- Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy
Eosinophilic esophagitis (EoE) is a chronic antigen-mediated inflammatory disease that affects the esophagus. In the last 20 years, a large number of epidemiological studies showed a significant increase in the incidence and prevalence of EoE, especially in developed countries. This phenomenon might correlate to the overall increase in pediatric allergic diseases or might be a result of improved medical awareness and knowledge through modern diagnostic instruments. Since 1993, when EoE was first recognized as a distinct clinical entity, several signs of progress in the pathophysiology of EoE were achieved. However, a few studies reported data on early risk factors for pediatric EoE and how these factors may interfere with genes. Currently, the most defined risk factors for EoE are male sex, Caucasian race, and atopic comorbidities. Other putative risk factors may include alterations in epithelial barrier function and fibrous remodeling, esophageal dysbiosis, variation in the nature and timing of oral antigen exposure, and early prescription of proton pump inhibitors and antibiotics. Notably, the timing and nature of food antigen exposure may be fundamental in inducing or reversing immune tolerance, but no studies are reported. This review summarized the current evidence on the risk factors that might contribute to the increasing development of EoE, focusing on the possible preventive role of early interventions.
Introduction
EoE is a chronic, antigen-mediated, inflammatory disease of the esophagus characterized by symptoms due to esophageal inflammation, dysmotility, and fibrosis (1, 2). EoE occurs in children and adults, and symptoms are often non-specific and depending on the age of onset (1, 2). While in toddlers and children EoE presents with inflammatory symptoms mimicking gastroesophageal reflux disease (GERD), in adolescents and adults EoE frequently appears with food impaction, dysphagia, odynophagia, or esophageal strictures, as a consequence of the ongoing fibrosis process (1, 2). EoE is a multifactorial disorder resulting from the combination of genetic predisposition, epithelial barrier dysfunction, environmental risk factors (Table 1), and allergen sensitization, leading to a T helper type 2 (Th2) atopic inflammation of the esophagus (2).
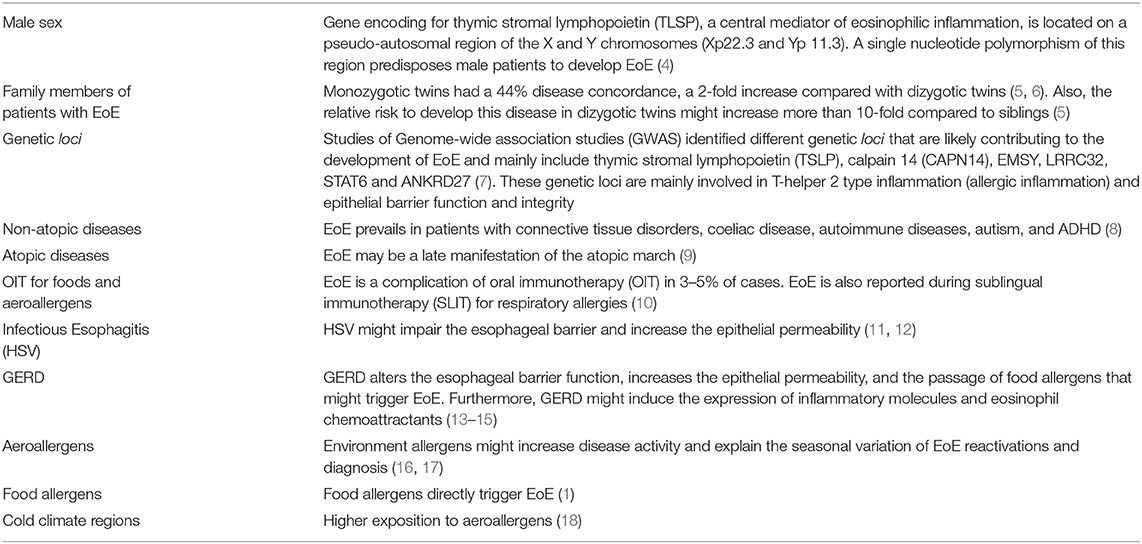
Table 1. Risk factors of eosinophilic esophagitis [adapted from Dellon and Hirano (3)].
Since 1993, when EoE was first recognized as a distinct clinical entity, several signs of progress in the pathophysiology of EoE were achieved; however, few studies reported data on early risk factors and how these factors might interfere with the genes in the disease onset and evolution. EoE is strictly associated with atopic disorders (asthma, atopic dermatitis, IgE mediated food allergy, allergic rhinitis), suggesting that EoE and allergic diseases share the same environmental risk factors and early life exposures.
We reviewed the recent evidence about the well-known risk factors of EoE, also reporting the less-investigated early exposures, to open future ideas of investigation in the limited field of prevention. Finally, we speculate about the possible strategies for EoE prevention.
Why is EoE a Modern Disease of Western Countries?
Recently, it was estimated that EoE affects 1/2,000 patients in the United States, with higher prevalence rate in adults (43.4/100,000; 95% CI, 22.5–71.2) than in children (29.5/100,000; 95% CI, 17.5–44.7), prevailing in Caucasian patients and male sex (Table 1) (1, 3, 19). In the last 20 years, a large number of epidemiological studies showed a significant increase of incidence and prevalence of EoE especially in children in Western Countries, varying widely across North America and Europe (19–21). This interesting phenomenon might be related to (1) an overall increased incidence of allergic and non-allergic diseases, (2) the chronic disease-course of EoE, and (3) the improved medical awareness and knowledge through modern diagnostic instruments (18). Although EoE is associated with some genetic polymorphisms (22, 23), this rapid increase in EoE frequency might indicate a prevalent role of environmental risk factors in disease development.
Hygienic Hypothesis, Dysbiosis, and Esophageal Infection
The hygienic hypothesis postulated for the first time in 1989 by Strachan (24), and recently reviewed (25), has explained the global rise of allergic and autoimmune diseases. Animal and human studies demonstrated that the increased frequency of allergic diseases in developed countries is a consequence of the modern hygienic conditions and fewer bacterial, viral, and parasitic infections during infancy and childhood (26). Although fundamental to reduce infectious diseases, an excessively hygienic environment in early life might induce adverse effects on the host microbiome, altering certain strains of necessary commensal bacteria (dysbiosis). Furthermore, microbial dysbiosis might arise from the modern lifestyle that is characterized by limited physical activity, low intake of fibers, a diet high in saturated fats, and more frequent use of antibiotics. An impaired microbiota might also result from early life events such as cesarean section, premature birth, early antibiotic exposure, and formula feeding (Table 2) (27). Patients with EoE showed differences in the esophageal microbiome and an increase of bacterial load compared to patients with GERD and healthy controls (28, 29, 62). Harris et al. have demonstrated that the esophageal microbiome in children with untreated and active EoE is characterized by the predominance of Haemophilus strain, compared to patients with disease-remission and healthy controls (29). Also, Benitez et al. characterized the bacterial composition of the oral and esophageal microenvironments from children with EoE and healthy controls, showing that specific bacterial strains (mainly Firmicutes) were more abundant in the esophagus compared to the oral cavity in EoE patients (62). These data suggest that eosinophilic inflammation might specifically alter the esophageal microbiota, and the oral microbiota could not be used as a surrogate for monitoring the disease activity.
