- 1Department of Gastroenterology, Hepatology, and Nutrition, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai, China
- 2Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China
- 3Ruili Maternal and Child Care Service Center, Ruili, China
Heiner syndrome (HS) is a food hypersensitivity disease that is mostly caused by cow's milk. The main features may include chronic or recurrent respiratory syndromes, pulmonary infiltrates on radiography, and even pulmonary hemosiderosis. However, gastrointestinal symptoms are rare in HS, which can lead to a misdiagnosis when the chief complaint is about the gastrointestinal system. Here, we report a case of HS complicated by severe hematochezia.
Introduction
Since the discovery of a pulmonary syndrome induced by cow's milk, only a few cases have been published. Heiner syndrome (HS) is caused by cow's milk, first described by Heiner et al. (1) in seven infants in 1962. HS can be resolved by removing cow's milk from the diet, and its manifestations include chronic respiratory symptoms, infiltrates on chest roentgenograph, failure to thrive, pulmonary hemosiderosis, and even iron deficiency anemia (2, 3). However, few gastrointestinal manifestations associated with HS have been reported in the available literature so far (4). Here, we report a case, whose chief presenting symptom was severe hematochezia instead of respiratory symptoms, which is rarely reported in cases of HS.
Case Report
A 4-month-old girl was referred to our hospital with continuous hematochezia since 10 days after birth, with hematochezia showing signs of aggravation 4 days before being examined. She was born full term (G1P1) without complications and was fed with the mother's milk and cow's milk. Physical examination showed a height of 59 cm, a weight of 4.25 kg, a fever at 38.1°C, and respiratory rates 70 times/min with rough breath sounds. The skin was pale and showed no rashes. Tonsils were not hypertrophic nor inflamed.
Laboratory studies showed the following: white blood cells (WBC), 16.31 (109 cells/L) (normal, 8–12); neutrophil, 31%, lymphocytes, 51%, and monocytes, 13% (normal: 3–8); eosinophils, 6% (normal: 1–5); C-reactive protein, 16 mg/L (normal: 0–5), red blood cells, 2.44 (1012 cells/L) (normal: 4–5.5); and hemoglobin, 70 g/L (normal: 110–160). Immunoglobulin E (IgE) antibodies to cow's milk is negative, and serum total IgE level was 35.5 IU/mL (normal: < 15). In addition, fecal occult blood was positive. The concentration of iron element in serum was 4.36 mmol/L (normal: 7.52–11.82). The PO2 in peripheral blood was 59 mmHg (<60), and PCO2 was normal, which indicated type I respiratory failure. Chest roentgenograms showed bilateral infiltrates and opacities in different lung lobes (Figure 1a). The CT scan showed interstitial lung disease (Figure 1b), and the oxyhemoglobin saturation (SpO2) could not be maintained without oxygen intake (the lowest SpO2 was 80%).
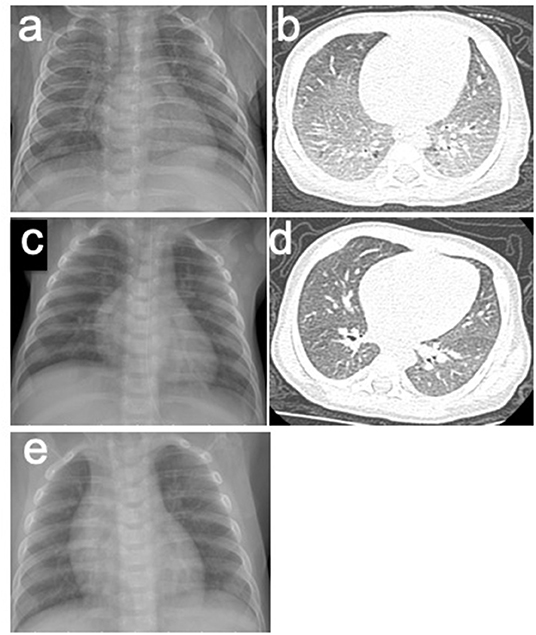
Figure 1. (a,b) X-rays and CT scans of the patient before the treatment of HS. (a) Chest roentgenogram showed bilateral infiltrates and opacities in different lung lobes. (b) Chest CT scan showed interstitial lung disease. (c,d) X-rays and CT scans of the patient after the treatment of HS. (c) Chest roentgenogram showed that pulmonary infiltrates cleared. (d) Chest CT scan was normal. (e) Chest roentgenograms in regular follow-up visit showed no infiltrates and opacities.
