- 1Department of Pediatrics, Fukushima Medical University School of Medicine, Fukushima, Japan
- 2Department of Advanced Cancer Immunotherapy, Fukushima Medical University School of Medicine, Fukushima, Japan
- 3Department of Blood Transfusion and Transplantation Immunology, Fukushima Medical University School of Medicine, Fukushima, Japan
- 4Department of Pediatrics, Sapporo Medical University School of Medicine, Sapporo, Japan
Background: Platelets participate in many physiological and pathological functions and some platelet parameters predict adult diseases. However, few studies report whether platelet parameters may reflect neonatal disease and mortality in a large cohort.
Objective: We aimed to investigate whether platelet parameters could predict bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), and NICU mortality.
Study Design and Methods: This retrospective cohort study examined records from 2006 to 2017 at the neonatal intensive care unit (NICU) of Fukushima Medical University Hospital. We retrospectively investigated platelet count, plateletcrit (PCT), mean platelet volume (MPV), and platelet distribution width (PDW) on the first day of life in preterm newborns born <32 weeks' gestation admitted to our NICU from 2006 to 2017. Receiver operating characteristic (ROC) and multiple regression analyses, along with Cox proportional hazard modeling, identified independent predictors of morbidities and mortality in preterm newborns.
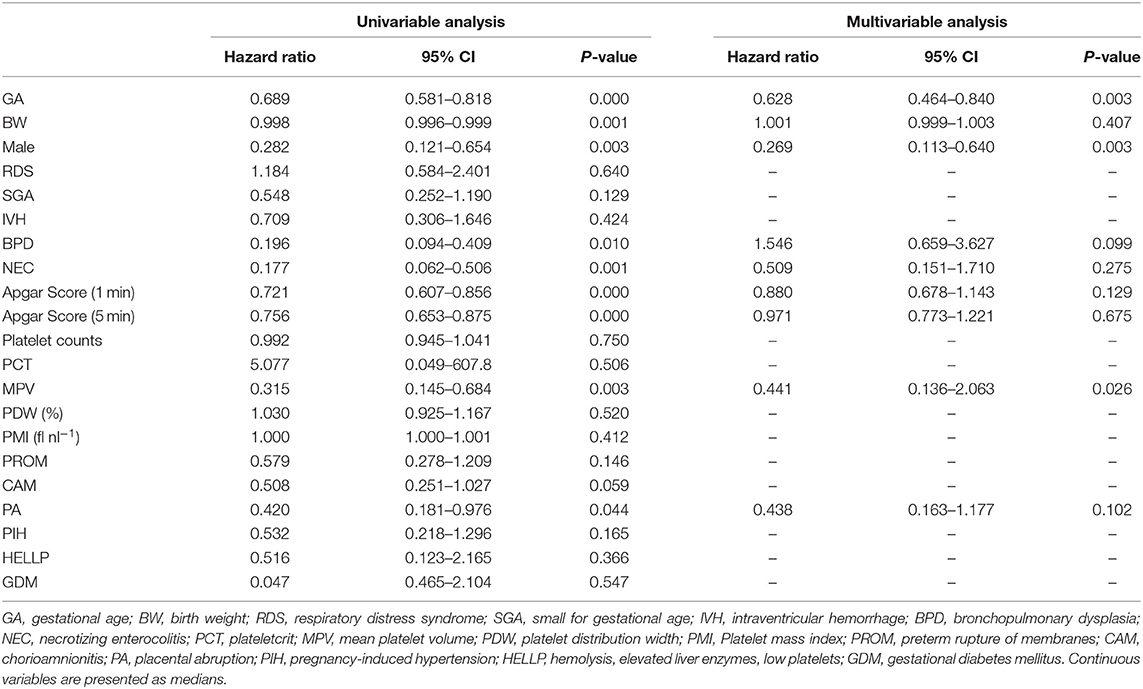
Results: Of 1,501 neonates admitted to our NICU, a total of 305 preterm newborns were included in this study. Gestational age, birth weight, and Apgar score were significantly lower in non-survivors than in survivors. Platelet count, PCT, PDW and PMI did not differ significantly between the two groups, whereas mean MPV in non-survivors was significantly higher than in survivors (10.5 fl vs. 10.0 fl, p = 0.001). Multivariate Cox hazard modeling showed that shorter GA [HR: 0.628, 95% CI: 0.464–0.840, p = 0.003], male sex [HR: 0.269, 95% CI: 0.113–0.640, p = 0.001], and MPV [HR: 1.469, 95% CI: 1.046–2.063, p = 0.026] independently predicted overall survival. Per receiver operating curve, an MPV threshold of 10.2 fl. MPV predicts prognosis in neonates with a sensitivity of 72.4% and a specificity of 58.6% (AUC = 0.685, 95% CI: 0.600–0.789, p = 0.001). Furthermore, multivariate analysis revealed that platelet parameters were not associated with BPD and NEC, whereas small for gestational age (SGA), Apgar score at 5 min, and low PCT were associated with intraventricular hemorrhage (IVH).
