- Department of Cardiology, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, Beijing, China
Previous studies have shown that children with Kawasaki disease (KD) who fail to respond to intravenous immunoglobulin (IVIG) therapy are at higher risk of developing coronary artery lesions (CALs). We aimed to conduct a meta-analysis to uncover the risk factors associated with IVIG resistance in children with KD. PubMed, Embase, and Cochrane Library databases were searched up to 31st October 2019, and 23 case-control studies were finally eligible, enrolling 2,053 patients of IVIG resistance and 16,635 patients of IVIG sensitivity. Potential factors were comprehensively analyzed by using stata15 software with a standard meta-analysis procedure and consequently found that in addition to patients with polymorphous rash or swelling of extremities symptoms had a tendency to be non-responders, IVIG resistance was more likely to occur in patients with severe anemia, hypoalbuminemia, decreased baseline platelet count, and elevated levels of erythrocyte sedimentation rate (ESR), total bilirubin, alanine aminotransferase (ALT) and neutrophils percentage. Particularly, male sex, hyponatraemia, increased aspartate aminotransferase (AST), and C-reactive protein (CRP) were confirmed as the risk factors favor IVIG resistance in Mongoloids from Asia countries, but not in Caucasians from non-Asia regions. In summary, we report several risk factors relevant to IVIG resistance in children with KD, which may provide guidance for the prediction of IVIG resistance. But a proposing of an optimal prediction system with high specificity and sensitivity needs further studies because of confounding factors.
Introduction
Kawasaki disease (KD) is an acute medium-sized vasculitis of unknown etiology, characterized by persistent fever and five typical clinical manifestations, including swelling of extremities, polymorphous rash, cervical lymphadenopathy, oral lesions, and bilateral conjunctivitis (1). It predominantly affects children younger than 5 years old and results in coronary artery lesions (CALs) such as ectasias or aneurysms in 25% of untreated patients (2), considering one of the most common cause of acquired heart disease in children in many countries (3). Timely treatment with intravenous immunoglobulin (IVIG) and oral aspirin has reduced the prevalence of CALs from 25% to about 4% (4). However, 10%–20% of patients with KD fail to respond or develop recrudescent fever 36–48 h after the first dose of IVIG (5), which are termed as IVIG resistance. Previous studies have shown that resistant patients are at higher risk of developing CALs (5, 6), and addition use of glucocorticoid or cyclosporine have been confirmed effectively reduce the incidence of CALs in children predicted with IVIG resistance before treatment according to different randomized clinical trails (7, 8). Therefore, it is of importance to predict targeted patients who will be IVIG non-responders so that they can benefit from the more aggressive therapy regarding CALs prevention.
The pathological development of KD is a systemic inflammatory process, during which the severity degree of inflammation is reflected in the duration of fever, more severe or clearer diagnostic clinical manifestations, and higherly activated laboratory parameters such as hemoglobin, baseline platelet count, percentage of neutrophils, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total bilirubin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum sodium and other inflammatory biomarkers, which once have been reported to differ remarkably between IVIG non-responders and responders before IVIG infusion. But these factors have no clear consensus on predicting IVIG resistance so far and the conflicting data may derived from ethnic and genetic backgrounds (9, 10).
This study was designed to perform a meta-analysis to identified risk factors associated with IVIG resistance in patients with KD. It may be helpful for prediction of IVIG non-responsiveness.
Materials and Methods
This meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (11).
Database Search
We searched databases including PubMed, Embase and Cochrane Library up to 31st October 2019 by using Medical Subject Headings (MeSH) terms or Emtree thesaurus terms combined with keywords, the search strategy was [“Mucocutaneous lymph node syndrome” OR “Kawasaki disease”] AND [“IVIG resistance” OR “IVIG non-responsiveness” OR “IVIG unresponsiveness”]. The language was restricted to English and a manual search was conducted using reference lists of original articles for further articles of interest.
Inclusion and Exclusion Criteria
Criteria for Inclusion
①Case-control study or cohort study; ②All subjects were children (aged 0–18 years) diagnosed with KD according to Japanese diagnostic criteria (12) or the 2017 American Heart Association common standards (4) and received IVIG infusion in the cumulative dose of 2 g/kg plus oral aspirin for the initial therapy; ③Odds ratio (OR) and 95% confidence interval (CI) provided for categorical variables in the original data or mean and standard deviation provided for continuous variables, and all the data provided were measured at the time of admission; ④Clear description of statistical methods and correct statistical analyses.
Criteria for Exclusion
①Animal studies; ②Reviews, duplicates, case reports, meta-analyses, conference abstracts or unpublished literatures; OR and 95% CI were not provided for categorical variables or mean and standard deviation were not provided directly or indirectly for continuous variables.
Data Extraction and Quality Assessment
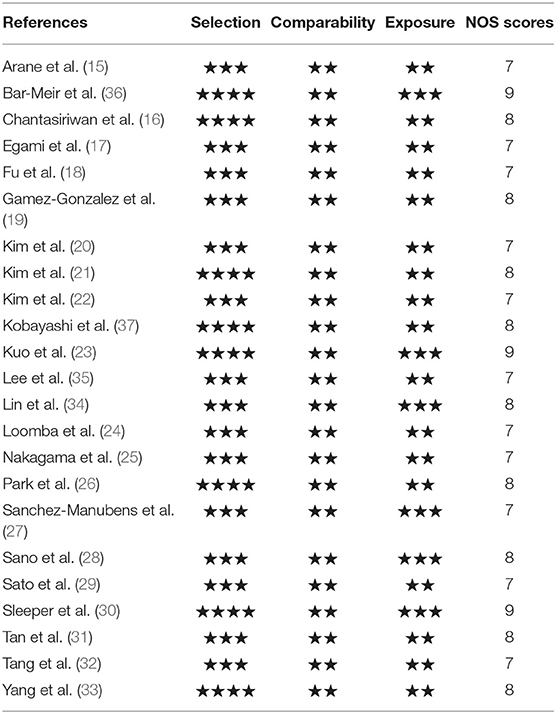
The data were independently gathered by 2 investigators on the basis of a predefined standard form. The data extracted from the studies included such details as the first author, publication year, region, sample size, clinical symptoms and laboratory indicators of cases and controls. We assessed quality of every included study with respect to cases and controls selection, comparability, and exposure using the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control or cohort studies (Supplementary Material). which has a total score of 9 stars, a study that awards ≥7 stars is considered high methodological quality, ≤ 3 as low quality, and 3–7 as moderate.
