget.8463
20. Perko R, Kang G, Sunkara A, Leung W, Thomas PG, Dallas MH. Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant. (2015) 21:130–6. doi: 10.1016/j.bbmt.2014.09.027
21. Li Z, Rubinstein SM, Thota R, Savani M, Brissot E, Shaw BE, et al. Immune-Mediated complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2016) 22:1368–75. doi: 10.1016/j.bbmt.2016.04.005
22. Admiraal R, Chiesa R, Bierings M, Versluijs AB, Hiwarkar P, Silva J, et al. Early CD4+ immune reconstitution predicts probability of relapse in pediatric AML after unrelated cord blood transplantation: importance of preventing in vivo T-cell depletion using thymoglobulin®. Biol Blood Marrow Transplant. (2015) 21:S206. doi: 10.1016/j.bbmt.2014.11.315
23. de Koning C, Prockop SE, Van roessel Ichelle Klein E, Boulad F, Kernan NA, et al. Early CD4+ T-cell reconstitution is an excellent predictor for survival and non-relapse mortality in pediatric and young adult patients who develop moderate to severe acute graft-versus-host-disease; a dual center validation. Biol Blood Marrow Transplant. (2020) 26:S188–9. doi: 10.1016/j.bbmt.2019.12.753
24. Admiraal R, de Koning CCH, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. (2017) 140:1643–50.e9. doi: 10.1016/j.jaci.2016.12.992
25. Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase iii clinical trial of anti-T-Lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-Matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. (2017) 35:4003–11. doi: 10.1200/JCO.2017.75.8177
26. Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TCG, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. (2015) 2:e194–203. doi: 10.1016/S2352-3026(15)00045-9
27. Mehta RS, Rezvani K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence. (2016) 7:901–16. doi: 10.1080/21505594.2016.1208866
28. Kontoyiannis DP. Infections following allogeneic stem cell transplantation: New concepts, improved insights, and renewed hope for better outcomes. Virulence. (2016) 7:898–900. doi: 10.1080/21505594.2016.1252019
29. Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. (2012) 14:1258–75. doi: 10.3109/14653249.2012.715243
30. Ciurea SO, Al Malki MM, Kongtim P, Fuchs EJ, Luznik L, Huang X-J, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. (2019) 55:12–24. doi: 10.1038/s41409-019-0499-z
31. Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. (2019) 134:924–34. doi: 10.1182/blood.2019001212
32. Lucarelli B, Merli P, Bertaina V, Locatelli F. Strategies to accelerate immune recovery after allogeneic hematopoietic stem cell transplantation. Expert Rev Clin Immunol. (2016) 12:343–58. doi: 10.1586/1744666X.2016.1123091
33. Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. (2012) 19:324–35. doi: 10.1097/MOH.0b013e328353bc7d
34. Admiraal R, Nierkens S, Bredius R, Bierings M, van Vliet I, Yurda ML, et al. Prospective open-label phase II trial of individualized anti-thymocyte globulin for improved T-cell reconstitution after pediatric allogeneic hematopoietic cell transplantation: the parachute-study. Biol Blood Marrow Transplant. (2020) 26:S33–S4. doi: 10.1016/j.bbmt.2019.12.577
35. Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke G-J, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. (2017) 4:e183–91. doi: 10.1016/S2352-3026(17)30029-7
36. Bonifazi F, Rubio M-T, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. (2020) 55:1093–102. doi: 10.1038/s41409-020-0792-x
37. Bartelink IH, Belitser SV, Knibbe CAJ, Danhof M, de Pagter AJ, Egberts TCG, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. (2013) 19:305–13. doi: 10.1016/j.bbmt.2012.10.010