Conclusion: This study demonstrates that low PCT predicts IVH, and MPV ≥ 10.2 fL correlates with mortality among infants born after <32 weeks' gestation.
Introduction
Platelets participate in many physiological and pathological functions and some platelet parameters predict adult diseases (1–3). High mean platelet volume (MPV) values are associated with coronary flow and high risks of recurrent stroke in adults (4, 5). Elevated MPV, platelet distribution width (PDW), and increased plateletcrit (PCT) are associated with mortality in diseases such as ischemic coronary artery disease, pneumonia, kidney disease, and cancer (6–9). Platelet activation and consumption are seen commonly in critical ill patients and indicate a poorer prognosis (10). MPV and PDW are easy-to-measure surrogates for platelet activation that is provoked inflammation. In particular, MPV can reflect platelet function better than a platelet count by itself. Likewise, previous studies have implicated high MPV in the first postpartum hours with increased risk of necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), and intravascular hemorrhage (IVH) in neonates (11, 12). On the other hand, platelet mass index (PMI), calculated by multiplying platelet count by MPV, is associated with retinopathy of prematurity, NEC, and IVH (13). However, few studies report whether platelet parameters (MPV, PDW, and PMI) may reflect neonatal disease such as BPD, NEC, and IVH in a large cohort. Furthermore, it has not been established whether MPV, PDW, or PMI correlate with mortality in preterm infants. The primary objective of this study was to evaluate the potential of platelet parameters to predict BPD, IVH, and NEC in preterm infants. The secondary objective was to evaluate the associations between platelet parameters and mortality.
Methods
Study Design and Population
This retrospective cohort study examined records from 2006 to 2017 at the neonatal intensive care unit (NICU) of Fukushima Medical University Hospital (FMU). The Ethics Committee of FMU, guided by local policy, national law, and the World Medical Association Declaration of Helsinki, approved this study without requiring informed consent from guardians. Neonates born <32 weeks' gestation were included in this study. Exclusion criteria were congenital anomalies and neonates not tested for platelet parameters within 12 h of birth.
Definition of BPD, IVH, and NEC
BPD was defined as the need for supplemental oxygen or supplemental ventilation at 36 weeks' postmenstrual age (14). IVH was defined on the basis of ultrasound scans on postnatal days 1, 4, 7, 14, 21, and 28, using Papille's classification (15). NEC was staged according to Bell's criteria, with pneumatosis intestinalis, hepatobiliary gas, and free intraperitoneal air on radiography as major factors (16).
Platelet Parameter Measurements
Blood samples were collected through umbilical cord or peripheral venipuncture to measure the PCT, MPV, PDW, and PMI of each newborn. Complete blood counts were measured using a Sysmex CS-5100 coagulation analyzer (Sysmex, Kobe, Japan) on admission. PMI was calculated as the product of platelet count and MPV.
Prenatal and Postnatal Risk Factors
Platelet parameters were compared with demographic variables including gender, birth weight, and gestational age. Furthermore, we considered pregnancy-induced hypertension (PIH); chorioamnionitis (CAM); preterm rupture of membranes (PROM); hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome); and placental abruption (PA) as possible prenatal risk factors, and small for gestational age (SGA, birth weight <10th percentile), respiratory distress syndrome (RDS), and Apgar score as factors affecting platelet parameters.
Statistical Analysis
Platelet parameters from medical records were rendered as median interquartile range (IQR) percentiles for continuous variables of non-normal distribution. Differences in categorical variables and continuous variables were assessed for significance using Chi-square and Mann-Whitney U-tests, respectively. The 1-year overall survivals were plotted on Kaplan-Meier curves, with differences assessed for significance by log-rank test. Correlations with p < 0.05 by univariable analysis were entered into a multivariable Cox proportional hazard regression model or multiple regression analysis to identify independent prognostic factors. A receiver operating characteristic (ROC) curve identified predictive MPV levels, along with their sensitivity and specificity.
SPSS for Mac, release 25.0 (SPSS, Chicago, IL) was used to perform the statistical analyses with P < 0.05 considered to be statistically significant.