Evidence on the role of the microbiome in EoE pathogenesis is still limited to a few studies. However, two possible hypotheses could explain the relationship between the gut microbiome and EoE: (1) early life risk factors might specifically influence the correct development of the esophageal microbiome, predisposing to EoE, (2) eosinophilic inflammation could lead to esophageal dysmotility and decrease the esophageal compliance; thus EoE itself might induce esophageal microbiome alteration (28). Both hypotheses might coexist in a vicious circle, and the first one opens the unexplored field of the early prevention of EoE. Currently, only a single study in a murine model showed the beneficial effect of the probiotic Lactococcus lactis NCC 2287 on the esophageal inflammation (63). Although raising evidence explained the pivotal role of the well-balanced gut microbiome in the correct development of the immune system (25), the precise mechanisms whereby hygienic environment and dysbiosis interact with each other and result in allergic and autoimmune disease is still understood (64). Moreover, further studies are needed to clarify the role of dysbiosis in EoE pathogenesis and to identify possible preventive strategies.
Infectious diseases might act as promotive or protective factors for atopic diseases, including the EoE. Studies reported the development of EoE after herpes simplex virus (HSV) infection in immunocompetent adults and children. These data suggest that HSV esophagitis might predispose to EoE, impairing the esophageal barrier, and increasing the epithelial permeability (11, 12).
In Western countries, the overall prevalence of Helicobacter pylori infection was decreased in the last decades, probably contributing to the rise of allergic diseases (65). Experiments in murine models demonstrated that the H. pylori infection early in life was protective against asthma through the induction of regulatory T cells (T-regs) (66). Furthermore, epidemiological data showed that the H. pylori infection was negatively associate with EoE, demonstrating the potential protective role in EoE pathogenesis (67–70). The decrease of H. pylori infection in Western countries might also be a consequence of better hygienic conditions; furthermore, its possible protective role might explain the lower prevalence of EoE in developing countries, where the infection is usually acquired in childhood. On the other hand, Molina-Infante et al. recently published the results of a large prospective case-control study conducted in 23 centers, and showed that the prevalence of H. pylori infection was not different between EoE cases and controls (37 vs. 40%; p = 0.3; OR 0.97; 95% CI 0.73–1.30), neither in children (42 vs. 46%; p = 0.1) nor in adults (36 vs. 38%; p = 0.4) (71). Therefore, there are already insufficient and conflicting data to support the protective role of H. pylori infection, and several issues are still open.
Diseases of Modern Life and Phenotypes of EoE
Recent advances in disease pathogenesis and prognosis have demonstrated that EoE could be classified in different phenotypes based on specific comorbidities. Epidemiological data demonstrated that EoE is so strongly associated with atopic comorbidities (asthma, allergic rhinitis, IgE-mediated food allergy, atopic dermatitis) (3, 9, 72) to follow allergic conditions in the atopic march, as a late manifestation (73). However, a significant number of EoE patients do not present allergic diseases, suggesting a possible non-atopic phenotype (2). Interestingly, several reports have suggested that EoE may be more frequently associated with some non-allergic disorders, including connective tissue disorders (74), autoimmune diseases (coeliac disease) (8), and contradictorily inflammatory bowel diseases (IBD) (8, 75–77), that are increased in the last decades, especially in Western countries (8). The pathogenetic mechanisms explaining the association between these non-atopic diseases and EoE are poorly understood and investigated. EoE and coeliac disease (CD) are two inflammatory diseases induced by food allergens. Although CD resulted more frequent in EoE patients than controls (5.6% of EoE, 0.9% of non-EoE, P < 0.0001) (8), Lucendo et al. did not find a common genetic basis between these two diseases (78). The frequency of the HLA DQ2 and DQ8 alleles predisposing to CD was not observed in adult EoE patients compared to controls (78). Also, type 1 diabetes, cystic fibrosis, adrenal insufficiency, autism, attention deficit hyperactivity disorder (ADHD) (8), and monogenic diseases (7) appear to be significantly associated with a non-atopic phenotype of EoE (2).
An increasing amount of evidence showed that children with esophageal atresia (EA) (30–32) or with diaphragmatic hernia (34) are at higher risk to develop EoE (33, 34, 79). Several risk factors have been associated with the development of EoE in children with EA, such as early life factors, early exposure to acid suppressants and antibiotics, GERD, esophageal dysmotility, and epithelial injury (79). Interestingly, Krishnan et al. demonstrated that children with EoE + EA share the same dysregulated genes (that encode for proteins involved in epithelial barrier functions and Th2 inflammation) compared to patients with EoE and without EA (33).
Although not widely demonstrated, another possible risk factor for EoE might be childhood exposure to caustic ingestion. Homan et al. reported a case of EoE development after caustic damage in a child with allergic comorbidity (35). The authors proposed two possible explanations for this association: (1) the caustic ingestion primarily triggered the eosinophilic inflammation of the esophagus or (2) after caustic damage the esophageal lesion might allow the trigger exposure (mainly food allergens) that might lead to EoE (35). Although fascinating, this report is characterized by some bias (child presented allergic diseases); however, further and extensive studies are required to confirm this data.
The diagnosis of gastroesophageal reflux disease (GERD) was also increased in the last two decades in Western countries (80), in parallel to allergic diseases, and, as a result of cow's milk allergy in the half of infants with refractory GER (81). Some authors reported that GERD might play a role in the pathogenesis of esophageal eosinophilia, more relevant in PPI-responsive cases (82). GERD, esophageal eosinophilia, and EoE are not mutually exclusive and may coexist in the same patient. However, there was no precise data about this association, and four mechanisms were proposed to explain it. (1) GERD causes esophageal eosinophilia in the absence of EoE, (2) GERD and EoE coexist but are unrelated, (3) EoE contributes to or causes GERD, (4) GERD contributes to or causes EoE (82). In patients with GERD, acid reflux alters the epithelial barrier of the esophagus, increasing the permeability and the passage of food allergens that might trigger EoE. Furthermore, acid reflux in GERD may induce the expression of inflammatory molecules and eosinophil chemoattractants (13, 83). On the other hand, eosinophilic inflammation produces different molecules (vasoactive intestinal peptide and interleukine-6) that might impair the esophageal peristalsis and delay the esophageal acid clearance (14). The subepithelial fibrosis, a delayed complication of EoE, might promote esophageal dysmotility (15). Further studies are needed to understand if this possible pathogenetic correlation might early predispose children with GERD to develop the EoE.
