
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 23 June 2020
Sec. Pediatric Cardiology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00338
 Xueping Gu1†
Xueping Gu1† Wenchun Lin2†
Wenchun Lin2† Yufen Xu3†
Yufen Xu3† Di Che3
Di Che3 Yaqian Tan3
Yaqian Tan3 Zhaoliang Lu3
Zhaoliang Lu3 Lei Pi3
Lei Pi3 Lanyan Fu3
Lanyan Fu3 Huazhong Zhou3
Huazhong Zhou3 Zhiyong Jiang1*
Zhiyong Jiang1* Xiaoqiong Gu1,3*
Xiaoqiong Gu1,3*Background: Kawasaki disease (KD) is a common cardiovascular disease in infants and young children, with fever, rash, and conjunctivitis as the main clinical manifestations, which can lead to the occurrence of coronary aneurysms. Intravenous immunoglobulin (IVIG) is the preferred treatment for KD patients, but 10–20% of patients are resistant to IVIG. Lipoprotein-associated phospholipase A 2 (Lp-PLA2) is a potential therapeutic target for coronary atherosclerotic heart disease, and the polymorphism of Phospholipase A2 Group VII (PLA2G7) is closely related to the activity of Lp-PLA2, of which rs1051931 is the strongest. Therefore, the rs1051931 polymorphism may be a predictor of IVIG resistance in KD patients.
Methods: A total of 760 KD cases, including 148 IVIG-resistant patients and 612 IVIG-responsive patients, were genotyped for rs1051931 in PLA2G7, we compared the effects of rs1051931 on IVIG treatment in KD patients by odds ratios (OR) and 95% confidence interval (CI).
Results: The homozygous mutation AA may be a protective factor for IVIG resistance in KD patients (adjusted OR = 3.47, 95% CI = 1.14–10.57, P = 0.0284) and is more evident in patients with KD aged <60 months (adjusted OR = 3.68, 95% CI = 1.10–12.28, P = 0.0399).
Conclusions: The PLA2G7 rs1051931 G>A polymorphism may be suitable as a biomarker for the diagnosis or prognosis of IVIG resistance in KD in a southern Chinese population.
Kawasaki disease (KD), known also as Kawasaki syndrome or mucocutaneous lymph node syndrome, is an acute systemic vasculitis that primarily affects infants and young children (1, 2). The disease was first described in Japan by Dr. Tomisaku Kawasaki in 1967 and is now recognized as the leading cause of acquired heart disease among children in industrialized countries, especially in Asian countries (3, 4). The cardiac complications of KD are coronary artery aneurysms, coronary artery dilatation, and even myocardial infarction (5). The pathogenesis of KD is still unclear, but the immune response, microbial infections, and genetic factors are considered to contribute to its development. Approximately 25% of untreated KD patients developed coronary artery complications (6). The standard treatment for KD is intravenous immunoglobulin (IVIG), which can reduce both fever duration and the incidence of coronary artery lesions (CAL). However, despite receiving IVIG treatment, fever persists in 10–15% of the patients (7), and patients with IVIG resistance have a higher risk of CAL (8). Thus, predicting IVIG resistance in patients with KD is very important.
PLA2G7 encodes plasma PAF acetylhydrolase (PAF-AH), an extracellular Lp-PLA2, whose activity is related to large-artery atherosclerotic etiology and recurrent stroke in transient ischaemic attack patients (9). The most interesting SNP of PLA2G7 is Ala379Val (rs1051931), which has been associated with circulating Lp-PLA2 and atherosclerotic disease (10, 11). The association of the rs1051931 G>A genetic polymorphism with IVIG insensitivity in patients with KD is unknown so far. In this study, we focused on whether the PLA2G7 rs1051931 G>A polymorphism was related to resistance to IVIG therapy in KD patients.