Statistical Analysis
Data analysis was performed by using Stata15 software. Cochrane Q test and I2 statistic were calculated to assess heterogeneity across studies. If the studies were shown to be homogeneous with P ≥ 0.10 and I2 <50%, the fixed effects model was selected, otherwise, the random effects model was applied (13). The pooled effects were presented with OR and corresponding 2-tailed 95% CI for dichotomous variables, or weighted mean difference (WMD) and corresponding 2-tailed 95% CI for continuous variables. The significance of the pooled effects were determined by the Z-test, all P-values were 2-tailed and a P < 0.05 was considered statistical significantly. Sensitivity analysis was conducted by omitting a single study involved in the meta-analysis in turn to identify the potential influence of each individual data on the pooled effects and to confirm that our results were not driven by any single study. Publication bias was estimated via funnel plot and Egger's test (14), a P > 0.05 was considered no significantly publication bias.
Results
Search Results
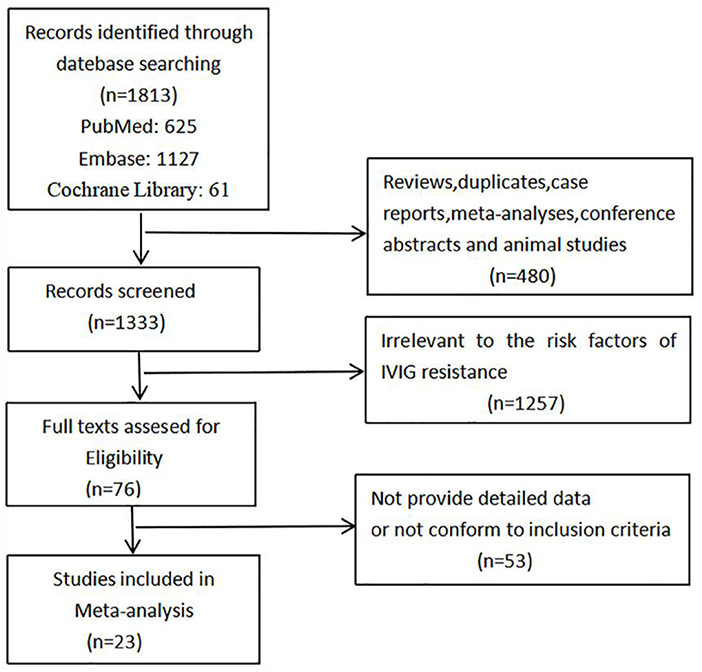
Of 1,813 articles initially searched, 480 reviews, duplicates, case reports, meta-analyses, conference abstracts and animal studies were firstly removed. After screening the titles and abstracts, 1,257 studies that irrelevant to the risk factors of IVIG resistance were excluded, and another 53 studies that did not provide detailed origin data or not in accordance with our inclusion criteria were excluded after assessing the full text. Finally, 23 studies were found to conform to our specific inclusion criteria and were included consequently in our meta-analysis. The studies selection process is shown in Figure 1.
Study Characteristics and Quality Assessment
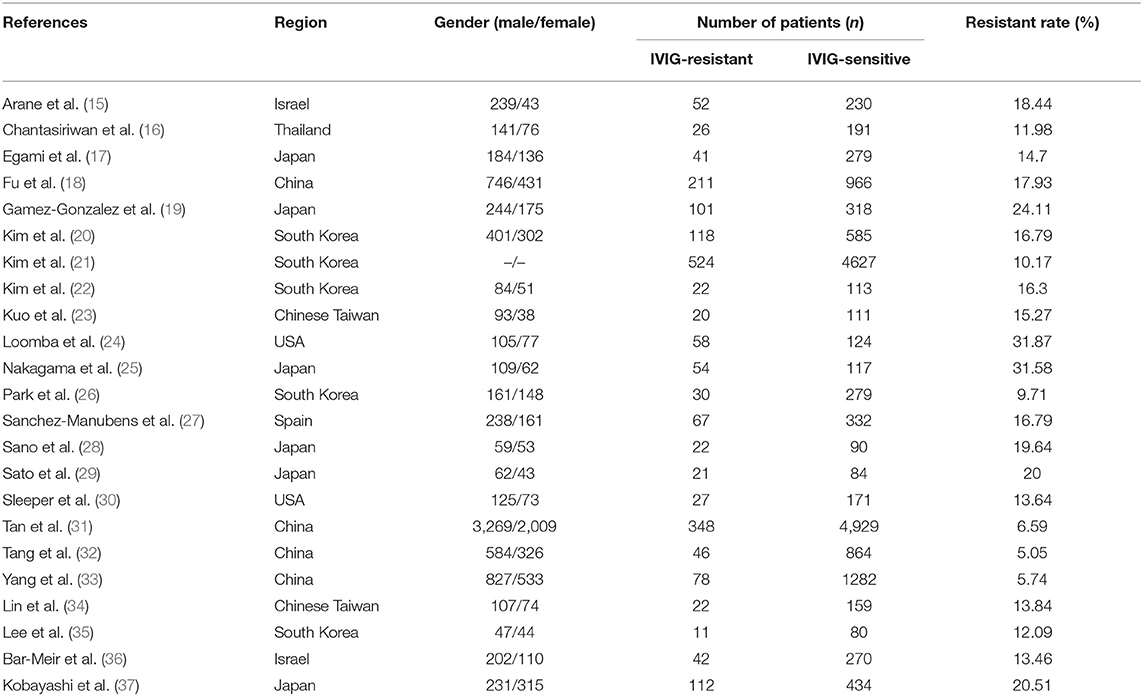
All the 23 included studies (15–37) were case-control studies, the general characteristics were summarized in Table 1. A total of 18,688 patients were enrolled, of which 2,053 were assigned in the IVIG-resistant group and 16,635 in the IVIG-sensitive group. These studies were conducted in different ethnic populations and different regions, including Mongoloids from Asia (Japan, China, Chinese Taiwan, South Korea, Thailand) and Caucasians from non-Asia (Spain, Israel, USA). The quality assessment found that all the included studies awarded ≥7 stars (Table 2), indicating that the studies are of high methodological quality and persuasive.