Interestingly, the 10–15% of children with EoE presented to the otolaryngologist before to be referred to the gastroenterologist (84), and the 33% of these patients required one or more otolaryngologic surgical interventions (20% bilateral myringotomy, 14% tonsillectomy, 18.5% adenoidectomy, 1.4% sinus irrigation, 3.3% bronchoscopy, and 1.4% laryngotracheoplasty), suggesting that EoE might overlap with otolaryngologic pathology (85).
Western Diet and Lifestyle
Although foods are the primary triggers of EoE, there are limited data about the role of the Western diet in the contribution of the EoE pathogenesis. Higher levels of fatty acids characterize the Western diet and could be related to the increased risk of developing allergic diseases. In a recent study in mice, Silva et al. demonstrated that high-fat diet and obesity aggravated the immune histopathological characteristics and increased inflammatory cells in the EoE experimental model (36). These fascinating data provide new insights about obesity as a possible risk factor, impairing EoE symptoms; however, further prospective studies are needed.
No studies evaluated tobacco exposure in children and adolescents with EoE. Only a recent case-control study of adult patients showed that smoking was inversely associated with EoE compared to controls (86).
Geographic Risk Factors and Vitamin D Levels
As previously reported and already described for other inflammatory gastrointestinal diseases, EoE prevails in specific geographic areas of the world. Prevalence rates of EoE were higher in Western regions of Europe, North America, and Australia than Asia and Africa (3). These geographic differences between Western countries (high prevalence) and Eastern countries (low prevalence) suggest that environmental factors might play a significant role in etiological mechanisms. The effects of people migration on the future development of EoE have not yet been investigated.
A few and conflicting studies evaluated the geographic distribution of EoE, based on the population density. An extensive US survey of Spergel et al. showed that EoE prevalence was higher in urban (0.58) and suburban (0.44) compared with rural settings (0.36, P < 0.0065) (87). Lee et al. demonstrated no significant difference in the incidence of EoE between people living in the rural area (50.9%) vs. patients from the urban ones (49.1%) (88). On the other hand, more recently, Jensen et al. found a strong inverse association between the population density and development of esophageal eosinophilia or EoE, demonstrating that EoE was more common in rural areas, in contrast with the hygienic hypothesis (89). A possible explanation of these results might be the geographic variation of specific environmental allergens.
Eosinophilic esophagitis prevails in cold climate zones, suggesting a possible association with specific aeroallergens (tree or grass pollens) and with low serum vitamin D levels (18). Increasingly significant evidence showed a link between vitamin D deficiency (maternal diet during pregnancy, early childhood diet, lack of exposure to sunlight) and risk of atopy, as described for asthma, allergic rhinitis, food allergy, and atopic dermatitis (37–39). This association is generally strongest in early life; in fact, interventional studies showed that the supplementation of vitamin D in utero and early life reduces the risk of recurrent wheeze and asthma (40–43). Although vitamin D enhances antimicrobial pathways, promotes peripheral immunological tolerance, and maintains mucosal barrier integrity, no studies have evaluated its possible preventive role in EoE development or its help in disease remission.
Climate zones might also affect the season of EoE diagnosis. Several single-center studies have evaluated the seasonality of symptoms and new diagnoses of EoE. In pediatric cohort studies, the seasonal exposure to aeroallergens increased the esophageal eosinophilic inflammation in children with EoE and allergic rhinitis (90, 91). However, the association between EoE relapse and season is still unclear, and available results were contradictory (16, 17, 92–97).
Early Life Risk Factors of EoE: State of Art
Early life is a critical period during the immune system and microbiota mature, becoming susceptible to early environmental exposures. A well-balanced microbiome is fundamental for the correct development of the immune system (98–100), and numerous early life exposures, including prenatal (maternal diseases, mother diet, and lifestyle), intrapartum (cesarean section, maternal fever, and infections, prematurity), and postnatal factors (early antibiotic and acid suppressants use, formula feeding), might impair the gut microbiome, and predispose to allergic diseases (101–109). The association between early impaired microbiota and risk of atopy is widely described for asthma, allergic rhinitis and food allergy (44, 45, 110). A few studies postulated that early life exposures might also predispose to EoE in childhood (Table 2). However, few studies focalized on early life exposures and their effects on the future development of EoE (27, 46–49). The available studies reported that formula feeding (27, 60), neonatal intensive care (NICU) admission, prematurity (47, 49), maternal fever (47), antibiotic and acid suppressants use in infancy (27, 49), cesarean delivery (27, 47) were putative early risk factors of EoE. The antibiotic and proton pump inhibitor (PPI) use in infancy showed the most consistent evidence of a positive correlation with the future development of EoE.
Effect of Early-Life Use of PPIs and Antibiotics
Although PPIs are used to treat GERD and esophageal eosinophilia, some studies paradoxically showed that the early PPI use might predispose to the development of autoimmune gastrointestinal diseases (celiac disease) (60), food allergies (13), and EoE (50). Physiologically, digestion of food proteins–and potential food allergens–begins into the stomach through pepsin proteinases, that are activated by the gastric acid milieu. PPI therapy might inactive proteinases and facilitate the digestive escape of food allergens, increasing the gastric pH. Also, PPI might increase the gastric mucosal permeability and the passage of allergens through the gastric mucosa, allowing their exposure to immune cells and the activation of atopic inflammation (51–53). Finally, PPI might alter the esophageal microbiota, and the modulation of immune response (54, 55). The risk to develop EoE after PPI therapy later in life has minimally been evaluated and could be higher after a long-term therapy (56, 111, 112). However, these data suggest that the immune system of infants might be more susceptible to PPI exposure, which might trigger the allergen-mediated inflammation of EoE. Since 1989 when the first PPI (Omeprazole) has been introduced into clinical practice, a worldwide escalation of PPI prescriptions was described at any age. Surprisingly, a pediatric study documented an 11-fold increase of new PPI prescriptions under 12 months of age in the last two decades (113).