We collected 148 IVIG-resistant patients and 612 IVIG-responsive patients with KD. The patients were derived from a portion of the KD cases collected from January 2012 to January 2017 at the Guangzhou Women and Children's Medical Center in Guangzhou, China. KD patients were diagnosed based on the American Heart Association's KD diagnostic criteria in 2004 (8). IVIG resistance is determined by persistent or recrudescent fever at least 36 h after completion of the first IVIG infusion (8). The KD patients, as outpatients with follow-ups and as inpatients, attended our hospital. This study was approved by the Guangzhou Women and Children's Medical Center Ethics Committee (2014073009), and with informed consent of the children and their families.
Genomic DNA was extracted from anticoagulant-containing peripheral blood collected from patients using the TIANamp Blood DNA Kit (DP318, TIANGEN Biotech, Beijing) according to the manufacturer's instructions. The procedures can be found in our previous paper (12). The PLA2G7 rs1051931 G>A polymorphism was genotyped with TaqMan method. Allele-specific probes were ordered from Applied Biosystems. PCR was performed in 384-well plates with an ABI-Q6 Sequence Detection System machine (Thermo Fisher Scientific). Additionally, in order to ensure the quality and accuracy of the genotyping results, we randomly selected 10% of the samples for repeated analysis, and the results were completely consistent.
We first examined the Hardy-Weinberg equilibrium (HWE) of the samples. Next, we use χ2 test to evaluate the significant differences between IVIG-resistant cases and IVIG-responsive cases in the frequency distributions and genotypes. Odds ratios (OR) and 95% confidence intervals (CI) were used to quantify the association between the PLA2G7 rs1051931 G>A polymorphism and the susceptibility of IVIG treatment in KD patients with adjustments for age and gender. The association between the PLA2G7 rs1051931 G>A polymorphism and resistance to IVIG treatment in KD cases was evaluated by age and gender stratification analysis. Statistical analyses were performed by SAS software (Version 9.4; SAS Institute, Cary, NC, USA).
A total of 148 IVIG-resistant cases and 612 IVIG-responsive cases were analyzed in this study. The demographics of participants are all shown in Figures 1A,B and Table 1. The mean ages were 29 months (29 ± 25, range from 1 to 125 months) for IVIG-resistant patients and 28 months (28 ± 23, range from 1 to 156 months) for IVIG-responsive patients. There showed no significant differences in age (P = 0.656) or gender (P = 0.5462) between the IVIG-resistant and IVIG-responsive KD patients.
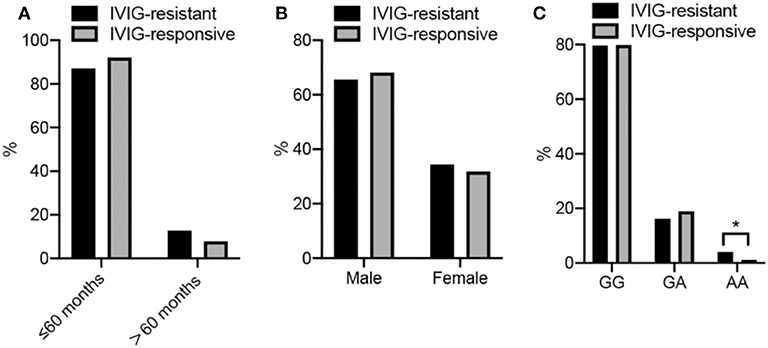
Figure 1. (A–C) Show the frequency disturbion of age, sex, and genotype in KD cases, respectly. More detailed data are described in the Tables.
The genotype distributions of the PLA2G7 rs1051931 G>A polymorphism in the IVIG-resistant and IVIG-responsive KD patients are shown in Figure 1C and Table 2. The genotype frequency distributions of the PLA2G7 rs1051931 polymorphisms were 79.73% (GG), 16.22% (GA), and 4.05% (AA) in the IVIG-resistant group and 79.90% (GG), 18.95% (GA), and 1.14% (AA) in the IVIG-responsive group. In comparison with IVIG-resistant and IVIG-responsive KD subjects, there were significant differences in the AA genotype of PLA2G7 rs1051931 (AA vs. GG: adjusted OR = 3.47, 95% CI = 1.14–10.57, P = 0.0284; AA vs. GG+GA: adjusted OR = 3.57, 95% CI = 1.18–10.84, P = 0.0247), which means KD patients with AA mutation were more resistant to IVIG (P = 0.0281).