The patient was suspicious of HS due to the chronic pulmonary syndrome and the possible history of cow's milk allergy. A low dose of methylprednisolone (1 mg/kg) and montelukast sodium were prescribed. Milk was eliminated from the diet, and amino acid formula was prescribed. Shortly after this, dyspnea significantly improved, and eosinophils decreased to 1%. Chest roentgenograms showed that pulmonary infiltrates cleared within 5 days (Figure 1c), and the CT scan was normal (Figure 1d). In addition, hematochezia was relieved, and hemoglobin was increased to 80 g/L. The diagnosis was further confirmed based on the positive response to the treatment and the elimination of milk. She was subjected to gastrointestinal endoscope, revealing chronic superficial gastritis (Figure 2a) and hyperplasia in the descending colon (Figure 2b). We did an endoscopic protractor biopsy on the hyperplasia. The examination showed granulation tissue infiltrated by acute and chronic inflammatory cells, including some eosinophils (at most three per high-power field) (Figure 2c). In addition, the iron-laden macrophages test in sputum or fasting gastric fluid was negative. She was discharged with low-dose methylprednisolone (4 mg qd), with the dose of methylprednisolone decreased every 2 weeks (4 mg qd→ 4 mg/2 mg qd→ 2 mg qd→ 2 mg qod).
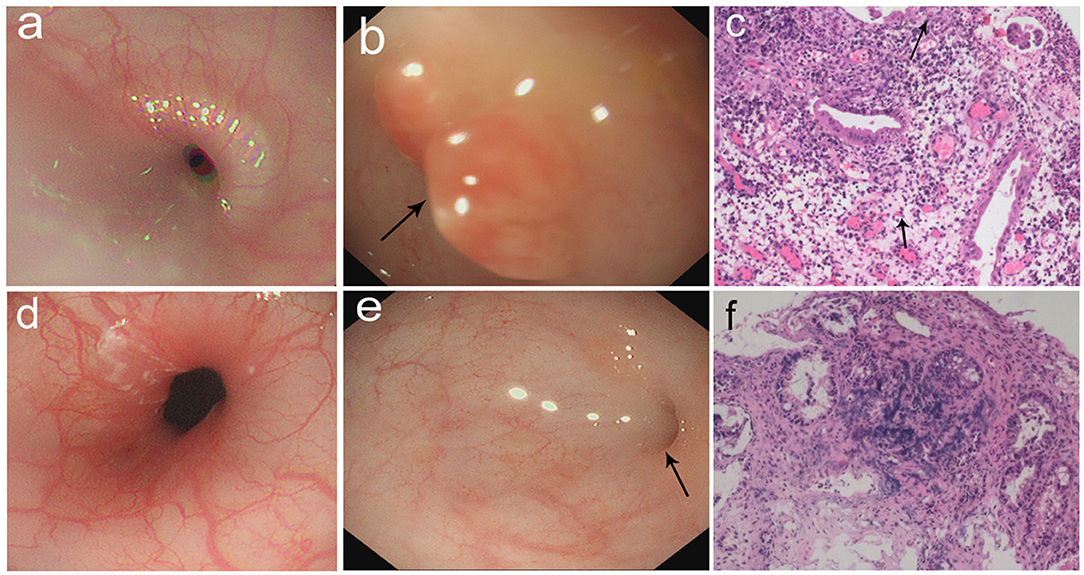
Figure 2. (a–c) Gastrointestinal endoscopic pictures and the pathological sections. (a) Gastroscopic pictures showed chronic superficial gastritis. (b) Enteroscopic pictures showed a hyperplasia in descending colon (arrow: hyperplasia). (c) The section of hyperplasia showed granulation tissue infiltrated mostly by acute and chronic inflammatory cells (arrow: eosinophils). (100×) (d–f) Gastrointestinal endoscopic pictures and the pathological sections in follow-up visit. (d) Gastroscopic pictures showed normal results. (e) Enteroscopic pictures showed inflammatory hyperplasia (arrow: hyperplasia). (f) The section of hyperplasia showed tissue infiltrated by inflammatory cells. (100×).
After 2 months, she came back for a regular follow-up. At this time, physical examination showed weight of 6.9 kg, no fever, and respiratory rates 26 times/min. Lungs were clear to auscultation. Skin was not pale and showed no rashes. Tonsils were not hypertrophic and not inflamed. SpO2 is more than 98%. Laboratory studies showed that WBC, C-reactive protein, red blood cells, and hemoglobin are all in a normal range. In addition, hematochezia disappeared, and fecal occult blood was negative. Chest roentgenograms showed neither infiltrates nor opacities (Figure 1e). Besides this, the gastroscope showed normal results (Figure 2d), and enteroscope showed inflammatory hyperplasia (Figure 2e). The light microscopic examination of the biopsy showed tissue infiltration by inflammatory cells (Figure 2f). In addition, the enteroscope examination and biopsy also showed that there was lymphonodular hyperplasia, which is uncommon in HS. It may be induced by the delayed-type food hypersensitivity (5).