Results
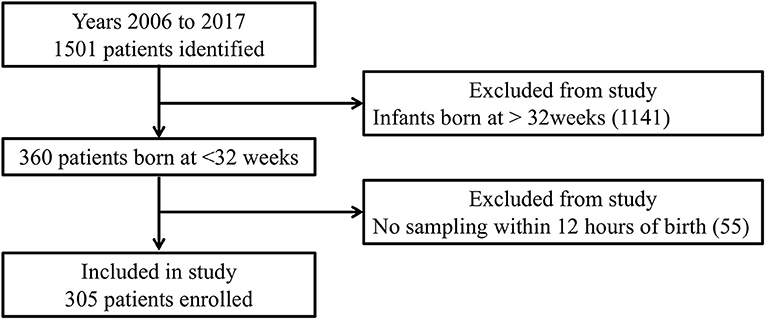
Of 1,501 neonates admitted to our NICU between January 2006 and December 2017, there were 305 born at <32 weeks' gestation who were assessed within 12 h postpartum (Figure 1). BPD, IVH, and NEC were diagnosed in 76, 54, and 10 of them, respectively. As shown in Table 1A, multivariate analysis showed that shorter GA was significantly correlated with BPD. Furthermore, as shown in Table 1B, SGA, lower PCT, and lower Apgar scores at 5 min were significantly correlated with IVH. In contrast, Table 1C shows no significant factors affecting NEC.
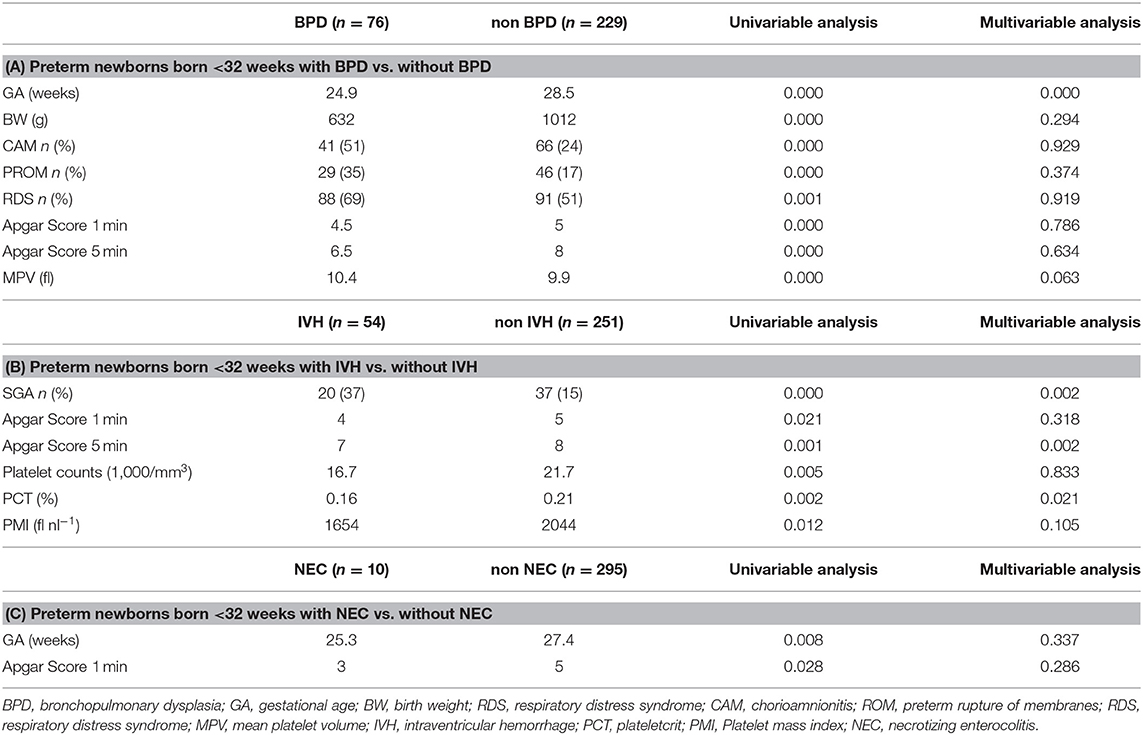
Table 1. The relationships between morbidities and clinical characteristics and platelet parameters in preterm newborns.
