
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 28 July 2020
Sec. Neonatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00366
This article is part of the Research TopicPrecision Medicine in NeonatesView all 12 articles
 Karel Allegaert1,2,3*
Karel Allegaert1,2,3* Anne Smits1,4
Anne Smits1,4 Tamara van Donge5
Tamara van Donge5 John van den Anker5,6,7
John van den Anker5,6,7 Kosmas Sarafidis8
Kosmas Sarafidis8 Elena Levtchenko1,9
Elena Levtchenko1,9 Djalila Mekahli1,9
Djalila Mekahli1,9Renal precision medicine in neonates is useful to support decision making on pharmacotherapy, signal detection of adverse (drug) events, and individual prediction of short- and long-term prognosis. To estimate kidney function or glomerular filtration rate (GFR), the most commonly measured and readily accessible biomarker is serum creatinine (Scr). However, there is extensive variability in Scr observations and GFR estimates within the neonatal population, because of developmental physiology and superimposed pathology. Furthermore, assay related differences still matter for Scr, but also exist for Cystatin C. Observations in extreme low birth weight (ELBW) and term asphyxiated neonates will illustrate how renal precision medicine contributes to neonatal precision medicine. When the Kidney Disease Improving Global Outcome (KDIGO) definition of acute kidney injury (AKI) is used, this results in an incidence up to 50% in ELBW neonates, associated with increased mortality and morbidity. However, urine output criteria needed adaptations to broader time intervals or weight trends, while Scr and its trends do not provide sufficient detail on kidney function between ELBW neonates. Instead, we suggest to use assay-specific centile Scr values to better describe postnatal trends and have illustrated its relevance by quantifying an adverse drug event (ibuprofen) and by explaining individual amikacin clearance. Term asphyxiated neonates also commonly display AKI. While oliguria is a specific AKI indicator, the majority of term asphyxiated cases are non-oliguric. Asphyxia results in a clinical significant—commonly transient—mean GFR decrease (−50%) with a lower renal drug elimination. But there is still major (unexplained) inter-individual variability in GFR and subsequent renal drug elimination between these asphyxiated neonates. Recently, the Baby-NINJA (nephrotoxic injury negated by just-in-time action) study provided evidence on the concept that a focus on nephrotoxic injury negation has a significant impact on AKI incidence and severity. It is hereby important to realize that follow-up should not be discontinued at discharge, as there are concerns about long-term renal outcome. These illustrations suggest that integration of renal (patho)physiology into neonatal precision medicine are an important tool to improve contemporary neonatal care, not only for the short-term but also with a positive health impact throughout life.
Precision medicine is defined as a structured approach to treat or prevent specific diseases based on the inter-individual variability in genes, physiology, and environment. This includes exploration of novel research approaches to improve the use of available information to support decision making about pharmacotherapy, signal detection of adverse (drug) events, or to improve individual prediction of short- and long-term prognosis. Neonatal renal precision medicine depends on the availability of reference intervals for any renal biomarker to support clinical decision making, tailor therapy, or support prognosis. This is still a major limitation, highlighted in the International Neonatal Consortium (INC) paper on safety, dosing, and pharmaceutical quality of medical products in neonates and during development of the neonatal adverse event severity scale. Severity grading for lab values—including kidney function—was omitted until reference values became available (1, 2).
Since maturational physiological changes are most prominent in early infancy, variability is their key feature. This is reflected in extensive inter- and intra-individual variability in serum creatinine (Scr), resulting in a cloud instead of extractable and interpretable information for clinicians. This “cloud” is illustrated in Figure 1, after plotting Scr observations (enzymatic assay) in (pre)term neonates collected in one Neonatal Intensive Care Unit (NICU) in the first 42 postnatal days of life (3). This reflects a pattern with an initial increase and subsequent decrease during postnatal life. As relevant, there is about a 4-fold difference in Scr observed for all consecutive days, so that improved understanding on reference values is needed to attain precision medicine.
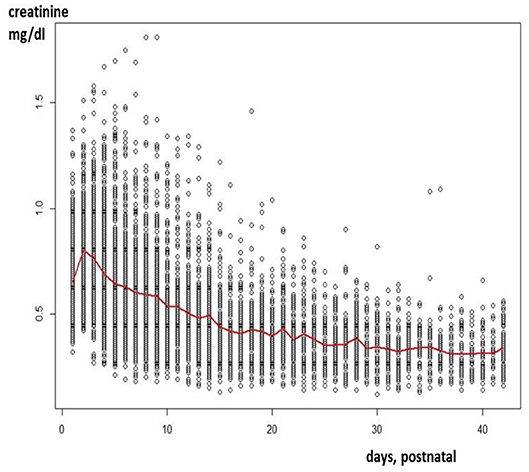
Figure 1. Creatinine values (enzymatic assay) as observed in a cohort of 1,140 neonates (gestational age 23–42 weeks) in the first 42 days of postnatal life (3).
This Scr variability is partly explained by maturational changes (e.g., birth weight, gestational age [GA], postnatal age) and non-maturational changes related to pathophysiology, e.g., perinatal asphyxia, co-medication, congenital anomalies of the kidney and urinary track (CAKUT), cardiac surgery with bypass, or extra-corporeal membrane oxygenation (4, 5). Postnatal kidney adaptation is proportional to the nephron number (GA driven) and renal perfusion (postnatal adaptation, mean arterial blood pressure) (6, 7). Nephrogenesis evolves as branching morphogenesis, similar to lung, pancreas, vascular tree, or retina. Neonates <36 GA weeks are still in active nephrogenesis and thus have an increased risk for decreased nephron endowment with lifelong impact (8). The main determinants of the increase in renal function in early life are circulatory changes, driven by the increase in proportional renal blood flow to cardiac output, from 2 to 25%. This increased renal blood flow is combined with dilation of the efferent and constriction of the afferent arterioli. This explains the significant impact of non-steroidal anti-inflammatory drugs (NSAIDs, −20 up to −40% of the glomerular filtration rate [GFR] in ELBW neonates, depending on the type and dose) or asphyxia (up to −40 to −50% of GFR).
Scr is the most commonly measured biomarker to estimate kidney function or GFR (creatinine clearance). However, before Scr can be used to estimate renal elimination capacity in neonates, issues that should be considered relate to physiology (renal tubular transport, hydration, muscle mass) and measurement (assay validity).
Creatinine at birth does not yet reflect neonatal but maternal Scr levels. Because of passive tubular back leak instead of active secretion, creatinine clearance does not yet fully reflect GFR. In contrast to later life, where creatinine clearance somewhat overestimates GFR due to active tubular secretion mediated by Organic Cation Transporter 2 (OCT-2), passive back leak occurs in early neonatal life (9, 10). Hydration is another issue, as early neonatal life is associated with weight reduction due to free water loss, usually associated with a sodium increase. Creatinine is a low molecular weight (133 g/mol) molecule produced by muscle catabolism (creatine to creatinine), reflecting muscle mass. As estimated by creatinine excretion in urine, muscle mass increased from 12% of birth weight at 25 weeks to 19% at 34 GA weeks and 24% at term (11). In contrast, the proportional muscle mass was estimated to remain stable (22–30%) without trend related to GA in autopsy findings (12).