Risk Factors
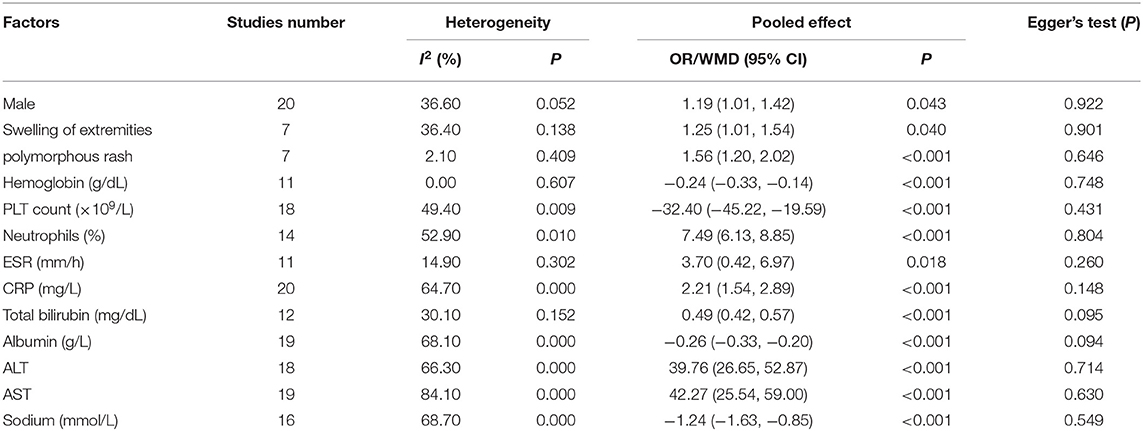
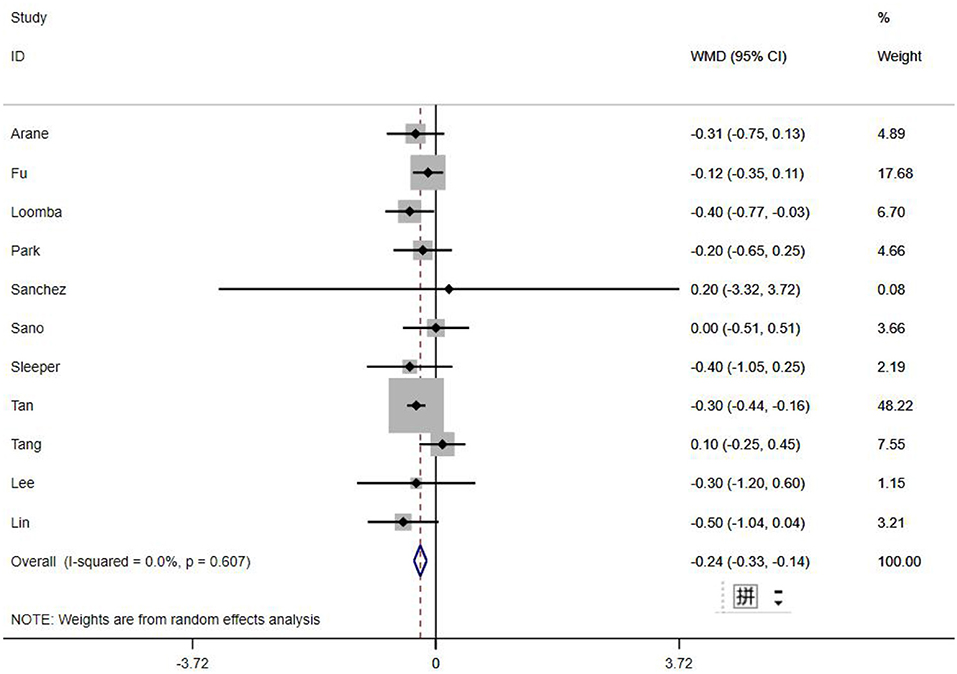
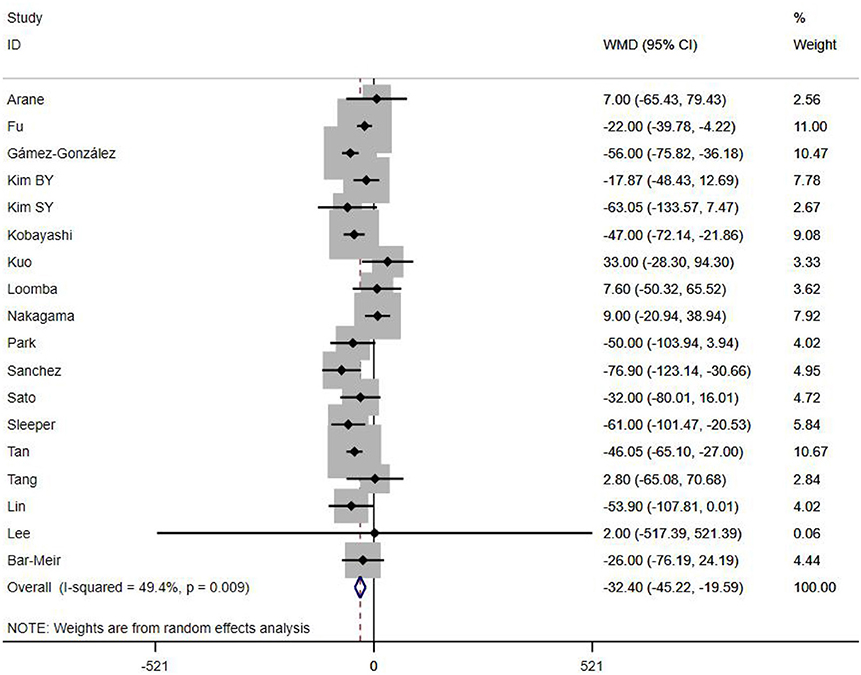
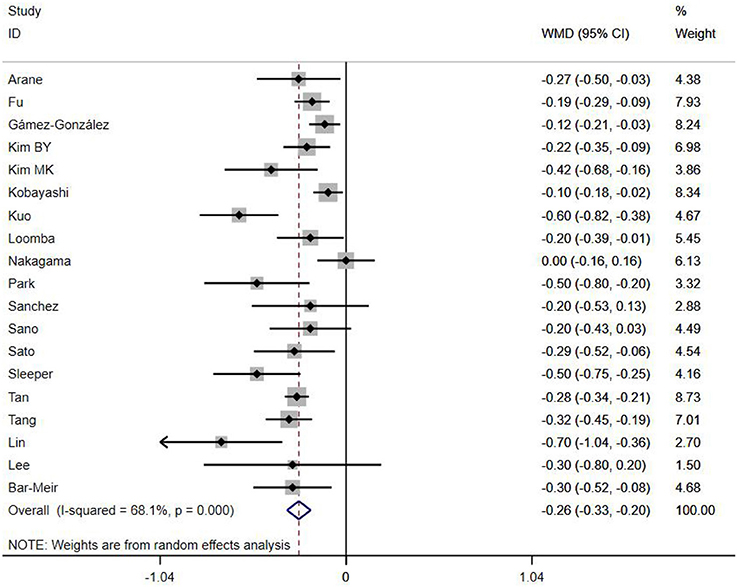
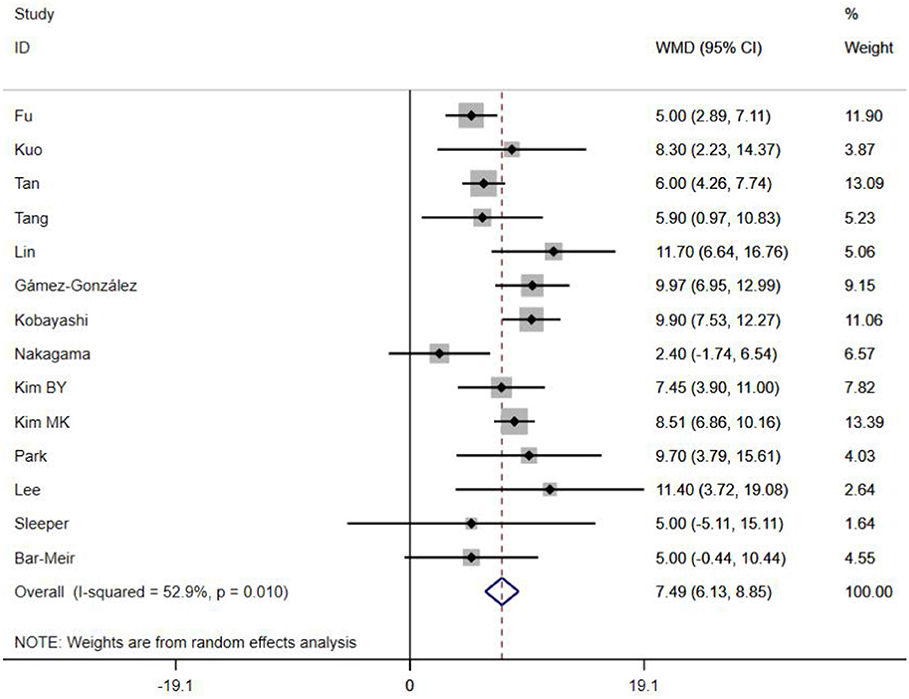
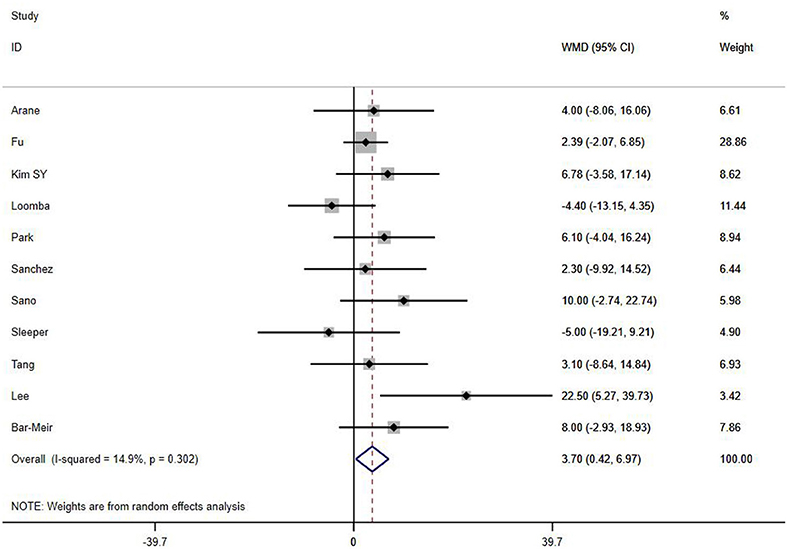
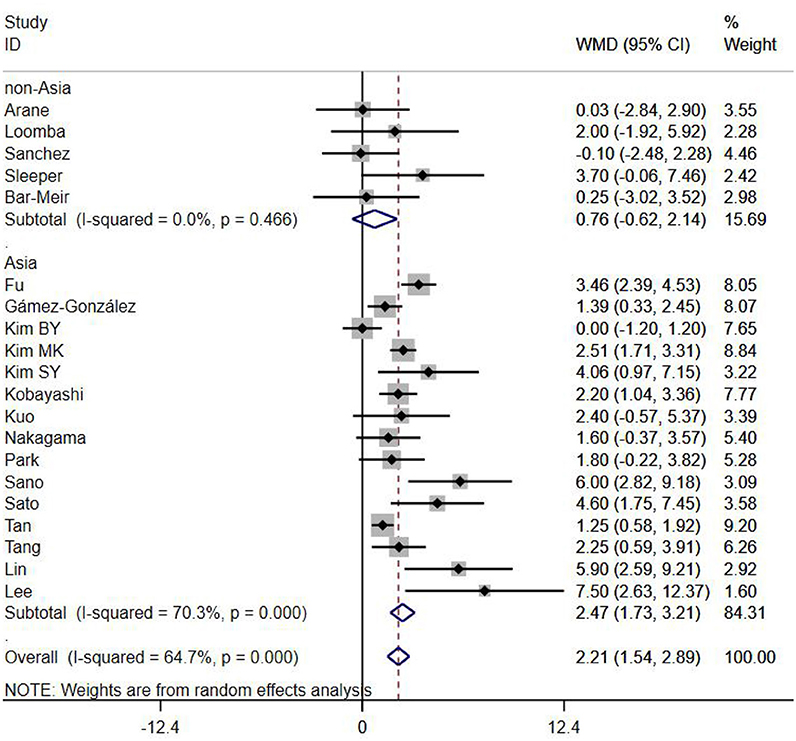
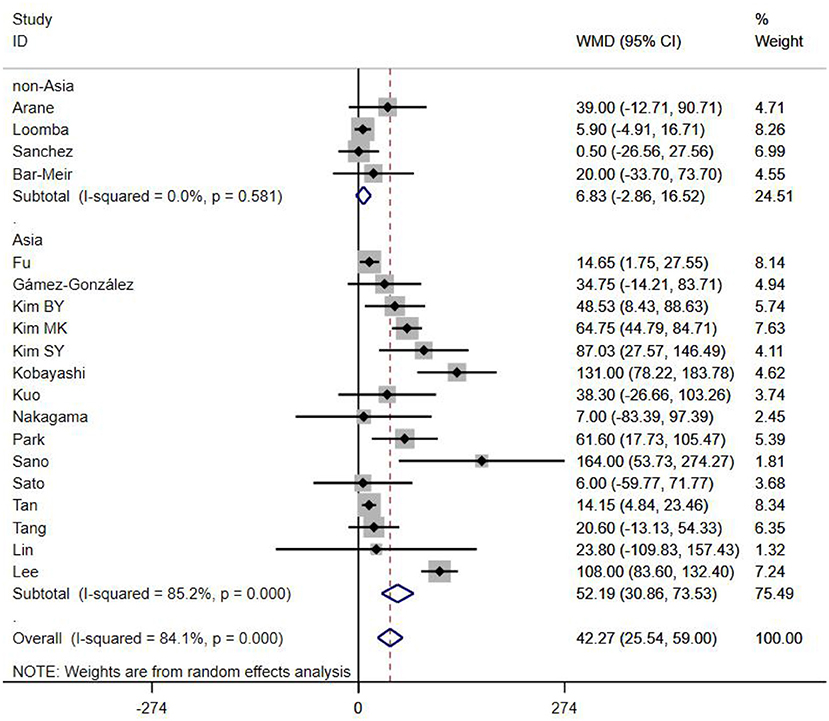
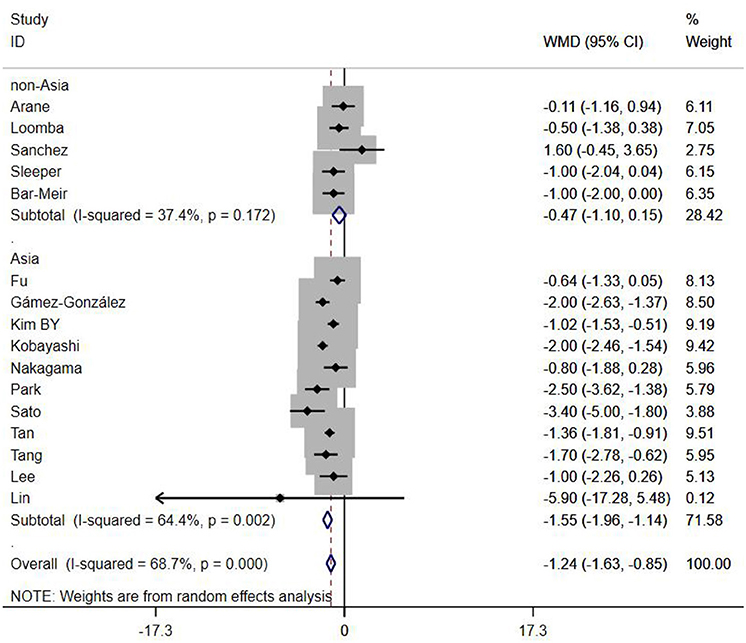
A total of 13 clinical and laboratory indicators were found associated with IVIG resistance, the results were summarized in Table 3. Our meta-analysis revealed that male patients were more likely to be IVIG non-responders (OR = 1.19, 95% CI: 1.01 to 1.42, P = 0.043) than females, and there was an increase in the