The use of antibiotics in pregnancy is related to the treatment of several infections, such as bacterial vaginosis and urinary tract infections. Also, intrapartum and peripartum antibiotic prophylaxis are fundamental to decrease the risk of Group B Streptococcus infection in positive mothers and newborns. However, antibiotics might alter the immature gut microbiome of the newborn. Studies in rodents demonstrated that the administration of antibiotics in pregnancy decreased the microbiota diversity and permanently altered the immunity (57, 58). In newborns, the early administration of antibiotics resulted in decreased Bifidobacterium and increased enterococci strains (59). The worldwide increase of antibiotics prescriptions, especially in infancy, might partially explain the rise of allergic diseases. Observational studies demonstrated that the early life antibiotic administration was associated with asthma, atopic dermatitis, and allergic rhinitis (114–116). As previously mentioned, Jensen et al. founded a significant association with early antibiotic use and the development of EoE in children (27). Although an exact cause-effect mechanism cannot be deducted, these data suggest that the early exposure to antibiotics potentially might alter the immature microbiome and the developing immune system, allowing the risk of EoE (117).
The worldwide increase of PPI and antibiotic prescriptions in early life, associated with their possible pathogenetic role in allergic disease and EoE, suggests a conscious and rational use of these drugs, especially in childhood.
Breastfeeding and Timing of Food Introduction in Children
Breastfeeding might be a possible factor that could prevent the development of food allergy through different mechanisms. Human milk shows potentially anti-allergic immune properties; in particular, the presence of maternal antibodies might prevent exposure to food allergens and induce oral immuno-tolerance (118). However, there is limited evidence on the direct correlation between breastfeeding and the development of EoE. In a pediatric case-control study, Jensen et al. identified a strong interaction between the calpain14 (CAPN14) gene variant (rs6736278) and breastfeeding, suggesting the possible protective role of human milk against EoE. CAPN14 is a cysteine protease and plays a fundamental role in the integrity of the esophageal epithelial barrier. Furthermore, its expression is only limited to the esophageal mucosa (119). CAPN14 expression was almost 4-fold increased in EoE patients compared to controls. Higher levels of CAPN14 expression are associated with the downregulation of desmoglein 1, filaggrin, and zonulin, which are pivotal proteins of the epithelial barrier (119). Although the exact mechanism of interaction between breastfeeding and CAPN14 is still unknown, human milk with its immunological properties might protect the esophagus from the epithelial barrier impairment and the development of EoE in patients with specific genotypes (120).
Over the last decade, food allergy research mainly focused on the timing of food introduction and oral tolerance. Murine models well-explained the concept of oral tolerance, and previous works showed how early and regular oral exposure to food allergens induced clinical tolerance and immunological changes. A large amount of evidence demonstrated that an early introduction of allergens might protect against the risk to develop IgE-mediated food allergy (61, 121, 122). In the last years, an increasing scientific interest focused on the diagnosis of non-IgE mediated food allergy, which often presents with a delayed onset of gastrointestinal symptoms. The EAT study evaluated data of non-IgE mediated symptoms (colic, vomiting, regurgitation, diarrhea, and constipation), demonstrating that infants in the early intervention arm reported significantly more non-IgE type symptoms than children in the standard intervention arm. However, rates of non-IgE mediated symptoms were equivalent in both groups at any time point, suggesting that the reporting of these symptoms did not depend on the introduction of the specific food allergen (121, 123). Further research is needed to understand if early food introduction could prevent non-IgE mediated food allergies, including EoE. Although the understanding of the EoE pathogenesis achieved notable progress, there are no published studies about the timing of food introduction in infancy and the future development of EoE.
Genetic Risk Factors
EoE has a strong familiar hereditability pattern. Monozygotic twins had a 44% disease concordance, a 2-fold increase compared with dizygotic twins (5, 6). These data underly a complex interplay between genic loci and environmental exposures, through epigenetic mechanisms that are partially understood (6). Also, the relative risk to develop this disease in dizygotic twins might increase more than 10-fold compared to siblings. The increased rate of EoE development in dizygotic twins could be attributed to the same early-life environmental factors, previously mentioned.
The inheritance mechanism of EoE could be related to the effects of multiple single nucleotide gene polymorphisms (SNPs) that increase disease risk, depending on the environmental exposures and disease risk-modifying factors (119, 124). Several studies, including candidate-gene identification and genome-wide association studies (GWAS), have identified different genetic loci that are likely contributing to the development of EoE and mainly include thymic stromal lymphopoietin (TSLP), calpain 14 (CAPN14), EMSY, LRRC32, STAT6, and ANKRD27 (7). These genetic loci are mainly involved in T-helper 2 type inflammation (allergic inflammation) and epithelial barrier function and integrity. Interestingly, EoE is also associated with several monogenic inherited diseases, especially with connective tissue disorders and skin diseases. Connective tissue disorders, such as Marfan and Ehlers Danlos Syndromes, share a common pathogenic mechanism through the dysregulation of the TGF-β signaling. Children with autosomal dominant Hyper-IgE Syndrome (HIES) and Netherton Syndrome have also significantly increased the incidence of EoE (125, 126). Defects in PTEN, dehydrogenase E1, and transketolase domain–containing 1 (DHTKD1) genes are also associated with EoE (127, 128).
How Could we Prevent EoE?
The rise of EoE diagnosis, especially in children, is an actual problem, and preventive strategies are needed to limit this phenomenon. Although there are no published studies about the prevention of EoE, we could speculate that possible strategies of primary prevention of EoE might be:
1. Sustaining breastfeeding in the first 6 months of life, especially in preterm babies and newborns from mothers that underwent cesarean section.
2. Limiting the uncontrolled prescriptions of acid suppressants and antibiotics only in specific and right circumstances.
3. Do not delay the introduction of food allergens in infants.
4. Providing adequate levels of vitamin D in infant and children, especially in those from cold climate regions.
5. Encouraging a well-balanced diet and a healthy lifestyle both in pregnant women both in children.
This work has several strengths. Firstly, this is a comprehensive review, summarizing the current knowledge on EoE risk factors, and focusing on the role of early exposures. Also, this review tried to answer to two main clinical issues: (1) the increased prevalence and incidence of EoE in Western countries, especially in children; (2) the lack of knowledge on early risk factors and possible preventive strategies.
There are several limitations. First of all, the lack of extensive and prospective studies evaluating the real burden of environmental risk factors, particularly the pathogenetic role of early exposures. Secondly, the vast majority of genetic and epidemiological studies were realized in Western Countries and mostly in the US. Finally, a few studies evaluated the gene-environmental interactions and the possible preventive strategies for EoE. Therefore, the lack of prospective and extensive studies from Eastern and developing Countries did not allow to draw reliable conclusions on the role of early risk factors and preventive strategies in EoE.
