
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 05 June 2020
Sec. Neonatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00289
This article is part of the Research Topic Experimental and Clinical Approaches in the Pursuit of Novel Therapeutic Strategies for Perinatal Brain Injury and its Neurological Sequelae View all 17 articles
 Jessie R. Maxwell1,2
Jessie R. Maxwell1,2 Amber J. Zimmerman2
Amber J. Zimmerman2 Nathaniel Pavlik1
Nathaniel Pavlik1 Jessie C. Newville2
Jessie C. Newville2 Katherine Carlin3
Katherine Carlin3 Shenandoah Robinson4
Shenandoah Robinson4 Jonathan L. Brigman2
Jonathan L. Brigman2 Frances J. Northington3
Frances J. Northington3 Lauren L. Jantzie3,5,6*
Lauren L. Jantzie3,5,6*Neonatal hypoxic-ischemic encephalopathy (HIE) remains a common problem world-wide for infants born at term. The impact of HIE on long-term outcomes, especially into adulthood, is not well-described. To facilitate identification of biobehavioral biomarkers utilizing a translational platform, we sought to investigate the impact of HIE on executive function and cognitive outcomes into adulthood utilizing a murine model of HIE. HIE mice (unilateral common carotid artery occlusion to induce ischemia, followed by hypoxia with a FiO2 of 0.08 for 45 min) and control mice were tested on discrimination and reversal touchscreen tasks (using their noses) shown to be sensitive to loss of basal ganglia or cortical function, respectively. We hypothesized that the HIE injury would result in deficits in reversal learning, revealing complex cognitive and executive functioning impairments. Following HIE, mice had a mild discrimination impairment as measured by incorrect responses but were able to learn the paradigm to similar levels as controls. During reversal, HIE mice required significantly more total trials, errors and correction trials across the paradigm. Analysis of specific stages showed that reversal impairments in HIE were driven by significant increases in all measured parameters during the late learning, striatal-mediated portion of the task. Together, these results support the concept that HIE occurring during the neonatal period results in abnormal neurodevelopment that persists into adulthood, which can impact efficient associated learning. Further, these data show that utilization of an established model of HIE coupled with touchscreen learning provides valuable information for screening therapeutic interventions that could mitigate these deficits to improve the long-term outcomes of this vulnerable population.
Neonatal hypoxic-ischemic encephalopathy (HIE) occurs in as many as 6 infants per 1,000 live births (1–4). While there is likely an acute event occurring around delivery, the injury observed in HIE is likely a combination of acute on chronic injury (5). Recent placental studies have found that chronic fetal vascular malperfusion appears to be associated with neonatal HIE, leading to the hypothesis that impaired fetal blood flow may result in the infant being more susceptible to brain injury from altered cerebral blood flow in the setting of an acute event (5, 6).
Children with a history of HIE are at high risk of abnormal cognitive development. Unfortunately, more than half of infants have abnormal neurodevelopment following HIE (1, 7). Additionally, even those infants with mild HIE, previously thought to have normal outcomes, have been observed to have abnormal neurodevelopment with deficits apparent in childhood including lower cognitive composite scores (1, 8). While executive function is an umbrella term used to describe the processes necessary to achieve goal-directed behavior, multiple domain specific deficits have been reported after HIE in children. A recent analysis reports that 22% of children with moderate HIE and no cerebral palsy were found to attend special education, compared to all of the control group attending mainstream school (9). Thus, it is paramount that we further investigate the long-term deficits in cognition these children may have following this perinatal injury.
Characterizing the spectrum of brain injury and identifying biobehavioral biomarkers are critical to predict outcomes and advance novel therapeutic interventions for children with HIE. Brain magnetic resonance imaging (MRI) was used in a term infant population with neonatal encephalopathy, and found evidence of acute insult without evidence of established injury or atrophy in 69–80% of the infants imaged (10–12), supporting the concept that the brain injury is occurring around the time of delivery. Specific regions of the brain seem to be more susceptible to injury in the setting of HIE, and include cerebral cortex, basal ganglia, putamen, thalamus, and brainstem (13–15). Additionally, cerebral white matter is also often injured (15) as well as the hippocampus (16).
Animal models have been utilized to characterize the brain injury following HIE more specifically, with rigorous characterization of the pathophysiology occurring during the newborn period. The Rice-Vannucci model is currently accepted as the standard model of term HIE. Studies have described the injury that occurs following this model, including cellular injury from mitochondrial dysfunction and oxidative stress, injury to the hippocampus, caudate-putamen, cerebral cortex, and thalamus (17–20). Currently, data defining the specific pillars of cognition impacted in adulthood by HIE is a gap in knowledge. Therefore, we sought to test the hypothesis that HIE would yield deficits in complex cognitive and executive functioning using a translational touchscreen approach sensitive enough to detect changes in functional outcome across many models of perinatal brain injury. Specifically, we tested the effects of neonatal HIE on a touchscreen platform to assess cognitive function in adulthood and we examined whether pairwise visual discrimination learning and reversal, known to be mediated by separate regions of the brain (21), were sensitive to long-term injury following HIE. Utilizing a touchscreen platform that incorporates stimuli, learning rules and response actions that mimic those used in human cognitive assessments such as the Cambridge Automated Neuropsychological Test Automated Battery (CANTAB), we investigated a validated, translational measure of cognitive function following HIE (22–24). Discrimination and reversal learning are heavily dependent on orbitofrontal-striatal connections in both humans and rodents (25, 26). Reversal learning is a paradigm to assess cognitive flexibility in the setting of changing stimulus-outcome or response-outcome (26). Different stages of learning can be assessed by looking at specific times during the reversal paradigm, including early (<50% correct) and late (>50% correct). Thus, the utilization of this testing paradigm allows for insight into specific characterization of cognitive deficits observed followed HIE in a preclinical model. We predicted that mice subject to HIE would have cognitive deficits as adults, defined by deficits in reversal learning, specifically the learning or later portion, reflective of the structural brain injury extensively published in this model (13, 17–20, 27–32).
