- Department of Anesthesiology, Critical Care and Pain Medicine, The Second Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
Background: We conducted this systematic review and meta-analysis to investigate the clinical effect of dexmedetomidine in preventing pediatric emergence agitation (EA) or delirium (ED) following anesthesia compared with placebo or other sedatives.
Methods: The databases of Pubmed, Embase, and Cochrane Library were searched until 8th January 2020. Inclusion criteria were participants with age<18 years and studies of comparison between dexmedetomidine and placebo or other sedatives. Exclusion criteria included adult studies; duplicate publications; management with dexmedetomidine alone; review or meta-analysis; basic research; article published as abstract, letter, case report, editorial, note, method, or protocol; and article presented in non-English language.
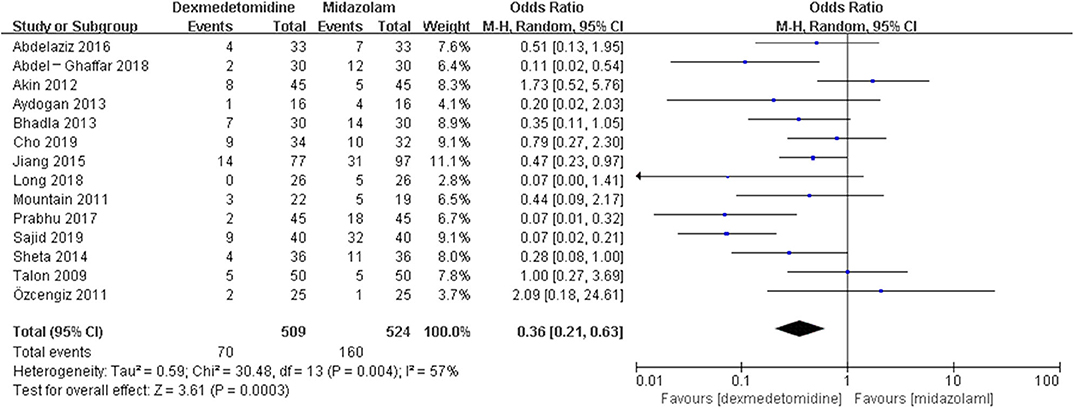
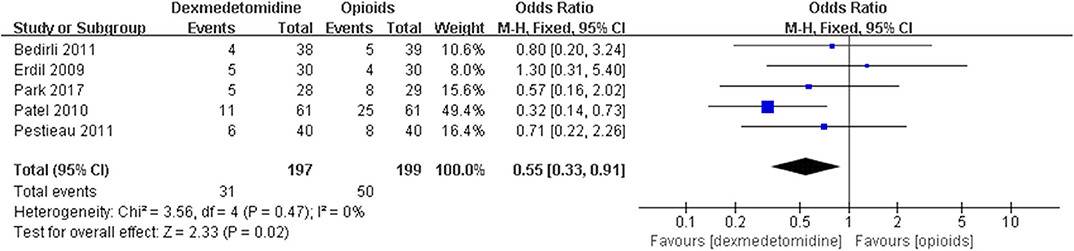
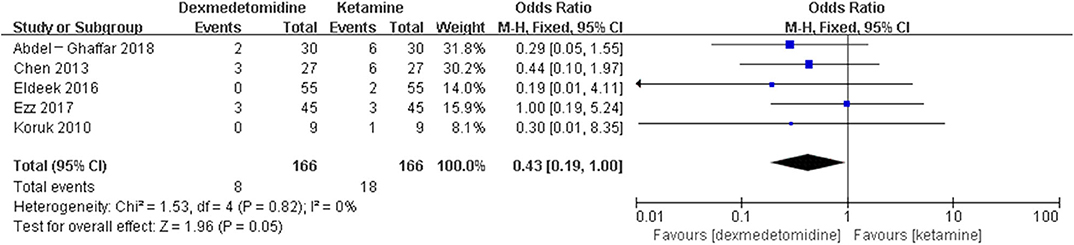
Results: Fifty-eight randomized controlled trials (RCTs) and five case-control trials (CCTs) including 7,714 patients were included. The results showed that dexmedetomidine significantly decreased the incidence of post-anesthesia EA or ED compared with placebo [OR = 0.22, 95% CI: (0.16, 0.32), I2 = 75, P < 0.00001], midazolam [OR = 0.36, 95% CI: (0.21, 0.63), I2 = 57, P = 0.0003], and opioids [OR = 0.55, 95% CI: (0.33, 0.91), I2 = 0, P = 0.02], whereas the significant difference was not exhibited compared with propofol (or pentobarbital) [OR = 0.56, 95% CI: (0.15, 2.14), I2 = 58, P = 0.39], ketamine [OR = 0.43, 95% CI: (0.19, 1.00), I2 = 0, P = 0.05], clonidine [OR = 0.54, 95% CI: (0.20, 1.45), P = 0.22], chloral hydrate [OR = 0.98, 95% CI: (0.26, 3.78), P = 0.98], melatonin [OR = 1.0, 95% CI: (0.13, 7.72), P = 1.00], and ketofol [OR = 0.55, 95% CI: (0.16, 1.93), P = 0.35].
Conclusion: Compared with placebo, midazolam, and opioids, dexmedetomidine significantly decreased the incidence of post-anesthesia EA or ED in pediatric patients. However, dexmedetomidine did not exhibit this superiority compared with propofol and ketamine. With regard to clonidine, chloral hydrate, melatonin, and ketofol, the results needed to be further tested due to the fact that only one trial was included for each control drug.
Introduction
Emergence agitation (EA) or delirium (ED) manifests as a series of sudden complex psychomotor disorders, characterized by perceptual disturbances, delusions, and disorientation following sedation or general anesthesia (1). So far, the specific mechanism of EA or ED has not been clear. The preschool children undergoing ophthalmology or otorhinolaryngology procedures under inhalation agents are susceptible population (2). According to some studies, the incidence of EA or ED after general anesthesia in children ranges from 10 to 80% (3) and significantly increases the occurrence of other complications after anesthesia, like self-injury, prolonged post-anesthesia care unit (PACU) stay, poor satisfaction of parents and care providers, and so on (4). Therefore, it is necessary to find effective measures to prevent or treat EA or ED.
Some studies have reported the pharmacological strategies to prevent EA or ED, including midazolam, propofol, ketamine, opioids, and α2 adrenergic receptor agonists (5–8). Activation of an α2 adrenergic receptor can contribute to pharmacological effects of sedation, analgesia, and anti-inflammation; thus, an α2 adrenergic receptor may be a target for prevention and treatment of EA or ED (9, 10). A study from Ydemann et al. (11) found that clonidine significantly decreased the incidence of postoperative agitation in children after sevoflurane anesthesia compared with placebo. Another commonly used α2 adrenergic receptor agonist dexmedetomidine shows a higher ratio of specificity for α2 receptor (α2:α1 1600:1) compared with clonidine (α2:α1 200:1) (12, 13). Although dexmedetomidine is used as an off-label drug in children, increasing studies about the effect of dexmedetomidine on EA and ED in pediatric patients have been completed. We conducted this meta-analysis for clinical trials to evaluate the effect of dexmedetomidine on EA or ED following sedation or general anesthesia in pediatric patients compared with placebo and other drugs.
Materials and Methods
This systematic review and meta-analysis was performed according to the guidelines of the 2009 PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) (Supplementary Table 1) (14).
Search Strategy and Study Selection
We searched the databases including “Pubmed,” “Embase,” and “Cochrane Library” through the PICOS (Population, Intervention, Comparison, Outcome, Study design) method until 8th January 2020. The entry words included “child” OR “children” OR “pediatric” AND “dexmedetomidine” OR “precedex” OR “MPV-1440” OR “MPV 1440” OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine” AND “agitation” OR “delirium,” and the search scope was “all fields.” Because all studies about the effect of dexmedetomidine vs. other drugs (placebo or other sedatives) on agitation or delirium in pediatric patients were eligible in this meta-analysis, we did not confine the search words of control drugs and study design. The inclusion criteria included the following: (1) participants with age<18 years; and (2) management with prophylactic dexmedetomidine and placebo or other sedatives. The exclusion criteria included the following: (1) participants with age≥18 years; (2) management with dexmedetomidine alone; (3) review or meta-analysis; (4) basic research; (5) article published as an abstract, letter, case report, editorial, note, method, or protocol; and (6) article presented in non-English language.
Data Analysis
The aim of this meta-analysis was to investigate whether dexmedetomidine had advantage in reducing the incidence of EA or ED following sedation or general anesthesia in pediatric patients compared with placebo or other sedatives.
Three authors were independently responsible for reviewing the titles, abstracts, or both and summarized the data of the included literatures. Another two authors were in charge of the data discrepancy adjustment.