association of both swelling of extremities (OR = 1.25, 95% CI: 1.01 to 1.54, P = 0.040) and polymorphous rash (OR = 1.56, 95% CI: 1.20 to 2.02, P < 0.001) with the odds of IVIG resistance. In terms of laboratory parameters, IVIG non-responders had significantly lower hemoglobin (Figure 2, WMD = −0.24, 95% CI: −0.33 to −0.14, P < 0.001), baseline platelet count (Figure 3, WMD = −32.4, 95% CI: −45.22 to −19.59, P < 0.001), albumin (Figure 4, WMD = −0.26, 95% CI: 0.33 to −0.20, P < 0.001) and serum sodium (WMD = −1.24, 95% CI = −1.63 to −0.85, P < 0.001) values than responders, while percentage of neutrophils (Figure 5, WMD = 7.49, 95% CI: 6.13 to 8.85, P < 0.001), ESR (Figure 6, WMD = 3.70, 95% CI: 0.42 to 6.97, P = 0.018), CRP (WMD = 2.21, 95% CI = 1.54–2.89, P < 0.001), total bilirubin (WMD = 0.49, 95% CI: 0.42 to 0.57, P < 0.001), AST(WMD = 42.27, 95% CI: 25.54 to 59.00, P < 0.001), and ALT (WMD = 39.76, 95% CI: 26.65 to 52.87, P < 0.001) values were significantly higher than responders.
We also identified several factors that were not relevant to IVIG resistance, including WBC (WMD = 0.33, 95% CI: −0.04 to 0.70, P = 0.078), age (WMD = 0.39, 95% CI: −0.90 to 1.68, P = 0.554), conjunctivitis (OR = 0.85, 95% CI: 0.62 to 1.15, P = 0.277), oral lesions (OR = 0.76, 95% CI: 0.56 to 1.03, P = 0.081) and cervical lymphadenopathy (OR = 0.99, 95% CI: 0.70 to 1.42, P = 0.974).