Scr values also depend on the assay, as the Jaffe assay is affected by specific constituents of neonatal serum like bilirubin or albumin concentrations. Harmonization through isotope dilution mass spectrometry (IDMS) traceability has reduced, but not eliminated, this inter-assay variability (9, 13). Scr measurements can subsequently be converted to estimated GFR, using the Schwartz formula (eGFR = k [L/Scr], Scr = μmol/l; k = 0.34 in preterm, 0.45 in term infants, L = length, cm). One should hereby be aware that this Schwartz formula has initially been validated (to inulin clearance) with the original, non-compensated Jaffe assay (9). Furthermore, length measurement has limitations in neonates (14). While lower k-values have been suggested when enzymatic assays are used, these studies have not included (pre)term neonates and infants (9). Recently, an eGFR specific to (pre)term neonates (median age 3 days postnatal age, compensated Jaffe) was suggested (eGFR= 2.32 × [weight (g)0.64/Scr (μmol/l)0.62]) following validation with inulin clearance (21.54 [SD 10.09] ml/min/1.73 m2). This formula performed somewhat better compared to the original neonatal Schwartz formula (15).
Cystatin C is an alternative to Scr to assess eGFR and is considered to be a more sensitive indicator for minor GFR changes. Cystatin C is a 130 amino acids containing small protein, generated by any cell with elimination by GFR. Reference values have been suggested, but Cystatin C also has assay-related issues like Scr. Figure 2 reflects assay-specific differences in mean umbilical cord blood Cystatin C values reported in 15 cohorts of healthy term neonates. In these 15 cohorts, Cystatin C was quantified by enzymatic immuno-assays (ELISA, n = 2), particle enhanced turbimetric immuno-assays (PETIA, n = 3), or particle enhanced nephelometric immuno-assays (PENIA, n = 10) (16–22). Since 2010, certified reference material for Cystatin C assay standardization (IDMS) has been available, but measurement bias still exists (23). This is relevant, since the majority of studies with Cystatin C in neonates were conducted in single units or with one assay. Within this setting, Cystatin C values are associated with maturational covariates (age, weight) or perinatal diseases, like congenital renal anomalies, sepsis, and septic shock, respiratory distress, hypotension, transient tachypnea of the newborn, or perinatal asphyxia, serving as a more sensitive indicator for renal dysfunction (16, 24, 25). However, clinicians should be cautious in extracting absolute values as reported for subsequent use in their specific setting as assay-related issues may exist. Of specific relevance to neonates, steroid administration or hypothyroidism may also affect Cystatin C (16, 26). Finally, Cystatin C was not retained in the recently published eGFR formula specific to (pre)term neonates (15).
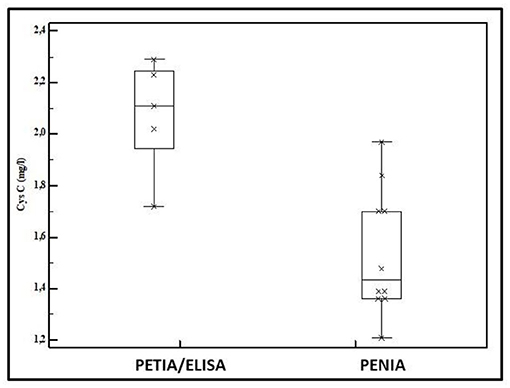
Figure 2. Mean Cystatin C values in umbilical cord blood as reported in 15 cohorts of healthy term neonates (2 enzymatic immuno-assay [ELISA], three particle enhanced turbimetric immuno-assay [PETIA], and 10 particle enhanced nephelometric immuno-assay [PENIA]) (16–22).
We therefore suggest development of age-dependent, assay specific Scr centiles to support clinical decisions and precision pharmacotherapy. We will focus on ELBW (<1,000 g) infants and on term neonates with perinatal asphyxia undergoing whole body hypothermia (WBH) to illustrate how a “Scr cloud” can be converted into a clinical decision tool. With these examples, we will illustrate how renal precision medicine is a crucial part of modern neonatal care. A similar approach can be considered for other subcategories like CAKUT newborns or neonates in need of cardiac bypass or extracorporeal membrane oxygenation.
• There is extensive variability in Scr and eGFR (Schwartz formula, Wilhelm-Bals formula) within the neonatal population, driven by (patho)physiology.
• Assay related differences exist for Scr and Cystatin C.
• We suggest to develop age-dependent, assay specific Scr centiles to support neonatal precision medicine.
Almost a decade ago, Jetton and Askenazi (27) suggested an AKI definition (neonatal modified KDIGO) specific for use in neonates. In essence, the definition is based on trends (increase) in Scr and in urine output to result in staging (stage 0–3) (Table 1). This definition was endorsed by a National Institute of Diabetes, Digestive, and Kidney (NIDDK) Diseases workshop. The attendees hereby concluded that this definition offered a reasonable starting point, but that further evaluation was needed (30, 31). In this context, a study protocol (Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates, AWAKEN) was put forward to assess its applicability and to report on neonatal AKI epidemiology (28).
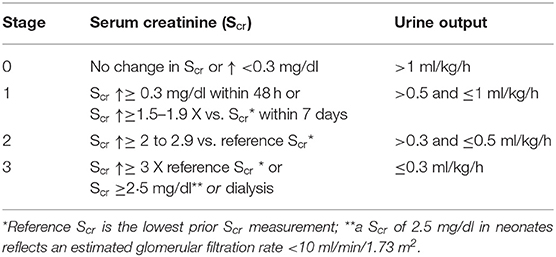
Table 1. Definition of neonatal Acute Kidney Injury (AKI) by serum creatinine and urine output (28, 29).
We highlight some results of this AWAKEN study specific to ELBW neonates to emphasize the limitations of the KDIGO definition: (i) the overall AKI incidence was 29.9%, but was 47.9% in preterm neonates (<29 weeks GA) (limited discriminating power); (ii) 8/24 of the contributing units still used a Jaffe assay (relevant differences in absolute values not considered in the definition); (iii) the median number of Scr counts was ≤3 and ≤5/patient respectively in 10 and 15/24 of these units (suggesting that there is not yet sufficient focus on renal function, even in AWAKEN units) (iv) urine output has been quantified in 24 h intervals with 1 ml/kg/h as pivotal finding (pragmatic, but not as suggested in the definition, Table 1), (v) despite these limitations, the AKI stage predicted mortality (adjusted Odds Ratio 3.7) but not length of stay in <29 weeks neonates (29). In secondary analyses specific in the most immature cohorts, an increased risk for bronchopulmonary dysplasia was observed in 29–32 GA cases (adjusted Odds Ratio 4.2), but not in <29 GA neonates (32). Early (within the first week of life) caffeine administration was associated with reduced AKI incidence (number needed to treat = 4.3) and severity (33). This can be explained by the adenosine related effects on the glomerular vascular tone. Finally, AKI was not limited to early neonatal life but also occurred beyond day 7 (9%). Risk factors were the presence of a patent ductus arteriosus with or without NSAIDs exposure, necrotizing enterocolitis, and sepsis (34).