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of New Mexico. All animal studies were carried out with standards of care and housing in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services. C57BL/6 male (n = 12) and female mice (n = 16) were used for a total of 28 mice.
To induce hypoxic-ischemic (HI) injury in a term-equivalent population, the Rice-Vannucci model was adapted for use in mice at postnatal day 10 (P10), as previously published (18, 29, 33–35). Briefly, mice were anesthetized with 3% isoflurane for induction and maintained with 1% isoflurane throughout the procedure and kept normothermic. The right common carotid artery was isolated and ligated via double suture to result in a permanent unilateral carotid ligation. The incision was closed with dermabond, and the mouse recovered for 1 h with their littermates. Mice with unilateral carotid ligation then underwent hypoxia for 45 min with an FiO2 of 0.08 in a humidified, temperature-controlled hypoxia chamber (29, 36, 37). Sham mice were anesthetized, with carotid artery isolated but not ligated. They were not exposed to hypoxia. Each litter consisted of both HI and sham mice. Following completion of the experiment, mice matured with their dams until weaning at P21.
All operant behavior was conducted in a chamber measuring 21.6 × 17.8 × 12.7 cm (model # ENV-307W, Med Associates, St. Albans, VT) housed within a sound- and light attenuating box (Med Associates, St. Albans, VT). A solid acrylic plate was used to cover the grid floor of the chamber to facilitate ambulation. A peristaltic pump delivered 30 μl of liquid strawberry milkshake (strawberry Nesquik mixed with skim milk) into a magazine as required. A house-light, tone generator and an ultra-sensitive lever was located on one end of the chamber, while a touch-sensitive screen (Conclusive Solutions, Sawbridgeworth, U.K.) was on the opposite side of the chamber covered by a black acrylic aperture plate, which creates two 7.5 × 7.5 cm touch areas separated by 1 cm and located at a height of 0.8 cm from the floor of the chamber. KLimbic Software Package v1.20.2 (Conclusive Solutions) controlled and recorded stimulus presentation and touches in the response windows.
Beginning at 8 weeks of age (~P60 or adolescent human age equivalent), all mice were handled daily and were food-restricted to 85% of their free-feeding body weight. Food restriction ensures animals are properly motivated to obtain the reward during task performance and did not begin until all mice had reached 20 grams in weight. While HI mice were slightly smaller after HI surgery and through the second and third postnatal week consistent with the model, all mice reached the same weight prior to starting the food restriction. Operant training began once mice reached food-restricted weight at ~10 weeks of age. Mice were first acclimated to the liquid reward by provision of ~30 μl/mouse in the home cage for 3 days and then habituated to retrieving reward in the operant chamber. Mice were allowed 30 min to freely retrieve rewards available in the magazine. Mice retrieving at least 10 rewards within 30 min were moved to lever press training. Here, mice could only obtain reward by responding on an ultrasensitive lever within the chamber. Reward delivery was accompanied by the presentation of secondary reinforcers: a 2-sec, 65 dB auditory tone and illumination of a magazine light. For each trial, mice were required to collect the delivered reward (measured by magazine beam-break) before another reward was available via an active lever response. Mice were required to lever-press and collect 30 rewards in under 30 min before moving to acquisition testing.
Pairwise discrimination and reversal was tested as previously described (38–41). Briefly, mice were first trained to discriminate 2 novel, approximately equally-luminescent stimuli, presented in the center of each window in a spatially pseudorandomized (left/right) manner, over 30-trial sessions (5-s inter-trial interval) using their nose (21, 42). The stimulus designated as correct was counterbalanced across mice and treatment. Responses at the correct stimulus window resulted in a 30 μl liquid reward, cued by a 1-s tone and illumination of the magazine. Responses at the incorrect stimulus window resulted in a forced timeout (10 s), signaled by illumination of the house-light. Correction trials following errors were presented with the correct stimulus presentation in the same window until a correct response was made. Discrimination criterion was ≥85% correct responding out of 30 trials, excluding correction trials, over 2 consecutive sessions. Reversal training began on the session after discrimination criterion was attained. Here, the designation of correct verses incorrect stimuli was reversed for each mouse. As for discrimination, there were 30-trial daily sessions until the mice reached a criterion of ≥85% correct responding (excluding correction trials) over 2 consecutive sessions. Figure 1 summarizes the surgical procedure completed at P10, followed by recovery and pretraining on the touchscreen platform at 8 weeks of age. Visual discrimination was then completed, in which one stimulus was the correct response. Upon reaching criterion, reversal testing occurred in which the previously correct stimulus was now incorrect.
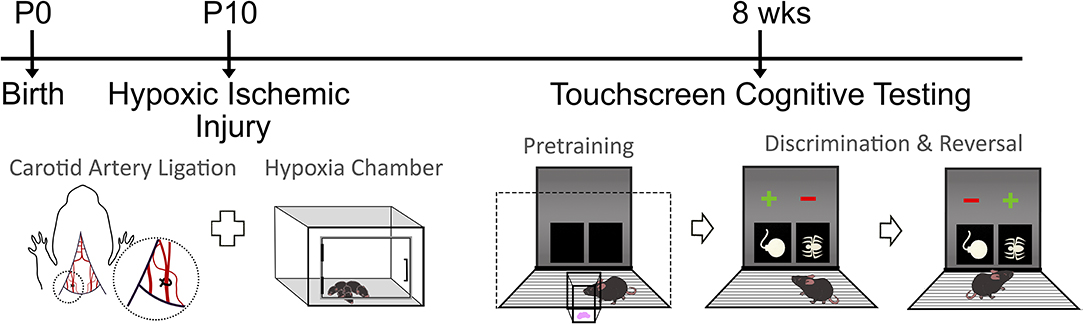
Figure 1. Timeline of experimental design. Mice randomized to the HIE group underwent the carotid artery ligation and hypoxia chamber exposure at postnatal day 10 (P10). Following recovery, the touchscreen cognitive testing started with pretraining, followed by discrimination and reversal tasks in both sham and HIE groups. During pretraining, mice became familiar with the chamber and the food reward system. In discrimination, one symbol was the correct answer, which would result in a food reward if chosen. During reversal, the symbol that was previously correct became the incorrect response.