Two authors were responsible for extracting the following information: (1) authors; (2) publication year; (3) number of the total participants in each study; (4) age range of all the participants; (5) country of publication; (6) procedures that the participants underwent; (7) time of dexmedetomidine or other sedative administration; (8) infusion speed or dosage of dexmedetomidine or other sedatives; and (9) number of patients with EA or ED following sedation or general anesthesia.
Two authors independently assessed the quality of included studies. The risk of bias of randomized controlled trials (RCTs) were assessed by the Cochrane Collaboration Risk of Bias Assessment tool including seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and others (bias due to vested financial interest and academic bias). If a trial had one or more of the items to be judged as high or unclear risk of bias, this trial was classified as having high risk (15). The bias risk of case-control trials (CCTs) was assessed by the Newcastle-Otawa Quality Assessment Scale (NOS) comprising three domains: selection, comparability, and outcome for cohort studies. There were four stars in the selection domain, two stars in the comparability domain, and three stars in the exposure domain. Trials with cumulative seven stars or more were considered to be of high quality, those with six stars were considered to be of moderate quality, and those with less than six stars were considered to be of low quality (Supplementary Table 2) (16). If the two authors had different assessment results, they consulted the third or the fourth one. Eventually, the authors reached consensus. All included trials were grouped based on different control drugs.
RevMan Review Manager version 5.3 (Cochrane collaboration, Oxford, UK) and Stata version 12.0 (Stata Corp, College Station, TX, USA) were used to perform statistical analyses. The values of I2 and the Mantel–Haenszel chi-square test (P-value for heterogeneity) were used to evaluate the heterogeneity of included studies. And the values of I2 < 40%, 40–60%, and >60% represented low, moderate, and high heterogeneity, respectively (17). If I2 > 50% or a P-value for heterogeneity <0.1 was identified, the method of random-effect model analysis was applied to analyze the data. Conversely, if I2 < 50% or a P-value for heterogeneity ≥0.1 was presented, the method of a fixed-effect model was used (18). The dichotomous outcome was reported as odds ratios (OR) with 95% confidence interval (CI). The statistical tests were two-sided, and a P-value for overall effect <0.05 was considered to have significant difference.
Sensitivity analysis was conducted to solve the problem of significant heterogeneity (I2 > 40%) through the method of subgroup analysis or one-by-one literature removal. Meta-regression was used to investigate the heterogeneity sources for the group with I2 > 40% according to possible risk factors. A subgroup analysis proceeded based on the risk factor with P < 0.05 by meta-regression analysis; conversely, the method of one-by-one literature removal was used if P-values of all risk factors were 0.05 or more.
Results
Study Location and Selection
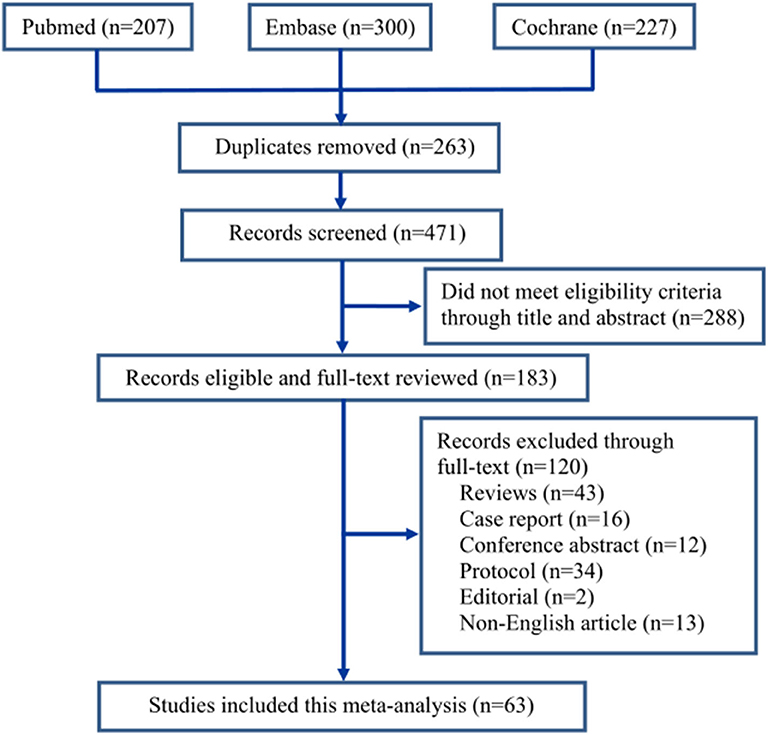
The screening process of the eligible literatures is shown in Figure 1. We obtained 207 trials from Pubmed, 300 from Embase, and 227 from Cochrane Library according to the inclusion criteria. Two hundred sixty-three trials were removed due to duplicates. Two hundred eighty-eight trials were excluded because they did not meet the eligibility criteria by browsing the titles and abstracts, and 120 trials were removed by browsing the full text. Eventually, 63 trials (19–81) including 7,714 patients were identified through our search strategy (Figure 1). All included trials were divided into nine groups based on control drugs: placebo (19–59), midazolam (19, 59–71), opioids (29, 45, 72–74), propofol (or pentobarbital) (22, 25, 42, 75, 76), ketamine (26, 60, 77–79), clonidine (80), chloral hydrate (81), melatonin (59), and ketofol (ketamine and propofol) (23). We assigned propofol and pentobarbital into the same group because both of them produced general anesthetic efficacy through directly activating the γ-aminobutyric acid A receptor of the central nervous system (82).
Characteristics of Included Trials