Although the current AKI definition may assist clinicians to recognize renal issues, there are still limitations to using this AKI tool for precision medicine in ELBW cases. As 47.9% of cases <29 weeks were classified as having AKI, the granularity needed for precision medicine is somewhat lost. The issues relate to both urine output and Scr as biomarkers of kidney function and AKI.
Continuous quantification of urine output is a technical burden and is even more difficult in the most immature neonates, as catheterization is invasive while sequential diaper weight is hampered by evaporation (up to 80% weight losses after 2 h of a 5 ml portion added to a diaper exposed in an incubator or under a radiant warmer) (35). This was already acknowledged by the AWAKEN study, since urine output was quantified by 24 h increments with 12% missing observations for urine output (29). Fluid overload and daily weight balance (change% = current weight – birthweight/current weight) in the 1st week of life were used as alternative markers. Based on these markers, a higher positive peak in the 1st week of life and a positive fluid balance on day 7 were associated with mechanical ventilation (36).
Scr itself is not an AKI biomarker, but rather an indicator of kidney function. As mentioned earlier, absolute values are affected by the assay (dependent on IDMS traceability), since Jaffe results are affected by some drugs and—more relevant to ELBW neonates—by bilirubin so that the median difference between the original Jaffe and an enzymatic assay is 0.12 to 0.27 mg/l, with always higher values for the Jaffe assay (37). Furthermore, the maturational Scr changes over postnatal age are extensive in ELBW neonates. There is an initial increase to peak on day 3, with a subsequent slow decrease over postnatal age (38, 39). To further illustrate this, we have summarized the postnatal Scr trends (10th, 25th, median, 75th, 90th, and 95th centile) over the first 28 days of life in an cohort of 217 ELBW cases (single unit, enzymatic assay, all exposed to caffeine) in Table 2 (38). If we focus on the median estimates, there is a clear increase from day 1 to 3 by 0.3 mg/dl, so that this median “normal” trend already qualifies for an AKI stage 1 classification. From a physiological point of view, it may even be reasonable to classify a relevant portion of ELBW as having AKI. However, by using a centile approach, more “granularity” in the data is provided to facilitate precision medicine.
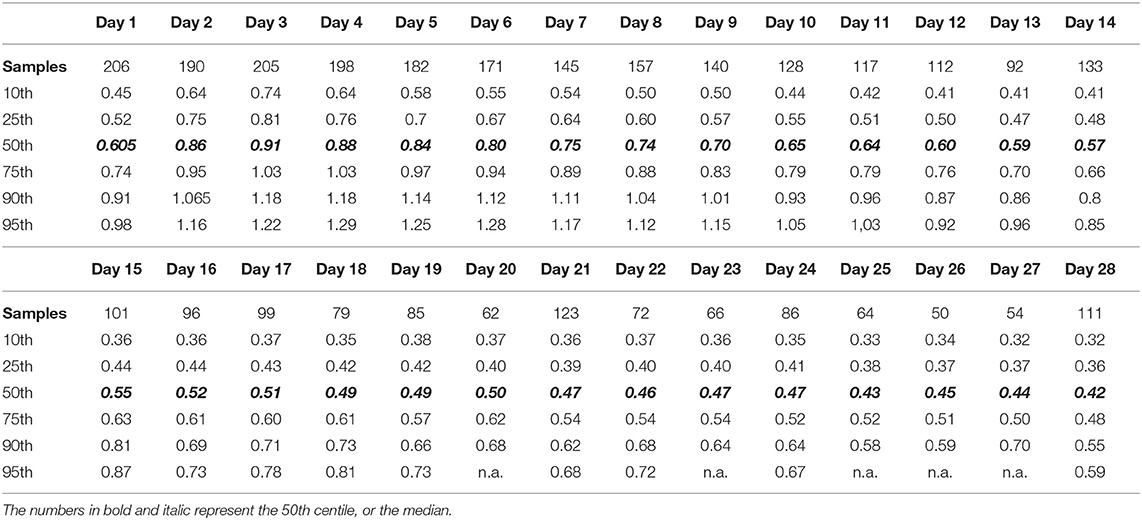
Table 2. Centiles (10th−95th) of serum creatinine values (enzymatic assay) in a cohort of 217 extremely low birth weight (ELBW) infants in the first 28 days of postnatal age (38).
Plotting individual observations or Scr trends over time in a single ELBW infant may facilitate recognition and quantification of an adverse drug event or may even facilitate precision pharmacotherapy, a concept somewhat similar to growth charts. To illustrate this, we compared mean Scr in ibuprofen-exposed ELBWs in the earlier mentioned cohort to these centiles (38). Figure 3 (visual presentation of Table 2 data) illustrates the reference Scr (gray lines) over postnatal age, with the plotted trend (black line) of median Scr observed in ELBW neonates exposed to ibuprofen. A shift of about 1 standard deviation in Scr 133 ibuprofen-exposed neonates is hereby observed (38, 39). On the other hand, we investigated how individual amikacin clearances are linked to Scr centiles (38). For aminoglycosides like amikacin, there is a strong correlation between clearance and GFR, also in neonates (40). Consequently, GA and ibuprofen affect amikacin clearance in early neonatal life (41). Integration of Scr centiles (<25th centile, 25–75th centile, or >75th centile) in this dataset further explained the individual amikacin clearance estimates (Figure 4) (38, 39). Along the same line, Cystatin C reference values (type of assay unclear, Modular Analytics ISE900 Analyzer, Germany) for ELBW (integrated in a cohort of very low birth weight, <1.5 kg) infants on day 1 and 3 [mean (SD) 1.77 (0.38) and 1.61 (0.37 mg/L, respectively)] have been suggested in the literature. In contrast to Scr values, observations were independent of the GA (42).
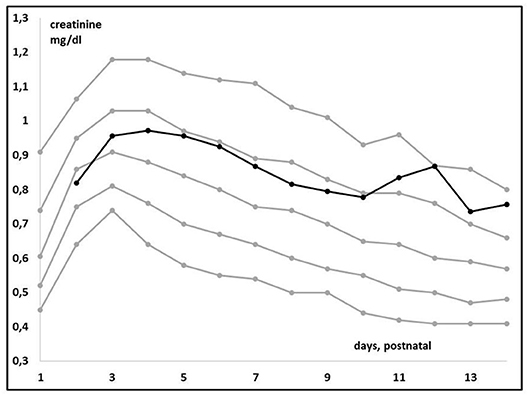
Figure 3. Trends in median creatinine values (enzymatic assay) in extreme low birth weight (ELBW) neonates when exposed to ibuprofen (n = 133, black line) compared to the reference centile trends (Table, gray lines) over time in the first 14 days of postnatal life (38).