For discrimination and reversal, the following dependent variables were analyzed: trials, errors, correction errors, reaction time (time from lever press initiation to screen touch) and magazine latency (time from screen touch to reward retrieval). In order to examine distinct phases of reversal (early perseverative and late learning) errors and correction errors for sessions where performance was below 50% and performance from 50% to criterion, were separately analyzed as previously described (25, 43). To analyze use of feedback for learning, correct and incorrect responses were further categorized based on previous trial outcome: correct responses were characterized as win-stay (following correct response) or lose-shift (following an error trial), while error trials were characterized as perseverative (following an error trial) or regressive (following correct response) as previously described (40). As assumptions for normality and equivalent variance were met, data were analyzed using unpaired t-tests followed by Bonferroni correction for multiple comparisons. Data is represented as mean ± standard error of the mean (SEM), with p < 0.05 designated as statistically significant.
Analysis of sex as a biological variable found no main effect of sex on any measure, and thus both sexes were combined by treatment for subsequent analysis [12 males (6 sham, 6 HIE) and 16 females (7 sham and 9 HIE) were used in total]. The average weights of the mice prior to food restriction was 20.01 grams ± 0.79. Examining pretraining, we found no significant differences between groups on any of the three stages (HIE: n = 15; Sham: n = 13). All mice in each group successfully completed visual discrimination to criterion. However, while the total number of trials during visual discrimination did not differ significantly between the HIE and sham groups (t(26) = 1.768, p = 0.09; Figure 2A), the HIE group had significantly more incorrect responses compared to sham (t(26) = 2.304, p = 0.03; Figure 2B). Additionally, no significant difference was noted in the number of correction trials during visual discrimination (t(26) = 1.540, p = 0.13; Figure 2C). There was also no significant difference in the reaction time (t(26) = 1.204, p = 0.24) or magazine latency (t(26) = 1.114. p = 0.28) between the two groups during visual discrimination (Figure 2D), suggesting that alterations in learning were not due to differences in motor behavior or motivation to work for reward.
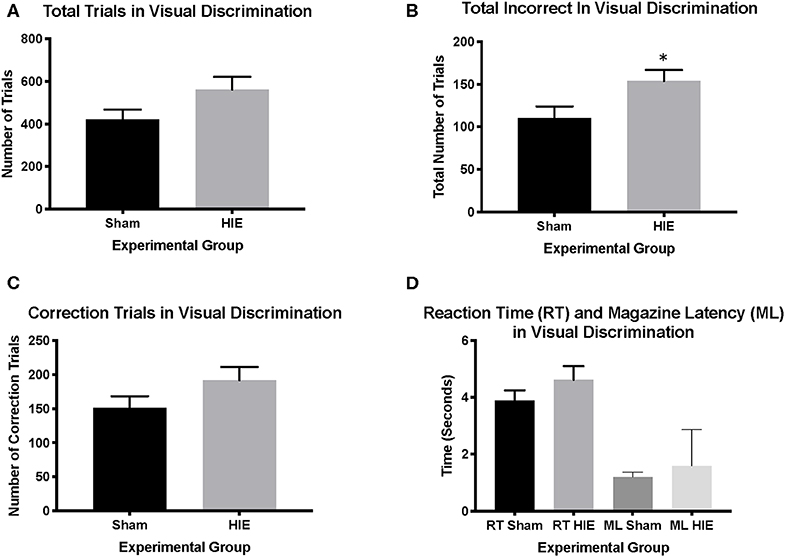
Figure 2. Visual discrimination revealed more incorrect trials in the HIE group compared to sham. During visual discrimination, the total number of trials between the sham and the HIE group were not significantly different (A). The HIE group did require significantly more incorrect trials to complete the visual discrimination component compared to sham (B). Although the HIE group had more correction trials during visual discrimination, this was not significant (C). There was no difference in either the reaction time or magazine latency between the two groups during visual discrimination (D) (n = 13–15, *p < 0.05). Data is represented as mean ± standard error of the mean (SEM).
Analysis of reversal performance revealed multiple significant differences between HIE and Sham control animals. HIE mice required significantly more total trials to complete the reversal compared to sham (t(26) = 2.196, p = 0.02, Figure 3A). Similarly, HIE made significantly more errors (t(26) = 2.118, p = 0.04, Figure 3B) and correction errors (t(26) = 2.494, p = 0.02, Figures 2, 3C) compared to sham control animals. Consistent with discrimination, HIE and sham did not differ significantly on either reaction time (t(26) = 0.911, p=0.37) or magazine latency (t(26) = 0.722, p = 0.48) across the reversal (Figure 3D). Analysis of reversal learning by stage revealed that HIE animals needed similar number of trials (t(26) = 1.948, p = 0.06) and made similar numbers errors (t(26) = 0.978, p = 0.33) and correction errors (t(26) = 1.384, p = 0.18) during the early, perseverative, stage of reversal (Figures 4A–C). However, during the learning phase of reversal testing, the HIE group needed significantly more trials (t(26) = 2.374, p = 0.03, Figure 4D), and made significantly more errors (t(26) = 3.021, p = 0.005, Figure 4E) and required more correction trials (t(26) = 2.723, p = 0.01; Figure 4F) vs. controls. While both the HIE and control group were able to complete the visual discrimination portion of the paradigm, the HIE group had more significant differences during the late, learning phase of reversal, a portion of testing sensitive to striatal-mediated functions.