There were 41 trials (19–59) including 3,600 patients in the placebo group, 14 trials (19, 59–71) including 1,033 patients in the midazolam group, 5 trials (29, 45, 72–74) including 396 patients in the opioids group, 5 trials (22, 25, 42, 75, 76) including 1,969 patients in the propofol (or pentobarbital) group, 5 trials (26, 60, 77–79) including 332 patients in the ketamine group, and 4 trials (23, 59, 80, 81) including 384 patients in the clonidine, chloral hydrate, melatonin, and ketofol group, respectively.
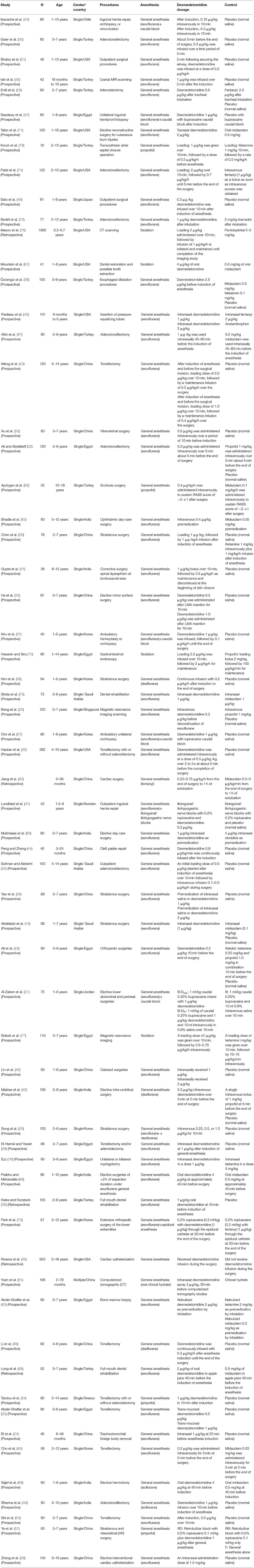
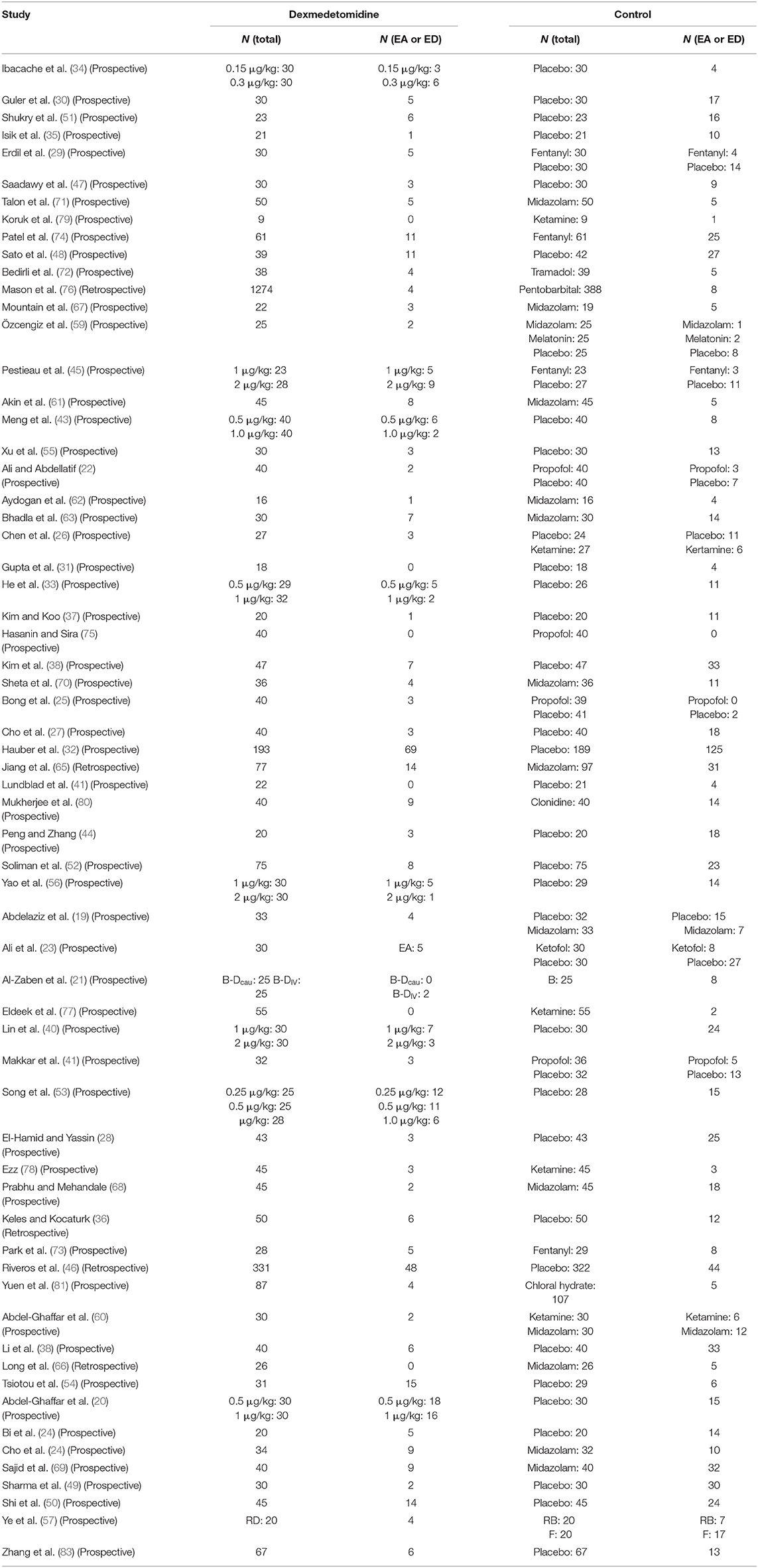
Table 1 demonstrated the basic information of all included trials; meanwhile, it was discovered that clinical heterogeneity might be associated with the study methods, the type of surgery, the number and age of participants, and the route, dosage, and timing of drug administration. Five trials in the included studies were CCTs (36, 46, 65, 66, 76). The patients in 17 trials (20, 22, 28–30, 32, 39, 43, 49, 50, 52, 54, 61, 64, 72, 74, 78) underwent otolaryngology surgeries, those in 5 trials (36, 44, 66, 67, 70) underwent dental or cleft palate surgeries, those in 9 trials (19, 26, 38, 40, 53, 55–57, 63) underwent ophthalmic surgeries, those in 11 trials (21, 27, 33, 34, 37, 41, 42, 47, 68, 69, 71) underwent general or urological surgeries, those in 4 trials (23, 31, 62, 73) underwent orthopedic surgeries, those in 1 trial (65) underwent cardiac surgery, those in 8 trials (24, 45, 46, 58–60, 75, 79) underwent invasive examination or treatment, those in 5 trials (25, 35, 76, 77, 81) underwent non-invasive examination or treatment, and those in 3 trials (48, 51, 80) underwent all kinds of outpatient surgeries. Different routes of drug administration were used: intranasal in 12 trials (19, 24, 28, 40, 45, 56, 58, 61, 71, 78, 80, 81), oral in 5 trials (36, 66–69), caudal or nerve block in 5 trials (21, 41, 47, 57, 73), inhalation in 1 trial (60), transmucosal in 1 trial (20), and intravenous in 39 trials. The strategy of drug administration was also different: (1) intravenous single dose in 20 trials (22, 23, 25, 29, 30, 32–35, 42, 48–50, 53–55, 59, 63, 64, 72), loading dose plus maintenance infusion in 10 trials (26, 31, 37, 43, 52, 74–77, 79), and only maintenance infusion in 6 trials (38, 39, 44, 51, 62, 65); and (2) administration onset before anesthesia in 29 trials (19, 20, 24, 28, 36, 40, 45, 46, 49, 55, 56, 58–61, 63, 66–71, 75–81), during anesthesia in 32 trials (21–23, 25–27, 29–35, 37–39, 41–44, 47, 48, 50–54, 57, 64, 72–74), and after anesthesia in 2 trials (62, 65). The number of patients with EA or ED in dexmedetomidine and control groups is shown in Table 2.
Bias Risk Assessment
Bias risk of 58 RCTs was assessed by the Cochrane Collaboration Risk of Bias Assessment tool. Random sequence generation was assessed as a low risk of bias in 57 studies (98%), allocation concealment was assessed in 36 studies (62%), blinding of participants was assessed in 38 studies (66%), blinding of outcome assessment was assessed in 34 studies (59%), incomplete outcome data were assessed in 58 studies (100%), and selective outcome reporting was assessed in 56 studies (97%). Nineteen RCTs (24, 26, 27, 32, 33, 37, 41, 45, 53, 54, 56, 58–61, 64, 67, 73, 81) were assessed to be of high quality (Supplementary Figures 1, 2). Bias risk of 5 CCTs (36, 46, 65, 66, 76) was assessed by NOS, and the number of stars was 7 from the study of Keles et al. (36), 8 from the study of Riveros et al. (46), 5 from the study of Jiang et al. (65), 5 from the study of Long et al. (66), and 8 from the study of Mason et al. (76), respectively. Therefore, 3 trials (36, 46, 76) were assessed to be of high quality because they obtained 7 stars or more (Supplementary Table 3).
Post-anesthesia Incidence of EA or ED
Different dosages of dexmedetomidine administration in each study were presented in nine trials (20, 21, 33, 34, 40, 43, 45, 53, 56). We chose the dexmedetomidine dosage with the highest incidence of EA or ED. We evaluated the effect of dexmedetomidine administration on EA or ED compared with placebo (19–59), midazolam (19, 59–71), opioids (29, 45, 72–74), propofol (or pentobarbital) (22, 25, 42, 75, 76), ketamine (26, 60, 77–79), and other sedatives (clonidine, chloral hydrate, melatonin) or ketofol (23, 59, 80, 81).
The random-effect model with OR was selected due to high I2 in the groups of placebo (I2 = 75%), midazolam (I2 = 57%), and propofol (or pentobarbital) (I2 = 58%), whereas the fixed-effect model with OR was selected because of low I2 in the group of opioids (I2 = 0%) and ketamine (I2 = 0%).