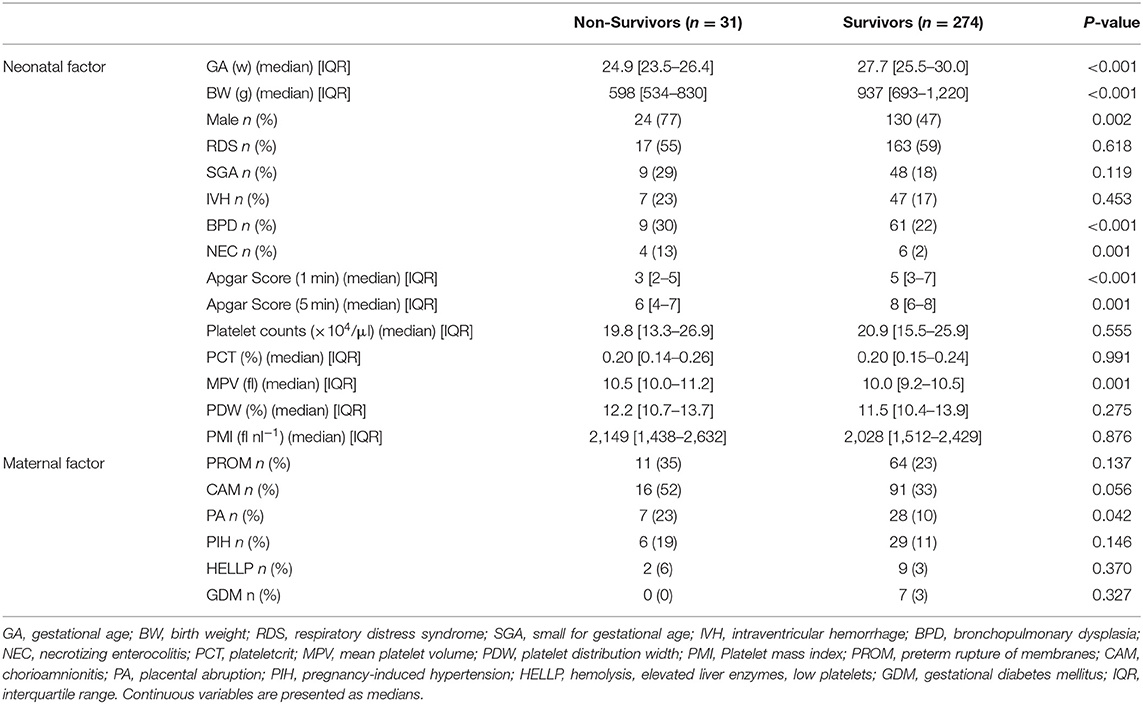
Table 2 describes non-survivors and survivors. Regrettably, 31 were non-survivors. Among non-survivors, gestational age, birth weight, and Apgar score were significantly lower than in survivors. Platelet counts, PCT, PDW and PMI were not significantly different between the two groups, whereas MPV in non-survivors was significantly higher than in survivors (10.5 vs. 10.0, p = 0.001) (Table 2).
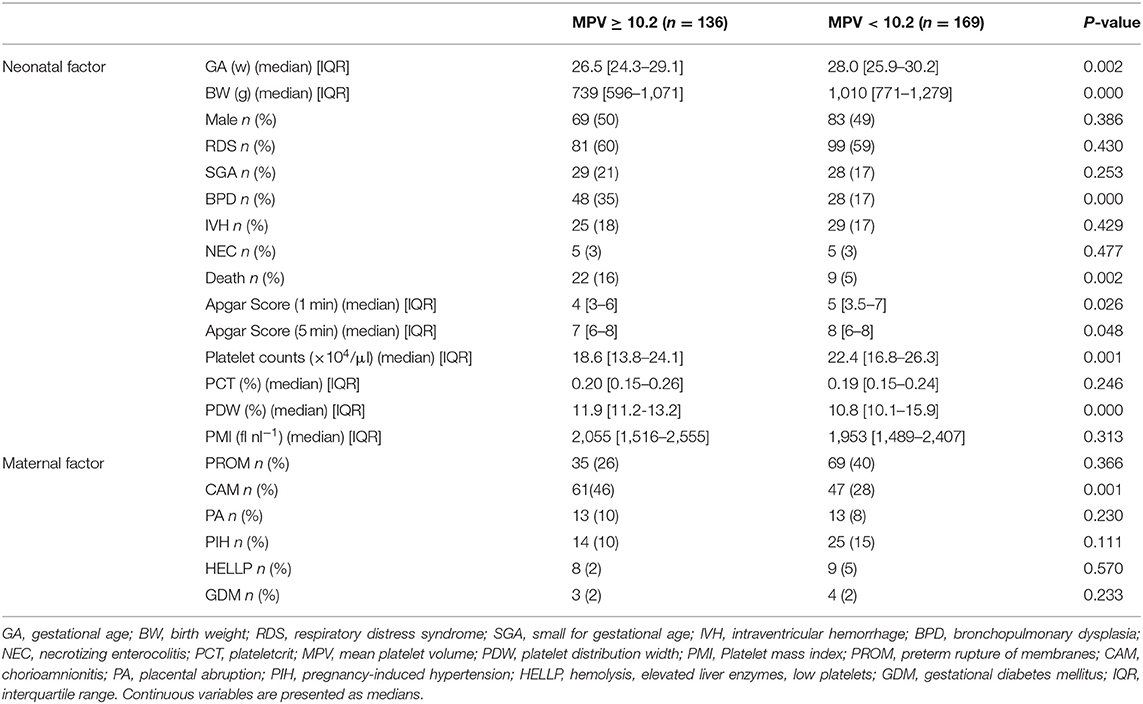
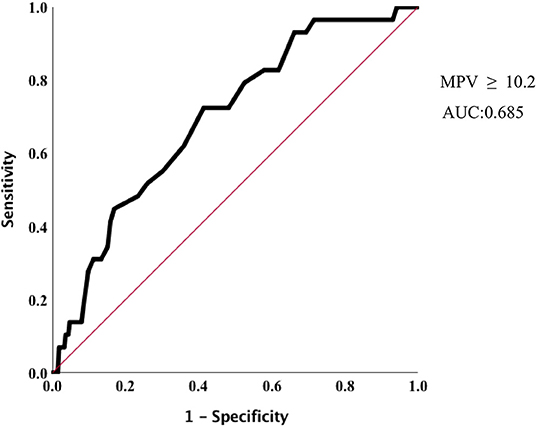
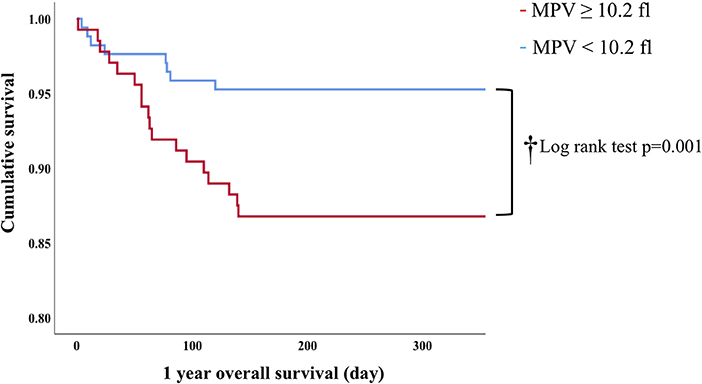
In Cox proportion univariable analysis, shorter GA, smaller BW, male sex, presence of PA and/or BPD, lower Apgar score, and MPV were significant predictors of overall survival (Table 3). Multivariable Cox hazard modeling showed that shorter GA [HR: 0.628, 95% CI: 0.464–0.840, p = 0.003], male sex [HR: 0.269, 95% CI: 0.113–0.640, p = 0.001], and MPV [HR: 1.469, 95% CI: 1.046–2.063, p = 0.026], independently predicted overall survival (Table 3). The ROC curve comprising Figure 2 shows than an MPV ≥ 10.2 fl is prognostic with a sensitivity of 72.4% and a specificity of 58.6% (AUC = 0.685, 95% CI: 0.600–0.789, p = 0.001). Thus, newborns were divided into two groups: MPV ≥ 10.2 fl (n = 136) and MPV < 10.2 fl (n = 169). The Kaplan-Meier curves of preterm neonates with MPV ≥ 10.2 and MPV < 10.2 were significantly different (Figure 3). As shown in Table 4, those with MPV ≥ 10.2 fl were lower in GA, BW, Apgar score and platelet counts; were higher in PDW; and had higher incidence of CAM and BPD. To further advance the prognostic value of MPV, we performed univariate analysis and multivariate Cox proportional hazard modeling to establish hazard ratios (HR).
Discussion
