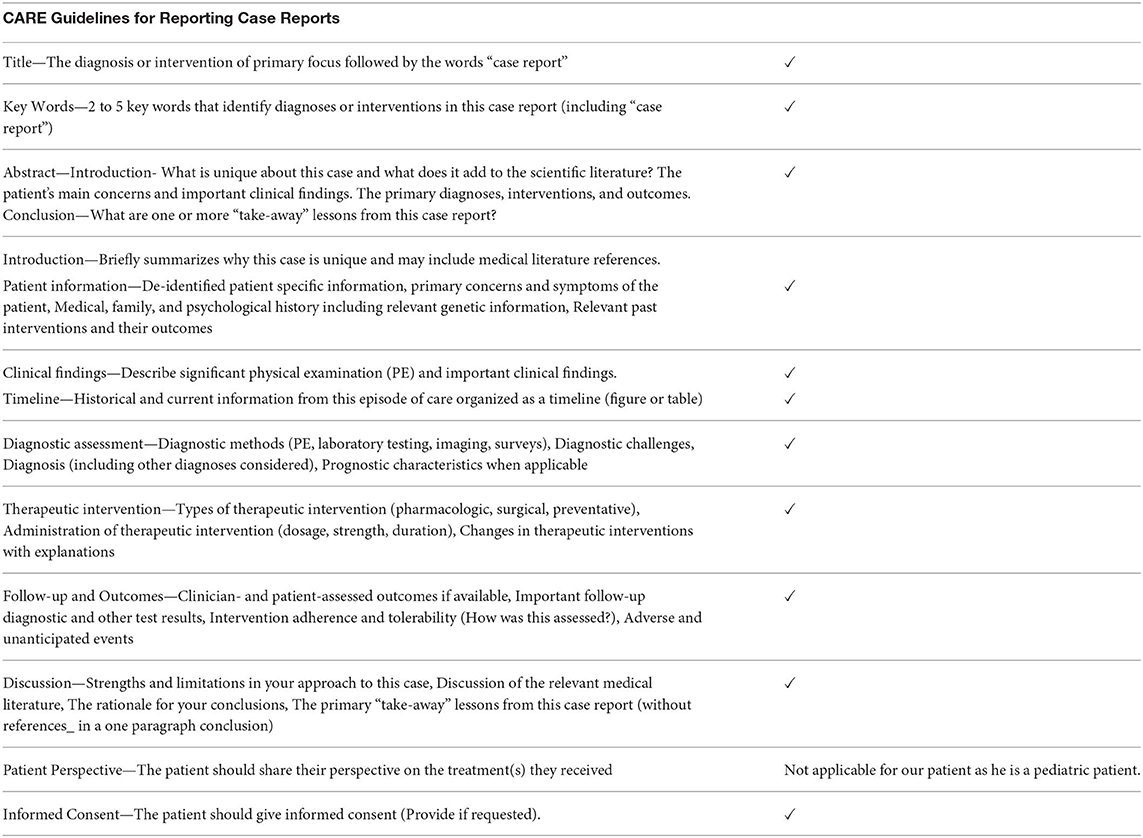
- 1University of Minnesota Medical Center, Minneapolis, MN, United States
- 2Division of Pediatric Hematology & Oncology, University of Minnesota, Minneapolis, MN, United States
- 3MHealth Fairview Pathology Service Line, Minneapolis, MN, United States
- 4Division of Pediatric Rheumatology, University of Minnesota, Minneapolis, MN, United States
Henoch-Schönlein purpura (HSP) is a common systemic vasculitis affecting children. It is managed in the outpatient setting and rarely associated with malignancy. We present a case of neuroblastoma in a 7-year-old boy diagnosed after suspected HSP. Our case highlights the importance of maintaining a broad differential diagnosis in children with atypical HSP and performing a skin biopsy with immunofluorescence when a rash is present.