Subgroup Analyses
Subgroups were selected based on different ethnic populations from different regions (Asian or non-Asian). All the high-risk factors with significant heterogeneity and more than 10 studies enrolled were analyzed, including male, baseline platelet count, percentage of neutrophils, CRP, albumin, ALT, AST, and serum sodium. It turned out that there were significant ethnicity-specific and region-specific differences in factors of male sex, elevation of CRP and AST, and decreased serum sodium, but not in baseline platelet count, percentage of neutrophils, albumin and ALT.
The summary WMDs for baseline platelet count were −21.87 (95% CI: −41.32, −2.42) and −35.73 (95% CI −68.47, −3.00) in Asian and non-Asian populations (P difference = 0.151). The WMDs for neutrophils percentage were 7.67 (95% CI: 6.22, 9.12) and 5.00 (95% CI: 0.21, 9.79) for the studies in Asian and non-Asian populations (P difference = 0.085). The WMDs for albumin and ALT were −0.25 (95% CI: −0.33, −0.18) vs. −0.29 (95% CI: −0.39, −0.18), and 45.98 (95% CI: 29.41, 62.56) vs. 21.30 (95% CI: 5.83, 36.77), respectively, for the studies in Asian and non-Asian populations, with P difference = 0.430 and 0.183.
It is noteworthy that Asian male patients were more likely to be non-responders (P = 0.027), but there was no significant difference between responders and non-responders in non-Asian patients (P = 0.507). As well as male sex, Asian patients with higher CRP, AST and lower serum sodium were more likely to be IVIG non-responders (All P < 0.001%), and no significant differences were observed between responders and non-responders in Caucasians from non-Asia regions (All P > 0.05) (Figures 7–9).
Sensitivity Analysis
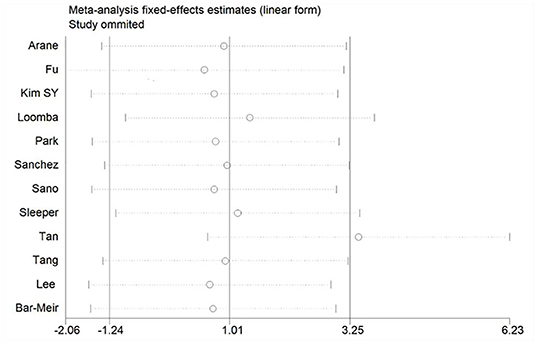
If there is significant heterogeneity among studies, addition or reduction of any one study may lead to remarkable change in results, so we used sensitivity analysis to verify the reliability of the meta-analysis findings. In addition to the discovery that Tan's study (31) had a great impact on pooled effect in the meta-analysis of ESR as shown in Figure 10 (The ESR data of this study was omitted in this meta-analysis), there was no significant change in the pooled OR and WMD after every single study was omitted, this indicated that our results were convinced because of good stability and not driving by any single study.
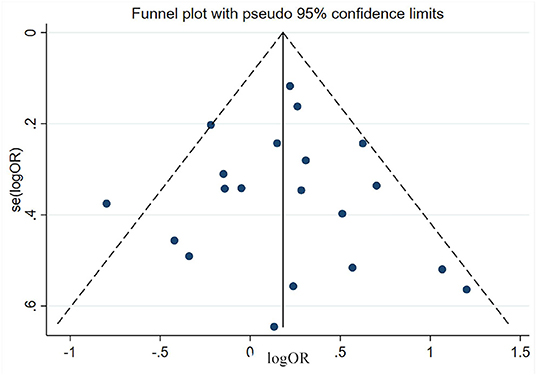
Estimation of Publication Bias
Publication bias was estimated via funnel plot and Egger's test. All the funnel plots showed generally symmetrical (e.g., the funnel plot of male sex shown in Figure 11) and all the p-value of Egger's test >0.05 (Table 3), which mean no significant publication bias was found in the meta-analyses of each risk factor.
Discussion
KD is an infection-related immune-mediated systemic inflammation, although the clinical phenotype of KD varies across individuals, the pathogenesis is basically definite. After an infection of unknown agents, immune cells (especially T cells) are activated. Then the hyperactivated immune cells produce massive cytokines, leading to a cytokine imbalance associated with further endothelial cell injury (38), CALs may begin to develop and progress in the early phase of the inflammation, so early prediction of IVIG resistance through clinical manifestations and laboratory parameters is indeed a wise method for the severely affected patients who need early intensive treatment.
In the present meta-analysis, we included 23 studies from Japan, South Korea, China, Chinese Taiwan, Thailand, Spain, Israel and the United States of America, to analyze the relationships between non-responsiveness and clinical or laboratory indicators that are associated with inflammation. Also, we have discussed the indicators between different ethnicities based on various genetic backgrounds. The results showed that in addition to patients with polymorphous rash or swelling of extremities symptoms had a tendency to be non-responders, IVIG resistance was more likely to occur in patients with severe anemia, hypoalbuminemia, decreased baseline platelet count, and increased ESR, total bilirubin, ALT and neutrophils percentage. In particular, male sex, hyponatraemia, elevated levels of AST and CRP were confirmed as the risk factors favor IVIG resistance in Mongoloid patients from Asia countries, but not in Caucasians from non-Asia regions. No significant difference was found in WBC count, age, conjunctivitis, oral lesions and cervical lymphadenopathy between responders and non-responders. Subgroup analyses showed that some factors differed by ethnicity. The difference of gender, CRP, AST, and serum sodium between Asian and non-Asian patients were significant. Male Asian patients with increased CRP, AST and decreased serum sodium were more likely to be IVIG non-responders, while the same phenomenons were not observed in Caucasians from non-Asia regions.