We further explored the association of the PLA2G7 rs1051931 polymorphism with IVIG resistance in KD in the stratified analysis by age and gender. For KD strikes predominantly children younger than 5 years of age (2), so we analyzed the rs1051931 GG/GA variant in children ≤60 months, the result showed that AA genotype may be more protective (adjusted OR = 3.68, 95% CI = 1.10–12.28, P = 0.0399) (Table 3). However, there showed no significant associations with other stratified analyses.
KD is the most common cause of acquired heart disease in infants and children, especially in developed countries. High-dose IVIG treatment significantly reduced the risk of CAL, but some children with KD failed to respond to initial IVIG therapy. Therefore, early identification of the risk factors of IVIG resistance is important. Our research showed that the PLA2G7 rs1051931 AA genotype was associated with a protective effect against IVIG resistance for KD and that the effect was more evident in children ≤60 months of age. To the best of my knowledge, this is the first study in which PLA2G7 rs1051931 G>A polymorphisms were found to be related to IVIG response in KD patients.
The mechanism of action of IVIG in KD is unclear. Potential explanations include immunologic blockade of the Fc receptor (13), interaction with dendritic cells (14)/T cells (15) and NK cells (16), an increase in the production of antibodies against the specific aetiologic agent (17), or downregulation of cytokine production (18). It is certainly plausible that IVIG interacts with many arms of the immune and vascular systems to achieve the downregulation of inflammation. The main purpose of IVIG treatment of KD is to guard against coronary artery damage and to reduce levels of tissue inflammation.
Experimental studies have shown that PLA2G7 may be an effective therapeutic target in humans and animals, assuming that this enzyme is related to oxidative modification of LDL and the development of arterial inflammation (19). PLA2G7 rs1051931 was nominally associated with Lp-PLA2 activity (20). The substitution of Ala by Val in A379V leads to twofold decrease in the affinity of Lp-PLA2 for its substrate, and thus reduces the degradation of PAF (21). The reduced activity of Lp-PLA2 due to potential genetic propensity may be an important factor that predisposes an individual toward IVIG resistance or involved in the pathogenesis of KD. PLA2G7 rs1051931 polymorphisms were found to be associated with coronary artery disease (11, 22). The SNP rs1051931 was not associated with any of the cardiovascular risk factors (23–26). However, the rs1051931 variant in coronary artery disease patients is associated with a high risk of myocardial infarction (27). A previous study showed that the PLA2G7 V279F polymorphism (G/T transversion) and consequent enzymatic deficiency is one of the factor for IVIG resistance in Japanese patients with acute KD (28), which is consistent with our results that mutant allele of PLA2G7 may involve in IVIG therapy for KD patients. According to these studies, PAF-AH could be important in protecting tissues by degrading PAF and oxidized phospholipids into biologically inactive molecules.
Although this is the first investigation of the association between the PLA2G7 rs1051931 G>A polymorphism and IVIG resistance in southern Chinese KD patients, our study has potential limitations that should be reviewed. First of all, this study was limited to a southern Chinese population, and cases from other ethnic groups were not assessed. Second, we examined only the rs1051931 G>A polymorphism; other potential SNPs of PLA2G7 were not included. Third, the number of IVIG resistant patients is insufficient, and further studies with larger sample size are needed to confirm our results.
Overall, we conclude that the A379V polymorphism in the PLA2G7 gene may be a potential factor for IVIG resistance in southern Chinese patients with KD, and it could be suitable as a biomarker for the diagnosis or prognosis of IVIG resistance in KD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
All authors contributed significantly to this work. XuG, ZJ, and XiG were primarily responsible for the overall project design and paper writing. WL and YX were responsible for performing the experiments. Data analysis was carried out by YX and DC. DC and XiG modified and polished the manuscript. The others give suggestion for this article. All authors have reviewed and approved the manuscript.