In conclusion, EoE is an emerging atopic disease that affects people at any age and characterized by symptoms due to esophageal inflammation, dysmotility, and fibrosis. As described for allergic diseases, several environmental risk factors and early-life exposures might interfere with genes, alter tolerance mechanisms, and activate the Th2 inflammation of EoE. Further studies are needed to identify risk factors of EoE, understand the interaction between genes and environment, finally find possible early preventive strategies.
Author Contributions
MV and MD reviewed the literature and wrote the manuscript. AL, SC, IB, and GM reviewed the literature and helped with the writing of the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work. Questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AA, arachidonic acid; ADHD, attention deficit hyperactivity disorder; CAPN14, calpain 14; DHA, docosahexaenoic acid; EA, esophageal atresia; EoE, eosinophilic esophagitis; EPA, eicosapentaenoic acid; GERD, gastroesophageal reflux disease; HSV, herpes simplex virus; NICU, neonatal intensive care unit; OIT, oral immunotherapy; PPI, proton pump inhibitor; PUFA, polinsatured fatty acid; SLIT, sublingual immunotherapy; Th2, T helper type 2; TLSP, thymic stromal lymphopoietin; T-regs, regulatory T cells.
References
1. Furuta GT, Katzka DA. Eosinophilic esophagitis. N Engl J Med. (2015) 373:1640–8. doi: 10.1056/NEJMra1502863
2. Ruffner MA, Cianferoni A. Phenotypes and endotypes in eosinophilic esophagitis. Ann Allergy Asthma Immunol. (2020) 124:233–9. doi: 10.1016/j.anai.2019.12.011
3. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. (2018) 154:319–32. doi: 10.1053/j.gastro.2017.06.067
4. Sherrill JD1, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. (2010) 126:160–5. doi: 10.1016/j.jaci.2010.04.037
5. Kottyan LC, Parameswaran S, Weirauch MT, Rothenberg ME, Martin LJ. The genetic etiology of eosinophilic esophagitis. J Allergy Clin Immunol. (2020) 145:9–15. doi: 10.1016/j.jaci.2019.11.013
6. Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. (2014) 134:1084–92. doi: 10.1016/j.jaci.2014.07.021
7. O'Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology. (2018) 154:333–45. doi: 10.1053/j.gastro.2017.06.065
8. Capucilli P, Cianferoni A, Grundmeier RW, Spergel JM. Comparison of comorbid diagnoses in children with and without eosinophilic esophagitis in a large population. Ann Allergy Asthma Immunol. (2018) 121:711–6. doi: 10.1016/j.anai.2018.08.022
9. Hill DA, Dudley JW, Spergel JM. The prevalence of eosinophilic esophagitis in pediatric patients with IgE-mediated food allergy. J Allergy Clin Immunol Pract. (2017) 5:369–75. doi: 10.1016/j.jaip.2016.11.020
10. Cafone J, Capucilli P, Hill DA, Spergel JM. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol. (2019) 19:350–7. doi: 10.1097/ACI.0000000000000537
11. Žaja Franulović O, Lesar T, Busic N, Tešović G. Herpes simplex primo-infection in an immunocompetent host with eosinophilic esophagitis. Pediatr Int. (2013) 55:38–41. doi: 10.1111/ped.12027
12. Squires KA, Cameron DJ, Oliver M, da Fonseca Junqueira JC. Herpes simplex and eosinophilic oesophagitis: the chicken or the egg? J Pediatr Gastroenterol Nutr. (2009) 49:246–50. doi: 10.1097/MPG.0b013e31817b5b73
13. Untersmayr E, Jensen-Jarolim E. The effect of gastric digestion on food allergy. Curr Opin Allergy Clin Immunol. (2006) 6:214–9. doi: 10.1097/01.all.0000225163.06016.93
14. Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. (2004) 287:1131–9. doi: 10.1152/ajpgi.00216.2004
15. Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. (2012) 303:1175–87. doi: 10.1152/ajpgi.00313.2012
16. Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. (2009) 104:828–33. doi: 10.1038/ajg.2008.169
17. Iwanczak B, Janczyk W, Ryzko J, Banaszkiewicz A, Radzikowski A, Jarocka-Cyrta E, et al. Eosinophilic esophagitis in children: frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci. (2011) 56:151–7. doi: 10.2478/v10039-011-0038-7
18. Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. Risk factors for eosinophilic esophagitis. Clin Exp Allergy. (2014) 44:1012–9. doi: 10.1111/cea.12363
19. Arias A, Perez-Martinez I, Tenias JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. (2016) 43:3–15. doi: 10.1111/apt.13441
20. Shaheen NJ, Mukkada V, Eichinger CS, Schofield H, Todorova L, Falk GW. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus. (2018) 31:doy015. doi: 10.1093/dote/doy015
21. Navarro P, Arias Á, Arias-González L, Laserna-Mendieta EJ, Ruiz-Ponce M, Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. (2019) 49:1116–25. doi: 10.1111/apt.15231
22. Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. (2015) 148:1143–57. doi: 10.1053/j.gastro.2015.02.002
23. Ruffner MA. Kennedy K, Cianferoni A. Pathophysiology of eosinophilic esophagitis: recent advances and their clinical implications. Expert Rev Clin Immunol. (2019) 15:83–95. doi: 10.1080/1744666X.2019.1544893
24. Strachan DP. Hay fever, hygiene, and household size. BMJ. (1989) 299:1259–60. doi: 10.1136/bmj.299.6710.1259
25. Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. (2015) 136:860–5. doi: 10.1016/j.jaci.2015.08.012
26. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. (2002) 347:911–20. doi: 10.1056/NEJMra020100
27. Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2013) 57:67–71. doi: 10.1097/MPG.0b013e318290d15a
28. Dellon ES. The esophageal microbiome in eosinophilic esophagitis. Gastroenterology. (2016) 151:364–5. doi: 10.1053/j.gastro.2016.06.026
29. Harris JK, Fang R, Wagner BD, Choe HN, Kelly CJ, Schroeder S, et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE. (2015) 10:e0128346. doi: 10.1371/journal.pone.0128346
30. Lardenois E, Michaud L, Schneider A, Onea M, Rebeuh J, Gottrand-Aumar M, et al. Prevalence of eosinophilic esophagitis in adolescents with esophageal atresia. J Pediatr Gastroenterol Nutr. (2019) 69:52–6. doi: 10.1097/MPG.0000000000002261