Discussion
Our patient mimicked a clinical picture of bacillary dysentery or inflammatory bowel disease with symptoms including hematochezia, diarrhea, and elevated WBC and C-reactive protein. The stool routine test had no significance besides being positive for fecal occult blood, and stool bacterial culture showed no pathogens such as Shigella, Salmonella, Vibrio cholerae, or pathogenic Escherichia coli. Results of tests and the pulmonary symptoms were not concordant with infection for this age group. In addition, the gastrointestinal endoscopic pictures showed non-specific inflammation, and histological sections showed infiltration of acute and chronic inflammatory cells, and some eosinophils, which indicated allergic proctocolitis. However, only allergic proctocolitis itself cannot account for the pulmonary symptoms in this patient.
The hematochezia after cow's milk diet, the increase in eosinophils, and the anemia indicated an allergy to cow's milk [cow's milk protein allergy (CMPA)]. In addition, the dramatic radiological and clinical improvements of pulmonary and gastrointestinal symptoms after the elimination of cow's milk and the treatment of methylprednisolone supported HS. It is noticeable that this patient had the chief complaint as severe hematochezia instead of respiratory symptoms, which is rarely reported in cases of HS. We also found that there was hyperplasia in intestinal mucosa under enteroscope and lymphonodular hyperplasia in biopsy, which are also rare in HS. It was reported that some people react to some food as though they were pathogens, which can cause hyperplasia with chronic inflammation in the mucosa (6). About the possible mechanisms, mast cells may be stimulated by the antigen–antibody complex and produce cytokines like interleukin-3 and interleukin-5, which promote allergy into a last phase (6, 7), and uncontrolled activated T-cell-mediated process also plays a significant role in hyperplasia and mucosal lesion (6, 8, 9). Lymphonodular hyperplasia can also be a potential source of rectal bleeding of infants (6, 10), and infants with severe rectal bleeding should be undergo an enteroscope examination and biopsy. If lymphonodular hyperplasia is found, delayed-type food allergy should be considered.
The precise mechanism which is responsible for HS is still poorly understood (11). It now seems that HS contains both IgE and non-IgE-mediated allergic responses (12). IgE-mediated reactions can develop when cow's milk-specific IgE antibodies residing on mast cells or basophils come into contact with and bind to the circulating food allergens, such as milk proteins, and then activate immune cells to release allergy mediators such as histamine and cytokines (13). IgE-mediated reactions are characterized by a rapid onset, which usually involves the formation of rashes, urticaria, and oral allergy symptoms. However, in non-IgE-mediated food allergic reactions, multiple inflammatory cells, such as macrophages or lymphocytes, play a significant role in the process (14–16). In addition, lymphonodular hyperplasia, regarded as a histological characteristic of mucosal immune response in the process, may also happen (17–19). In non-IgE-mediated food allergy, disorders in the body become evident after hours or days and predominantly manifest in the gastrointestinal tract, as opposed to the skin. Non-IgE-mediated gastrointestinal allergic disorders contain many diseases, such as enteropathy, enterocolitis, and proctocolitis. The combination of IgE and non-IgE-mediated allergic reactions have been established in the pathogenesis of some diseases, such as eosinophilic esophagitis and gastroenteritis. The exact roles of IgE and non-IgE-mediated allergies during the process of HS needs further investigation as well as specific confirmatory tests aimed to diagnose HS beyond clinical symptoms (3).
Conclusion
To summarize, HS should be worth considering in any young child who has an unexplained chronic pulmonary infiltrate, respiratory failure, and an evidence of CMPA, even though the chief complaint of the patient is hematochezia and not focused on the respiratory system. This allergy may affect many systems, so extending the focus beyond the chief complaint is imperative. The diagnosis would be supported by dramatic radiological and clinical improvements after the elimination of cow's milk and the treatment of methylprednisolone. Although HS is rare in the pediatric population, this syndrome should be suspected in those who have pediatric pulmonary syndromes and CMPA. Therefore, insufficient awareness about HS is probably a major reason for its misdiagnosis.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
This study was reviewed and approved by the Ethical Review Commitment of Shanghai Children's Hospital.
Author Contributions
X-YL drafted the manuscript. TZ revised the manuscript. X-YL, X-RH, J-WZ, and Y-MX collected data and pictures. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81870373), the Shanghai Hospital Development Center New Frontier Technology Joint Research Project (Grant No. SHDC12017115), and the Shanghai Municipal Commission of Health and Family Planning Key Project (Grant No. 2017ZZ02019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Heiner DC, Sears JW, Kniker WT. Multiple precipitins to cow's milk in chronic respiratory disease. A syndrome including poor growth, gastrointestinal symptoms, evidence of allergy, iron deficiency anemia, and pulmonary hemosiderosis. Am J Dis Child. (1962) 103:634–54. doi: 10.1001/archpedi.1962.02080020649003
2. Sim J, Kim H, Lee H, Ahn K, Lee SI. Etiology of hemoptysis in children: a single institutional series of 40 cases. Allergy Asthma Immunol Res. (2009)1:41–4. doi: 10.4168/aair.2009.1.1.41
3. Yavuz S, Karabay-Bayazit A, Yilmaz M, Gonlusen G, Anarat A. Crescentic glomerulonephritis in a child with Heiner syndrome. Turk J Pediatr. (2014) 56:661–4.