38. de Koning C, Langenhorst J, van Kesteren C, Lindemans CA, Huitema ADR, Nierkens S, et al. Innate immune recovery predicts CD4+ T-cell reconstitution after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2019) 25:819–26. doi: 10.1016/j.bbmt.2018.10.013
39. Van Roessel I, Prockop SE, Klein E, Boulad F, Scaradavou A, Spitzer B, et al. Early CD4+ T cell reconstruction as predictor for outcomes after allogeneic hematopoietic cell transplantation in pediatric and young adult patients: a validation cohort analyses. Biol Blood Marrow Transplant. (2020) 26:S302–3. doi: 10.1016/j.bbmt.2019.12.400
40. Bartelink IH, Lalmohamed A, van Reij EML, Dvorak CC, Savic RM, Zwaveling J, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. (2016) 3:e526–e36. doi: 10.1016/S2352-3026(16)30114-4
41. Langenhorst JB, van Kesteren C, van Maarseveen EM, Dorlo TPC, Nierkens S, Lindemans CA, et al. Fludarabine exposure in the conditioning prior to allogeneic hematopoietic cell transplantation predicts outcomes. Blood Adv. (2019) 3:2179–87. doi: 10.1182/bloodadvances.2018029421
42. Langenhorst JB, Dorlo TPC, van Maarseveen EM, Nierkens S, Kuball J, Boelens JJ, et al. Population pharmacokinetics of fludarabine in children and adults during conditioning prior to allogeneic hematopoietic cell transplantation. Clin Pharmacokinet. (2019) 58:627–37. doi: 10.1007/s40262-018-0715-9
43. Nunes NS, Kanakry CG. Mechanisms of graft-versus-host disease prevention by post-transplantation cyclophosphamide: an evolving understanding. Front Immunol. (2019) 10:2668. doi: 10.3389/fimmu.2019.02668
44. Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. (2012) 39:683–693. doi: 10.1053/j.seminoncol.2012.09.005
45. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. (2016) 375:143–53. doi: 10.1056/NEJMoa1601202
46. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. (2013) 14:1014–22. doi: 10.1038/ni.2703
47. Soares MV, Azevedo RI, Ferreira IA, Bucar S, Ribeiro AC, Vieira A, et al. Naive and stem cell memory T cell subset recovery reveals opposing reconstitution patterns in CD4 and CD8 T cells in chronic graft vs. host disease. Front Immunol. (2019) 10:334. doi: 10.3389/fimmu.2019.00334
48. Haddad E, Logan BR, Griffith LM, Buckley RH, Parrott RE, Prockop SE, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery. Blood. (2018) 132:1737–1749. doi: 10.1182/blood-2018-03-840702
49. Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. (2014) 110:2560–8. doi: 10.1038/bjc.2014.162
50. Reading JL, Gálvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol Rev. (2018) 283:194–212. doi: 10.1111/imr.12657
51. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. (2002) 295:2097–100. doi: 10.1126/science.1068440
52. Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. (2005) 105:3051–7. doi: 10.1182/blood-2004-07-2974
53. Wilkinson AN, Chang K, Kuns RD, Henden AS, Minnie SA, Ensbey KS, et al. IL-6 dysregulation originates in dendritic cells and mediates graft-versus-host disease via classical signaling. Blood. (2019) 134:2092–106. doi: 10.1182/blood.2019000396
54. Ladányi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. (2007) 56:1459–69. doi: 10.1007/s00262-007-0286-3
55. Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. (2015) 194:2985–91. doi: 10.4049/jimmunol.1403134
56. Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. (2010) 127:77–84. doi: 10.1016/j.imlet.2009.09.003
57. Shang B, Liu Y, Jiang S, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. (2015) 5:15179. doi: 10.1038/srep15179
58. Whangbo JS, Antin JH, Koreth J. The role of regulatory T cells in graft-versus-host disease management. Expert Rev Hematol. (2020) 13:141–4. doi: 10.1080/17474086.2020.1709436
59. Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. (1999) 163:5211–8.