Introduction
Henoch-Schönlein purpura (HSP) is the most common vasculitis affecting children (1). It is an immunoglobulin (Ig)A-mediated leukocytoclastic vasculitis in which IgA-containing immune complexes deposit in the small vessels of the skin, gastrointestinal tract, and kidneys. HSP is more common in children than adults and although the cause is not clear, it has been associated with preceding infection—especially group A streptococcal infections, staphylococcal infections, and parainfluenza (2). Several solid tumors and hematologic malignancies have been reported to induce or be associated with adult-onset HSP, but the association is rare and poorly understood (3–7). Malignancy is even less commonly associated with childhood-onset HSP (8–10). Here we present a case of a young boy who presented with clinical features of HSP who was ultimately diagnosed with neuroblastoma.
Case Report
A 7-year-old boy with a history of a port wine stain and dysfunctional elimination syndrome presented to his local medical center with a 3-day history of an erythematous, non-blanching rash on the legs, arthralgias involving the lower extremities, and pain in his abdomen, groin, and scrotum. At this visit, it was noted that he had a temperature of 100.4°F, face, penis, and scrotum swelling, and scattered, non-blanching purpuric lesions on the lower legs and groin. Urinalysis done at this visit showed positive protein (not quantified), 0–5 white blood cells (WBC), and 0–2 red blood cells (RBC). Other lab results were normal as follows: creatinine (Cr) 0.42 mg/dl, WBC 7.0 10 e9/L with a normal differential, hemoglobin (hgb) 10.6 g/dl, mean corpuscular volume (MCV) 82 fl, and platelets (plts) 249 10 e9/L. Based upon these clinical findings, he was diagnosed with HSP and instructed to follow up with his primary care provider (PCP). Supportive measures and reassurance were provided. Two weeks later, he continued to experience worsening arthralgias and reported intermittent fevers, but he had some improvement in his rash. The PCP prescribed a 3-week tapered dose of prednisolone starting at 10 mg per day, and he began showing signs of improvement. However, during his third week of treatment with prednisolone, he again presented to the emergency department with new and worsening symptoms including progressive fatigue, persistent fever with temperature up to 102°F, and intermittent joint pain, with episodes of vomiting and epistaxis. Repeat labs showed a mild normocytic anemia (hgb 10 g/dL) and elevated C-reactive protein (CRP) of 7.33 mg/dL (normal < 0.5 mg/dL).
A few days later, ~2 months after the initial onset of his symptoms, the patient was evaluated by pediatric rheumatology for his ongoing symptoms. He had recently completed the 3-week tapered course of prednisolone. His rash and abdominal pain had resolved. On exam, he appeared significantly fatigued, grimaced with range of motion of the neck and shoulders, and cringed when his hips were abducted. No joint effusions were noted. A diagnosis of recalcitrant HSP was considered, along with the possibility of another systemic vasculitis, inflammatory bowel disease, systemic juvenile idiopathic arthritis, macrophage activation syndrome, and malignancy. Further labs were obtained and results included the following: CRP 7.1 mg/dL (normal < 0.5 mg/dL), erythrocyte sedimentation rate (ESR) 78 mm/h, elevated lactate dehydrogenase (LDH) (345 U/L), worsening microcytic anemia (hgb 8.7 g/dl), hypoalbuminemia (3.0 g/dL), a normal uric acid (2.2 mg/dL), and an overall normal urinalysis (UA) without hematuria but with protein 10 mg/dl, 6 WBC, and 1 RBC. A peripheral blood smear, performed to rule out an oncologic disorder, demonstrated a mild leukoerythroblastic reaction, suggesting a bone marrow infiltration process (Figure 1). The patient was subsequently referred to pediatric oncology for further evaluation.
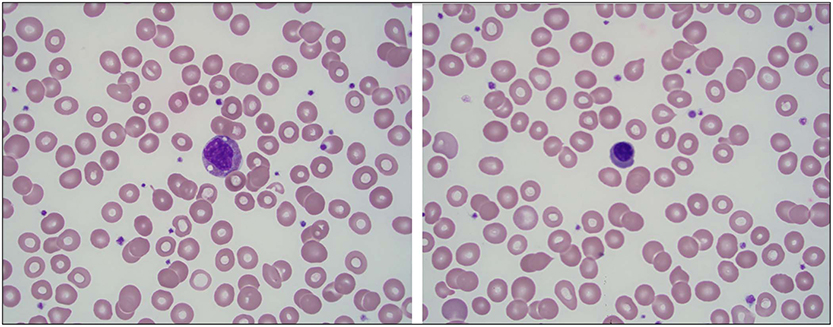
Figure 1. Mild leukoerythroblastic reaction seen on peripheral blood smear with slightly left-shifted granulocyte series and rare circulating nucleated red blood cells. (A) A granulocyte precursor is seen at the center of the field (100×). (B) A nucleated red blood cell is seen at the center of the field (100×).