Changes in these indicators can be explained by the pathophysiology of KD. KD inflammatory reaction changes the redox state of serum, with elevated inducible nitric oxide synthase (39), which would alter the homeostasis of red blood cells (RBCs) and result in a type of premature in these cells that lead to anemia and thrombus. RBC aging, inflammation and thrombus inevitably result in increased ESR. Intensive inflammation and immune reaction lead to serious vascular permeability and liver damage, resulting in albumin leakage and transaminases elevation. It is confirmed that cytokines such as plasma IL-6 and tumor necrosis factor-α (TNF-α) participate in inflammation of KD in the acute phase and were markedly increased in IVIG non-responders compared with responders (40–42), and the release of ADH is promoted by IL-6 and TNF-αduring inflammation (43), so the probable pathophysiological for hyponatremia may be relevant to inappropriate release of ADH. Overall, anemia, decreased serum albumin and sodium, elevated levels of neutrophils percentage and acute phase reactants such as ESR and CRP largely represent a more severe degree of inflammation and intensive immune response.
There are several limitations of this study should be noted. Firstly, the presence of confounding factors may reduce the accuracy of prediction and treatment guidance. The intensity of KD inflammation gradually increases in the acute stage and reach the peak, then decreases and enters to the convalescent stage (44), and the immune reaction before the peak may be responsible for tissue cell injury while immune reaction after the peak may be responsible for tissue cell repair (38), so inflammatory indices change throughout this process over time. Obviously, fever duration is a confounding factor predicting IVIG resistance. Besides, some laboratory values varied according to age, personal immunity and organ or tissues involvement in individuals. Secondly, the definition of IVIG resistance is not completely uniform in all the included studies, the observation periods after IVIG are different (24, 36, or 36–48 h). Thirdly, there are some differences in the infusion modalities of IVIG, some patients treated with IVIG 2 g/kg as a single infusion, while others received IVIG 1 g/kg for 2 days or 400 mg/kg for 5 consecutive days, this may lead to slightly different sensitivity to IVIG due to dose-response effects. As well as limitations, our meta-analysis also has significant aspects. We included various studies involving different ethnic populations from all over the world to ensure the applicability of our findings and to investigate a wide range of risk factors for IVIG resistance, and we had a sufficient sample size to carry out Egger's test for most factors and found no apparent publication bias.
In conclusion, some parameters were demonstrated associated with IVIG resistance, and clinicians should aware an increased likelihood that the patient may fail to respond to initial IVIG therapy when such factors present, but further studies are needed because of confounding factors in data analysis.
Data Availability Statement
All datasets analyzed for this study are included in the article/Supplementary Material.
Author Contributions
GL and ZD designed the study. GL contributed to literatures search, data collection, statistical analysis, and drafting the manuscript. SW contributed to literature search and data collection. ZD performed manuscript review. All authors have read and approved the content of the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00187/full#supplementary-material
References
1. Burns JC, Glode MP. Kawasaki syndrome. Lancet. (2004) 364:533–44. doi: 10.1016/S0140-6736(04)16814-1
2. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. (1996) 94:1379–85. doi: 10.1161/01.CIR.94.6.1379
3. Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child. (2014) 99:74–83. doi: 10.1136/archdischild-2012-302841
4. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
5. Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. (2008) 153:117–21. doi: 10.1016/j.jpeds.2007.12.021
6. Kibata T, Suzuki Y, Hasegawa S, Matsushige T, Kusuda T, Hoshide M, et al. Coronary artery lesions and the increasing incidence of Kawasaki disease resistant to initial immunoglobulin. Int J Cardiol. (2016) 214:209–15. doi: 10.1016/j.ijcard.2016.03.017
7. Hamada H, Suzuki H, Onouchi Y, Ebata R, Terai M, Fuse S, et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA) : a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet. (2019) 393:1128–37. doi: 10.1093/rheumatology/kez063.031
8. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study) : a randomised, open-label, blinded-endpoints trial. Lancet. (2012) 379:1613–20. doi: 10.1016/S0140-6736(11)61930-2
9. Assari R, Aghighi Y, Ziaee V, Sadr M, Rezaei A, Rahmani F, et al. Interleukin-4 cytokine single nucleotide polymorphisms in Kawasaki disease: a case-control study and a review of knowledge. Int J Rheum Dis. (2018) 21:266–70. doi: 10.1111/1756-185X.12968
10. Kuo HC, Wong HS, Chang WP, Chen BK, Wu MS, Yang KD, et al. Prediction for intravenous immunoglobulin resistance by using weighted genetic risk score identified from genome-wide association study in Kawasaki disease. Circ Cardiovasc Genet. (2017) 10:e001625. doi: 10.1161/CIRCGENETICS.116.001625
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
12. Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. (2005) 47:232–4. doi: 10.1111/j.1442-200x.2005.02033.x
13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
14. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
15. Arane K, Mendelsohn K, Mimouni M, Mimouni F, Koren Y, Brik SD, et al. Japanese scoring systems to predict resistance to intravenous immunoglobulin in Kawasaki disease were unreliable for Caucasian Israeli children. Acta Paediatr. (2018) 107:2179–84. doi: 10.1111/apa.14418
16. Chantasiriwan N, Silvilairat S, Makonkawkeyoon K, Pongprot Y, Sittiwangkul R. Predictors of intravenous immunoglobulin resistance and coronary artery aneurysm in patients with Kawasaki disease. Paediatr Int Child Health. (2018) 38:209–12. doi: 10.1080/20469047.2018.1471381
17. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2006) 149:237–40. doi: 10.1016/j.jpeds.2006.03.050