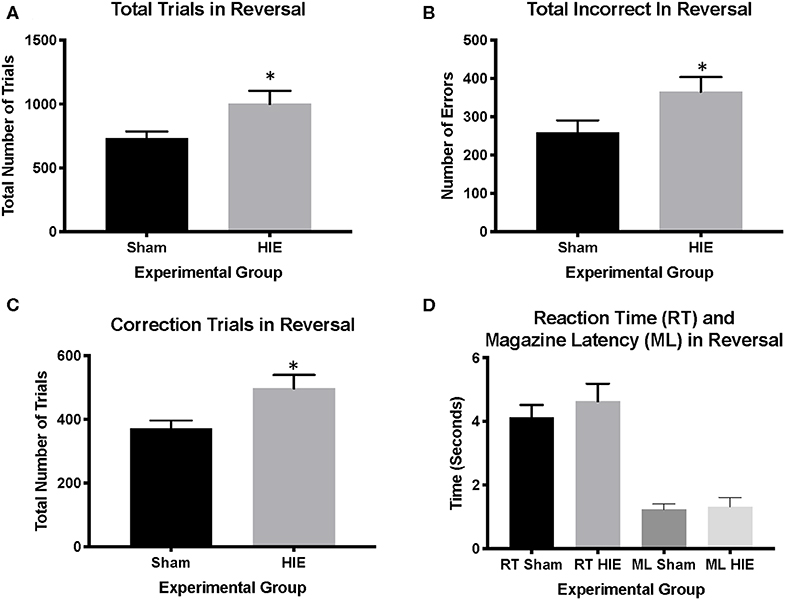
Figure 3. HIE induces reversal learning Deficits. The HIE group required significantly more trials to complete reversal compared to the sham group (A), as well as more incorrect responses during reversal (B). Additionally, the HIE group completed significantly more correction trials compared to the sham group (C). There was no difference in either the reaction time or magazine latency between the two groups during reversal (D) (n = 13–15, *p < 0.05). Data is represented as mean ± standard error of the mean (SEM).
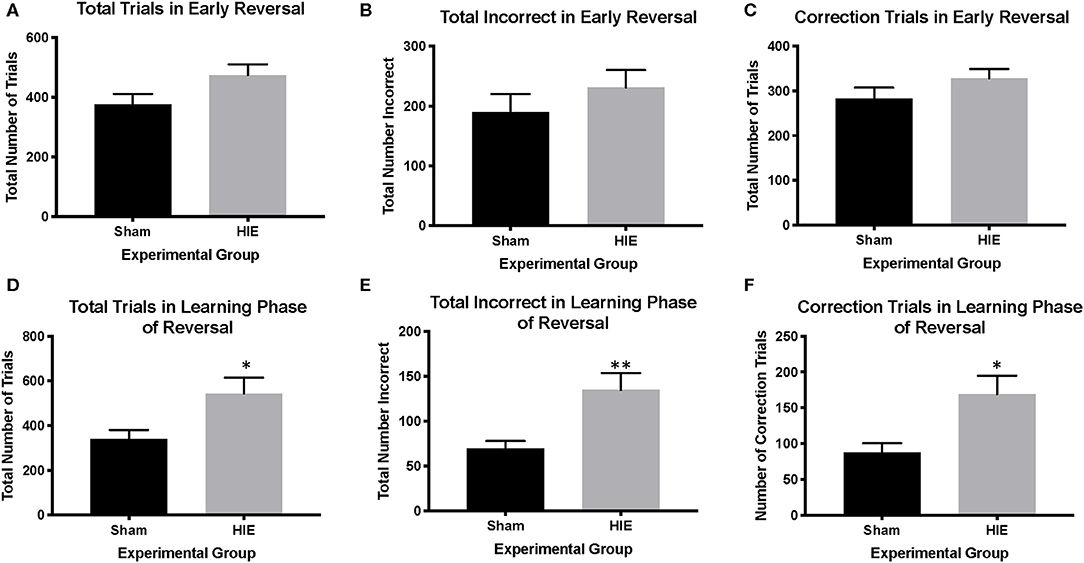
Figure 4. HIE yields distinct early phase and learning phase reversal deficits. In the early phase of reversal, there were no significant differences between HIE and sham in the total trials completed, the total incorrect, and the number of correction trials (A–C). However, in the learning phase of reversal, the HIE group had significantly more total trials (D), more incorrect trials (E) and more correction trials (F) compared to sham (n = 13–15, *p < 0.05, **p < 0.01). Data is represented as mean ± standard error of the mean (SEM).
