- 1Department of Oral Medicine, Central Hospital of Yingkou, Yingkou, China
- 2Disease Control and Prevention Center, Shenyang, China
Background: Our goal was to analyze the value of the pretreatment neutrophil-to-lymphocyte ratio (NLR) in the prognosis of pediatrics with parotid mucoepidermoid carcinoma (MEC).
Methods: Patients (≤ 18 years old) undergoing surgical treatment for primary parotid MEC were enrolled from multiple clinical centers retrospectively. The χ2-test was used to analyze the associations between clinicopathological variables and the NLR. The main study endpoints were recurrence-free survival (RFS) and disease-specific survival (DSS). The prognostic value of NLR was assessed by Kaplan–Meier method and Cox model analysis.
Results: There were 88 patients included in total, with mean NLR of 2.32 (range, 1.8–6.0). Histologic tumor grade and tumor stage were associated with the NLR significantly. The 10-year RFS rates were 98 and 81% for patients with an NLR < 2.32 and patients with an NLR ≥ 2.32, respectively, the difference was significant (p = 0.010). The 10-year DSS rate was 97 and 81% for patients with an NLR < 2.32 and patients with an NLR ≥ 2.32, respectively; the difference was not significant (p = 0.072). The independence of NLR in predicting the RFS was further confirmed in Cox model analysis.
Conclusion: The NLR significantly affects the prognosis in pediatrics with primary parotid MEC.
Introduction
Parotid cancer is relatively uncommon in pediatrics (1–3), and the most common pathologic subtype is mucoepidermoid carcinoma (MEC), but because of the rarity of this disease, it is challenging to develop a consensus treatment. Moreover, there is some difference between pediatric and adult patients with parotid MEC regarding clinicopathological characteristics and disease prognosis (4), and reported predictors for recurrence and death in pediatrics include high tumor stage, high histological grade, and adverse pathologic characteristics (5–10).
There are important roles of interactions between the tumor cells and tumor microenvironment on cancer progression (11–13). The peripheral neutrophil-to-lymphocyte ratio (NLR) is an accurate and reliable inflammatory indicator. A number of previous authors have concluded that worse survival in solid cancers is possibly predicted by pretreatment high NLR (14, 15). However, whether there is similar phenomenon in parotid MEC remains unclear. Considering there is different immature lymphatic defense system in pediatrics, therefore, we aimed to assess the significance of the pretreatment NLR in the prognosis of pediatrics with parotid MEC.
Materials and Methods
Our hospital institutional research committee had approved this study, and written informed consent was obtained from all the legal guardians for pediatric patients (≤ 18 years). Our research was performed based on the Declaration of Helsinki.
Pediatric patients (≤ 18 years old) with surgically treated primary parotid MEC were retrospectively enrolled between January 1991 and September 2018 in four hospitals in Liaoning. Related patient information regarding age, sex, TNM stage, operation, pathology, intraparotid node (IPN) metastasis, histologic tumor grade, recurrence, and death was extracted and analyzed. The histologic tumor grade and the tumor stage were defined according to the World health Organization 2017 classification and the American Joint Committee on Cancer seventh edition staging system, respectively. All pathologic sections were re-reviewed by at least two head and neck pathologists.
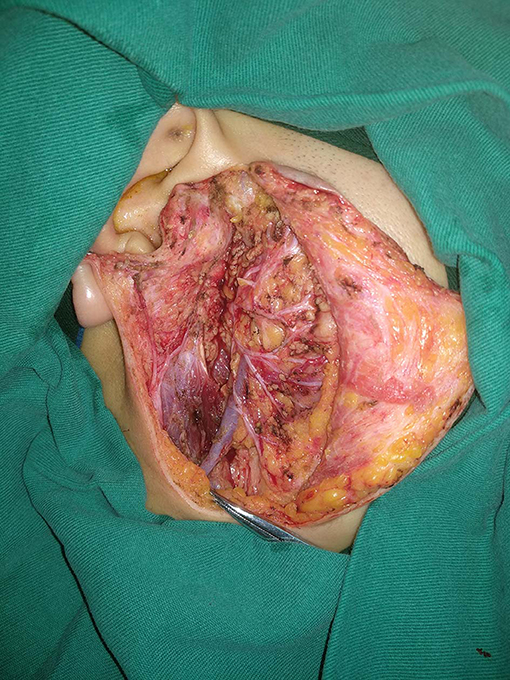
In our department, preoperative ultrasound, computed tomography, and/or magnetic resonance imaging examinations were routinely performed; fine-needle biopsy was selectively performed in the case of differential diagnosis from normal lymph nodes. Operation types of partial parotidectomy, superficial parotidectomy, and total parotidectomy were performed based on pathologic characteristics and the surgeon's experience (Figure 1). Facial nerve was tried to be preserved in every case. Adjuvant treatment was suggested if there was presence of positive margin, advanced tumor stage, and pathologic cervical lymph node metastasis.