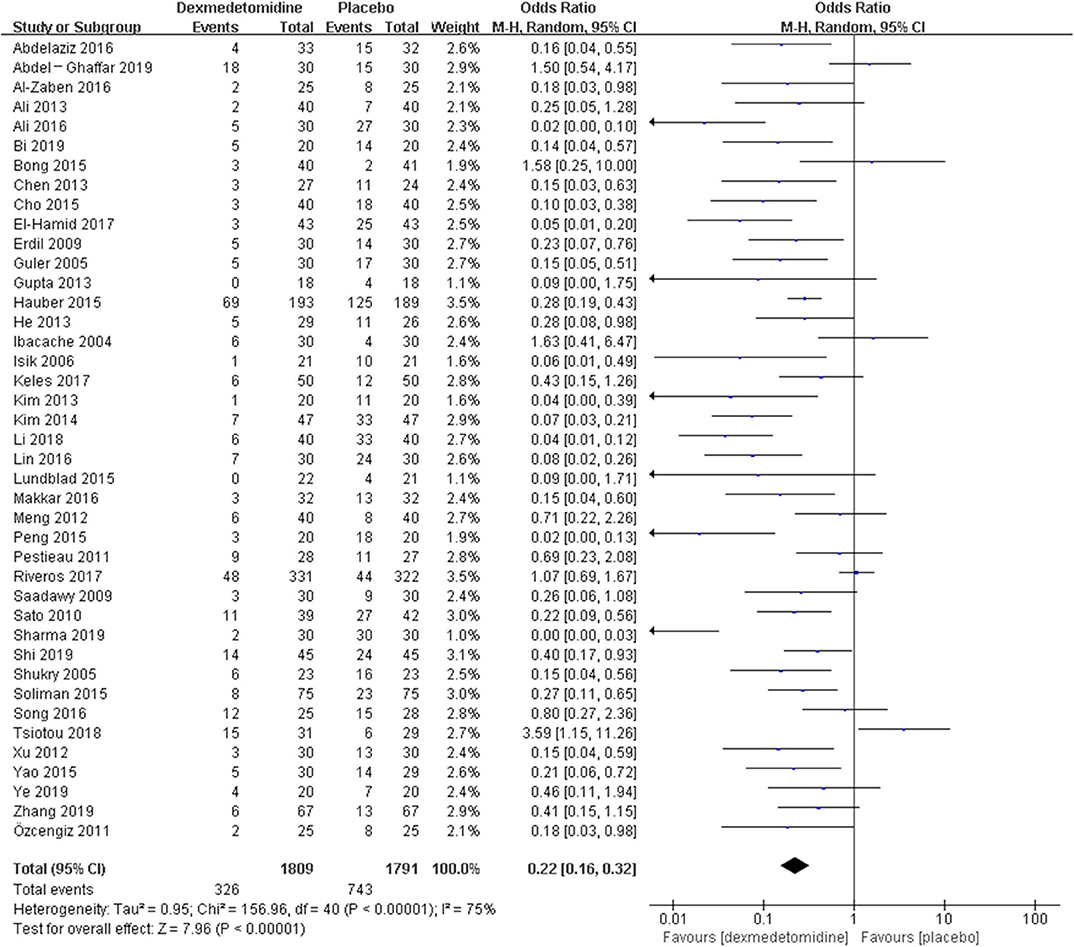
The pooled results demonstrated significant difference in the incidence of EA or ED after anesthesia in the groups of placebo [OR = 0.22, 95% CI: (0.16, 0.32), I2 = 75%, P for effect <0.00001] (Figure 2), midazolam [OR = 0.36, 95% CI: (0.21, 0.63), I2 = 57%, P for effect = 0.0003] (Figure 3), and opioids [OR = 0.55, 95% CI: (0.33, 0.91), I2 = 0, P for effect = 0.02] (Figure 4). However, no significant difference was exhibited in the groups of propofol (or pentobarbital) [OR = 0.56, 95% CI: (0.15, 2.14), I2 = 58%, P for effect = 0.39] (Figure 5) and ketamine [OR = 0.43, 95% CI: (0.19, 1.00), I2 = 0, P for effect = 0.05] (Figure 6).
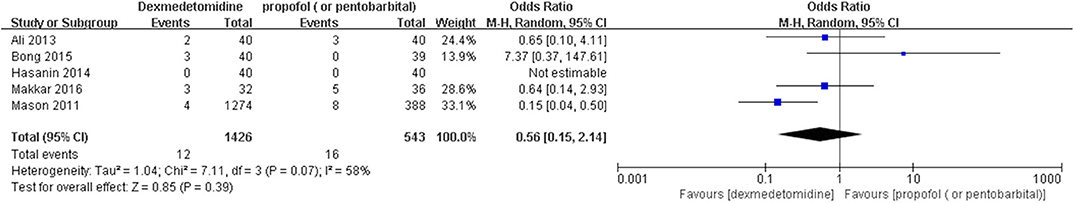
Figure 5. Comparison of pediatric EA or ED between dexmedetomidine and propofol (or pentobarbital) groups.
With regard to other control sedatives or drug combination, no heterogenicity was presented because only one literature was retrieved for each group. The results did not demonstrate significant difference in the incidence of EA or ED after anesthesia when comparing dexmedetomidine with clonidine [OR = 0.54, 95% CI: (0.20, 1.45), P for effect = 0.22], chloral hydrate [OR = 0.98, 95% CI: (0.26, 3.78), P for effect = 0.98], melatonin [OR = 1.0, 95% CI: (0.13, 7.72), P for effect = 1.00], and ketofol [OR = 0.55, 95% CI: (0.16, 1.93), P for effect = 0.35].
Sensitivity Analysis
Meta-regression was performed to investigate the heterogeneity sources by assessing the potential factors including the year of publication, study methods, the country of authors, the time of drug administration, the type of surgery, routes of drug administration, the bias risk of the study, and the range of patients' age for the groups of placebo and midazolam. Unexpectedly, all P-values of these risk factors were over 0.05 (Supplementary Tables 4, 5). Afterward, the method of one-by-one literature removal was used. Seven trials (20, 23, 39, 44, 46, 49, 54) were found to be the main sources of heterogeneity in the placebo group (I2 dropped from 75 to 36%) and two trials (61, 69) in the midazolam group (I2 dropped from 57 to 28%). Due to a small number of included trials in the group of propofol (or pentobarbital), the method of one-by-one literature removal was directly used to lower the heterogeneity. When we removed the retrospective trial from Mason et al. (76), the value of I2 in the propofol (or pentobarbital) group dropped from 58 to 13%, and the changes suggested that this retrospective trial was the main source of significant heterogeneity.
The post hoc analysis was performed by the fixed-effects model with OR, and the pooled results were consistent with those prior to the sensitivity analysis—placebo group: [OR = 0.24, 95% CI: (0.18, 0.31), I2 = 36%, P for effect <0.00001] (Figure 7); midazolam group: [OR = 0.37, 95% CI: (0.26, 0.52), I2 = 28%, P for effect <0.00001] (Figure 8); propofol (or pentobarbital) group: [OR = 1.06, 95% CI: (0.39, 2.85), I2 = 13%, P for effect = 0.92] (Figure 9).
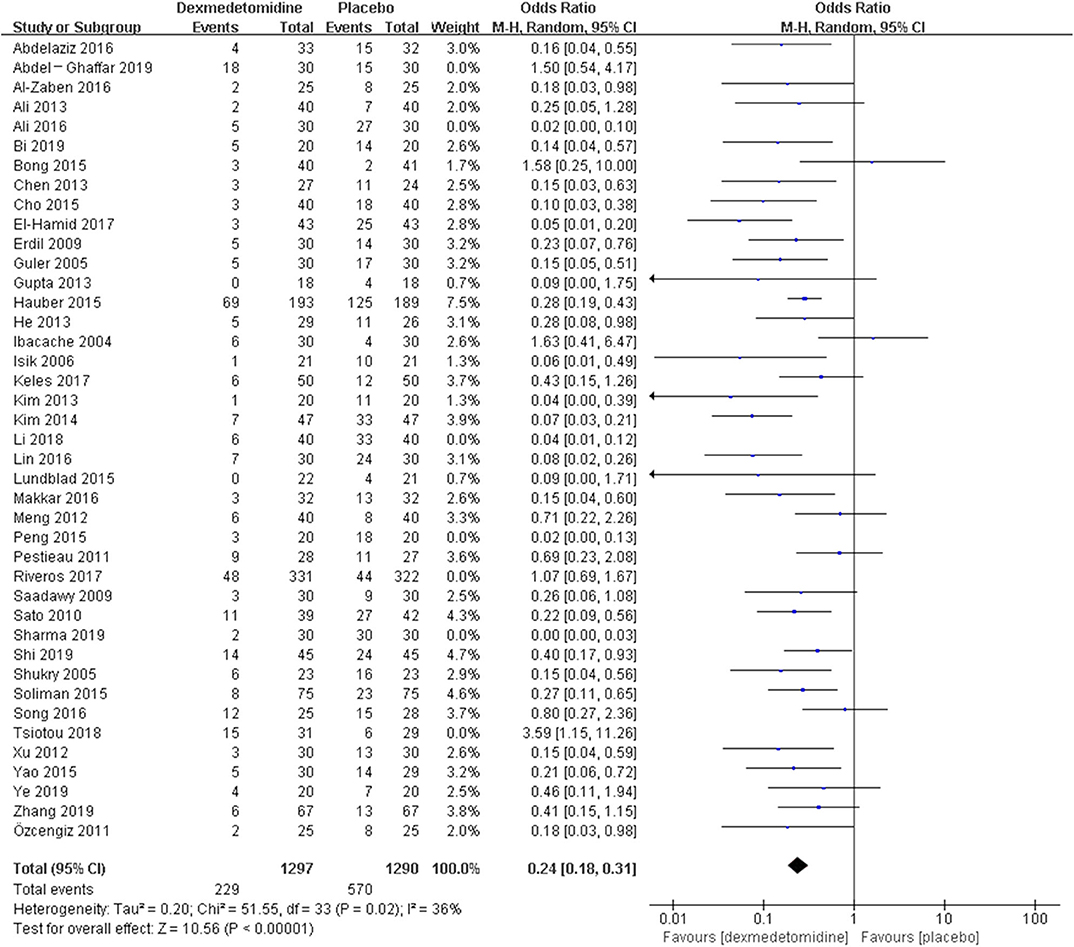
Figure 7. Comparison of pediatric EA or ED between dexmedetomidine and placebo groups after sensitivity analysis.