While clinical studies have shown significant cognitive delays at 18 to 24 months of age following HIE as determined by lower mental developmental index scores and subnormal intelligence quotient scores at age 6 to 7, outcome studies beyond this time are extremely limited (44). Here, we investigated the long-term impact of HIE on learning and cognitive flexibility in adult mice following a neonatal HIE insult utilizing a translational touchscreen task. We found that HIE at term equivalent stage of development was sufficient to produce permanent deficits in learning, both in discrimination and late stage reversal learning. To date, this is the first report of deficits of cognition using a translational touchscreen platform and the first evidence establishing the long-term cognitive deficits that can occur following HIE in rodents. Prior studies in animals however have focused on sensory motor impairments and basic assessments of reflexes. For example, Y-maze testing and object location task after HIE, without and with therapeutic hypothermia, revealed a lower exploratory preference after HIE. The memory impairments were not altered by therapeutic hypothermia (33). In a different study, three developmental reflexes (righting, cliff aversion and geotaxis) were assessed 24 h after HIE in mice, and correlated with Morris Water Maze testing 8 weeks later (45). The Morris Water Maze testing of navigational learning and memory supported neurofunction deficits, leading the authors to conclude that sensorimotor reflex performance in the acute phase of HIE may have predictive values for long-term outcome (45). Given that these tests rely on aversive motivation, we chose to utilize the touchscreen testing platform which relies on positive motivation (food) as a means to test the hypothesis that HIE yield complex impairments in cognition and executive function that are specific to brain regions susceptible to injury. Additionally, touchscreen offers sophisticated, reproducible analysis of multiple pillars of behavior using a platform that directly corresponds to human testing (46–48).
Children with a history of HIE are at high risk of abnormal cognitive development. However, few studies have utilized animal models of HIE to examine the long-term impacts on cognitive function, with testing occurring into adulthood. Notably, mice at the conclusion of touchscreen training are over 150 days old. Utilizing a touchscreen platform, we found multiple deficits in learning, both during an initial pairwise discrimination, and during late stage reversal, when animals learn the new response rule to a high degree of consistency. HIE did not globally impair response learning, as mice were able to learn initiation and response behaviors in the absence of a discrimination during pretraining at levels similar to control. During discrimination, HIE animals showed consistently lower performance on all measures, although they were only significantly worse than control as measured by first-presentation correction errors. Importantly, secondary latency measures, such as reaction time and the time to retrieve a reward, did not differ across groups, suggesting that HIE did not globally impair motor behavior, or motivation to work for food reward. While they did have more incorrect responses on their way to completing the discrimination task, all HIE mice were able to perform at a high criterion level, allowing us to test them on reversal learning.
During reversal, the HIE group demonstrated a global impairment as measured by number of trials, errors and correction errors across the entire paradigm. However, the impairment was not typified by a cortical-mediated loss of flexibility. Reversal learning, and particularly the early, perseverative phase, has consistently been shown to be mediated by the orbitofrontal cortex (OFC) across species (26, 49–52). In the current study, HIE did not significantly differ on any measure during the perseverative phase (39, 40, 53). Rather, analysis of learning stage revealed that the reversal impairment was driven by significant increases in trials, errors, and correction trials during the later stages of reversal, when performance has reached or exceeded chance levels. Together, the increase in incorrect responses during initial learning together with impaired late-stage reversal suggest an impairment in the acquisition of well-trained stimulus-response contingencies, which is mediated by subcortical structures such as the dorsal striatum (dS) (25, 39). The efficient balance of associative learning and behavioral flexibility is mediated by cortico-striatal-thalamic loops (54) and single unit recording and imaging studies have demonstrated that the striatum plays a critical role in the representation of reward-action relationships required to efficiently guide choices (25, 55, 56). Interestingly, the touchscreen results of impaired learning implicate the dorsal striatum, in alignment with evidence that human infants with term HIE are more likely to have injury to the basal ganglia on present on neuroimaging evaluation.
Our study provides evidence of a significant and selective deficits in associative learning in mice with HIE. Upon acquiring a pairwise visual discrimination, when the reinforcement contingencies of the learned association were reversed, HIE mice were significantly impaired compared to shams. This was due to deficient learning of the new association rather than impaired reversal per se. Both groups performed at equivalent levels during reversal sessions when performance was low and perseveration high (i.e., <50%), while HIE mice committed more trials and errors during sessions when performance was largely learning related (i.e., >50%). This deficit is in contrast to previously published touchscreen assessments in animal models of perinatal brain injury (38, 41, 57). Specifically, adult rats perinatal brain injury secondary to chorioamnionitis have a perseverative phenotype and significant deficit in cognitive control defined by a reversal deficit (38). Similarly, adult rats suffering severe traumatic brain injury in infancy perseverate, lack cognitive flexibility and struggle to pass reversal learning criteria (41). The extensive white matter brain injury and orbitofrontocortical decoupling observed in both chorioamnionitis and traumatic brain injury may partially explain these findings. Interestingly, animals with perinatal exposure to methadone have a mixed phenotype of executive dysfunction with rats committing significantly more correction errors both during the perseverative phase and during the later learning phase compared to saline control animals (57). Together, these data indicate that adult rats exposed to perinatal methadone are impaired in both early and late reversal learning, consistent with global learning and executive control dysfunction (57) and widespread structural brain injury. In contrast to methadone exposure, prenatal alcohol exposure in mice results in behavioral inflexibility during early reversal testing, with persistent aberrant lateral orbital frontal cortex to dorsolateral striatum signaling (58). Taken together, these data indicate that the timing of injury (prenatal, perinatal or postnatal) has an impact on the type of cognitive deficits observed. While there are similarities in the injuries discussed such as inflammation, each injury results in a distinct behavioral phenotype. Notably, impaired performance as observed in HIE mice are not due to non-specific motivation or sensorimotor related performance as evidenced by normal scores on reaction response times and reward retrieval latency. Thus, although each injury model was different, the touchscreen platform is sensitive enough to detect distinct differences in cognitive domains following different types of perinatal brain injury.