The NLR was calculated within 2 weeks before the initial treatment by the ratio of the absolute neutrophil count to the absolute lymphocyte count (14–21). The previous reported cutoff values varied from 1.98 to 5 (14–20), and the standard cutoff value remains unknown. The cutoff value was defined as the mean value of the NLR in current study.
The associations between the NLR and the clinicopathological variables were analyzed using the χ2 test. The main study interests were the recurrence-free survival (RFS) and disease-specific survival (DSS). The survival time of RFS was calculated from the date of surgery to the date of first disease recurrence or the last follow-up, and the survival time of DSS was calculated from the date of surgery to the date of cancer-caused death or the last follow-up. The RFS and DSS rates were analyzed by the Kaplan–Meier method. The factors that were significant in univariate analysis were then analyzed in the Cox model to find out the independent prognostic factors. All reported p-values were two-sided, and a p < 0.05 was considered to be significant; all statistical analyses were performed by SPSS 20.0, IBM, USA.
Results
There were 88 patients (53 female and 35 male patients) in total enrolled in the study with mean age of 14.2 years (range, 6–18 years), including 23 patients from the Affiliated First Hospital of China Medical University, 18 patients from the Affiliated Stomatology Hospital of China Medical University, 42 patients from the Affiliated Shengjing Hospital of China Medical University, and 5 patients from the Central Hospital of Yingkou. A history of blood malignancy was reported in 12 patients, and the mean duration between the diagnosis of previous blood malignancy and the diagnosis of parotid MEC was 8.0 years, with a range from 5 to 11 years. Thirty-five patients had T1 disease, 38 patients had T2 disease, 10 patients had T3 disease, and 5 patients had T4 disease. Operations of superficial parotidectomy, partial parotidectomy, and total parotidectomy were performed in 26, 13, and 49 patients, respectively. Six patients had facial nerve branches resected because of tumor invasion. Eighty patients had a clear margin. Neck dissection was performed in 23 patients, of whom 10 patients had metastatic neck lymph nodes; the mean number of positive nodes was 1.1, with a range from 1 to 3. Lymphovascular invasion and perineural invasion were presented in 8 and 10 patients, respectively. Low-, moderate-, and high-grade diseases were noted in 67, 14, and 7 patients, respectively.
A total of 77 patients had IPN information, and 16 patients had IPN metastases. The mean positive lymph nodes diameter was 0.8 cm with a range from 0.4 to 1.9 cm. The mean number of positive IPNs was 1.1, with a range from 1 to 3.