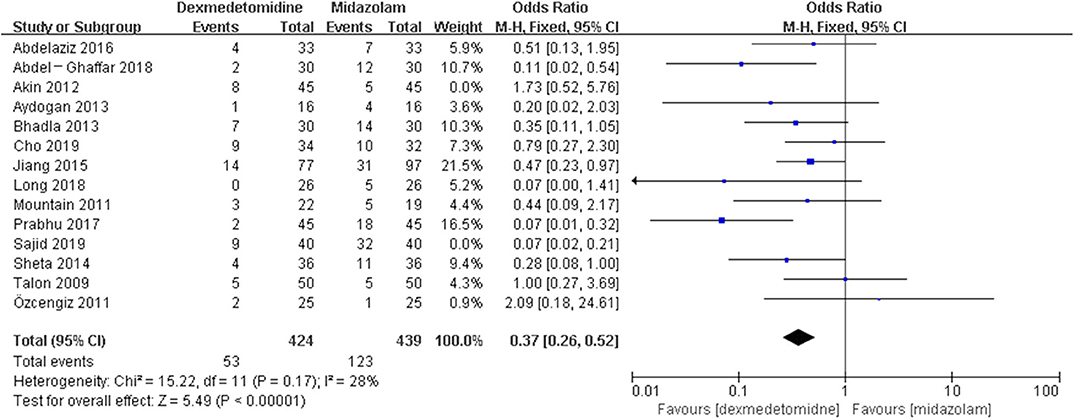
Figure 8. Comparison of pediatric EA or ED between dexmedetomidine and midazolam groups after sensitivity analysis.
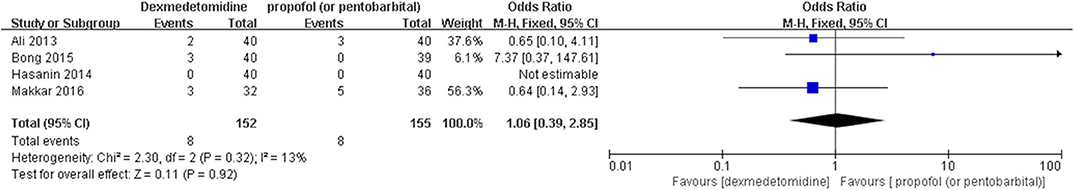
Figure 9. Comparison of pediatric EA or ED between dexmedetomidine and propofol (or pentobarbital) groups after sensitivity analysis.
Discussion
This meta-analysis included 58 RCTs and 5 CCTs that compared the prophylactic effect of dexmedetomidine vs. placebo or other sedatives on post-anesthesia EA or ED in pediatric patients undergoing medical procedures. The results showed that dexmedetomidine strikingly decreased the incidence of post-anesthesia EA or ED compared with placebo, midazolam, or opioids, whereas the significant difference was not exhibited compared with propofol (or pentobarbital), ketamine, clonidine, chloral hydrate, melatonin, and ketofol, respectively.
Currently, the specific predisposing causes of EA or ED following medical procedures in children remain unclear. Children undergoing general anesthesia are prone to suffer post-anesthesia EA or ED due to their immature central nervous system, preoperative fear and anxiety about unfamiliar surroundings, and postoperative pain (84–86). In addition, the children undergoing inhalation anesthesia through sevoflurane, isoflurane, or desflurane may suffer from a high incidence of post-anesthesia EA or ED (87, 88). Various medications have been used to prevent EA or ED in pediatric patients, like benzodiazepines, opioids, propofol, ketamine, clonidine, dexmedetomidine, and so on (11, 89–93).
Dexmedetomidine, as a highly selective α2 adrenergic receptor agonist, can produce pharmacological effects of anti-anxiety, sedation, and analgesia without overt respiratory and circulatory inhibition in a routine dose (94, 95). Meanwhile, dexmedetomidine can improve the cognitive function in children during recovery from general anesthesia (96) and contributes to dose-dependent inhibition of EA or ED after medical procedures (97). The optimal dose (ED95) of dexmedetomidine for preventing EA was 0.30 μg/kg (95% CI: 0.21–1.00 μg/kg) (83). An animal experiment demonstrated that dexmedetomidine could enhance spatial learning and memory in neonatal rats under physiological conditions through promoting hippocampal neurogenesis (98). In this meta-analysis, nine trials had different dexmedetomidine groups according to different dosages (20, 33, 34, 40, 43, 45, 53, 56) or administration routes of this drug (21). Patients in the control groups of these nine trials were treated with a placebo (20, 21, 33, 34, 40, 43, 45, 53, 56), and patients in another control group in the study from Pestieau et al. received fentanyl treatment (45). We chose the dexmedetomidine group with higher incidence of EA or ED. Therefore, the pooled results were more convincing in the powerful prophylactic effect of dexmedetomidine on the occurrence of EA or ED in children compared with placebo and opioids.
Dexmedetomidine can be administered in a variety of ways, like intravenous, transnasal, oral, inhalation, caudal or nerve block, and so on; thus, pediatric patients can easily accept it. The pooled results of 53 trials comparing dexmedetomidine with placebo and midazolam showed that dexmedetomidine could work in various ways and was superior to placebo or midazolam in inhibiting EA or ED in children. However, compared with propofol (or pentobarbital) or ketamine, dexmedetomidine did not demonstrate its superiority in reducing pediatric EA or ED following anesthesia. The possible explanations included the following: (1) the efficacy of propofol (or pentobarbital) or ketamine in suppressing EA or ED occurrence was no less than that of dexmedetomidine; and (2) the number of relevant prospective studies needed to be further increased. Because only one article was included, we could not perform meta-analysis for trials in the group of clonidine, chloral hydrate, melatonin, or ketofol.
In this meta-analysis, high heterogenicity was detected in trials comparing dexmedetomidine with placebo (I2 = 75%), midazolam (I2 = 57%), and propofol (or pentobarbital) (I2 = 58%), respectively. Subgroup analysis is an effective method to solve large heterogenicity among studies (99). We suggested some possible risk factors associated with overt heterogenicity including the year of publication, study methods, the country of authors, the time of drug administration, the type of surgery, routes of drug administration, the bias risk of the study, and the range of patients' age. Meta-regression was used to identify heterogenicity sources. If the P-value of meta-regression was <0.05 through analyzing one risk factor, the subgroup analysis was performed based on this risk factor (99, 100). However, in this meta-analysis, all P-values of meta-regression were more than 0.05 through analyzing all possible risk factors in the placebo and midazolam groups. Hence, we considered that significant heterogeneity may be the result of a combination of multiple factors. The meta-analysis by a random-effect model can decrease the effect of significant heterogeneity on the results, although this method does not solve heterogeneity (101). In addition, the method of trial exclusion is also an effective method to solve large heterogenicity for meta-analysis (102). When we excluded seven trials (20, 23, 39, 44, 46, 49, 54) in the placebo group, two trials (61, 69) in the midazolam group, and one trial (76) in the propofol (or pentobarbital) group, all values of I2 dropped to below 40%. Interestingly, the pooled results were consistent with those prior to sensitivity analysis.
It is necessary to elaborate the strengths and limitations of our meta-analysis. Firstly, this meta-analysis presented a comprehensive and up-to-date analysis of dexmedetomidine vs. placebo or other sedatives in pediatric patients. Sixty-three included trials with unlimited study methods (RCTs and CCTs) and various administration routes and dosages were grouped according to control drugs; thus, the pooled outcomes revealed the effect of dexmedetomidine on pediatric EA or ED more comprehensively. Secondly, sensitivity analysis was conducted in groups with high heterogeneity to remove the influence of heterogeneity on the overall results. Thirdly, this meta-analysis provided several directions for future clinical studies about the effect of dexmedetomidine on EA or ED in children. In addition, some limitations should be taken into account in this meta-analysis. Foremost, 39 RCTs and 2 CCTs in 63 included trials were assessed to be high bias risk, and so many trials with high-risk bias would affect the results. Additionally, the age gap of participants in 9 trials (46, 52, 54, 58, 64, 71, 72, 75, 79) was over 10 years, and a large age gap might be an important risk factor associated with the unreliability of outcomes. Lastly, non-uniform definitions of EA or ED were an additional limitation of this meta-analysis. There were five strategies diagnosing EA or ED in included trials, i.e., three-point scale, four-point scale, five-point scale, pediatric Anesthesia Emergence Delirium (PAED) scale, and the Confusion Assessment Method for the ICU.