A unilateral bone marrow biopsy showed extensive involvement by a non-hematopoietic malignancy with features suggestive of neuroblastoma. Follow-up computerized tomography (CT) imaging revealed a heterogeneous, partially calcified tumor in the right hepatorenal recess with widespread metastatic disease in the axial spine without spinal canal infiltration. A biopsy of the primary tumor identified poorly differentiated neuroblastoma, n-myc non-amplified. Urine catecholamines were elevated. A metaiodobenzylguanidine (MIBG) scan demonstrated diffuse uptake in the primary tumor and diffuse osseous metastatic disease. Given the presence of metastatic disease, he was classified as having International Neuroblastoma Risk Group (INRG) Stage M disease (International Neuroblastoma Staging System Stage 4). He completed five cycles of chemotherapy followed by tandem autologous hematopoietic stem cell transplantation, radiation and immunotherapy as per Children's Oncology Group (COG) standard of care for high-risk neuroblastoma therapy (11). His end of therapy evaluations revealed no evidence of disease and he remains in remission. A summary of his clinical course can be seen in Figure 2.
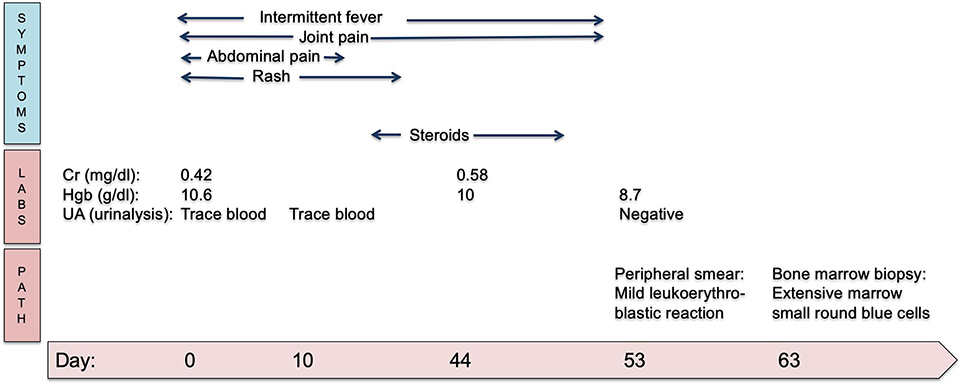
Figure 2. Timeline in days of clinical symptoms of HSP and eventual progression to neuroblastoma diagnosis. Cr, creatinine; Hgb, hemoglobin; UA, urinalysis.
Discussion
This case report (see Appendix) highlights an example of a patient with HSP who was later diagnosed with neuroblastoma. The phenomenon of HSP associated with malignancy has been described in the literature but typically in older males with a median age of 60 (6, 12). This patient presented with classic features of HSP including lower extremity purpuric rash, abdominal pain and arthralgias, and scrotal swelling (1). However, he also had atypical features that prompted further evaluation for an underlying disease. These atypical features included recurrent fever, severe limb pain out of proportion to that typically seen in HSP and without arthritis, a high degree of systemic inflammation, and long length of course.
HSP is the most common type of vasculitis in childhood and is often managed by general pediatricians (13). HSP is diagnosed based upon clinical features of palpable purpura or petechiae with lower limb predominance and at least one of the following: abdominal pain, typical skin or renal histopathology, arthritis or arthralgia, and renal involvement (14). Although this classification criteria is highly sensitive (100%) and specific (87%), it is important for providers to maintain a differential diagnosis in patients presenting with these features (14). Our patient met diagnostic criteria with purpuric rash, abdominal pain, and arthralgia. Additionally, he presented with transient scrotal swelling, a recognized manifestation of HSP although not pathognomonic (14). The combination of severe limb pain, fevers, and systemic inflammation in a child should prompt consideration of hematologic malignancy (15). Neuroblastoma is a tumor of primitive neuroblasts of the embryonic neural crest, occurring anywhere along the sympathetic nervous system with a primary site often in the adrenal medulla. Rare cases of metastatic cutaneous lesions of neuroblastoma are noted in the literature and are typically associated with neonatal or infantile neuroblastoma (16). Symptoms of neuroblastoma may include abdominal pain, vomiting, constipation, nausea, and bone pain among others. In this patient's case, his skin eruption, abdominal pain and arthralgia may have been solely caused by the neuroblastoma.