31. Stave Salgado KV, Rocca AM. Eosinophilic esophagitis and esophageal atresia: coincidence or causality? Arch Argent Pediatr. (2018) 116:61–9. doi: 10.5546/aap.2018.eng.e61
32. Krishnan U. Eosinophilic esophagitis in children with esophageal atresia. Eur J Pediatr Surg. (2015) 25:336–44. doi: 10.1055/s-0035-1559815
33. Krishnan U, Lijuan C, Andrew GJ, Rothenberg ME, Wen T. Analysis of eosinophilic esophagitis in children with repaired congenital esophageal atresia. J Allergy Clin Immunol. (2019) 143:1455–64. doi: 10.1016/j.jaci.2018.08.040
34. Licari A, Castagnoli R, Marseglia GL. Eosinophilic esophagitis after congenital diaphragmatic hernia. Ital J Pediatr. (2016) 42:96. doi: 10.1186/s13052-016-0307-y
35. Homan M, Orel R, Liacouras C. Caustic ingestion: a possible cause of eosinophilic esophagitis? Pediatrics. (2013) 131:e1284–7. doi: 10.1542/peds.2012-2582
36. Silva FMCE, Oliveira EE, Ambrósio MGE, Ayupe MC, Souza VP, Gameiro J, et al. High-fat diet-induced obesity worsens TH2 immune response and immunopathologic characteristics in murine model of eosinophilic oesophagitis. Clin Exp Allergy. (2020) 50:244–55. doi: 10.1111/cea.13533
37. D'Auria E, Barberi S, Cerri A, Boccardi D, Turati F, Sortino S, et al. Vitamin D status and body mass index in children with atopic dermatitis: a pilot study in Italian children. Immunol Lett. (2017) 181:31–5. doi: 10.1016/j.imlet.2016.11.004
38. Pacheco-Gonzalez RM, Garcia-Marcos PW, Garcia-Marcos L. Vitamin D and atopic dermatitis. Mini Rev Med Chem. (2015) 15:927–34. doi: 10.2174/1389557515666150519110209
39. Licari A, Marseglia GL, Ciprandi G. Vitamin D3 in children with allergic asthma in clinical practice. Pediatr Pulmonol. (2019) 54:225–7. doi: 10.1002/ppul.24229
40. Kelly RS, Chawes BL, Guo F, Zhang L, Blighe K, Litonjua AA, et al. The role of the 17q21 genotype in the prevention of early childhood asthma and recurrent wheeze by vitamin D. Eur Respir J. (2019) 54:1900761. doi: 10.1183/13993003.00761-2019
41. Wolsk HM, Chawes BL, Litonjua AA, Hollis BW, Waage J, Stokholm J, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: a combined analysis of two randomized controlled trials. PLoS ONE. (2017) 12:186657. doi: 10.1371/journal.pone.0186657
42. Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, O'Connor G, Sandel M, et al. Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: Secondary analyses from the vitamin D antenatal asthma reduction trial. J Allergy Clin Immunol. (2017) 140:1423–9. doi: 10.1016/j.jaci.2017.01.013
43. Hibbs AM, Ross K, Kerns LA, Wagner C, Fuloria M, Groh-Wargo S, et al. Effect of vitamin D supplementation on recurrent wheezing in black infants who were born preterm: the D-wheeze randomized clinical trial. JAMA. (2018) 319:2086–94. doi: 10.1001/jama.2018.5729
44. Bager P, Melbye M, Rostgaard K, Benn CS, Westergaard T. Mode of delivery and risk of allergic rhinitis and asthma. J Allergy Clin Immunol. (2003) 111:51–6. doi: 10.1067/mai.2003.34
45. Celedon JC, Fuhlbrigge A, Rifas-Shiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy. (2004) 34:1011–6. doi: 10.1111/j.1365-2222.2004.01994.x
46. Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. (2014) 2:475–7. doi: 10.1016/j.jaip.2014.02.018
47. Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol. (2018) 141:214–22. doi: 10.1016/j.jaci.2017.05.018
48. Jensen ET, Gupta SK. Early life factors and eosinophilic esophagitis: building the evidence. J Pediatr Gastroenterol Nutr. (2018) 67:549–50. doi: 10.1097/MPG.0000000000002136
49. Witmer CP, Susi A, Min SB, Nylund CM. Early infant risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2018) 67:610–5. doi: 10.1097/MPG.0000000000002123
50. Merwat SN, Spechler SJ. Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol. (2009) 104:1897–902. doi: 10.1038/ajg.2009.87
51. Hopkins AM, McDonnell C, Breslin NP, O'Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol. (2002) 54:341–7. doi: 10.1211/0022357021778583
52. Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, Jain V, et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. (2008) 28:1317–25. doi: 10.1111/j.1365-2036.2008.03824.x
53. Gabello M, Valenzano MC, Zurbach EP, Mullin JM. Omeprazole induces gastric transmucosal permeability to the peptide bradykinin. World J Gastroenterol. (2010) 16:1097–103. doi: 10.3748/wjg.v16.i9.1097
54. Theisen J, Nehra D, Citron D, Johansson J, Hagen JA, Crookes PF, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. (2000) 4:50–4. doi: 10.1016/S1091-255X(00)80032-3
55. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. (2006) 23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x
56. Orel R, Murch S, Amil Dias J, Vandenplas Y, Homan M. Eosinophilic esophagitis that develops during therapy with proton pump inhibitors: case series and possible mechanisms. Acta Gastroenterol Belg. (2016) 79:245–50. doi: 10.4166/kjg.2016.67.4.178
57. Tormo-Badia N, Hakansson A, Vasudevan K, Molin G, Ahrne S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the off-spring. Scand J Immunol. (2014) 80:250–60. doi: 10.1111/sji.12205
58. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. (2014) 158:705–21. doi: 10.1016/j.cell.2014.05.052
59. Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. (2009) 56:80–7. doi: 10.1111/j.1574-695X.2009.00553.x
60. Lebwohl B, Spechler SJ, Wang TC, Green PH, Ludvigsson JF. Use of proton pump inhibitors and subsequent risk of celiac disease. Dig Liver Dis. (2014) 46:36–40. doi: 10.1016/j.dld.2013.08.128
61. Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the learning early about peanut allergy (LEAP) screening study. J Allergy Clin Immunol. (2013) 131:1–12. doi: 10.1016/j.jaci.2012.09.015
62. Benitez AJ, Hoffmann C, Muir AB, Dods KK, Spergel JM, Bushman FD, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. (2015) 3:23. doi: 10.1186/s40168-015-0085-6
63. Holvoet S, Doucet-Ladevèze R, Perrot M, Barretto C, Nutten S, Blanchard C. Beneficial effect of Lactococcus lactis NCC 2287 in a murine model of eosinophilic esophagitis. Allergy. (2016) 71:1753–61. doi: 10.1111/all.12951