Conclusion
In conclusion, compared with placebo, midazolam, and opioids, dexmedetomidine significantly decreased the incidence of post-anesthesia EA or ED in pediatric patients. However, dexmedetomidine did not exhibit this superiority when compared with propofol and ketamine. With regard to clonidine, chloral hydrate, melatonin, or ketofol, the results needed to be further tested due to the fact that there was only one trial in each study.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
XW and JL designed this meta-analysis and supervised the acquisition and analysis of the data. YR, RZ, and XJ were independently responsible for reviewing the titles, abstracts, or both and summarized the data of the included literatures. RZ and XJ conducted statistical analysis of the data. YR wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00329/full#supplementary-material
Supplementary Figure 1. Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Supplementary Figure 2. Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Supplementary Table 1. The guidelines of the 2009 PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses).
Supplementary Table 2. The Newcastle-Otawa Quality Assessment Scale (NOS).
Supplementary Table 3. The bias risk of CCTs by Newcastle-Otawa Quality Assessment Scale (NOS).
Supplementary Table 4. Meta-regression (dexmedetomidine vs. placebo): estimate of between-study variance % residual variation due to heterogeneity.
Supplementary Table 5. Meta-regression (dexmedetomidine vs. midazolam): estimate of between-study variance % residual variation due to heterogeneity.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: American Psychiatric Publishing (2000).
2. Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. (2003) 96:1625–30. doi: 10.1213/01.ANE.0000062522.21048.61
3. Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. (2007) 104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8
4. Dexter F, Macario A, Manberg PJ, Lubarsky DA. Computer simulation to determine how rapid anesthetic recovery protocols to decrease the time for emergence or increase the phase I postanesthesia care unit bypass rate affect staffing of an ambulatory surgery center. Anesth Analg. (1999) 88:1053–63. doi: 10.1097/00000539-199905000-00016
5. Cho EJ, Yoon SZ, Cho JE, Lee HW. Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology. (2014) 120:1354–61. doi: 10.1097/ALN.0000000000000181
6. Aouad MT, Yazbeck-Karam VG, Nasr VG, El-Khatib MF, Kanazi GE, Bleik JH. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. (2007) 107:733–8. doi: 10.1097/01.anes.0000287009.46896.a7
7. Ng KT, Sarode D, Lai YS, Teoh WY, Wang CY. The effect of ketamine on emergence agitation in children: a systematic review and meta-analysis. Paediatr Anaesth. (2019) 29:1163–72. doi: 10.1111/pan.13752
8. Pickard A, Davies P, Birnie K, Beringer R. Systematic review and meta-analysis of the effect of intraoperative α-adrenergic agonists on postoperative behaviour in children. Br J Anaesth. (2014) 112:982–90. doi: 10.1093/bja/aeu093
9. Nguyen V, Tiemann D, Park E, Salehi A. Alpha-2 Agonists. Anesthesiol Clin. (2017) 35:233–45. doi: 10.1016/j.anclin.2017.01.009
10. Rong H, Zhao Z, Feng J, Lei Y, Wu H, Sun R, et al. The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun. (2017) 64:195–207. doi: 10.1016/j.bbi.2017.03.006
11. Ydemann M, Nielsen BN, Henneberg S, Jakobsen JC, Wetterslev J, Lauritsen T, et al. Intraoperative clonidine for prevention of postoperative agitation in children anaesthetised with sevoflurane (PREVENT AGITATION): a randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health. (2018) 2:15–24. doi: 10.1016/S2352-4642(17)30127-X
12. Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. (2017) 56:893–913. doi: 10.1007/s40262-017-0507-7
13. Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. (1988) 150:9–14. doi: 10.1016/0014-2999(88)90744-3
14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Koster G, Wetterslev J, Gluud C, Zijlstra JG, Scheeren TW, van der Horst IC, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. (2015) 41:203–21. doi: 10.1007/s00134-014-3604-1
16. Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. (2017) 23:6500–15. doi: 10.3748/wjg.v23.i35.6500
17. Aziz O, Athanasiou T, Darzi A. Minimally invasive conduit harvesting: a systematic review. Eur J Cardiothorac Surg. (2006) 29:324–3. doi: 10.1016/j.ejcts.2005.11.032
18. Chen P, Wu X, Wang Z, Li Z, Tian X, Wang J, et al. Effects of levosimendan on mortality in patients undergoing cardiac surgery: a systematic review and meta-analysis. J Card Surg. (2018) 33:322–9. doi: 10.1111/jocs.13716
19. Abdelaziz HMM, Bakr RH, Kasem AA. Effect of intranasal dexmedetomidine or intranasal midazolam on prevention of emergence agitation in pediatric strabismus surgery: a randomized controlled study. Egypt J Anaesth. (2016) 32:285–91. doi: 10.1016/j.egja.2015.11.009
20. Abdel-Ghaffar HS, Abdel-Wahab AH, Roushdy MM. Oral trans-mucosal dexmedetomidine for controlling of emergence agitation in children undergoing tonsillectomy: a randomized controlled trial. Rev Bras Anestesiol. (2019) 69:469–76. doi: 10.1016/j.bjane.2019.07.001
21. Al-Zaben KR, Qudaisat IY, Alja'bari AN, Ababneh OA, Yousef AM, Al-Shudifat AM. The effects of caudal or intravenous dexmedetomidine on postoperative analgesia produced by caudal bupivacaine in children: a randomized controlled double-blinded study. J Clin Anesth. (2016) 33:386–94. doi: 10.1016/j.jclinane.2016.04.049
22. Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth. (2013) 7:296–300. doi: 10.4103/1658-354X.115363
23. Ali WA, Mohammed AK, Elshorbagy HM. Dexmedetomidine versus ketofol effect on the incidence of emergence agitation associated with sevoflurane-based anesthesia in children undergoing orthopedic surgery. Egypt J Anaesth. (2016) 32:277–84. doi: 10.1016/j.egja.2016.01.004
24. Bi Y, Ma Y, Ni J, Wu L. Efficacy of premedication with intranasal dexmedetomidine for removal of inhaled foreign bodies in children by flexible fiberoptic bronchoscopy: a randomized, double-blind, placebo-controlled clinical trial. BMC Anesthesiol. (2019) 19:219. doi: 10.1186/s12871-019-0892-6
25. Bong CL, Lim E, Allen JC, Choo WL, Siow YN, Teo PB, et al. A comparison of single-dose dexmedetomidine or propofol on the incidence of emergence delirium in children undergoing general anaesthesia for magnetic resonance imaging. Anaesthesia. (2015) 70:393–9. doi: 10.1111/anae.12867
26. Chen JY, Jia JE, Liu TJ, Qin MJ, Li WX. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth. (2013) 60:385–92. doi: 10.1007/s12630-013-9886-x
27. Cho JE, Kim JY, Park SJ, Kil HK. The effect of 1 μg/kg dexmedetomidine combined with high-volume/low-concentration caudal ropivacaine in children undergoing ambulatory orchiopexy. Biol Pharm Bull. (2015) 38:1020–25. doi: 10.1248/bpb.b15-00086
28. El-Hamid AMA, Yassin HM. Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy and/or adenoidectomy. Saudi J Anaesth. (2017) 11:137–43. doi: 10.4103/1658-354X.203020
29. Erdil F, Demirbilek S, Begec Z, Ozturk E, Ulger MH, Ersoy MO. The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth Intensive Care. (2009) 37:571–6. doi: 10.1177/0310057X0903700405
30. Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. (2005) 15:762–6. doi: 10.1111/j.1460-9592.2004.01541.x
31. Gupta N, Rath GP, Prabhakar H, Dash HH. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol. (2013) 25:271–8. doi: 10.1097/ANA.0b013e31828cb6c0
32. Hauber JA, Davis PJ, Bendel LP, Martyn SV, McCarthy DL, Evans MC, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesthesia Anal. (2015) 121:1308–15. doi: 10.1213/ANE.0000000000000931
33. He L, Wang X, Zheng S, Shi Y. Effects of dexmedetomidine infusion on laryngeal mask airway removal and postoperative recovery in children anaesthetised with sevoflurane. Anaesth Intensive Care. (2013) 41:328–33. doi: 10.1177/0310057X1304100309
34. Ibacache ME, Munoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. (2004) 98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E
35. Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth. (2006) 16:748–53. doi: 10.1111/j.1460-9592.2006.01845.x
36. Keles S, Kocaturk O. The effect of oral dexmedetomidine premedication on preoperative cooperation and emergence delirium in children undergoing dental procedures. Biomed Res Int. (2017) 2017:6742183. doi: 10.1155/2017/6742183
37. Kim JM, Koo BN. Low dose-dexmedetomidine reduces emergence agitation in children after desflurane anesthesia undergoing strabismus surgery. Eur J Anaesthesiol. (2013) 30:158. doi: 10.1097/00003643-201306001-00492
38. Kim NY, Kim SY, Yoon HJ, Kil HK. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J. (2014) 55:209–15. doi: 10.3349/ymj.2014.55.1.209
39. Li H, Zhang L, Shi M, Yang S, Li S, Gao S. Impact of dexmedetomidine on pediatric agitation in the postanesthesia care unit. J Perianesth Nurs. (2018) 33:53–7. doi: 10.1016/j.jopan.2016.03.005
40. Lin Y, Chen Y, Huang J, Chen H, Shen W, Guo W, et al. Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J Clin Anesth. (2016) 33:289–95. doi: 10.1016/j.jclinane.2016.04.027
41. Lundblad M, Marhofer D, Eksborg S, Lönnqvist PA. Dexmedetomidine as adjunct to ilioinguinal/iliohypogastric nerve blocks for pediatric inguinal hernia repair: an exploratory randomized controlled trial. Paediatr Anaesth. (2015) 25:897–905. doi: 10.1111/pan.12704
42. Makkar JK, Bhatia N, Bala I, Dwivedi D, Singh PM. A comparison of single dose dexmedetomidine with propofol for the prevention of emergence delirium after desflurane anaesthesia in children. Anaesthesia. (2016) 71:50–7. doi: 10.1111/anae.13230
43. Meng QT, Xia ZY, Luo T, Wu Y, Tang LH, Zhao B, et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int J Pediatr Otorhinolaryngol. (2012) 76:1036–41. doi: 10.1016/j.ijporl.2012.03.028
44. Peng W, Zhang T. Dexmedetomidine decreases the emergence agitation in infant patients undergoing cleft palate repair surgery after general anesthesia. BMC Anesthesiol. (2015) 15:145. doi: 10.1186/s12871-015-0124-7
45. Pestieau SR, Quezado ZM, Johnson YJ, Anderson JL, Cheng YI, McCarter RJ, et al. The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatr Anaesth. (2011) 21:1128–35. doi: 10.1111/j.1460-9592.2011.03615.x
46. Riveros R, Makarova N, Riveros-Perez E, Chodavarapu P, Saasouh W, Yilmaz HO, et al. Utility and clinical profile of dexmedetomidine in pediatric cardiac catheterization procedures: a matched controlled analysis. Semin Cardiothor Vasc Anesth. (2017) 21:330–40. doi: 10.1177/1089253217708035
47. Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. (2009) 53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x
48. Sato M, Shirakami G, Tazuke-Nishimura M, Matsuura S, Tanimoto K, Fukuda K. Effect of single-dose dexmedetomidine on emergence agitation and recovery profiles after sevoflurane anesthesia in pediatric ambulatory surgery. J Anesth. (2010) 24:675–82. doi: 10.1007/s00540-010-0976-4
49. Sharma K, Kumar M, Gandhi R. Effect of single-dose dexmedetomidine on intraoperative hemodynamics and postoperative recovery during pediatric adenotonsillectomy. Anesth Essays Res. (2019) 13:63–7. doi: 10.4103/aer.AER_178_18
50. Shi M, Miao S, Gu T, Wang D, Zhang H, Liu J. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des Devel Ther. (2019) 13:897–905. doi: 10.2147/DDDT.S196075
51. Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. (2005) 15:1098–4. doi: 10.1111/j.1460-9592.2005.01660.x
52. Soliman R, Alshehri A. Effect of dexmedetomidine on emergence agitation in children undergoing adenotonsillectomy under sevoflurane anesthesia: a randomized controlled study. Egyptian Journal of Anaesthesia. (2015) 31:283–9. doi: 10.1016/j.egja.2015.04.006
53. Song IA, Seo KS, Oh AY, Baik JS, Kim JH, Hwang JW, et al. Dexmedetomidine injection during strabismus surgery reduces emergence agitation without increasing the oculocardiac reflex in children: a randomized controlled trial. PLoS ONE. (2016) 11:e0162785. doi: 10.1371/journal.pone.0162785
54. Tsiotou AG, Malisiova A, Kouptsova E, Mavri M, Anagnostopoulou M, Kalliardou E. Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr Anaesth. (2018) 28:632–638. doi: 10.1111/pan.13397
55. Xu L, Shen J, Zhou H. The application of dexmedetomidine in children undergoing vitreoretinal surgery. J Anesth. (2012) 26:556–61. doi: 10.1007/s00540-012-1354-1
56. Yao Y, Qian B, Lin Y, Wu W, Ye H, Chen Y. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr Anaesth. (2015) 25:492–8. doi: 10.1111/pan.12574
57. Ye W, Hu Y, Wu Y, Zhu Z, Jin X, Hu Z. Retrobulbar dexmedetomidine in pediatric vitreoretinal surgery eliminates the need for intraoperative fentanyl and postoperative analgesia: a randomized controlled study. Indian J Ophthalmol. (2019) 67:922–27. doi: 10.4103/ijo.IJO_1905_18
58. Zhang S, Zhang R, Cai M, Zhang K, Zhang M, Zheng J. Intranasal dexmedetomidine premedication in children with recent upper respiratory tract infection undergoing interventional cardiac catheterisation: a randomised controlled trial. Eur J Anaesthesiol. (2020) 37:85–90. doi: 10.1097/EJA.0000000000001097
59. Özcengiz D, Gunes Y, Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesthesia. (2011) 25:184–8. doi: 10.1007/s00540-011-1099-2
60. Abdel-Ghaffar HS, Kamal SM, El Sherif FA, Mohamed SA. Comparison of nebulised dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. BMC Anesthesiol. (2018) 121:445–52. doi: 10.1016/j.bja.2018.03.039
61. Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth. (2012) 22:871–6. doi: 10.1111/j.1460-9592.2012.03802.x
62. Aydogan MS, Korkmaz MF, Ozgül U, Erdogan MA, Yucel A, Karaman A, et al. Pain, fentanyl consumption, and delirium in adolescents after scoliosis surgery: dexmedetomidine vs midazolam. Paediatr Anaesth. (2013) 23:446–52. doi: 10.1111/pan.12128
63. Bhadla S, Prajapati D, Louis T, Puri G, Panchal S, Bhuva M. Comparison between dexmedetomidine and midazolam premedication in pediatric patients undergoing ophthalmic day-care surgeries. Anesth Essays Res. (2013) 7:248–56. doi: 10.4103/0259-1162.118982
64. Cho EA, Cha YB, Shim JG, Ahn JH, Lee SH, Ryu KH. Comparison of single minimum dose administration of dexmedetomidine and midazolam for prevention of emergence delirium in children: a randomized controlled trial. J Anesth. (2019) 34:59–65. doi: 10.1007/s00540-019-02705-6
65. Jiang L, Ding S, Yan H, Li Y, Zhang L, Chen X, et al. A retrospective comparison of dexmedetomidine versus midazolam for pediatric patients with congenital heart disease requiring postoperative sedation. Pediatr Cardiol. (2015) 36:993–9. doi: 10.1007/s00246-015-1110-z
66. Long D, Keles S, Kocaturk O. Comparison of oral dexmedetomidine and midazolam for premedication and emergence delirium in children after dental procedures under general anesthesia: a retrospective study. BMJ Open. (2018) 12:647–53. doi: 10.2147/DDDT.S163828
67. Mountain BW, Smithson L, Cramolini M, Wyatt TH, Newman M. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. Aana J. (2011) 79:219–24.
68. Prabhu MK, Mehandale SG. Comparison of oral dexmedetomidine versus oral midazolam as premedication to prevent emergence agitation after sevoflurane anaesthesia in paediatric patients. Indian J Anaesth. (2017) 61:131–6. doi: 10.4103/0019-5049.199852
69. Sajid B, Mohamed T, Jumaila M. A comparison of oral dexmedetomidine and oral midazolam as premedicants in children. J Anaesthesiol Clin Pharmacol. (2019) 35:36–40. doi: 10.4103/joacp.JOACP_20_18
70. Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth. (2014) 24:181–9. doi: 10.1111/pan.12287
71. Talon MD, Woodson LC, Sherwood ER, Aarsland A, McRae L, Benham T. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res. (2009) 30:599–605. doi: 10.1097/BCR.0b013e3181abff90
72. Bedirli N, Akcabay M, Emik U. The effects of intraoperative single dosage tramadol or dexmedetomidine on postoperative analgesia, sedation and emerge reactions in pediatric patients undergoing adenotonsillectomy. Reg Anesth Pain Med. (2011) 36:E152.