4. Moissidis I, Chaidaroon D, Vichyanond P, Bahna SL. Milk-induced pulmonary disease in infants (Heiner syndrome). Pediatr Allergy Immunol. (2005) 16:545–52. doi: 10.1111/j.1399-3038.2005.00291.x
5. Iacono G, Ravelli A, Di Prima L, Scalici C, Bolognini S, Chiappa S, et al. Colonic lymphoid nodular hyperplasia in children: relationship to food hypersensitivity. Clin Gastroenterol Hepatol. (2007) 5:361–6. doi: 10.1016/j.cgh.2006.12.010
6. Ohtsuka Y. Food intolerance and mucosal inflammation. Pediatr Int. (2015) 57:22–9. doi: 10.1111/ped.12546
7. Caffarelli C, Romanini E, Caruana P, Street ME, de' Angelis G. Clinical food hypersensitivity: the relevance of duodenal immunoglobulin E-positive cells. Pediatr Res. (1998) 44:485–90. doi: 10.1203/00006450-199810000-00004
8. MacDonald TT, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. (1988) 167:1341–9. doi: 10.1084/jem.167.4.1341
9. Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. (1997) 158:1582–90.
10. Kaplan B, Benson J, Rothstein F, Dahms B, Halpin T. Lymphonodular hyperplasia of the colon as a pathologic finding in children with lower gastrointestinal bleeding. J Pediatr Gastroenterol Nutr. (1984) 3:704–8. doi: 10.1097/00005176-198411000-00012
11. Sigua JA, Zacharisen M. Heiner syndrome mimicking an immune deficiency. WMJ. (2013) 112:215–7; quiz 8.
12. Stafford HA, Polmar SH, Boat TF. Immunologic studies in cow's milk-induced pulmonary hemosiderosis. Pediatr Res. (1977) 11:898–903. doi: 10.1203/00006450-197708000-00009
13. Chehade M. IgE and non-IgE-mediated food allergy: treatment in 2007. Curr Opin Allergy Clin Immunol. (2007) 7:264–8. doi: 10.1097/ACI.0b013e32814a5607
14. Ruffner MA, Spergel JM. Non-IgE-mediated food allergy syndromes. Ann Allergy Asthma Immunol. (2016) 117:452–4. doi: 10.1016/j.anai.2016.04.014
15. Nowak-Wegrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. (2015) 135:1114–24. doi: 10.1016/j.jaci.2015.03.025
16. Jyonouchi H. Non-IgE mediated food allergy - update of recent progress in mucosal immunity. Inflamm Allergy Drug Targets. (2012) 11:382–96. doi: 10.2174/187152812803250971
17. Bellanti JA, Zeligs BJ, Malka-Rais J, Sabra A. Abnormalities of Th1 function in non-IgE food allergy, celiac disease, and ileal lymphonodular hyperplasia: a new relationship? Ann Allergy Asthma Immunol. (2003) 90(6 Suppl. 3):84–9. doi: 10.1016/S1081-1206(10)61667-5
18. Kokkonen J, Karttunen TJ, Niinimaki A. Lymphonodular hyperplasia as a sign of food allergy in children. J Pediatr Gastroenterol Nutr. (1999) 29:57–62. doi: 10.1097/00005176-199907000-00015
Keywords: Heiner syndrome, hematochezia, cow's milk, food hypersensitivity disease, pulmonary infiltrates
Citation: Liu X-Y, Huang X-R, Zhang J-W, Xiao Y-M and Zhang T (2020) Hematochezia in a Child With Heiner Syndrome. Front. Pediatr. 7:551. doi: 10.3389/fped.2019.00551
Received: 10 September 2019; Accepted: 17 December 2019;
Published: 28 January 2020.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Matjaž Homan, University Medical Centre Ljubljana, SloveniaCorentin Babakissa, Université de Sherbrooke, Canada
Copyright © 2020 Liu, Huang, Zhang, Xiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Zhang, emhhbmd0QHNoY2hpbGRyZW4uY29tLmNu
 Xiang-Yu Liu1,2
Xiang-Yu Liu1,2 Yong-Mei Xiao
Yong-Mei Xiao Ting Zhang
Ting Zhang