Importantly, the impairments in learning during discrimination and reversal seen after HIE persist into adulthood. While more studies have reviewed outcomes of infants at a young age, few have observed this cohort into adolescence or adulthood (16). There has been greater recognition that HIE can have long-lasting impact on developmental trajectories, and studies have begun to assess the neurodevelopmental outcomes of children following HIE in the newborn period (9, 44, 59). Interestingly, children diagnosed with severe neonatal encephalopathy were found to be more than one grade behind the expected level for their age, with children diagnosed with moderate neonatal encephalopathy having difficulties in reading, spelling and mathematics (16). A recent prospective case-control study in the United Kingdom reported significantly lower mean full-scale IQ at 6–8 years of age after an HIE event, when compared to controls (59). Children with HIE also have significant differences in verbal comprehension, perceptual reasoning, working memory and processing speed (59). Similarly, a systematic review of five published studies reported a higher proportion of cognitive impairments at school age in children with a history of HIE, specifically in the area of executive functioning (9). The current results underline the idea that following infants diagnosed with neonatal encephalopathy is critically important, as they are at high risk of not only having difficulties at school age, but also into adulthood.
This study had limitations. While our data indicate significant differences in the HIE group, there is known variability of injury that occurs with the use of the Rice-Vannucci model (60). This variability can be beneficial, as it mimics the variation of injury observed in infants following HIE. Additionally, no environmental enrichment was used in the study, which could impact the neurodevelopmental outcomes (61, 62). Both sexes were utilized in this study, which adds to the generalizability of the results. The touchscreen platform is more robust and less vulnerable to environmental confounders than other modes of testing such as the Morris Water Maze. The rigor and reproducibility of the touchscreen testing, with the sensitivity of the measures obtained, allows for differences in performance from therapeutic interventions to be readily detected. Correlating outcomes on this touchscreen assessment of diverse pillars of cognition with pathology, lesion size, and multi-modal high-resolution neuroimaging could be investigated as a future direction. Finally, we did not include treatment within this study, such as hypothermia. Investigation the effect of hypothermia on the cognitive impairment reported here would be an essential element for future investigation as hypothermia is the only approved treatment for HIE in humans.
In sum, this is the first report that HIE in rodents is sufficient to cause long-lasting impairments in basal-ganglia mediated learning processes. This approach provides an important model platform for studies trialing adjunctive therapies to improve outcomes after HIE. Future studies should examine whether therapeutic hypothermia with additional therapies can mitigate the specific cognitive deficits that occur following HIE.
The datasets generated for this study are available on request to the corresponding author.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee (IACUC) of the University of New Mexico.
LJ conceptualized the hypothesis, designed and supervised the experiments. JM, AZ, NP, JN, KC, and LJ performed the experiments. JM, SR, JB, FN and LJ interpreted the data. JM, AZ, SR, FN, and LJ wrote the manuscript. All authors contributed to manuscript revision and approved the final version.
This study was supported by generous funding from the National Institutes of Health R01HL139492 to LJ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge the contributions of Haikun Zhang and Tracylyn R. Yellowhair.
1. Finder M, Boylan GB, Twomey D, Ahearne C, Murray DM, Hallberg B. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. (2019) 174:48–55. doi: 10.1001/jamapediatrics.2019.4011
2. Goswami IR, Whyte H, Wintermark P, Mohammad K, Shivananda S, Louis D, et al. Characteristics and short-term outcomes of neonates with mild hypoxic-ischemic encephalopathy treated with hypothermia. J Perinatol. (2019) 40:275–83. doi: 10.1038/s41372-019-0551-2
3. Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. (2010) 86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010
4. Shankaran S. Neonatal encephalopathy: treatment with hypothermia. J Neurotrauma. (2009) 26:437–43. doi: 10.1089/neu.2008.0678
5. Wu YW, Goodman AM, Chang T, Mulkey SB, Gonzalez FF, Mayock DE, et al. Placental pathology and neonatal brain MRI in a randomized trial of erythropoietin for hypoxic-ischemic encephalopathy. Pediatr Res. (2019) 87:1. doi: 10.1038/s41390-019-0493-6
6. Volpe JJ. Placental assessment provides insight into mechanisms and timing of neonatal hypoxic-ischemic encephalopathy. J Neonatal Perinatal Med. (2019) 12:113–6. doi: 10.3233/NPM-190270
7. Chiang MC, Lien R, Chu SM, Yang PH, Lin JJ, Hsu JF, et al. Serum lactate, brain magnetic resonance imaging and outcome of neonatal hypoxic ischemic encephalopathy after therapeutic hypothermia. Pediatr Neonatol. (2016) 57:35–40. doi: 10.1016/j.pedneo.2015.04.008
8. Lodygensky GA, Battin MR, Gunn AJ. Mild neonatal encephalopathy-how, when, and how much to treat?. JAMA Pediatr. (2018) 172:3–4. doi: 10.1001/jamapediatrics.2017.3044
9. Schreglmann M, Ground A, Vollmer B, Johnson MJ. Systematic review: long-term cognitive and behavioural outcomes of neonatal hypoxic-ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr. (2019) 109:20–30. doi: 10.1111/apa.14821