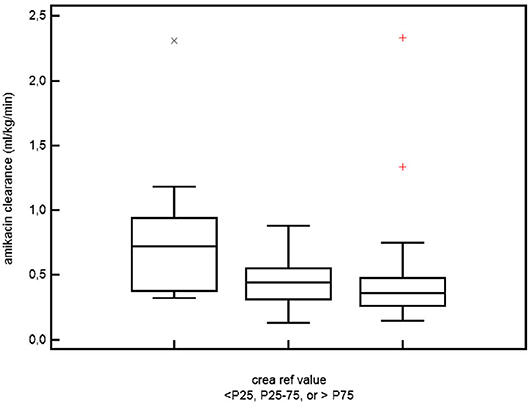
Figure 4. The relation between serum creatinine and amikacin clearance in ELBW neonates. Differences in amikacin clearance in ELBW neonates who had a serum creatinine reference value either <25th, between 25th and 75th, or >75th centile for ELBW neonates and for the specific postnatal day (38, 39, 41) (permission for re-use of the figure has been granted, Rightslink).
• Using the KDIGO-AKI definition results in an AKI incidence of about 50% in ELBW neonates. Similar to other populations, AKI is associated with increased mortality and morbidity.
• For pragmatic reasons, the criteria on urine output were converted to more extensive time intervals or weight trends.
• We suggest to use assay-specific Scr centiles to better describe the normal postnatal trend and its variability. We illustrated that this approach facilitates recognition and quantification of adverse drug events (ibuprofen, Figure 3) or to explain individual amikacin clearance (Figure 4).
Perinatal asphyxia is a multi-organ disease, with moderate to severe encephalopathy as pivotal finding to initiate WBH in term neonates (4). This also includes AKI. As part of precision medicine, quantifying the incidence, extent, and variability of AKI and its covariates is relevant to tailor fluid administration and pharmacotherapy (4, 43). AKI has also been identified as prognostic factor for adverse neurological outcome and death (44, 45).
AKI occurs in neonates following perinatal asphyxia, but the incidence varies and in part depends on the case mix. In 36 neonates with asphyxia (Apgar score 5 min <7), AKI was documented in 1/11 (9%) with moderate and 12/25 (56%) with severe asphyxia (46). When we focus on WBH cases, AKI (according to the modified neonatal KDIGO criteria) was diagnosed in 39–42% (47, 48). Oliguria (<1 ml/kg/h for 12 h) was observed in 11% WBH neonates (49). In the most recent Cochrane meta-analysis, there was a trend to a lower incidence of renal impairment (urine output <0.5 ml/kg/h for ≥24 h + Scr >1 mg/dl) for neonates undergoing WBH (38.5 vs. 45%, risk ratio and 95% CI, 0.87, 0.74–1.02), without effect on oliguria (<1 mg/kg/h, 23 vs. 24%) (50).
Table 3 provides an overview on Scr observations (including assay) from day 1 to 10 as reported in cohorts of (pre)term asphyxia neonates (including criteria) in the era before WBH. Compared to the Gupta and Kaur cohorts, the other cohorts had more restrictive inclusion criteria and likely better reflect the neonates that currently qualify for WBH (19, 44, 51–54). These data remain valuable as some neonates may miss the 6-h therapeutic window or to subsequently compare such data with WBH-related observations. In these studies, different definitions for AKI or kidney failure were used (Table 3).
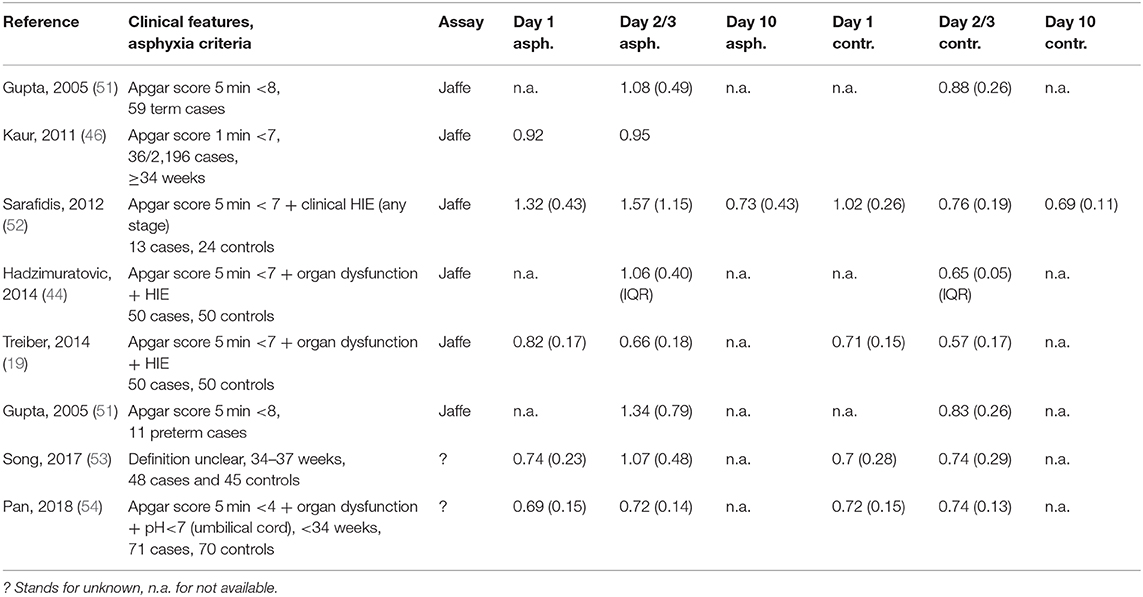
Table 3. Overview of serum creatinine observations (assay mentioned, mean, and standard deviation or interquartile range, all converted to mg/dl) on day 1, 2, or 3 and day 10 in term and preterm (light gray) asphyxiated neonates (criteria provided) and gestational age-matched controls (19, 44, 46, 51–54).
In the Gupta cohort, 47% of asphyxia neonates were classified as having “renal failure” [blood urea >40 mg/dl, Scr >1 mg/dl (Jaffe) or oliguria (<0.5 ml/kg/h)] (51). Mean blood urea and Scr on day 3 of postnatal life were significantly higher (+60 and 30%, respectively) in asphyxiated neonates, while the standard deviation (SD) for both biochemical markers doubled, indicating an increase in patient variability. This variability was in part explained by the HIE stage (mean [SD] Scr for HIE stage 0, 1, 2, or 3 was 0.9 [0.2], 1.1 [0.4], 1.3 [0.8], 1.4 [0.6] mg/dl, respectively). Interestingly, the urine output was comparable between cases and controls while oliguria was rare (7/33 renal failure cases), while oliguric vs. non-oliguric renal failure was associated with higher mortality (43 vs. 8%). During follow-up, urine output normalized from day 4–6 onwards, urea and Scr from day 7–9 onwards (51). In the more recently reported AWAKEN cohort (113 WBH neonates, AKI incidence 42%), oliguria was more commonly observed in AKI cases (isolated oliguria in 47, 26% mixed with Scr thresholds, Table 1) (47). This confirms the complexity of AKI diagnosis, and the need to simultaneously assess both diuresis and Scr or Cystatin C to tailor clinical care and pharmacotherapy. Kaur et al. reported on mean Scr (Jaffe) values 24–36 h and 72–96 h in asphyxiated neonates (0.92 and 0.95 mg/dl), with significant differences between AKI and non-AKI neonates (1.49 vs. 0.8 mg/dl and 1.65 vs. 0.81 mg/dl) for both time intervals (46).