This study was supported by the Guangdong Natural Science Fund, China, grant numbers 2016A030313836; the Guangzhou Science and Technology Program Project, China, grant numbers 201510010287, 201510010159, and 201607010011; the Guangzhou Medical and Health Technology Projects, China, grant numbers 20171A011260 and 20161A010030. Trial Registration Number: ChiCTR-EOC-17013266. The Mechanism of Coronary Artery Lesions and Effect of Treatment in Children with Kawasaki Disease: A Clinical Cohort Study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thanks for the clinical samples provided by the Clinical Biological Resource Bank of Guangzhou Women and Children's Medical Center.
1. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. (2004) 110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78
2. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. (2016) 67:1738–49. doi: 10.1016/j.jacc.2015.12.073
3. Burns JC, Glode MP. Kawasaki syndrome. Lancet. (2004) 364:533–44. doi: 10.1016/S0140-6736(04)16814-1
4. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. (2015) 100:1084–8. doi: 10.1136/archdischild-2014-307536
5. Singh S, Jindal AK, Pilania RK. Diagnosis of Kawasaki disease. Int J Rheum Dis. (2018) 21:36–44. doi: 10.1111/1756-185X.13224
6. Wang CL, Wu Y-T, Liu C-A, Kuo H-C, Yang KD. Kawasaki disease: infection, immunity and genetics. Pediatr Infect Dis J. (2005) 24:998–1004. doi: 10.1097/01.inf.0000183786.70519.fa
7. Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. (1998) 17:1144–8. doi: 10.1097/00006454-199812000-00009
8. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
9. Kono N, Arai H. Platelet-activating factor acetylhydrolases: an overview and update. Biochim Biophys Acta Mol Cell Biol Lipids. (2018) 1864:922–31. doi: 10.1016/j.bbalip.2018.07.006
10. Abuzeid AM, Hawe E, Humphries SE, Talmud PJ. Association between the Ala379Val variant of the lipoprotein associated phospholipase A2 and risk of myocardial infarction in the north and south of Europe. Atherosclerosis. (2003) 168:283–8. doi: 10.1016/S0021-9150(03)00086-8
11. Liu PY, Li YH, Wu HL, Chao TH, Tsai LM, Lin LJ, et al. Platelet-activating factor-acetylhydrolase A379V (exon 11) gene polymorphism is an independent and functional risk factor for premature myocardial infarction. J Thromb Haemost. (2006) 4:1023–8. doi: 10.1111/j.1538-7836.2006.01895.x
12. Che D, Li J, Fu L, Pi L, Rong X, Wang Y, et al. The rs1625579 T>G polymorphism in the gene confers a risk of early- onset Kawasaki disease in a southern Chinese population. Infect Drug Resist. (2018) 11:1055–60. doi: 10.2147/IDR.S174140
13. Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. (2011) 475:110–3. doi: 10.1038/nature10134
14. Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood. (2013) 122:1419–27. doi: 10.1182/blood-2012-11-468264
15. Maddur MS, Vani J, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin. J Allergy Clin Immunol. (2011) 127:823–30. doi: 10.1016/j.jaci.2010.12.1102
16. Finberg RW, Newburger JW, Mikati MA, Heller AH, Burns JC. Effect of high doses of intravenously administered immune globulin on natural killer cell activity in peripheral blood. J Pediatr. (1992) 120:376–80. doi: 10.1016/S0022-3476(05)80900-X
17. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. (2001) 345:747–55. doi: 10.1056/NEJMra993360