18. Fu PP, Du ZD, Pan YS. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J. (2013) 32:e319–23. doi: 10.1097/INF.0b013e31828e887f
19. Gamez-Gonzalez LB, Hamada H, Cisneros CM, Honda T, Yasukawa K, Takanashi JI. Vital signs as predictor factors of intravenous immunoglobulin resistance in patients with Kawasaki disease. Clin Pediatr. (2018) 57:1148–53. doi: 10.1177/0009922818759320
20. Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon IS, et al. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. (2016) 46:542–9. doi: 10.4070/kcj.2016.46.4.542
21. Kim MK, Song MS, Kim GB. Factors predicting resistance to intravenous immunoglobulin treatment and coronary artery lesion in patients with Kawasaki disease: analysis of the Korean nationwide multicenter survey from 2012 to 2014. Korean Circ J. (2018) 48:71–9. doi: 10.4070/kcj.2017.0136
22. Kim SY, Han MY, Cha SH, Jeon YB. N-terminal pro-brain natriuretic peptide (NT proBNP) as a predictive indicator of initial intravenous immunoglobulin treatment failure in children with Kawasaki disease: a retrospective study. Pediatr Cardiol. (2013) 34:1837–43. doi: 10.1007/s00246-013-0724-2
23. Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. (2010) 99:1578–83. doi: 10.1111/j.1651-2227.2010.01875.x
24. Loomba RS, Raskin A, Gudausky TM, Kirkpatrick E. Role of the Egami score in predicting intravenous mmmunoglobulin resistance in Kawasaki disease among different ethnicities. Am J Ther. (2016) 23:e1293–9. doi: 10.1097/MJT.0000000000000045
25. Nakagama Y, Inuzuka R, Hayashi T, Shindo T, Hirata Y, Shimizu N, et al. Fever pattern and C-reactive protein predict response to rescue therapy in Kawasaki disease. Pediatr Int. (2016) 58:180–4. doi: 10.1111/ped.12762
26. Park HM, Lee DW, Hyun MC, Lee SB. Predictors of nonresponse to intravenous immunoglobulin therapy in Kawasaki disease. Korean J Pediatr. (2013) 56:75–9. doi: 10.3345/kjp.2013.56.2.75
27. Sanchez-Manubens J, Anton J, Bou R, Iglesias E, Calzada-Hernandez J, Borlan S, et al. Role of the Egami score to predict immunoglobulin resistance in Kawasaki disease among a western mediterranean population. Rheumatol Int. (2016) 36:905–10. doi: 10.1007/s00296-016-3499-y
28. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. (2007) 166:131–7. doi: 10.1007/s00431-006-0223-z
29. Sato S, Kawashima H, Kashiwagi Y, Hoshika A. Inflammatory cytokines as predictors of resistance to intravenous immunoglobulin therapy in Kawasaki disease patients. Int J Rheum Dis. (2013) 16:168–72. doi: 10.1111/1756-185X.12082
30. Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. (2011) 158:831–5.e3. doi: 10.1016/j.jpeds.2010.10.031
31. Tan XH, Zhang XW, Wang XY, He XQ, Fan C, Lyu TW, et al. A new model for predicting intravenous immunoglobin-resistant Kawasaki disease in Chongqing: a retrospective study on 5277 patients. Sci Rep. (2019) 9:1722. doi: 10.1038/s41598-019-39330-y
32. Tang Y, Yan W, Sun L, Huang J, Qian W, Ding Y, et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in an East China population. Clin Rheumatol. (2016) 35:2771–6. doi: 10.1007/s10067-016-3370-2
33. Yang S, Song R, Zhang J, Li X, Li C. Predictive tool for intravenous immunoglobulin resistance of Kawasaki disease in Beijing. Arch Dis Child. (2019) 104:262–7. doi: 10.1136/archdischild-2017-314512
34. Lin MT, Chang CH, Sun LC, Liu HM, Chang HW, Chen CA, et al. Risk factors and derived formosa score for intravenous immunoglobulin unresponsiveness in Taiwanese children with Kawasaki disease. J Formos Med Assoc. (2016) 115:350–5. doi: 10.1016/j.jfma.2015.03.012
35. Lee SM, Lee JB, Go YB, Song HY, Lee BJ, Kwak JH. Prediction of resistance to standard intravenous immunoglobulin therapy in Kawasaki disease. Korean Circ J. (2014) 44:415–22. doi: 10.4070/kcj.2014.44.6.415
36. Bar-Meir M, Kalisky I, Schwartz A, Somekh E, Tasher D. Prediction of resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatric Infect Dis Soc. (2018) 7:25–9. doi: 10.1093/jpids/piw075
37. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. (2006) 113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865
38. Lee KY, Rhim JW, Kang JH. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med J. (2012) 53:262–75. doi: 10.3349/ymj.2012.53.2.262
39. Straface E, Marchesi A, Gambardella L, Metere A, Tarissi DJI, Viora M, et al. Does oxidative stress play a critical role in cardiovascular complications of Kawasaki disease? Antioxid Redox Signal. (2012) 17:1441–6. doi: 10.1089/ars.2012.4660
40. Agarwal S, Agrawal DK. Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol. (2017) 13:247–58. doi: 10.1080/1744666X.2017.1232165
41. Hu P, Jiang GM, Wu Y, Huang BY, Liu SY, Zhang DD, et al. TNF-alpha is superior to conventional inflammatory mediators in forecasting IVIG nonresponse and coronary arteritis in Chinese children with Kawasaki disease. Clin Chim Acta. (2017) 471:76–80. doi: 10.1016/j.cca.2017.05.019
42. Wu Y, Liu FF, Xu Y, Wang JJ, Samadli S, Wu YF, et al. Interleukin-6 is prone to be a candidate biomarker for predicting incomplete and IVIG nonresponsive Kawasaki disease rather than coronary artery aneurysm. Clin Exp Med. (2019) 19:173–81. doi: 10.1007/s10238-018-00544-5
43. Kim JH, Park JH, Eisenhut M, Yu JW, Shin JI. Inflammasome activation by cell volume regulation and inflammation-associated hyponatremia: a vicious cycle. Med Hypotheses. (2016) 93:117–21. doi: 10.1016/j.mehy.2016.05.018
Keywords: Kawasaki disease, intravenous immunoglobulin resistance, risk factors, meta-analysis, children
Citation: Liu G, Wang S and Du Z (2020) Risk Factors of Intravenous Immunoglobulin Resistance in Children With Kawasaki Disease: A Meta-Analysis of Case-Control Studies. Front. Pediatr. 8:187. doi: 10.3389/fped.2020.00187
Received: 13 January 2020; Accepted: 30 March 2020;
Published: 21 April 2020.
Edited by:
Mamoru Ayusawa, Nihon University Itabashi Hospital, JapanReviewed by:
Kyung-Yil Lee, The Catholic University of Korea, South KoreaWei Wang, Fourth Military Medical University, China
Copyright © 2020 Liu, Wang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongdong Du, ZHV6aG9uZ2RvbmcmI3gwMDA0MDsxMjYuY29t
 Gengying Liu
Gengying Liu Shunyu Wang
Shunyu Wang Zhongdong Du
Zhongdong Du