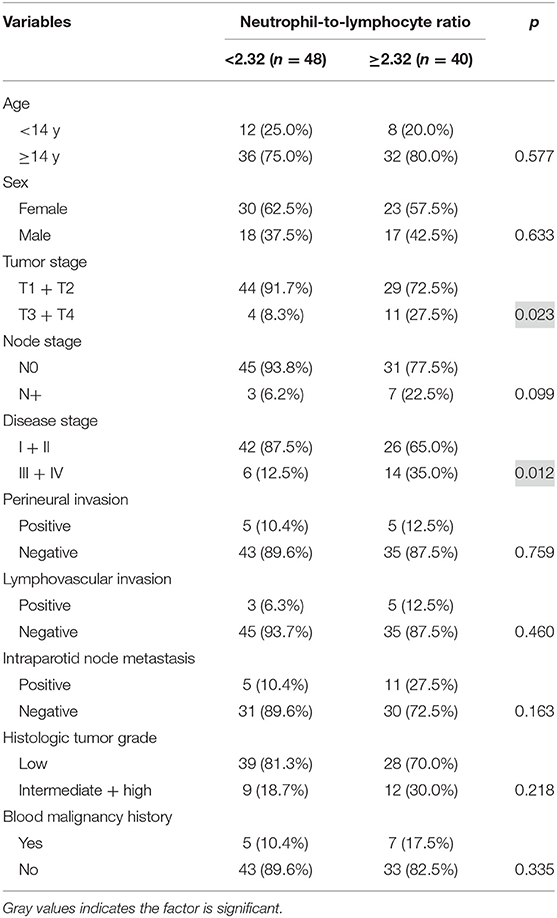
The NLR varied from 1.8 to 6.0 with mean value of 2.32. Table 1 presents the associations between clinicopathological variables and NLR. Histologic tumor grade and tumor stage were associated with the NLR significantly (both p < 0.05) (Table 1).
During our follow-up with mean time of 88.4 (range, 18–173) months, 21 patients received adjuvant radiotherapy, and 4 patients received adjuvant chemotherapy. Recurrence occurred in 8 patients: 4 cases locally, 2 cases locoregionally, 1 case regionally, and 1 case distantly. The 10-year RFS rates were 98 and 81% for patients with an NLR < 2.32 and patients with an NLR ≥ 2.32, respectively, the difference was significant (p = 0.010; Figure 2). Cox proportional hazards model analysis reported the independence of the NLR in affecting the RFS (Table 2).
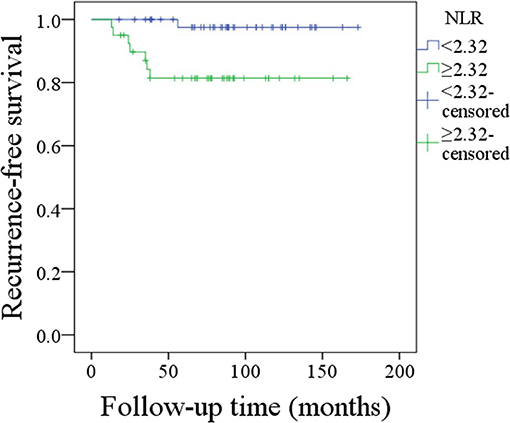
Figure 2. Recurrence-free survival of patients with different neutrophil-to-lymphocyte ratios (p = 0.010).
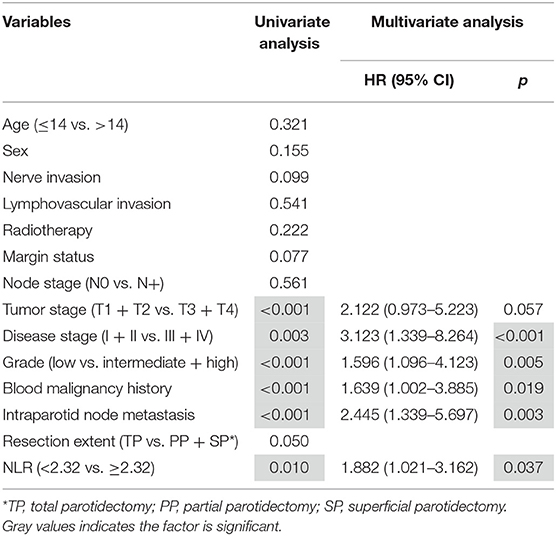
Table 2. Predictors for recurrence-free survival in pediatric patients with parotid mucoepidermoid carcinoma.
Disease-caused death occurred in 5 patients, and the 10-year overall DSS rate was 91%. The 10-year DSS rate was 97% and 81% for patients with an NLR < 2.32 and patients with an NLR ≥ 2.32, respectively, the difference was not significant (p = 0.072; Figure 3). Cox proportional hazards model analysis reported the independence of histologic tumor grade, disease stage, malignancy history, and IPN metastasis status in predicting the DSS (Table 3).