In the Sarafidis cohort, AKI (any Scr > 1.5 mg/dl, or increase >0.3 mg/dl from day 1, Jaffe) was observed in 8/13 (61%). The Apgar score at 5 min was significantly lower in subsequent AKI neonates, while other indicators of asphyxia severity (inotropics, ventilation, anti-epileptic drugs, HIE moderate/severe, mortality) also associated with AKI (52). Scr (day 1, 3, and 10) were significantly higher in asphyxiated neonates (1.32, 1.57, and 0.73 mg/dl) compared to controls (1.02, 0.76, and 0.69 mg/dl) with normalization on postnatal day 10. There is also a broader range (SD higher), reflecting higher inter-patient variability in renal impairment in asphyxia cases. In the Sarafidis cohort, several other biomarkers of renal GFR or tubular damage were also quantified. Serum Cystatin C (ELISA) was marginally increased only on day 1 in the asphyxiated neonates, while urine Cystatin C and Neutrophil Gelatinase-associated lipocalin were significantly increased in cases until day 10, suggesting earlier restoration of glomerular than tubular impairment (52). In the Hadzimuratovic cohort, Scr (Jaffe) and Cystatin C (turbimetric assay) was significantly higher (63 and 49%, respectively) in asphyxia cases. In contrast to the findings of Sarafidis, both the absolute Cystatin C values and trends remained different, perhaps reflecting assay-related difference. The HIE stage (I vs. III) in part explained the Scr variability on day 3. Finally, Treiber et al. also reported on renal biomarkers (Scr, Jaffe; Cystatin C, nephelometry) at birth (umbilical cord) and on day 3 in a cohort of asphyxia neonates. Interestingly, Scr and Cystatin C were significantly higher from delivery onwards. Umbilical Cystatin C was the most sensitive marker of asphyxia (receiver operating characteristic curve = 0.918) (Table 3).
A specific focus on preterm neonates is warranted. These patients do not qualify for WBH but can still display asphyxia-related AKI (Table 3). Gupta et al. (51) documented that the mean Scr is significantly higher (+60%) in asphyxiated (Apgar score 5 min <8) preterms. Song et al. (53) reported on Scr (assay unclear) values in near-term (34–37 GA) asphyxia (definition unclear, n = 48) and age-matched controls, and observed a significant decrease (−20%) in eGFR in asphyxia cases. Pan et al. included 71 preterms (<34 weeks) with asphyxia (pH <7 + Apgar at 5 min <4 + multi organ dysfunction) and 70 preterm controls, and collected samples at 24, 48, and 96 h. Scr was significantly higher at 96 h (83.5 vs. 62.9 μmol/l) and eGFR was consistently lower at 24 and 48 h (−30 and −20%, respectively) (54). Cystatin C (PENIA) had good distinguishability between asphyxiated and non-asphyxiated preterms, irrespective (<28, 28–32, or ≥32 weeks subgroups) of GA, and further discriminated between mild, moderate, and severe asphyxia. In contrast, Scr (assay unclear) was not discriminative (55).
AKI affects the renal and non-renal outcome in asphyxia neonates. AKI is associated with a 4.6-fold higher mortality risk in the AWAKEN study, and this higher mortality risk also holds true for post-asphyxiated neonates with AKI (29, 51). When considering the renal outcome after discharge, Hadzimuratovic et al. (44) and Gupta et al. (51) reported on normalization of renal findings at 1 and 6 months, respectively in asphyxia cases (44, 51). AKI also associates with non-renal outcome, like prolonged hospital stay (48), prolonged mechanical ventilation (47), or abnormal brain imaging findings (73 vs. 46%) at the end of the first week of life (56). Post-asphyxial renal injury (urine output + Scr) was a prognostic factor for neurological outcome at the end of the 1st year of life (44). However, the absence of AKI neither guarantees a positive outcome (45).
Once AKI has been identified, precision medicine involves fluid and electrolyte management, drug choice (avoid nephrotoxic drugs, potential nephro-protective interventions), and for those drugs, dose selection.
In the earlier mentioned AWAKEN study, the daily %-weight change from birth weight in the 1st week of postnatal life was used to reflect the fluid balance in 645 critically ill term neonates. A higher peak fluid balance and higher fluid balance over the first week of life were independently associated with mechanical ventilation on day 7. A negative fluid balance was observed in 53% of neonates (21 vs. 41% in ventilated vs. non-ventilated neonates). Those with AKI had a consistently higher fluid balance throughout the 1st week of life (36). Suggested nephro-protective interventions include “low dose dopamine” or methylxanthines, like theophylline (57). At present, there is no robust evidence for the use of low dose dopamine to protect kidney function, while there is meta-analytic evidence that prophylactic theophylline (single intravenous dose, 5 mg/kg) results in a significant lower AKI incidence (OR 0.24) in asphyxiated neonates (58). Despite the evidence, neonatologists remain reluctant to administer theophylline as this increases metabolic activity of the brain, while hypothermia and sedation are used to reduce metabolic (cerebral) activity.
With respect to precision pharmacotherapy, it is important to realize that the mean difference in Scr or eGFR between asphyxiated neonates (either or not undergoing WBH) compared to term controls is clinical significant (−40 to −50%) and further adds to maturational (weight, postnatal) changes. This is reflected by the impact of asphyxia on drugs exclusively cleared by renal elimination (4). To illustrate this, amikacin clearance trends in early neonatal life based on pooling of reported datasets were plotted (Figure 6). There is a maturational trend in clearance, related to birth weight and postnatal age (day 1, 2, 3, 4, as indicated by different colors) compared to a subgroup of WBH neonates. These differences indicate mean differences in clearance, but do not cover the additional unexplained between-individual variability (59). Essentially, there is a shift in the Gauss curve for Scr or eGFR toward renal impairment, but mean differences do not fully cover the between individual variability. For this type of drugs, this means that the time interval between consecutive administrations should be extended (in general from 24 to 36 h, so compensating for the 40–50% decrease in clearance) but therapeutic drug monitoring remains compulsory as there still will be toxic trough levels in 14–25% of cases (59).