The present study shows that elevated MPV at birth correlates with unfavorable outcomes for NICU patients born after <32 weeks' gestation. While precise pathophysiologic mechanisms remain elusive, platelet numbers and average size may increase in conjunction with inflammatory and thrombotic conditions (17). Young platelets are generally larger than old ones, and the presence of more young platelets indicates increased production in response to consumption, which in turn may be provoked by inflammation. Thus, an elevated MPV may be indicative of oxidative stress in newborns (18). In short, MPV in preterm newborns can inform clinicians of possible hypercoagulative states, increased inflammatory response, and oxidative stress. Among these, a possible explanation for the relationship between MPV and mortality is inflammatory response. It is known that inflammatory response is significantly associated with adverse clinical outcomes in ICU patients. Platelet parameters such as MPV are immediate indicators of platelet activation that is driven by inflammatory processes.
In adults, some studies showed that the initial MPV, as a predictor of mortality in critically ill patients, was not significantly associated with in-hospital mortality (19), although other studies showed that elevated MPV during hospitalization was a predictor of mortality in critically ill adults (9, 20). High MPV was suggested to be associated with overall survival in various diseases such as cancer, cardiac disease, septic shock, kidney injury, and patients hospitalized in a respiratory intensive care unit (21–25). On the other hand, subsequent MPV and PDW are suggested to be associated with mortality in critically ill children receiving mechanical ventilation, however initial admission MPV was not associated with mortality in those children (17).