10. Cabaj A, Bekiesinska-Figatowska M, Madzik J. MRI patterns of hypoxic-ischemic brain injury in preterm and full term infants - classical and less common MR findings. Pol J Radiol. (2012) 77:71–6. doi: 10.12659/PJR.883379
11. Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. (2003) 361:736–42. doi: 10.1016/S0140-6736(03)12658-X
12. Krishnan P, Shroff M. Neuroimaging in neonatal hypoxic ischemic encephalopathy. Indian J Pediatr. (2016) 83:995–1002. doi: 10.1007/s12098-016-2042-1
13. Gerner GJ, Newman EI, Burton VJ, Roman B, Cristofalo EA, Leppert M, et al. Correlation between white matter injury identified by neonatal diffusion tensor imaging and neurodevelopmental outcomes following term neonatal asphyxia and therapeutic hypothermia: an exploratory pilot study. J Child Neurol. (2019) 34:556–66. doi: 10.1177/0883073819841717
14. Jisa KA, Clarey DD, Peeples ES. Magnetic resonance imaging findings of term and preterm hypoxic-ischemic encephalopathy: a review of relevant animal models and correlation to human imaging. Open Neuroimag J. (2018) 12:55–65. doi: 10.2174/1874440001812010055
15. Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol. (2012) 72:156–66. doi: 10.1002/ana.23647
16. van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. (2007) 166:645–54. doi: 10.1007/s00431-007-0437-8
17. Aggarwal M, Burnsed J, Martin LJ, Northington FJ, Zhang J. Imaging neurodegeneration in the mouse hippocampus after neonatal hypoxia-ischemia using oscillating gradient diffusion MRI. Magn Reson Med. (2014) 72:829–40. doi: 10.1002/mrm.24956
18. Fang J, Chavez-Valdez R, Flock DL, Avaritt O, Saraswati M, Robertson C, et al. An inhibitor of the mitochondrial permeability transition pore lacks therapeutic efficacy following neonatal hypoxia ischemia in mice. Neuroscience. (2019) 406:202–11. doi: 10.1016/j.neuroscience.2019.02.030
19. Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol. (2008) 64:535–46. doi: 10.1002/ana.21517
20. Tsuji S, Di Martino E, Mukai T, Tsuji S, Murakami T, Harris RA, et al. Aggravated brain injury after neonatal hypoxic ischemia in microglia-depleted mice. J Neuroinflammation. (2020) 17:111. doi: 10.1186/s12974-020-01792-7
21. Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR, et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. (2013) 8:1961–84. doi: 10.1038/nprot.2013.122
22. MacQueen DA, Minassian A, Kenton JA, Geyer MA, Perry W, Brigman JL, et al. Amphetamine improves mouse and human attention in the 5-choice continuous performance test. Neuropharmacology. (2018) 138:87–96. doi: 10.1016/j.neuropharm.2018.05.034
23. Nithianantharajah J, Grant SG. Cognitive components in mice and humans: combining genetics and touchscreens for medical translation. Neurobiol Learn Mem. (2013) 105:13–9. doi: 10.1016/j.nlm.2013.06.006
24. Nithianantharajah J, McKechanie AG, Stewart TJ, Johnstone M, Blackwood DH, St Clair D, et al. Bridging the translational divide: identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Sci Rep. (2015) 5:14613. doi: 10.1038/srep14613
25. Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, et al. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. (2013) 16:1101–10. doi: 10.1038/nn.3457
26. Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: an updated perspective. Neuroscience. (2017) 345:12–26. doi: 10.1016/j.neuroscience.2016.03.021
27. Carrasco M, Perin J, Jennings JM, Parkinson C, Gilmore MM, Chavez-Valdez R, et al. Cerebral autoregulation and conventional and diffusion tensor imaging magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol. (2018) 82:36–43. doi: 10.1016/j.pediatrneurol.2018.02.004
28. McNally MA, Chavez-Valdez R, Felling RJ, Flock DL, Northington FJ, Stafstrom CE. Seizure susceptibility correlates with brain injury in male mice treated with hypothermia after neonatal hypoxia-ischemia. Dev Neurosci. (2019) 69:1–10. doi: 10.1159/000496468
29. Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. (2011) 69:743–58. doi: 10.1002/ana.22419
30. Salas J, Reddy N, Orru E, Carson KA, Chavez-Valdez R, Burton VJ, et al. The role of diffusion tensor imaging in detecting hippocampal injury following neonatal hypoxic-ischemic encephalopathy. J Neuroimaging. (2019) 29:252–9. doi: 10.1111/jon.12572
31. Salas J, Tekes A, Hwang M, Northington FJ, Huisman T. Head ultrasound in neonatal hypoxic-ischemic injury and its mimickers for clinicians: a review of the patterns of injury and the evolution of findings over time. Neonatology. (2018) 114:185–97. doi: 10.1159/000487913
32. Wu D, Martin LJ, Northington FJ, Zhang J. Oscillating-gradient diffusion magnetic resonance imaging detects acute subcellular structural changes in the mouse forebrain after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. (2019) 39:1336–48. doi: 10.1177/0271678X18759859
33. Diaz J, Abiola S, Kim N, Avaritt O, Flock D, Yu J, et al. Therapeutic hypothermia provides variable protection against behavioral deficits after neonatal hypoxia-ischemia: a potential role for brain-derived neurotrophic factor. Dev Neurosci. (2017) 39:257–72. doi: 10.1159/000454949
34. Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. (1981) 9:131–41. doi: 10.1002/ana.410090206
35. Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. (2005) 27:81–6. doi: 10.1159/000085978
36. Jantzie LL, Talos DM, Selip DB, An L, Jackson MC, Folkerth RD, et al. Developmental regulation of group I metabotropic glutamate receptors in the premature brain and their protective role in a rodent model of periventricular leukomalacia. Neuron Glia Biol. (2010) 6:277–88. doi: 10.1017/S1740925X11000111