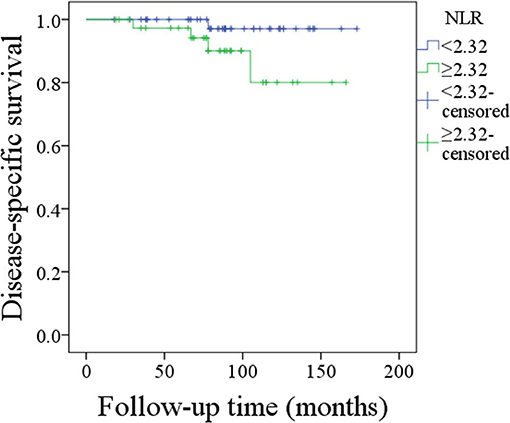
Figure 3. Disease-specific survival of patients with different neutrophil-to-lymphocyte ratios (p = 0.072).
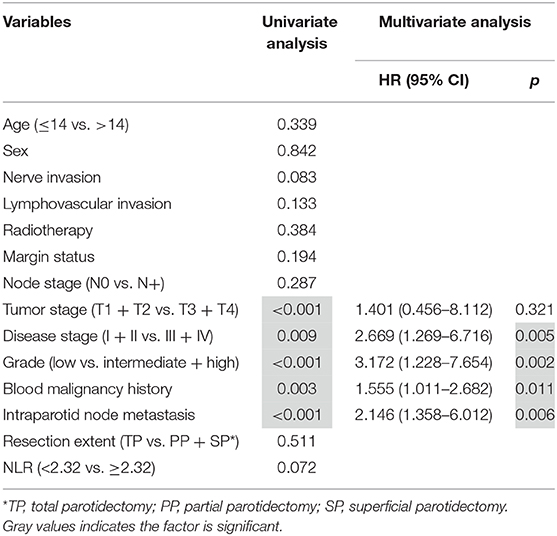
Table 3. Predictors for disease-specific survival in pediatric patients with parotid mucoepidermoid carcinoma.
Discussion
The most important finding in the current study was that histologic tumor grade and tumor stage were associated with the NLR significantly, and also an NLR ≥ 2.32 meant worse disease prognosis would be expected. The finding could benefit all the pediatric surgeons and head and neck surgeons in better controlling the cancer.
The usefulness of the NLR was only previously analyzed in allergic conditions, inflammatory disorders, and infectious diseases in children (22–24). Vasquez et al. (25) first found that an NLR >2 was an independent indicator of worse overall survival in pediatric sarcomas; significant associations were noted between a high NLR and metastatic disease or poor histological response. We are the first to evaluate the significance of the NLR in pediatric patients with parotid MEC, and a high NLR predicted a decreased RFS rate in multivariate analysis. With regard to adult patients, there were only two articles focusing on this question (16, 17). Damar et al. (16) found that compared with patients with malignant salivary gland tumor the NLR was significantly lower in patients with benign salivary tumors, and the NLR significantly differed with histologic tumor grade. The finding was consistent with ours; it was also described that there was significant association between the NLR and disease stage. Kawakita et al. (17) described that compared to salivary duct carcinoma patients with baseline NLR, an NLR > 2.5 meant a nearly two-fold increase in the risk of death in overall survival.
The exact mechanisms for explaining the associations between the NLR and clinicopathologic variables or prognosis remain unclear, but based on previous evidence some possible explanations can be inferred. The pretreatment NLR is an indicator for the immune system and systemic inflammation. Several angiogenic factors and cytokines, which play an important role in promoting tumor development, can be generated by neutrophils (26). On the other hand, hematological markers might be associated with cancer cachexia; it carried poor survival (17, 27). In contrast, lymphocytes are associated with immune surveillance and play an important role of eliminating cancer cells (28). Therefore, the NLR might act as a predictor for the prognosis.