• Oliguria is a specific indicator of AKI, but the majority of AKI cases are non-oliguric. Assessment of the fluid balance is an alternative. This confirms the complexity of AKI diagnosis, and the need to simultaneously assess both diuresis and Scr or Cystatin C to tailor clinical care and pharmacotherapy.
• Asphyxia with WBH results in a clinical significant—often transient—mean decrease in eGFR (−40 to −50%), with GA and HIE stage as additional covariates (Table 3).
• This mean decrease in GFR affects renal drug elimination. However, there is still large (unexplained) inter-individual variability in GFR (mean vs. SD) and renal drug clearance in asphyxiated neonates (Figures 5, 6).
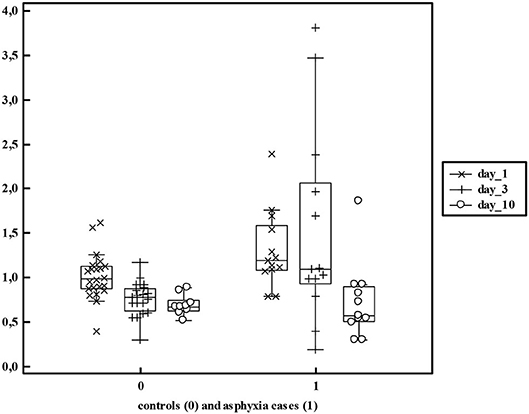
Figure 5. Serum creatinine values in non-asphyxiated (n = 24) and asphyxiated neonates (n = 13) as reported by Sarafidis et al. (52) reflecting both the mean differences and additional variability (>5 fold) within these cohorts.
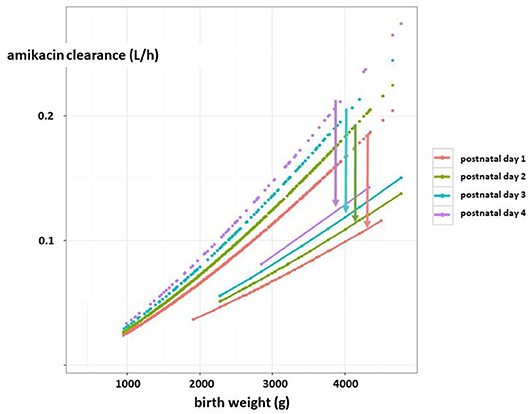
Figure 6. Estimates of amikacin clearance (l/h) trends in early neonatal life based on pooling of reported datasets (dashed lines). There is a maturational trend in clearance, related to birth weight (g) and postnatal age (day 1, 2, 3, 4, as reflected by the colors) compared to a subgroup of term neonates undergoing therapeutic hypothermia as treatment for perinatal asphyxia (solid lines). The arrows indicate the difference in clearance between both cohorts for the respective postnatal age (in days) (4, 59) [this figure has earlier been published in reference (4), permission for re-use has been granted, first author Anne Smits].
Using ELBW and asphyxia as case examples, we illustrated that kidney function and AKI are relevant to contemporary neonatal care. In ELBW, AKI is associated with increased mortality and morbidity while Scr centiles were used to recognize and quantify adverse drug events and to explain individual amikacin clearance. Asphyxia associated AKI affects mortality and morbidity. WBH is associated with a significant mean (−40 to −50%) GFR decrease. Of relevance, there is still important variability in GFR decrease around this mean decrease, so that the mean decrease does not predict well the GFR decrease in an individual neonate. Although these data strongly suggest that better integration of renal precision is important to improve contemporary neonatal care, the AWAKEN study showed that the median number of Scr measurements was ≤3 and ≤5/patient in 10 and 15/24 of the units, suggesting that further improvements can be made (29). There are some elegant illustrations on how these improvements can be implemented and how a focus on renal aspect indeed improves neonatal care.
In the Baby NINJA study, 476 individual events of high-risk nephrotoxic drug exposure were observed. During these events, a daily Scr was obtained until 2 days after exposure or after end of AKI. Within this framework, there was a reduction in exposure (16.4 to 9.6/1,000 patient days), a reduction in drug-associated AKI (30.9 to 11%), and in AKI intensity (9.1 to 2.9/100 susceptible patient days) (60). Implementation of AKI guidelines in a single NICU resulted in improvements in recognition, diagnosis, and subsequent follow-up of AKI (61). This matters, as in ELBW infants, exposure to nephrotoxic drugs is common (87%) with gentamicin (86%), indomethacin (43%), and vancomycin (25%) as most commonly administered drugs (62). It is hereby important to highlight that follow-up of these populations remains important after hospital discharge as there are concerns on the long-term renal outcome, most pronounced in former ELBW cases (63). Research should focus on perinatal risk factors associated with impaired GFR in long-term outcome studies, but is hampered by single center cohorts, small samples sizes, and heterogeneity of GFR assessment tools (64). The diagnosis of AKI remains complex with integrated assessment both of diuresis and Scr or Cystatin C to tailor clinical care and pharmacotherapy. We therefore state that further integration of renal (patho)physiology into neonatal precision medicine and pharmacotherapy may not only result in better short-term outcome but also may have impact throughout pediatric life and beyond.
KA initiated the project. AS and KS hereby had a specific focus on the section on acute kidney injury following perinatal asphyxia. TD and JA had a specific focus on the section of acute kidney injury. EL and DM provided crucial nephrologic expertise on these sections and on the introduction and discussion section. All authors approved the final version of the paper.
TD and JA are funded by the Eckenstein-Geigy foundation, EL by F.W.O. Vlaanderen, grant 1801110N. The research activities of AS are supported by the Clinical Research and Education Council, University Hospitals Leuven.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Ward RM, Benjamin D, Barrett JS, Allegaert K, Portman R, Davis JM, et al. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr Res. (2017) 81:692–711. doi: 10.1038/pr.2016.221
2. Salaets T, Turner MA, Short M, Ward RM, Hokuto I, Ariagno RL, et al. Development of a neonatal adverse event severity scale through a Delphi consensus approach. Arch Dis Child. (2019) 104:1167–73. doi: 10.1136/archdischild-2019-317399
3. Allegaert K. Creatinine assays in early infancy: how to aim for a moving target. In: Patel VB, Preedy VR, editors. Biomarkers in Kidney Disease. Dordrecht: Springer Science+Business Media. (2015). p. 271–300.