HSP is not traditionally felt to be a paraneoplastic syndrome of neuroblastoma and to our knowledge there is only a single published case report describing an incidental finding of neuroblastoma on CT scan for abdominal pain in a 5-year-old child diagnosed and treated for HSP (10). While both this patient and our patient presented similarly, our patient's rash resolved prior to evaluation by pediatric oncology and diagnosis of neuroblastoma. Our patient also had additional concerning symptoms such as intermittent fever and limb pain that were felt to be atypical for HSP.
Although the mechanism is not completely understood, there is concern that neoplastic antigens could lead to the formation of immune complexes, which induce the lesions of HSP (3). Podjasek et al. noted, in a patient cohort of 15 individuals with cutaneous small-vessel vasculitis secondary to solid-organ malignancy, the absence of papillary dermal inflammation and edema, lymphocytes and plasma cells on histopathology was found to be a statistically significant finding (4). This patient did not have a skin biopsy at the time of diagnosis of HSP, however, the features of this patient's rash, along with these other clinical features, were concerning for HSP. Nevertheless, a skin biopsy could have potentially distinguished a classic HSP, IgA-mediated leukocytoclastic vasculitis from another malignancy-induced small vessel vasculitis.
The association between malignancy and vasculitis in this patient remains unclear—the HSP may have developed independently, as a paraneoplastic syndrome, or as a manifestation of his metastatic disease. A limitation of this case, however, is that the authors of this case did not see the rash firsthand. By the time the patient presented to the pediatric rheumatology clinic, the rash had resolved. The classification criteria for HSP requires that a patient have palpable purpura or petechiae predominant in the lower extremities. We trust that this patient's local provider accurately assessed the rash as non-blanching and purpuric. In this case, the purpuric rash was suggestive of HSP. Unfortunately, a photo was not available for inclusion in this case report.
Although HSP is generally self-limited and managed in the primary care setting, patients who meet the diagnostic criteria for HSP should still be assessed closely for atypical findings such as severe joint pain or limb pain, persistent fevers, and significantly elevated inflammatory markers. Additionally, early universal treatment with corticosteroids has not been shown to prevent renal or gastrointestinal complications of HSP and thus should be weighed against the potential to mask signs of acute infection or malignancy (17). This case highlights the importance of maintaining a broad differential in the setting of recalcitrant HSP. Providers should utilize laboratory markers and peripheral smear findings to guide the diagnostic work-up, and exercise caution when prescribing steroids for atypical presentations of vasculitis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
Written informed consent was obtained from the parents for publication of this case report.
Author Contributions
ZA, AF, EG, KS, and CC conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
COG, Children's Oncology Group; Cr, creatinine; F, Fahrenheit; WBC, white blood cell; N, neutrophil; L, lymphocyte; M, monocyte; E, eosinophil; Hgb, hemoglobin; MCV, mean corpuscular volume; Plts, platelets; INR, international normalized ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; MIBG, metaiodobenzylguanidine; PCP, primary care provider; UA, urinalysis.
References
1. Gedalia A. Henoch-Schönlein purpura. Curr Rheumatol Rep. (2004) 6:195–202. doi: 10.1007/s11926-004-0068-2
2. Weiss PF, Klink AJ, Luan X, Feudtner C. Temporal association of Streptococcus, Staphylococcus, and parainfluenza pediatric hospitalizations and hospitalized cases of Henoch-Schönlein purpura. J Rheumatol. (2010) 37:2587–94. doi: 10.3899/jrheum.100364
3. Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein Purpura associated with malignancy. J Eur Acad Dermatol Venereol. (2009) 23:394–401. doi: 10.1111/j.1468-3083.2008.03065.x
4. Podjasek JO, Wetter DA, Pittelkow MR, Wada DA. Henoch-Schönlein purpura associated with solid-organ malignancies: three case reports and a literature review. Acta Derm Venereol. (2012) 92:388–92. doi: 10.2340/00015555-1288
5. Fox MC, Carter S, Khouri IF, Giralt SA, Prieto VG, Nash JW, et al. Adult Henoch-Schönlein purpura in a patient with myelodysplastic syndrome and a history of follicular lymphoma. Cutis. (2008) 81:131–7.