The issue of IPN metastasis in pediatric parotid MEC was rarely assessed. We first describe that IPN metastasis predicts worse RFS and DSS. There were similar findings in adults (29–32). Lim et al. (29) might have been the first to identify patients with cN0 neck disease, but their patients with IPN metastasis were more likely to develop locoregional recurrence than their patients who did not have IPN metastasis. Klussmann et al. (30) noted that there was an additional significant risk for tumor recurrence related to the involvement of the IPNs in 55 patients with pN+ disease. Nisa et al. (31) reported that in patients with IPN metastasis decreased disease-free survival could be foreseen. Therefore, IPN metastasis was associated with worse RFS and DSS in pediatrics as well as adults. The underlying mechanism might be that first there were superficial and deep lobe lymph nodes in parotid; recurrence would be aroused owing to unresected positive IPN tissue left by un-total parotidectomy. Second, the parotid lymph nodes are not part of any neck lymph node levels, but the IPNs might act as a predictor for neck lymph node metastasis.
We noted a history of malignancy-predicted worse RFS and DSS. A secondary parotid malignancy in pediatric patients has been reported by case reports (33), and owing to the extreme rarity, the prognosis of those cancers remains unknown. Védrine et al. (5) reported there was no significant difference regarding pathologic tumor grade and tumor location between patients with primary and secondary parotid MEC, but patients with secondary parotid MEC had less advanced stage than patients with primary disease. However, the two groups had similar overall survival, DSS, and disease-free survival. There were only five deaths in the current study; worse DSS was noted in patients with a history of malignancy. Previous chemotherapy, which had a significant adverse impact on the lymphatic system, might be partially responsible.
The prognostic significance of CRTC1/3-MAML2 fusion in MEC has been analyzed. Okumura et al. (34) reported that in early-stage MEC patients positive for CRTC1/3-MAML2 fusions, an excellent prognosis may be achieved without adjuvant radiotherapy when the tumors are completely resected without tumor spillage. However, Birkeland et al. (35) found a high rate of CRTC1/3-MAML2 gene fusions in a large cohort of MEC patients, but the authors did not note any correlation between fusion status and tumor grade or survival. Moreover, whether a similar phenomenon occurs in pediatric patients remains unclear, and more high-quality studies are required to illustrate clearly.
Limitations in the current study must be stated: first, the statistical power was decreased by our inherent bias in retrospective study and our relatively small sample size. Second, neutrophil and lymphocyte counts are easily influenced by infections or inflammation; they are nonspecific parameters. Third, because of differences in diagnostic ability by the different pathologists in four hospitals, there might be undetected IPN metastases.
Conclusion
In summary, the pretreatment NLR is significantly associated with survival in pediatric patients with parotid MEC.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The Zhengzhou University institutional research committee approved our study, and all of the legal guardians, including parents, provided written informed consent for any patient under the age of 18 years old. This study was conducted in accordance with the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations.
Author Contributions
All authors make significant contribution in data collection, analysis, and manuscript writing and revision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
MEC, mucoepidermoid carcinoma; IPN, intraparotid node; NLR, neutrophil-to-lymphocyte ratio; RFS, recurrence-free survival; DSS, disease-specific survival.