64. Spechler SJ. Speculation as to why the frequency of eosinophilic esophagitis is Increasing. Curr Gastroenterol Rep. (2018) 20:26. doi: 10.1007/s11894-018-0633-x
65. Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. (2007) 167:821–7. doi: 10.1001/archinte.167.8.821
66. Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. (2011) 121:3088–93. doi: 10.1172/JCI45041
67. Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH, et al. Inverse association of esophageal eosinophilia with helicobacter pylori based on analysis of a US pathology database. Gastroenterology. (2011) 141:1586–92. doi: 10.1053/j.gastro.2011.06.081
68. von Arnim U, Wex T, Link A, Messerschmidt M, Venerito M, Miehlke S, et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther. (2016) 43:825–30. doi: 10.1111/apt.13560
69. Furuta K, Adachi K, Aimi M, Ishimura N, Sato S, Ishihara S, et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr. (2013) 53:60–2. doi: 10.3164/jcbn.13-15
70. Elitsur Y, Alrazzak BA, Preston D, Demetieva Y. Does Helicobacter pylori protect against eosinophilic esophagitis in children? Helicobacter. (2014) 19:367–71. doi: 10.1111/hel.12129
71. Molina-Infante J, Gutierrez-Junquera C, Savarino E, Penagini R, Modolell I, Bartolo O, et al. Upper GI tract study group from the spanish gastroenterological association (AEG). Helicobacter pylori infection does not protect against eosinophilic esophagitis: results from a large multicenter case-control study. Am J Gastroenterol. (2018) 113:972–9. doi: 10.1038/s41395-018-0035-6
72. Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. (2003) 125:1660–9. doi: 10.1053/j.gastro.2003.09.024
73. Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract. (2018) 6:1528–33. doi: 10.1016/j.jaip.2018.05.010
74. Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. (2013) 132:378–86. doi: 10.1016/j.jaci.2013.02.030
75. Talathi S, Knight T, Dimmitt R, Mestre J, Jester T. Concurrent eosinophilic esophagitis in pediatric patients with inflammatory bowel disease: a case series. Ann Allergy Asthma Immunol. (2019) 123:313–6. doi: 10.1016/j.anai.2019.06.010
76. Sonnenberg A, Turner KO, Genta RM. Comorbid occurrence of eosinophilic esophagitis and inflammatory bowel disease. Clin Gastroenterol Hepatol. (2020). doi: 10.1016/j.cgh.2020.02.015. [Epub ahead of print].
77. Limketkai BN, Shah SC, Hirano I, Bellaguarda E, Colombel JF. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut. (2019) 68:2152–60. doi: 10.1136/gutjnl-2018-318074
78. Lucendo AJ1, Arias Á, Pérez-Martínez I, López-Vázquez A, Ontañón-Rodríguez J, González-Castillo S, et al. Adult patients with eosinophilic esophagitis do not show an increased frequency of the HLA-DQ2/DQ8 genotypes predisposing to celiac disease. Dig Dis Sci. (2011) 56:1107–11. doi: 10.1007/s10620-010-1383-2
79. Krishnan U. Eosinophilic esophagitis in esophageal atresia. Front Pediatr. (2019) 7:497. doi: 10.3389/fped.2019.00497
80. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. (2014) 63:871–80. doi: 10.1136/gutjnl-2012-304269
81. Pensabene L, Salvatore S, D'Auria E, Parisi F, Concolino D, Borrelli O, et al. Cow's milk protein allergy in infancy: a risk factor for functional gastrointestinal disorders in children? Nutrients. (2018) 10:1716. doi: 10.3390/nu10111716
82. Cheng E, Souza RF, Spechler SJ. Eosinophilic esophagitis: interactions with gastroesophageal reflux disease. Gastroenterol Clin North Am. (2014) 43:243–56. doi: 10.1016/j.gtc.2014.02.004
83. Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. (2004) 99:13–22. doi: 10.1046/j.1572-0241.2003.04018.x
84. Smith LP, Chewaproug L, Spergel JM, Zur KB. Otolaryngologists may not be doing enough to diagnose pediatric eosinophilic esophagitis. Int J Pediatr Otorhinolaryngol. (2009) 73:1554–7. doi: 10.1016/j.ijporl.2009.07.023
85. Kelly EA, Linn D, Keppel KL, Noel RJ, Chun RH. Otolaryngologic surgeries are frequent in children with eosinophilic esophagitis. Ann Otol Rhinol Laryngol. (2015) 124:355–60. doi: 10.1177/0003489414558108
86. Koutlas NT, Eluri S, Rusin S, Perjar I, Hollyfield J, Woosley JT, et al. Impact of smoking, alcohol consumption, and NSAID use on risk for and phenotypes of eosinophilic esophagitis. Dis Esophagus. (2018) 31:1–7. doi: 10.1093/dote/dox111
87. Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. (2011) 52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f
88. Lee YJ, Redd M, Bayman L, Frederickson N, Valestin J, Schey R. Comparison of clinical features in patients with eosinophilic esophagitis living in an urban and rural environment. Dis Esophagus. (2015) 28:19–24. doi: 10.1111/dote.12164
89. Jensen ET, Hoffman K, Shaheen NJ, Genta RM, Dellon ES. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol. (2014) 109:668–75. doi: 10.1038/ajg.2014.47
90. Ram G, Lee J, Ott M, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy Asthma Immunol. (2015) 115:224–8. doi: 10.1016/j.anai.2015.07.004
91. Reed CC, Iglesia EGA, Commins SP, Dellon ES. Seasonal exacerbation of eosinophilic esophagitis histologic activity in adults and children implicates role of aeroallergens. Ann Allergy Asthma Immunol. (2019) 122:296–301. doi: 10.1016/j.anai.2018.12.013
92. Moawad FJ, Veerappan GR, Lake JM, Maydonovitch CL, Haymore BR, Kosisky SE, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. (2010) 31:509–15. doi: 10.1111/j.1365-2036.2009.04199.x
93. Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. (2007) 41:451–3. doi: 10.1097/01.mcg.0000248019.16139.67
94. Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: epidemiology, clinical presentation and seasonal variation. J Gastroenterol. (2013) 48:81–5. doi: 10.1007/s00535-012-0608-x
95. Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: the experience in children living in rural communities. J Clin Gastroenterol. (2013) 47:287–8. doi: 10.1097/MCG.0b013e31826df861