6. Pertuiset E, Lioté F, Launay-Russ E, Kemiche F, Cerf-Payrastre I, Chesneau AM. Adult Henoch-Schönlein purpura associated with malignancy. Semin Arthritis Rheum. (2000) 29:360–7. doi: 10.1053/sarh.2000.6988
7. Fain O, Hamidou M, Cacoub P, Godeau B, Wechsler B, Pariès J, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. (2007) 57:1473–80. doi: 10.1002/art.23085
8. Funato M, Kaneko H, Kubota K, Ozeki M, Kanda K, Orii K, et al. Pediatric acute lymphoblastic leukemia mimicking Henoch-Schönlein purpura. Pediatr Int. (2011) 53:766–8. doi: 10.1111/j.1442-200X.2011.03445.x
9. Sivak LE, Virshup DM. Occurrence of Henoch-Schönlein purpura in a child with Wilms' tumor. Med Pediatr Oncol. (1995) 24:213–4. doi: 10.1002/mpo.2950240314
10. Dong Q, Cao S, Zhang H, Geng H. Henoch-Schönlein purpura associated with a neuroblastoma: report of one case and a review of the literature. Intractable Rare Dis Res. (2012) 1:167–9. doi: 10.5582/irdr.2012.v1.4.167
11. Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA. (2019) 322:746–55. doi: 10.1001/jama.2019.11642
12. Zurada JM, Ward KM, Grossman ME. Henoch-Schönlein purpura associated with malignancy in adults. J. Am. Acad. Dermatol. (2006) 55 (Suppl. 5):S65–70. doi: 10.1016/j.jaad.2005.10.011
13. Oni L, Sampath S. Childhood IgA vasculitis. (Henoch Schonlein purpura)-advances and knowledge gaps. Front Pediatr. (2019) 7:257. doi: 10.3389/fped.2019.00257
14. Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria Ann Rheum Dis. (2010) 69:798–806. doi: 10.1136/ard.2009.116657
15. Clarke RT, Van den Bruel A, Bankhead C, Mitchell CD, Phillips B, Thompson MJ. Clinical presentation of childhood leukaemia: a systematic review and meta-analysis. Arch. Dis. Child. (2016) 101:894–901. doi: 10.1136/archdischild-2016-311251
16. Mondì V, Piersigilli F, Salvatori G, Auriti C. The skin as an early expression of malignancies in the neonatal age: a review of the literature and a case series. Biomed Res Int. (2015) 2015:809406. doi: 10.1155/2015/809406
17. Bluman J, Goldman RD. Henoch-Schönlein purpura in children: limited benefit of corticosteroids. Can Fam Physician. (2014) 60:1007–10.
Appendix
Keywords: neuroblastoma, Henoch-Schönlein purpura, rash, pediatrics, case report
Citation: Alfath Z, Ferdjallah A, Greengard E, Askari SK, Sadak KT and Correll CK (2020) Henoch-Schönlein Purpura Presenting in Association With Neuroblastoma: A Case Report. Front. Pediatr. 8:77. doi: 10.3389/fped.2020.00077
Received: 05 November 2019; Accepted: 17 February 2020;
Published: 28 February 2020.
Edited by:
Jérémie F. Cohen, Necker-Enfants Malades Hospital, FranceReviewed by:
Maryam Piram, CHU de Bicêtre, FranceJoseph Lam, University of British Columbia, Canada
Copyright © 2020 Alfath, Ferdjallah, Greengard, Askari, Sadak and Correll. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asmaa Ferdjallah, YXNtYWFAdW1uLmVkdQ==
 Zineb Alfath
Zineb Alfath Asmaa Ferdjallah
Asmaa Ferdjallah Emily Greengard2
Emily Greengard2 Karim T. Sadak
Karim T. Sadak Colleen K. Correll
Colleen K. Correll