References
1. Chiaravalli S, Guzzo M, Bisogno G, De Pasquale MD, Migliorati R, De Leonardis F, et al. Salivary gland carcinomas in children and adolescents: the Italian TREP project experience. Pediatr Blood Cancer. (2014) 61:1961–8. doi: 10.1002/pbc.25139
2. Cockerill CC, Gross BC, Contag S, Rein S, Moore EJ, Olsen KD, et al. Pediatric malignant salivary gland tumors: 60 year follow up. Int J Pediatr Otorhinolaryngol. (2016) 88:1–6. doi: 10.1016/j.ijporl.2016.05.021
3. Fang QG, Shi S, Li ZN, Zhang X, Liu FY, Sun CF. Epithelial salivary gland tumors in children: a twenty-five-year experience of 122 patients. Int J Pediatr Otorhinolaryngol. (2013) 77:1252–4. doi: 10.1016/j.ijporl.2013.04.034
4. Sultan I, Rodriguez-Galindo C, Al-Sharabati S, Guzzo M, Casanova M, Ferrari A. Salivary gland carcinomas in children and adolescents: a population-based study, with comparison to adult cases. Head Neck. (2011) 33:1476–81. doi: 10.1002/hed.21629
5. Védrine PO, Coffinet L, Temam S, Montagne K, Lapeyre M, Oberlin O, et al. Mucoepidermoid carcinoma of salivary glands in the pediatric age group: 18 clinical cases, including 11 second malignant neoplasms. Head Neck. (2006) 28:827–33. doi: 10.1002/hed.20429
6. Morse E, Fujiwara RJT, Husain Z, Judson B, Mehra S. Pediatric salivary cancer: epidemiology, treatment trends, and association of treatment modality with survival. Otolaryngol Head Neck Surg. (2018) 159:553–63. doi: 10.1177/0194599818771926
7. Qureshi SS, Bhagat M, Singhal N, Tathe N, Kembhavi S, Laskar S, et al. Clinical characteristics and treatment outcomes of primary and recurrent malignancy involving the salivary glands in children. Head Neck. (2016) 38:852–6. doi: 10.1002/hed.24114
8. Radomski S, Dermody S, Harley EH Jr. Clinical characteristics and outcomes of major salivary gland malignancies in children. Laryngoscope. (2018) 128:1126–32. doi: 10.1002/lary.26946
9. Rebours C, Couloigner V, Galmiche L, Casiraghi O, Badoual C, Boudjemaa S, et al. Pediatric salivary gland carcinomas: Diagnostic and therapeutic management. Laryngoscope. (2017) 127:140–7. doi: 10.1002/lary.26204
10. Xu B, Aneja A, Ghossein R, Katabi N. Salivary gland epithelial neoplasms in pediatric population: a single-institute experience with a focus on the histologic spectrum and clinical outcome. Hum Pathol. (2017) 67:37–44. doi: 10.1016/j.humpath.2017.07.007
11. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. (2005) 7:211–7. doi: 10.1016/j.ccr.2005.02.013
12. Wei J, Meng L, Hou X, Qu C, Wang B, Xin Y, et al. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. (2018) 11:167–77. doi: 10.2147/CMAR.S188655
13. Zhao JJ, Zhou ZQ, Wang P, Chen CL, Liu Y, Pan QZ, et al. Orchestration of immune checkpoints in tumor immune contexture and their prognostic significance in esophageal squamous cell carcinoma. Cancer Manag Res. (2018) 10:6457–68. doi: 10.2147/CMAR.S181949
14. Kano S, Homma A, Hatakeyama H, Mizumachi T, Sakashita T, Kakizaki T, et al. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. (2017) 39:247–53. doi: 10.1002/hed.24576
15. Kapoor S. Neutrophil to lymphocyte ratio and its association with tumor prognosis in systemic malignancies. J Surg Oncol. (2013) 107:560. doi: 10.1002/jso.23291
16. Damar M, Dinç AE, Erdem D, Aydil U, Kizil Y, Eravci FC, et al. Pretreatment neutrophil-lymphocyte ratio in salivary gland tumors is associated with malignancy. Otolaryngol Head Neck Surg. (2016) 155:988–96. doi: 10.1177/0194599816659257
17. Kawakita D, Tada Y, Imanishi Y, Beppu S, Tsukahara K, Kano S, et al. Impact of hematological inflammatory markers on clinical outcome in patients with salivary duct carcinoma: a multi-institutional study in Japan. Oncotarget. (2017) 8:1083–91. doi: 10.18632/oncotarget.13565
18. Fang Q, Liu F, Seng D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manag Res. (2019) 11:1081–5. doi: 10.2147/CMAR.S192788