60. Koehn BH, Saha A, McDonald-Hyman C, Loschi M, Thangavelu G, Ma L, et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood. (2019) 134:1670–82. doi: 10.1182/blood.2019001950
61. Kim Y-D, Park S-M, Ha HC, Lee AR, Won H, Cha H, et al. HDAC inhibitor, CG-745, enhances the anti-cancer effect of anti-PD-1 immune checkpoint inhibitor by modulation of the immune microenvironment. J Cancer. (2020) 11:4059–72. doi: 10.7150/jca.44622
62. Fan Q, Liu H, Liang X, Yang T, Fan Z, Huang F, et al. Superior GVHD-free, relapse-free survival for G-BM to G-PBSC grafts is associated with higher MDSCs content in allografting for patients with acute leukemia. J Hematol Oncol. (2017) 10:135. doi: 10.1186/s13045-017-0503-2
63. Zhou H, Zhan F, Zhang H, Gu J, Mu X, Gao J, et al. The proportion of CD19+CD24hiCD27+ regulatory B cells predicts the occurrence of acute allograft rejection in liver transplantation. Ann Transl Med. (2019) 7:465. doi: 10.21037/atm.2019.08.05
64. Wu H, Xia L, Jia D, Zou H, Jin G, Qian W, et al. PD-L1+ regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Mol Immunol. (2020) 119:83–91. doi: 10.1016/j.molimm.2020.01.008
65. Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol. (2013) 34:169–73. doi: 10.1016/j.it.2012.10.007
66. Mathew NR, Baumgartner F, Braun L, O'Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. (2018) 24:282–91. doi: 10.1038/nm.4484
67. Finkelstein SE, Carey T, Fricke I, Yu D, Goetz D, Gratz M, et al. Changes in dendritic cell phenotype after a new high-dose weekly schedule of interleukin-2 therapy for kidney cancer and melanoma. J Immunother. (2010) 33:817–27. doi: 10.1097/CJI.0b013e3181ecccad
68. DiCarlo J, Agarwal-Hashmi R, Shah A, Kim P, Craveiro L, Killen R, et al. Cytokine and chemokine patterns across 100 days after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. (2014) 20:361–9. doi: 10.1016/j.bbmt.2013.11.026
69. Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. (2015) 2:e21–9. doi: 10.1016/S2352-3026(14)00035-0
70. Adom D, Rowan C, Adeniyan T, Yang J, Paczesny S. Biomarkers for allogeneic HCT outcomes. Front Immunol. (2020) 11:673. doi: 10.3389/fimmu.2020.00673
71. Luft T, Benner A, Jodele S, Dandoy CE, Storb R, Gooley T, et al. EASIX in patients with acute graft-versus-host disease: a retrospective cohort analysis. Lancet Haematol. (2017) 4:e414–23. doi: 10.1016/S2352-3026(17)30108-4
72. Van Gool A, Corrales F, Colović M, Krstić D, Oliver-Martos B, Martínez-Cáceres E, Jakasa I, et al. Analytical techniques for multiplex analysis of protein biomarkers. Expert Rev Proteomics. (2020) 17:257–73. doi: 10.1080/14789450.2020.1763174
73. Keustermans GCE, Hoeks SBE, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. (2013) 61:10–7. doi: 10.1016/j.ymeth.2013.04.005
Keywords: immune monitoring, immune reconstitution, hematopoietic (Stem) cell transplantation (HCT), cellular therapies, harmonization
Citation: Boelens JJ, Hosszu KK and Nierkens S (2020) Immune Monitoring After Allogeneic Hematopoietic Cell Transplantation: Toward Practical Guidelines and Standardization. Front. Pediatr. 8:454. doi: 10.3389/fped.2020.00454
Received: 05 March 2020; Accepted: 30 June 2020;
Published: 21 August 2020.
Edited by:
Emmanuel Katsanis, University of Arizona, United StatesReviewed by:
Leo D. Wang, City of Hope National Medical Center, United StatesGeoff D. E. Cuvelier, CancerCare Manitoba, Canada
Copyright © 2020 Boelens, Hosszu and Nierkens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaap Jan Boelens, Ym9lbGVuc2omI3gwMDA0MDttc2tjYy5vcmc=
 Jaap Jan Boelens
Jaap Jan Boelens Kinga K. Hosszu
Kinga K. Hosszu Stefan Nierkens2
Stefan Nierkens2