There are few reports about the relationship between MPV and morbidities among large cohorts of premature newborns. Cekmez et al. (11) found that elevated MPV measured within 2 h of birth was associated with BPD, IVH, and NEC using univariate analysis in 44 BPD, 42 IVH, and 21 NEC preterm newborns, but they did not perform multivariate analysis. In our study, univariate analysis also suggested that MPV at birth was higher in BPD vs. non-BPD, but multivariate analysis showed no relationship between BPD and MPV. On the other hand, Dani et al., also suggested that elevated MPV at 24 to 48 h of life was higher in BPD than in non-BPD groups, however, there was no significant difference of MPV between IVH and non-IVH groups (12). Korkmaz et al. also suggested that platelet counts, MPV, and PMI at 12 h postpartum were not associated with IVH (26). In this study, PCT is an independent risk factor for IVH. To date, previous research suggested that PCT had a predictive value for RDS in preterm newborns (27). However, few studies investigated the relationship between PCT and IVH. Further studies are needed to investigate the mechanism(s) relating PCT with IVH.
Our study has several limitations. First, we could not assess the changes of MPV values during early postnatal life. Previous studies reported that BPD correlated with MPV changes at 24 to 48 h of life. However, to avoid phlebotomy, we did not perform complete blood counts at 48 h. Second, we could not investigate the relationship between platelet indices and sepsis because few of our preterm infants had sepsis at birth. Other studies associated sepsis with elevated MPV in neonates (28–30). Third, an interesting finding in our study is that there is no association between MPV and preterm morbidities (BPD/NEC) that might be related to inflammation or oxidative stress, only with mortality. We could not elucidate any pathologic mechanism of this phenomenon. Some studies also suggest that among critically ill patients, including children and adults, higher MPV is strong predictor of mortality. However, mechanisms have not been elucidated. Our study is limited by its retrospective nature and the lack of clear causality between platelet parameters and mortality. Future prospective studies and multicenter collaboration may be needed to address this relationship.
Finally, we could not assess the relationship between serum lactate at birth and subsequent mortality. While there are missing data in this study, lactate and pH values in cord or venous blood at birth were not associated with mortality (not shown). Some studies reported that high lactate levels and low pH in cord blood within the first day of life predict mortality in premature infants (31, 32). Conversely, other studies found no association between umbilical cord acidemia and adverse outcomes in those born at <32 weeks (33, 34). Gaudier et al., reported that 1 and 5 min Apgar scores were better predictors of survival than umbilical artery blood gases in 1,073 infants weighing 500–1,000 g at birth (35). However, in this study, no correlation emerged between mortality and Apgar scores. Wedzicha et al. reported that there was a negative correlation between MPV and PaO2 (r = −0.70) in patients with chronic air-flow obstruction (36). Thus, perinatal hypoxia may have a direct effect on platelet production and elevated MPV.
In conclusion, the present study demonstrates that MPV ≥ 10.2 fl at birth is an independent risk associated with neonatal mortality, and lower PCT was a predictor of IVH. The mechanisms behind the association between high MPV and mortality in preterm infants warrant further investigation.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Z29oYXlhdG8yNTI1QGdtYWlsLmNvbQ==.
Ethics Statement
The studies involving human participants were reviewed and approved by Institution of the ethics committee of Fukushima Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
HG and HO conceived and coordinated the study and wrote the paper. HG designed the research. HG, MC, NK, ST, TI, HM, NM, YK, and MH collected and analyzed the data. KN and HG edited the manuscript and provided general guidance. All authors reviewed the results and approved the final version of the manuscripts.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Kyohei Miyazaki, Kenichi Sato, Kei Ogasawara, and Maki Sato for their productive discussions and comments on the manuscript, as well as for their technical help.
References
1. Zheng YG, Yang T, Xiong CM, He JG, Liu ZH, Gu Q, et al. Platelet distribution width and mean platelet volume in idiopathic pulmonary arterial hypertension. Heart Lung Circ. (2015) 24:566–72. doi: 10.1016/j.hlc.2014.11.025
2. Cui MM, Li N, Liu X, Yun ZY, Niu Y, Zhang Y, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. (2017) 7:3456. doi: 10.1038/s41598-017-03772-z
3. Gu X, Fu X, Wang Y, Zhang W, Fan W, Jiang Y, et al. Comparison of ticagrelor and high-dose clopidogrel on the platelet functions in patients with inadequate response to clopidogrel. Am J Cardiovasc Dis. (2017) 17:1–8.