19. Cheng G, Liu F, Niu X, Fang Q. Role of the pretreatment neutrophil to lymphocyte ratio in the survival of primary parotid cancer patients. Cancer Manag Res. (2019) 11:2281–6. doi: 10.2147/CMAR.S195413
20. Fang Q, Li P, Qi J, Luo R, Chen D, Zhang X. Value of lingual lymph node metastasis in patients with squamous cell carcinoma of the tongue. Laryngoscope. (2019) 129:2527–30. doi: 10.1002/lary.27927
21. Seng D, Fang Q, Li P, Liu F, Liu S. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in pediatric parotid cancer. Front Pediatr. (2019) 7:207. doi: 10.3389/fped.2019.00207
22. Aktar F, Tekin R, Bektas MS. Diagnostic role of inflammatory markers in pediatric Brucella arthritis. Ital J Pediatr. (2016) 42:3. doi: 10.1186/s13052-016-0211-5
23. Dogru M, Yesiltepe Mutlu RG. The evaluation of neutrophil-lymphocyte ratio in children with asthma. Allergol Immunopathol. (2016) 44:292–6. doi: 10.1016/j.aller.2015.09.005
24. Eryilmaz A, Basal Y, Tosun A. The neutrophil to lymphocyte ratios of our pediatric patients with Bell's palsy. Int J Pediatr Otorhinolaryngol. (2015) 79:2374–7. doi: 10.1016/j.ijporl.2015.10.047
25. Vasquez L, León E, Beltran B, Maza I, Oscanoa M, Geronimo J. Pretreatment neutrophil-to-lymphocyte ratio and lymphocyte recovery: independent prognostic factors for survival in pediatric sarcomas. J Pediatr Hematol Oncol. (2017) 39:538–46. doi: 10.1097/MPH.0000000000000911
26. Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. (2013) 23:159–70. doi: 10.1016/j.semcancer.2013.02.004
27. Liu F, Cheng GY, Fang QG, Sun Q. Natural history of untreated squamous cell carcinoma of the head and neck. Clin Otolaryngol. (2019) 44:200–03. doi: 10.1111/coa.13260
28. Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. (2012) 107:864–73. doi: 10.1038/bjc.2012.347
29. Lim CM, Gilbert MR, Johnson JT, Kim S. Clinical significance of intraparotid lymph node metastasis in primary parotid cancer. Head Neck. (2014) 36:1634–7. doi: 10.1002/hed.23507
30. Klussmann JP, Ponert T, Mueller RP, Dienes HP, Guntinas-Lichius O. Patterns of lymph node spread and its influence on outcome in resectable parotid cancer. Eur J Surg Oncol. (2008) 34:932–7. doi: 10.1016/j.ejso.2008.02.004
31. Nisa L, Salmina C, Dettmer MS, Arnold A, Aebersold DM, Borner U, et al. Implications of intraglandular lymph node metastases in primary carcinomas of the parotid gland. Laryngoscope. (2015) 125:2099–106. doi: 10.1002/lary.25342
32. Feng YP, Liu F, Cheng GY, Fang QG, Niu XY, He W. Significance of intraparotid node metastasis in predicting local control in primary parotid cancer. Laryngoscope. (2019) 129:2309–12. doi: 10.1002/lary.27701
33. Prasannan L, Pu A, Hoff P, Weatherly R, Castle V. Parotid carcinoma as a second malignancy after treatment of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. (1999) 21:535–8. doi: 10.1097/00043426-199911000-00017
34. Okumura Y, Murase T, Saida K, Fujii K, Takino H, Masaki A, et al. Postoperative radiotherapy for T1/2N0M0 mucoepidermoid carcinoma positive for CRTC1/3-MAML2 fusions. Head Neck. (2018) 40:2565–73. doi: 10.1002/hed.24856
Keywords: salivary gland cancer, pediatric cancer, salivary cancer, neutrophil-to-lymphocyte ratio, mucoepidermoid carcinoma
Citation: Gao H, Gao Q and Sun J (2020) Significance of Pretreatment Neutrophil-to-Lymphocyte Ratio in Mucoepidermoid Carcinoma of Pediatrics: A Multicenter Study. Front. Pediatr. 8:96. doi: 10.3389/fped.2020.00096
Received: 21 December 2019; Accepted: 24 February 2020;
Published: 27 March 2020.
Edited by:
Qigen Fang, Henan Provincial Cancer Hospital, Shenyang, ChinaReviewed by:
Jie Fan, Capital Medical University, ChinaWent Li, First Affiliated Hospital of Zhengzhou University, China
Zhenning Li, University at Buffalo, United States
Copyright © 2020 Gao, Gao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Gao, NzE1NDI2NTExQHFxLmNvbQ==; Jinlan Sun, c2pseWtAMTI2LmNvbQ==
 Hua Gao
Hua Gao Qing Gao2
Qing Gao2