96. Elias MK, Kopacova J, Arora AS, Dierkhising RA, Enders FT, Katzka DA, et al. The diagnosis of esophageal eosinophilia is not increased in the summer months. Dysphagia. (2015) 30:67–73. doi: 10.1007/s00455-014-9574-1
97. van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. (2013) 25:47–52. doi: 10.1111/nmo.12009
98. Walker A. Intestinal colonization and programming of the intestinal immune response. J Clin Gastroenterol. (2014) 48:8–11. doi: 10.1097/MCG.0000000000000230
99. Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. (2015) 77:220–8. doi: 10.1038/pr.2014.160
100. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. (2012) 336:489–93. doi: 10.1126/science.1219328
101. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. (2011) 128:646–52. doi: 10.1016/j.jaci.2011.04.060
102. Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. (2014) 44:842–50. doi: 10.1111/cea.12253
103. Arboleya S, Sanchez B, Milani C, Duranti S, Solis G, Fernandez N, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. (2015) 166:538–44. doi: 10.1016/j.jpeds.2014.09.041
104. Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. (2015) 45:632–43. doi: 10.1111/cea.12487
105. Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. (2016) 123:983–93. doi: 10.1111/1471-0528.13601
106. Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. (2010) 86:13–5. doi: 10.1016/j.earlhumdev.2010.01.004
107. Cassidy-Bushrow AE, Sitarik A, Levin AM, Lynch SV, Havstad S, Ownby DR, et al. Maternal group B Streptococcus and the infant gut microbiota. J Dev Orig Health Dis. (2016) 7:45–53. doi: 10.1017/S2040174415001361
108. Chernikova DA, Koestler DC, Hoen AG, Housman ML, Hibberd PL, Moore JH, et al. Fetal exposures and perinatal influences on the stool microbiota of premature infants. J Matern Fetal Neonatal Med. (2015) 29:99–105. doi: 10.3109/14767058.2014.987748
109. Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. (2010) 51:77–84. doi: 10.1097/MPG.0b013e3181d1b11e
110. Codispoti CD, Levin L, LeMasters GK, Ryan P, Reponen T, Villareal M, et al. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. (2010) 125:1054–60. doi: 10.1016/j.jaci.2010.02.004
111. Veerappan GR, Perry JL, Duncan TJ, Baker TP, Maydonovitch C, Lake JM, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol. (2009) 7:420–6. doi: 10.1016/j.cgh.2008.10.009
112. Moawad FJ, Maydonovitch CL, Lake JM, Veerappan GR. PPIs may not predispose to eosinophilic esophagitis. Am J Gastroenterol. (2010) 105:468–9. doi: 10.1038/ajg.2009.617
113. Chen IL, Gao WY, Johnson AP, Niak A, Troiani J, Korvick J, et al. Proton pump inhibitor use in infants: FDA reviewer experience. J Pediatr Gastroenterol Nutr. (2012) 54:8–14. doi: 10.1097/MPG.0b013e31823890b4
114. Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. CHEST J. (2007) 131:1753–9. doi: 10.1378/chest.06-3008
115. Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. (2009) 123:1003–10. doi: 10.1542/peds.2008-1146
116. Kummeling I, Stelma FF, Dagnelie PC, Snijders BE, Penders J, Huber M, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA birth cohort study. Pediatrics. (2007) 119:225–31. doi: 10.1542/peds.2006-0896
117. Jensen ET, Bertelsen RJ. Assessing early life factors for eosinophilic esophagitis: lessons from other allergic diseases. Curr Treat Options Gastroenterol. (2016) 14:39–50. doi: 10.1007/s11938-016-0083-1
118. du Toit G, Tsakok T, Lack S, Lack G. Prevention of food allergy. J Allergy Clin Immunol. (2016) 137:998–1010. doi: 10.1016/j.jaci.2016.02.005
119. Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. (2014) 5:5593. doi: 10.1038/ncomms6593
120. Jensen ET, Kuhl JT, Martin LJ, Langefeld CD, Dellon ES, Rothenberg ME. Early-life environmental exposures interact with genetic susceptibility variants in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. (2018) 141:632–7. doi: 10.1016/j.jaci.2017.07.010
121. Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breastfed infants. N Engl J Med. (2016) 374:1733–43. doi: 10.1056/NEJMoa1514210
122. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. (2015) 372:803–13. doi: 10.1056/NEJMoa1414850
123. Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Flohr C, et al. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. (2016) 137:1477–86. doi: 10.1016/j.jaci.2015.12.1322
124. Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. (2010) 42:289–91. doi: 10.1038/ng.547
125. Arora M, Bagi P, Strongin A, Heimall J, Zhao X, Lawrence MG, et al. Gastrointestinal manifestations of STAT3-Deficient Hyper-IgE syndrome. J Clin Immunol. (2017) 37:695–700. doi: 10.1007/s10875-017-0429-z
126. Paluel-Marmont C, Bellon N, Barbet P, Leclerc-Mercier S, Hadj-Rabia S, Dupont C, et al. Eosinophilic esophagitis and colonic mucosal eosinophilia in Netherton syndrome. J Allergy Clin Immunol. (2017) 139:2003–5. doi: 10.1016/j.jaci.2016.10.045
127. Henderson CJ, Ngeow J, Collins MH, Martin LJ, Putnam PE, Abonia JP, et al. Increased prevalence of eosinophilic gastrointestinal disorders in pediatric PTEN hamartoma tumor syndromes. J Pediatr Gastroenterol Nutr. (2014) 58:553–60. doi: 10.1097/MPG.0000000000000253
Keywords: eosinophilic esophagitis, allergy, risk factors, early life exposures, food allergens, microbiome, prevention
Citation: Votto M, Marseglia GL, De Filippo M, Brambilla I, Caimmi SME and Licari A (2020) Early Life Risk Factors in Pediatric EoE: Could We Prevent This Modern Disease? Front. Pediatr. 8:263. doi: 10.3389/fped.2020.00263
Received: 20 February 2020; Accepted: 27 April 2020;
Published: 29 May 2020.
Edited by:
Enza D'Auria, University of Milan, ItalyReviewed by:
Matjaž Homan, University Medical Centre Ljubljana, SloveniaVictor Manuel Navas-López, Hospital Materno-Infantil, Spain
Copyright © 2020 Votto, Marseglia, De Filippo, Brambilla, Caimmi and Licari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia Licari, YS5saWNhcmkmI3gwMDA0MDtzbWF0dGVvLnB2Lml0
 Martina Votto
Martina Votto Gian Luigi Marseglia
Gian Luigi Marseglia Maria De Filippo
Maria De Filippo Ilaria Brambilla
Ilaria Brambilla Amelia Licari
Amelia Licari