73. Park SJ, Shin S, Kim SH, Kim HW, Kim SH, Do HY, et al. Comparison of dexmedetomidine and fentanyl as an adjuvant to ropivacaine for postoperative epidural analgesia in pediatric orthopedic surgery. Yonsei Med J. (2017) 58:650–7. doi: 10.3349/ymj.2017.58.3.650
74. Patel A, Davidson M, Tran MCJ, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. (2010) 111:1004–10. doi: 10.1213/ANE.0b013e3181ee82fa
75. Hasanin AS, Sira AM. Dexmedetomidine versus propofol for sedation during gastrointestinal endoscopy in pediatric patients. Egypt J Anaesth. (2014) 30:21–6. doi: 10.1016/j.egja.2013.09.006
76. Mason KP, Prescilla R, Fontaine PJ, Zurakowski D. Pediatric CT sedation: comparison of dexmedetomidine and pentobarbital. AJR Am J Roentgenol. (2011) 196:W194–8. doi: 10.2214/AJR.10.5045
77. Eldeek AM, Elfawal SM, Allam MG. Sedation in children undergoing magnetic resonance imaging comparative study between dexmedetomidine and ketamine. Egypt J Anaesth. (2016) 32:263–8. doi: 10.1016/j.egja.2016.04.007
78. Ezz HAA. Preoperative intranasal dexmedetomidine versus intranasal ketamine for prevention of emergence agitation after sevoflurane in myringotomy patients: a randomized clinical trial. Egypt J Anaesth. (2017) 33:141–6. doi: 10.1016/j.egja.2017.03.001
79. Koruk S, Mizrak A, Kaya Ugur B, Ilhan O, Baspinar O, Oner U. Propofol/dexmedetomidine and propofol/ketamine combinations for anesthesia in pediatric patients undergoing transcatheter atrial septal defect closure: a prospective randomized study. Clin Ther. (2010) 32:701–9. doi: 10.1016/j.clinthera.2010.04.010
80. Mukherjee A, Das A, Basunia SR, Chattopadhyay S, Kundu R, Bhattacharyya R. Emergence agitation prevention in paediatric ambulatory surgery: a comparison between intranasal Dexmedetomidine and Clonidine. J Res Pharm Pract. (2015) 4:24–30. doi: 10.4103/2279-042X.150051
81. Yuen VM, Li BL, Cheuk DK, Leung MKM, Hui TWC, Wong IC, et al. A randomised controlled trial of oral chloral hydrate vs. intranasal dexmedetomidine before computerised tomography in children. Anaesthesia. (2017) 72:1191–5. doi: 10.1111/anae.13981
82. Olsen RW. Analysis of γ-aminobutyric acid (GABA) type A receptor subtypes using isosteric and allosteric ligands. Neurochem Res. (2014) 39:1924–41. doi: 10.1007/s11064-014-1382-3
83. Zhang YZ, Wang X, Wu JM, Song CY, Cui XG. Optimal dexmedetomidine dose to prevent emergence agitation under sevoflurane and remifentanil anesthesia during pediatric tonsillectomy and adenoidectomy. Front Pharmacol. (2019) 10:1091. doi: 10.3389/fphar.2019.01091
84. Porter S, Holly C, Echevarria M. Infants with delirium: a primer on prevention, recognition, and management. Pediatr Nurs. (2016) 42:223–9.
85. Banchs RJ, Lerman J. Preoperative anxiety management, emergence delirium, and postoperative behavior. Anesthesiol Clin. (2014) 32:1–23. doi: 10.1016/j.anclin.2013.10.011
86. Kim JS, Lee HS, Park DH, Seok S, Kim TK, Lee HS, et al. Effect of size and location of nevi on postoperative pain and emergence agitation in children undergoing nevi excision. J Clin Med. (2019) 8:E106. doi: 10.3390/jcm8010106
87. Costi D, Cyna AM, Ahmed S, Stephens K, Strickland P, Ellwood J, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev. (2014) 2014:CD007084. doi: 10.1002/14651858.CD007084.pub2
88. Jildenstål PK, Rawal N, Hallén JL, Berggren L, Jakobsson JG. Routines for reducing the occurrence of emergence agitation during awakening in children, a national survey. Springerplus. (2014) 3:572. doi: 10.1186/2193-1801-3-572
89. Anderson BJ, Exarchos H, Lee K, Brown TC. Oral premedication in children: a comparison of chloral hydrate, diazepam, alprazolam, midazolam and placebo for day surgery. Anaesth Intensive Care. (1990) 18:185–93. doi: 10.1177/0310057X9001800205
90. Cray SH, Dixon JL, Heard CM, Selsby DS. Oral midazolam premedication for paediatric day case patients. Paediatr Anaesth. (1996) 6:265–70. doi: 10.1111/j.1460-9592.1996.tb00448.x
91. Bilgen S, Köner Ö, Karacay S, Sancar NK, Kaspar EC, Sözübir S. Effect of ketamine versus alfentanil following midazolam in preventing emergence agitation in children after sevoflurane anaesthesia: a prospective randomized clinical trial. J Int Med Res. (2014) 42:1262–71. doi: 10.1177/0300060514543039
92. Wu X, Cao J, Shan C, Peng B, Zhang R, Cao J, et al. Efficacy and safety of propofol in preventing emergence agitation after sevoflurane anesthesia for children. Exp Ther Med. (2019) 17:3136–40. doi: 10.3892/etm.2019.7289
93. Fang XZ, Gao J, Ge YL, Zhou LJ, Zhang Y. Network meta-analysis on the efficacy of dexmedetomidine, midazolam, ketamine, propofol, and fentanyl for the prevention of sevoflurane-related emergence agitation in children. Am J Ther. (2016) 23:e1032–42. doi: 10.1097/MJT.0000000000000321
94. Faritus SZ, Khazaee-Koohpar M, Ziyaeifard M, Mehrabanian MJ. Oral dexmedetomidine versus midazolam as anesthetic premedication in children undergoing congenital heart surgery. Anesth Pain Med. (2015) 5:e25032. doi: 10.5812/aapm.5(3)2015.25032
95. Bellon M, Le Bot A, Michelet D, Hilly J, Maesani M, Brasher C, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for postoperative pain management: a meta-analysis of published studies. Pain Ther. (2016) 5:63–80. doi: 10.1007/s40122-016-0045-2
96. Jia ZM, Hao HN, Huang ML, Ma DF, Jia XL, Ma B. Influence of dexmedetomidine to cognitive function during recovery period for children with general anesthesia. Eur Rev Med Pharmacol Sci. (2017) 21:1106–11.
97. Chen F, Wang C, Lu Y, Huang M, Fu Z. Efficacy of different doses of dexmedetomidine as a rapid bolus for children: a double-blind, prospective, randomized study. BMC Anesthesiol. (2018) 18:103. doi: 10.1186/s12871-018-0562-0
98. Zhang Y, Gao Q, Wu Z, Xue H, Liu B, Zhao P. Dexmedetomidine promotes hippocampal neurogenesis and improves spatial learning and memory in neonatal rats. Drug Des Devel Ther. (2019) 13:4439–49. doi: 10.2147/DDDT.S228220
99. Brunetti ND, Santoro F, Correale M, De Gennaro L, Conte G, Di Biase M. Incidence of atrial fibrillation is associated with age and gender in subjects practicing physical exercise: a meta-analysis and meta-regression analysis. Int J Cardiol. (2016) 221:1056–60. doi: 10.1016/j.ijcard.2016.07.133
100. Weymann A, Sabashnikov A, Ali-Hasan-Al-Saegh S, Popov AF, Jalil Mirhosseini S, Baker WL, et al. Predictive role of coagulation, fibrinolytic, and endothelial markers in patients with atrial fibrillation, stroke, and thromboembolism: a meta-analysis, meta-regression, and systematic review. Med Sci Monit Basic Res. (2017) 23:97–140. doi: 10.12659/MSMBR.902557
101. Moreno E, Vázquez-Polo FJ, Negrín MA. Bayesian meta-analysis: the role of the between-sample heterogeneity. Stat Methods Med Res. (2018) 27:3643–3657. doi: 10.1177/0962280217709837
Keywords: dexmedetomidine, pediatric, agitation, delirium, meta-analysis
Citation: Rao Y, Zeng R, Jiang X, Li J and Wang X (2020) The Effect of Dexmedetomidine on Emergence Agitation or Delirium in Children After Anesthesia—A Systematic Review and Meta-Analysis of Clinical Studies. Front. Pediatr. 8:329. doi: 10.3389/fped.2020.00329
Received: 25 March 2020; Accepted: 20 May 2020;
Published: 14 July 2020.
Edited by:
Saskia N. De Wildt, Radboud University Nijmegen, NetherlandsReviewed by:
Oguz Dursun, Akdeniz University, TurkeyPhuc Huu Phan, Vietnam National Hospital of Pediatrics, Vietnam
Copyright © 2020 Rao, Zeng, Jiang, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaocou Wang, wangxiaocou@126.com
†These authors have contributed equally to this work
 Yuquan Rao
Yuquan Rao Xiaocou Wang
Xiaocou Wang