4. Abitbol CL, DeFreitas MJ, Strauss J. Assessment of kidney function in preterm infants: lifelong implications. Pediatr Nephrol. (2016) 31:2213–22. doi: 10.1007/s00467-016-3320-x
5. Filler G, Lee M. Educational review: measurement of GFR in special populations. Pediatr Nephrol. (2018) 33:2037–46. doi: 10.1007/s00467-017-3852-8
6. Luyckx VA, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the low birth weight and nephron number working group. Lancet. (2017) 390:424–8. doi: 10.1016/S0140-6736(17)30576-7
7. Smits A, Annaert P, van Cruchten S, Allegaert K. A physiology-based pharmacokinetic framework to support drug development and dose precision during therapeutic hypothermia in neonates. Front Pharmacol. (2020) 11:587. doi: 10.3389/fphar.2020.00587
8. Raffaeli G, Pokorna P, Allegaert K, Mosca F, Cavallaro G, Wildschut ED, et al. Drug disposition and pharmacotherapy in neonatal ECMO: from fragmented data to integrated knowledge. Front Pediatr. (2019) 7:360. doi: 10.3389/fped.2019.00360
9. Kastl JT. Renal function in the fetus and the neonate - the creatinine enigma. Semin Fetal Neonatal Med. (2017) 22:83–9. doi: 10.1016/j.siny.2016.12.002
10. Schreuder MF, Bueters RR, Allegaert K. The interplay between drugs and the kidney in premature neonates. Pediatr Nephrol. (2014) 29:2083–91. doi: 10.1007/s00467-013-2651-0
11. Modi N, Hutton JL. Urinary creatinine excretion and estimation of muscle mass in infants of 25-34 weeks gestation. Acta Paediatr Scand. (1990) 79:1156–62. doi: 10.1111/j.1651-2227.1990.tb11404.x
12. Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a Physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. (2015) 21:5688–98. doi: 10.2174/1381612821666150901110533
13. Allegaert K, Pauwels S, Smits A, Crèvecoeur K, van den Anker J, Mekahli D, et al. Enzymatic isotope dilution mass spectrometry (IDMS) traceable serum creatinine is preferable over Jaffe in neonates and young infants. Clin Chem Lab Med. (2014) 52:e107–9. doi: 10.1515/cclm-2013-1035
14. Wood AJ, Raynes-Greenow CH, Carberry AE, Jeffery HE. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J Paediatr Child Health. (2013) 49:199–203. doi: 10.1111/jpc.12119
15. Wilhelm-Bals A, Combescure C, Chehade H, Daali Y, Parvex P. Variables of interest to predict glomerular filtration rate in preterm newborns in the first days of life. Pediatr Nephrol. (2020) 35:703–12. doi: 10.1007/s00467-019-04257-z
16. Allegaert K, Mekahli D, van den Anker J. Cystatin C in newborns: a promising renal biomarker in search for standardization and validation. J Matern Fetal Neonatal Med. (2015) 28:1833–8. doi: 10.3109/14767058.2014.969236
17. Treiber M, Pečovnik-Balon B, Gorenjak M. Cystatin C versus creatinine as a marker of glomerular filtration rate in the newborn. Wien Klin Wochenschr. (2006) 118(Suppl 2):66–70. doi: 10.1007/s00508-006-0555-8
18. Treiber M, Pečovnik Balon B, Gorenjak M. A new serum cystatin formula for estimating glomerular filtration rat in newborns. Pediatr Nephrol. (2015) 30:1297–305. doi: 10.1007/s00467-014-3029-7
19. Treiber M, Gorenjak M, Pecovnik Balon B. Serum cystatin-C as marker of acute kidney injury in the newborn after perinatal hypoxia/asphyxia. Ther Apher Dial. (2014) 18:57–67. doi: 10.1111/1744-9987.12054
20. Madise-Wobo AD, Gbelee OH, Solarin A, Animasahun BA, Njokanma OF. Serum cystatin C levels in healthy Nigerian neonates: is there a need for normative values in Nigerian babies? Saudi J Kidney Dis Transpl. (2017) 28:1247–55. doi: 10.4103/1319-2442.220881
21. Bahar A, Yilmaz Y, Unver S, Gocmen I, Karademir F. Reference values of umbilical cord and third-day cystatin C levels for determining glomerular filtration rates in newborns. J Int Med Res. (2003) 31:231–5. doi: 10.1177/147323000303100310
22. Novo AC, Sadeck Ldos S, Okay TS, Leone CR. Longitudinal study of Cystatin C in healthy term newborns. Clinics (São Paulo). (2011) 66:217–20. doi: 10.1590/S1807-59322011000200006
23. Bargnoux AS, Piéroni L, Cristol JP, Kuster N, Delanaye P, Carlier MC, et al. Multicenter evaluation of Cystatin C measurement after assay standardization. Clin Chem. (2017) 63:833–41. doi: 10.1373/clinchem.2016.264325
24. Kim BB, Chung SH, Yoon HS, Hahn WH, Bae CW, Choi YS. Decreased Cystatin C-estimated glomerular filtration rate is correlated with prolonged hospital stay in transient tachypnea of newborn infants. Pediatr Neonatol. (2016) 57:195–200. doi: 10.1016/j.pedneo.2015.08.006
25. El-Gammacy TM, Shinkar DM, Mohamed NR, Al-Halag AR. Serum Cystatin C as an early predictor of acute kidney injury in preterm neonates with respiratory distress syndrome. Scand J Clin Lab Invest. (2018) 78:352–7. doi: 10.1080/00365513.2018.1472803
26. Foster J, Reisman W, Lepage N, Filler G. Influence of commonly used drugs on the accuracy of cystatin C-derived glomerular filtration rate. Pediatr Nephrol. (2006) 21:235–8. doi: 10.1007/s00467-005-2075-6
27. Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. (2012) 24:191–6. doi: 10.1097/MOP.0b013e32834f62d5
28. Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr. (2016) 19:68. doi: 10.3389/fped.2016.00068
29. Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. (2017) 1:184–94. doi: 10.1016/S2352-4642(17)30069-X
30. Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics. (2015) 136:e463–73. doi: 10.1542/peds.2014-3819
31. Zappitelli M, Ambalavanan N, Askenazi DJ, Moxey-Mims MM, Kimmel PL, Star RA, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. (2017) 82:569–73. doi: 10.1038/pr.2017.136
32. Starr MC, Boohaker L, Eldredge LC, Menon S, Griffin R, Mayock DE, et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks' gestation. Am J Perinatol. (2020) 37:341–8. doi: 10.1055/s-0039-3400311
33. Harer MW, Askenazi DJ, Boohaker LJ, Carmody JB, Griffin RL, Guillet R, et al. Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates: results from the AWAKEN study. JAMA Pediatr. (2018) 172:e180322. doi: 10.1001/jamapediatrics.2018.0322
34. Charlton JR, Boohaker L, Askenazi D, Brophy PD, Fuloria M, Gien J, et al. Late onset neonatal acute kidney injury: results from the AWAKEN study. Pediatr Res. (2019) 85:339–48. doi: 10.1038/s41390-018-0255-x