18. Rowley AH, Shulman ST. Kawasaki syndrome. Clin Microbiol Rev. (1998) 11:405–14. doi: 10.1128/CMR.11.3.405
19. Corson MA. Darapladib: an emerging therapy for atherosclerosis. Ther Adv Cardiovasc Dis. (2010) 4:241–8. doi: 10.1177/1753944710375820
20. Schnabel R, Dupuis J, Larson MG, Lunetta KL, Robins SJ, Zhu Y, et al. Clinical and genetic factors associated with lipoprotein-associated phospholipase A2 in the Framingham Heart Study. Atherosclerosis. (2009) 204:601–7. doi: 10.1016/j.atherosclerosis.2008.10.030
21. Kruse S, Mao XQ, Heinzmann A, Blattmann S, Roberts MH, Braun S, et al. The Ile198Thr and Ala379Val variants of plasmatic PAF-acetylhydrolase impair catalytical activities and are associated with atopy and asthma. Am J Hum Genet. (2000) 66:1522–30. doi: 10.1086/302901
22. Sutton BS, Crosslin DR, Shah SH, Nelson SC, Bassil A, Hale AB, et al. Comprehensive genetic analysis of the platelet activating factor acetylhydrolase (PLA2G7) gene and cardiovascular disease in case-control and family datasets. Hum Mol Genet. (2008) 17:1318–28. doi: 10.1093/hmg/ddn020
23. Casas JP, Ninio E, Panayiotou A, Palmen J, Cooper JA, Ricketts SL, et al. PLA2G7 genotype, lipoprotein-associated phospholipase A2 activity, and coronary heart disease risk in 10 494 cases and 15 624 controls of European Ancestry. Circulation. (2010) 121:2284–93. doi: 10.1161/CIRCULATIONAHA.109.923383
24. Hoffmann MM, Winkler K, Renner W, Winkelmann BR, Seelhorst U, Wellnitz B, et al. Genetic variants and haplotypes of lipoprotein associated phospholipase A2 and their influence on cardiovascular disease (The Ludwigshafen Risk and Cardiovascular Health Study). J Thromb Haemost. (2009) 7:41–8. doi: 10.1111/j.1538-7836.2008.03216.x
25. Grallert H, Dupuis J, Bis JC, Dehghan A, Barbalic M, Baumert J, et al. Eight genetic loci associated with variation in lipoprotein-associated phospholipase A2 mass and activity and coronary heart disease: meta-analysis of genome-wide association studies from five community-based studies. Eur Heart J. (2012) 33:238–51. doi: 10.1093/eurheartj/ehr372
26. Maiolino G, Lenzini L, Pedon L, Cesari M, Seccia TM, Frigo AC, et al. Lipoprotein-associated phospholipase A2 single-nucleotide polymorphisms and cardiovascular events in patients with coronary artery disease. J Cardiovasc Med. (2015) 16:29–36. doi: 10.2459/JCM.0000000000000057
27. Li L, Qi L, Lv N, Gao Q, Cheng Y, Wei Y, et al. Association between lipoprotein-associated phospholipase A2 gene polymorphism and coronary artery disease in the Chinese Han population. Ann Hum Genet. (2011) 75:605–11. doi: 10.1111/j.1469-1809.2011.00666.x
Keywords: rs1051931, polymorphism, PLA2G7, immunoglobulin therapy, Kawasaki disease
Citation: Gu X, Lin W, Xu Y, Che D, Tan Y, Lu Z, Pi L, Fu L, Zhou H, Jiang Z and Gu X (2020) The rs1051931 G>A Polymorphism in the PLA2G7 Gene Confers Resistance to Immunoglobulin Therapy in Kawasaki Disease in a Southern Chinese Population. Front. Pediatr. 8:338. doi: 10.3389/fped.2020.00338
Received: 14 August 2019; Accepted: 21 May 2020;
Published: 23 June 2020.
Edited by:
Oswin Grollmuss, Université Paris-Sud, FranceReviewed by:
Zhijun Dai, Zhejiang University, ChinaCopyright © 2020 Gu, Lin, Xu, Che, Tan, Lu, Pi, Fu, Zhou, Jiang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Jiang, MTAzMTc2MDY0M0BxcS5jb20=; Xiaoqiong Gu, Z3V4aWFvcWlvbmdAZ3djbWMub3Jn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.