4. Pizzuli L, Yang A, Martin JF, Luderitz B. Changes in platelet size and count in unstable angina compared to stable angina and non-cardiac chest pain. Eur Heart J. (1998) 19:80–4. doi: 10.1053/euhj.1997.0747
5. Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, La-louschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischaemic cerebrovascular events? Stroke. (2004) 35:1688–91. doi: 10.1161/01.STR.0000130512.81212.a2
6. Golwala ZM, Shah H, Gupta N, Sreenivas V, Puliyel JM. Mean Platelet Volume (MPV), Platelet Distribution Width (PDW), Platelet Count and Plateletcrit (PCT) as predictors of in-hospital paediatric mortality: a case-control Study. Afr Health Sci. (2016) 16:356–62. doi: 10.4314/ahs.v16i2.3
7. Peng F, Li Z, Yi C, Guo Q, Yang R, Long H, et al. Platelet index levels and cardiovascular mortality in incident peritoneal dialysis patients: a cohort study. Platelets. (2017) 28:576–84. doi: 10.1080/09537104.2016.1246716
8. Tian C, Song J, He D, Wu J, Sun Z, Sun Z. Predictive value of mean platelet volume/platelet count for prognosis in acute myocardial infarction. Int Heart J. (2018) 59:286–92. doi: 10.1536/ihj.17-212
9. Gorelik O, Tzur I, Barchel D, Almoznino-Sarafian D, Swarka M, Beberashvili I, et al. A rise in mean platelet volume during hospitalization for community-acquired pneumonia predicts poor prognosis: a retrospective observational cohort study. BMC Pulm Med. (2017) 17:137. doi: 10.1186/s12890-017-0483-6
10. Zampieri FG, Ranzani OT, Sabatoski de Souza HP, Barbeiro H, da Neto LMC, et al. An increase in mean platelet volume after admission is associated with higher mortality in critically ill patients. Ann Intens Care. (2014) 4:20. doi: 10.1186/s13613-014-0020-1
11. Cekmez F, Tanju IA, Canpolat FE, Aydinoz S, Aydemir G, Karademir F, et al. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharmacol Sci. (2013) 17:134–7.
12. Dani C, Poggi C, Barp J, Berti E, Fontanelli G. Mean platelet volume and risk of bronchopulmonary dysplasia and intraventricular hemorrhage in extremely preterm infants. Am J Perinatol. (2011) 28:551–6. doi: 10.1055/s-0031-1274503
13. Okur N, Buyuktiryyaki M, Uras N, Oncel MY, Ertekin O, Canpolat FE, et al. Platelet mass index in very preterm infants: can it be used as parameter for neonatal morbidities? J Matern Fetal Neonatal Med. (2016) 29:3218–22. doi: 10.3109/14767058.2015.1121475
14. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
15. Papille LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
16. Bell MJ, Temberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decision based upon clinical staging. Ann Surg. (1978) 187:1–7. doi: 10.1097/00000658-197801000-00001
17. Ye S, Zhang Y, Zhang C, Xu D. Are platelet volume indices related to mortality in hospitalized children on mechanical ventilation? J Int Med Res. (2018) 46:1197–208. doi: 10.1177/0300060517737211
18. Mohsen L, Akmal DM, Ghonaim EKE, Riad NM. Role of mean platelet volume and ischemia modified albumin in evaluation of oxidative stress and its association with postnatal complications in infants of diabetic mothers. J Matern Fetal Neonatal Med. (2018) 3:1819–23. doi: 10.1080/14767058.2017.1330329
19. Tajarernmuang P, Phrommintikul A, Limsukon A, Pothirat C, Chittawatanarat K. The role of mean plate-let volume as a predictor of mortality in critically ill patients: a systematic review and meta-analysis. Crit Care Res Pract. (2016) 2016:4370834. doi: 10.1155/2016/4370834
20. Yildiz A, Yigit A, Benli AR. The prognostic role of platelet to lymphocyte ratio and mean platelet volume in critically ill patients. Eur Rev Med Pharmacol Sci. (2018) 22:2246–52. doi: 10.26355/eurrev_201804_14811
21. Zhang Z, Xu X, Ni H, Deng H. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. J Crit Care. (2014) 29: 885.e1–6. doi: 10.1016/j.jcrc.2014.04.020
22. Gu M, Zhai Z, Huang L, Zheng W, Zhou Y, Zhu R, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer. (2016) 23:752–60. doi: 10.1007/s12282-015-0635-6