35. Cooke RJ, Werkman S, Watson D. Urine output measurement in premature infants. Pediatrics. (1989) 83:116–8.
36. Selewski DT, Gist KM, Nathan AT, Goldstein SL, Boohaker LJ, Akcan-Arikan A, et al. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr Res. (2020) 87:550–7. doi: 10.1038/s41390-019-0579-1
37. Allegaert K, Kuppens M, Mekahli D, Levtchenko E, Vanstapel F, Vanhole C, et al. Creatinine reference values in ELBW infants: impact of quantification by Jaffe or enzymatic method. J Matern Fetal Neonatal Med. (2012) 25:1678–81. doi: 10.3109/14767058.2012.657277
38. Smits A, Annaert P, Allegaert K. Drug disposition and clinical practice in neonates: cross talk between developmental physiology and pharmacology. Int J Pharm. (2013) 452:8–13. doi: 10.1016/j.ijpharm.2012.03.035
39. George I, Mekahli D, Rayyan M, Levtchenko E, Allegaert K. Postnatal trends in creatinemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol. (2011) 26:1843–9. doi: 10.1007/s00467-011-1883-0
40. De Cock RF, Allegaert K, Sherwin CM, Nielsen EI, de Hoog M, van den Anker JN, et al. A neonatal amikacin covariate model can be used to predict ontogeny of other drugs eliminated through glomerular filtration in neonates. Pharm Res. (2014) 31:754–67. doi: 10.1007/s11095-013-1197-y
41. Allegaert K, Cossey V, Langhendries JP, Naulaers G, Vanhole C, Devlieger H, et al. Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol Neonate. (2004) 86:207–11. doi: 10.1159/000079618
42. Demirel G, Celik IH, Canpolat FE, Erdever O, Biyikli Z, Dilmen U. Reference values of serum cystatin C in very low-birthweight premature infants. Acta Paediatr. (2013) 102:e4–7. doi: 10.1111/apa.12041
43. Lutz IC, Allegaert K, de Hoon JN, Marynissen H. Pharmacokinetics during therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy: a literature review. BMJ Pediatr Open. (2020) 4:e000685. doi: 10.1136/bmjpo-2020-000685
44. Hadzimuratovic E, Skrablin S, Hadzimuratovic A, Dinarevic SM. Postasphyxial renal injury in newborns as a prognostic factor of neurological outcome. J Matern Fetal Neonatal Med. (2014) 27:407–10. doi: 10.3109/14767058.2013.818646
45. Cavallin F, Rubin G, Vidal E, Cainelli E, Bonadies L, Suppiej A, et al. Prognostic role of acute kidney injury on long-term outcome in infants with hypoxic-ischemic encephalopathy. Pediatr Nephrol. (2020) 35:477–83. doi: 10.1007/s00467-019-04406-4
46. Kaur S, Jain S, Saha A, Chawla D, Parmar VR, Basu S, et al. Evaluation of glomerular and tubular renal function in neonates with birth asphyxia. Ann Trop Paediatr. (2011) 31:129–34. doi: 10.1179/146532811X12925735813922
47. Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr. (2013) 162:725–9.e1. doi: 10.1016/j.jpeds.2012.10.002
48. Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol. (2019) 34:169–76. doi: 10.1007/s00467-018-4068-2
49. Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. (2013) 31:CD003311. doi: 10.1002/14651858.CD003311.pub3
50. Diederen CMJ, van Bel F, Groenendaal F. Complications during therapeutic hypothermia after perinatal asphyxia: a comparison with trial data. Ther Hypothermia Temp Manag. (2018). doi: 10.1089/ther.2017.0046
51. Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP. Renal failure in asphyxiated neonates. Indian Pediatr. (2005) 42:928–34.
52. Sarafidis K, Tsepkentzi E, Agakidou E, Diamanti E, Taparkou A, Soubasi V, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol. (2012) 27:1575–82. doi: 10.1007/s00467-012-2162-4
53. Song Y, Sun S, Yu Y, Li G, Song J, Zhang H, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin for renal injury in asphyxiated preterm infants. Exp Ther Med. (2017) 13:1245–8. doi: 10.3892/etm.2017.4107
54. Pan JJ, Sun ZY, Zhou XY, Hu YH, Cheng R, Chen XQ, et al. Is neutrophil gelatinase-associated lipocalin a gooddiagnostic marker for renal injury in asphyxiated preterm infants? J Res Med Sci. (2018) 23:90. doi: 10.4103/jrms.JRMS_112_18
55. Yang Y, Wu Y, Pan JJ, Cheng R. Change of cystatin C values in preterm infants with asphyxia-From two centers of China. J Clin Lab Anal. (2017) 31:e22070. doi: 10.1002/jcla.22070
56. Sarkar S, Askenazi DJ, Jordan BK, Bhagat I, Bapuraj JR, Dechert RE, et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res. (2014) 75:431–5. doi: 10.1038/pr.2013.230
57. Jetton JG, Sorenson M. Pharmacological management of acute kidney injury and chronic kidney disease in neonates. Semin Fetal Neonatal Med. (2017) 22:109–15. doi: 10.1016/j.siny.2016.09.002
58. Bellos I, Pandita A, Yachha M. Effectiveness of theophylline administration in neonates with perinatal asphyxia: a meta-analysis. J Matern Fetal Neonatal Med. (2019). 1–9. doi: 10.1080/14767058.2019.1673722. [Epub ahead of print].
59. Cristea S, Smits A, Kulo A, Knibbe CAJ, van Weissenbruch M, Krekels EHJ, et al. Amikacin pharmacokinetics to optimize dosing in neonates with perinatal asphyxia treated with hypothermia. Antimicrob Agents Chemother. (2017) 61:e01282–17. doi: 10.1128/AAC.01282-17
60. Stoops C, Stone S, Evans E, Dill L, Henderson T, Griffin R, et al. Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr. (2019) 215:223–8.e6. doi: 10.1016/j.jpeds.2019.08.046
61. Vincent K, Murphy HJ, Ross JR, Twombley KE. Acute kidney injury guidelines are associated with improved recognition and follow-up for neonatal patients. Adv Neonatal Care. (2019). doi: 10.1097/ANC.0000000000000664
62. Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. (2014) 27:1485–90. doi: 10.3109/14767058.2013.860522
63. Gilarska M, Raaijmakers A, Zhang ZY, Staessen JA, Levtchenko E, Klimek M, et al. Extremely low birth weight predisposes to impaired renal health: a pooled analysis. Kidney Blood Press Res. (2019) 44:897–906. doi: 10.1159/000502715
Keywords: creatinine, Cystatin C, precision medicine, acute kidney injury, newborn, nephron number
Citation: Allegaert K, Smits A, van Donge T, van den Anker J, Sarafidis K, Levtchenko E and Mekahli D (2020) Renal Precision Medicine in Neonates and Acute Kidney Injury: How to Convert a Cloud of Creatinine Observations to Support Clinical Decisions. Front. Pediatr. 8:366. doi: 10.3389/fped.2020.00366
Received: 01 May 2020; Accepted: 02 June 2020;
Published: 28 July 2020.
Edited by:
Yogen Singh, Cambridge University Hospitals NHS Foundation Trust, United KingdomReviewed by:
Sajeev Job, Cambridge University Hospitals, United KingdomCopyright © 2020 Allegaert, Smits, van Donge, van den Anker, Sarafidis, Levtchenko and Mekahli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karel Allegaert, a2FyZWwuYWxsZWdhZXJ0QHV6bGV1dmVuLmJl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.