37. Jantzie LL, Todd KG. Doxycycline inhibits proinflammatory cytokines but not acute cerebral cytogenesis after hypoxia-ischemia in neonatal rats. J Psychiatry Neurosci. (2010) 35:20–32. doi: 10.1503/jpn.090061
38. Jantzie LL, Oppong AY, Conteh FS, Yellowhair TR, Kim J, Fink G, et al. Repetitive neonatal erythropoietin and melatonin combinatorial treatment provides sustained repair of functional deficits in a rat model of cerebral palsy. Front Neurol. (2018) 9:233. doi: 10.3389/fneur.2018.00233
39. Marquardt K, Josey M, Kenton JA, Cavanagh JF, Holmes A, Brigman JL. Impaired cognitive flexibility following NMDAR-GluN2B deletion is associated with altered orbitofrontal-striatal function. Neuroscience. (2019) 404:338–52. doi: 10.1016/j.neuroscience.2019.01.066
40. Marquardt K, Sigdel R, Brigman JL. Touch-screen visual reversal learning is mediated by value encoding and signal propagation in the orbitofrontal cortex. Neurobiol Learn Mem. (2017) 139:179–88. doi: 10.1016/j.nlm.2017.01.006
41. Robinson S, Winer JL, Chan LAS, Oppong AY, Yellowhair TR, Maxwell JR, et al. Extended erythropoietin treatment prevents chronic executive functional and microstructural deficits following early severe traumatic brain injury in rats. Front Neurol. (2018) 9:451. doi: 10.3389/fneur.2018.00451
42. Turner KM, Simpson CG, Burne TH. BALB/c mice can learn touchscreen visual discrimination and reversal tasks faster than C57BL/6 mice. Front Behav Neurosci. (2017) 11:16. doi: 10.3389/fnbeh.2017.00016
43. Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, et al. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. (2010) 20:1955–63. doi: 10.1093/cercor/bhp266
44. Pappas A, Shankaran S, McDonald SA, Vohr BR, Hintz SR, Ehrenkranz RA, et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics. (2015) 135:e624–34. doi: 10.1542/peds.2014-1566
45. Ten VS, Bradley-Moore M, Gingrich JA, Stark RI, Pinsky DJ. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav Brain Res. (2003) 145:209–19. doi: 10.1016/S0166-4328(03)00146-3
46. Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. (2012) 62:1191–203. doi: 10.1016/j.neuropharm.2011.04.011
47. Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem. (2008) 15:516–23. doi: 10.1101/lm.987808
48. Cotter J, Vithanage N, Colville S, Lyle D, Cranley D, Cormack F, et al. Investigating domain-specific cognitive impairment among patients with multiple sclerosis using touchscreen cognitive testing in routine clinical care. Front Neurol. (2018) 9:331. doi: 10.3389/fneur.2018.00331
49. Hamilton DA, Brigman JL. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes Brain Behav. (2015) 14:4–21. doi: 10.1111/gbb.12191
50. Kennerley SW, Behrens TE, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. (2011) 14:1581–9. doi: 10.1038/nn.2961
51. Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. (2013) 16:1140–5. doi: 10.1038/nn.3440
52. Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nat Neurosci. (2015) 18:620–7. doi: 10.1038/nn.3982
53. Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. (2008) 15:50–4. doi: 10.1101/lm.777308
54. Middleton FA, Strick PL. Basal-ganglia 'projections' to the prefrontal cortex of the primate. Cereb Cortex. (2002) 12:926–35. doi: 10.1093/cercor/12.9.926
55. Bergstrom HC, Lipkin AM, Lieberman AG, Pinard CR, Gunduz-Cinar O, Brockway ET, et al. Dorsolateral striatum engagement interferes with early discrimination learning. Cell Rep. (2018) 23:2264–72. doi: 10.1016/j.celrep.2018.04.081
56. Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. (2009) 12:333–41. doi: 10.1038/nn.2261
57. Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, et al. Prenatal opioid exposure: the next neonatal neuroinflammatory disease. Brain Behav Immun. (2020) 84:45–58. doi: 10.1016/j.bbi.2019.11.007
58. Marquardt K, Cavanagh JF, Brigman JL. Alcohol exposure in utero disrupts cortico-striatal coordination required for behavioral flexibility. Neuropharmacology. (2020) 162:107832. doi: 10.1016/j.neuropharm.2019.107832
59. Lee-Kelland R, Jary S, Tonks J, Cowan FM, Thoresen M, Chakkarapani E. School-age outcomes of children without cerebral palsy cooled for neonatal hypoxic-ischaemic encephalopathy in 2008-2010. Arch Dis Child Fetal Neonatal Ed. (2019) 105:8–13. doi: 10.1136/archdischild-2018-316509
60. Edwards AB, Feindel KW, Cross JL, Anderton RS, Clark VW, Knuckey NW, et al. Modification to the rice-vannucci perinatal hypoxic-ischaemic encephalopathy model in the P7 rat improves the reliability of cerebral infarct development after 48 hours. J Neurosci Methods. (2017) 288:62–71. doi: 10.1016/j.jneumeth.2017.06.016
61. Bailoo JD, Murphy E, Boada-Sana M, Varholick JA, Hintze S, Baussiere C, et al. Effects of cage enrichment on behavior, welfare and outcome variability in female mice. Front Behav Neurosci. (2018) 12:232. doi: 10.3389/fnbeh.2018.00232
Keywords: biobehavioral biomarker, HIE, touchscreen, learning acquisition, cognitive flexibility, reversal learning
Citation: Maxwell JR, Zimmerman AJ, Pavlik N, Newville JC, Carlin K, Robinson S, Brigman JL, Northington FJ and Jantzie LL (2020) Neonatal Hypoxic-Ischemic Encephalopathy Yields Permanent Deficits in Learning Acquisition: A Preclinical Touchscreen Assessment. Front. Pediatr. 8:289. doi: 10.3389/fped.2020.00289
Received: 10 January 2020; Accepted: 07 May 2020;
Published: 05 June 2020.
Edited by:
Changlian Zhu, Third Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Ana A. Baburamani, King's College London, United KingdomCopyright © 2020 Maxwell, Zimmerman, Pavlik, Newville, Carlin, Robinson, Brigman, Northington and Jantzie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren L. Jantzie, TEphbnR6aWVAamhtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.