23. Sun XP, Li BY, Li J, Zhu WW, Hua Q. Impact of mean platelet volume on long-term mortality in Chinese patients with ST-elevation myocardial infarction. Sci Rep. (2016) 16:21350. doi: 10.1038/srep21350
24. Kim CH, Kim SJ, Lee MJ, Kwon YE, Kim YL, Park KS, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS ONE. (2015) 10:e0119437. doi: 10.1371/journal.pone.0119437
25. Sezgi C, Taylan M, Kaya H, Selimoglu Sen H, Abakay O, Demir M, et al. Alterations in platelet count and mean platelet volume as predictor of patient outcome in the respiratory intensive care unit. Clin Respir J. (2015) 9:403–8. doi: 10.1111/crj.12151
26. Korkmaz L, Bastug O, Ozdemir A, Ceylan M, Gunes T, Ozturk MA, et al. Can platelet mass index be a parameter to predict intraventricular hemorrhage in very-birth -weight newborns? Am J Perinatol. (2018) 36:1188–97. doi: 10.1055/s-0038-1676535
27. Dundar B, Cakmak B, Ozgen G, Tasgoz FN, Guclu T, Ocakoglu G. Platelet indices in preterm premature rupture of membranes and their relation with adverse neonatal outcomes. J Obstet Gynaecol Res. (2018) 44:67–73. doi: 10.1111/jog.13484
28. Hanaganahalli SB, Sreeram S, Bompada M, Kuppannagari SK, Suresh PK, Philipos CS. Is MPV a predictive marker for neonatal sepsis? A pilot study. J Pediatr Hematol Oncol. (2018) 40:548–52. doi: 10.1097/MPH.0000000000001272
29. Aydemir C, Aydemir H, Kokturk F, Kulah C, Mungan AG. The cut-off levels of procalcitonin and C-reactive protein and the kinetics of mean platelet volume in preterm neonates with sepsis. BMC Pediatr. (2018) 18:253. doi: 10.1186/s12887-018-1236-2
30. Shaaban HA, Safwat N. Mean platelet volume in preterm: a predictor of early onset neonatal sepsis. J Matern Fetal Neonatal Med. (2018) 22:1–6. doi: 10.1093/qjmed/hcy200.235
31. Randolph DA, Nolen TL, Ambalavanan N, Carlo WA, Peralta-Carrcelen M, Das A, et al. Outcomes of extremely low birth weight infants with acidosis at birth. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F263–8. doi: 10.1136/archdischild-2013-304179
32. Hussain F, Gilshenan K, Gray PH. Does lactate level in the first 12 hours of life predict mortality in extremely premature infants. J Paediatr Child Health. (2009) 45:263–7. doi: 10.1111/j.1440-1754.2009.01488.x
33. Deshpande SA, Platt M. Association between blood lactate and acid-base status and mortality in ventilated babies. Arch Dis Child Fetal Neonatal Ed. (1997) 76:F15–20. doi: 10.1136/fn.76.1.F15
34. Tuten A, Dincer E, Topcuoglu S, Sancak S, Akar S, Toptan HH, et al. Serum lactate levels and perfusion index: are these prognostic factors on mortality and morbidity in very low-birth weight infants? J Matern Fetal Neonatal Med. (2017) 30:1092–5. doi: 10.1080/14767058.2016.1205019
35. Gaudier FL, Goldenberg RL, Nelson KG, Peralta-Carcelen M, DuBard MB, Hauth JC. Influence of acid-base status at birth and Apgar Scores on survival 500–1000 g infants. Obstet Gynecol. (1996) 87:175–80. doi: 10.1016/0029-7844(95)00407-6
Keywords: platelet parameters, mean platelet volume, premature neonates, plateletcrit, mortality
Citation: Go H, Ohto H, Nollet KE, Takano S, Kashiwabara N, Chishiki M, Maeda H, Imamura T, Kawasaki Y, Momoi N and Hosoya M (2020) Using Platelet Parameters to Anticipate Morbidity and Mortality Among Preterm Neonates: A Retrospective Study. Front. Pediatr. 8:90. doi: 10.3389/fped.2020.00090
Received: 08 November 2019; Accepted: 21 February 2020;
Published: 13 March 2020.
Edited by:
Arjan Te Pas, Leiden University, NetherlandsReviewed by:
Satoshi Kusuda, Tokyo Women's Medical University, JapanRonny Knol, Erasmus Medical Center, Netherlands
Copyright © 2020 Go, Ohto, Nollet, Takano, Kashiwabara, Chishiki, Maeda, Imamura, Kawasaki, Momoi and Hosoya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hayato Go, Z29oYXlhdG8yNTI1QGdtYWlsLmNvbQ==
 Hayato Go
Hayato Go Hitoshi Ohto2
Hitoshi Ohto2 Shunya Takano
Shunya Takano Nozomi Kashiwabara
Nozomi Kashiwabara Takashi Imamura
Takashi Imamura