- 1Children's Healthcare of Atlanta, Atlanta, GA, United States
- 2Department of Biology, University of Georgia, Athens, GA, United States
- 3Department of Biostatistics, Emory University, Atlanta, GA, United States
Purpose: Endoscopic dextranomer/hyaluronic acid (Dx/HA) injection is a common treatment for vesicoureteral reflux (VUR) with excellent reported short-term clinical success rates. Long-term outcomes are less well-defined. We assessed long-term outcomes and parental satisfaction after Dx/HA injection for primary VUR with >5-year follow-up.
Materials and Methods: Families of all patients who underwent Dx/HA injection for primary VUR at our institution between 2008 and 2012 were contacted for telephone interview. Data collected by phone included parental satisfaction and presence and severity of UTIs pre-operatively and post-operatively. Patient demographics, radiographic VUR data, need for secondary surgery, and surgical indications were obtained through chart review.
Results: Five hundred and seventy-five patients underwent Dx/HA injection for primary VUR between 2008 and 2012. Ninety-nine (17.2%) of these patients' parents were successfully contacted and interviewed. Median follow-up time from surgery to survey was 8.4 (IQR 6.8–9.6) years. Secondary surgery was performed in 13/99 (13.1%), most commonly repeat Dx/HA injection. Seven patients (7.1%) underwent secondary Dx/HA injection for persistent VUR without UTIs at a median of 0.35 (IQR 0.33–0.77) years post-operatively. Five patients (5.1%) underwent Dx/HA injection (n = 3) or ureteral reimplantation (n = 2) for VUR with febrile UTIs (fUTIs) at a median of 2.2 (IQR 1.3–5.1) years. One patient had ureteral reimplantation for symptomatic obstruction 2.8 years after initial surgery. Only 3/99 (3.0%) required open or laparoscopic surgery after Dx/HA injection. Eighty-three families (84.7%) reported ≥1 fUTIs pre-operatively. Of these, only 9/83 (10.8%) reported fUTIs post-operatively, for an overall clinical success rate of 89.2%. Clinical success was 93.1% in patients whose pre-operative fUTIs were treated outpatient and 80.0% in those hospitalized at least once for fUTI treatment pre-operatively. Ninety-four percent of parents were highly satisfied, 2.4% partially satisfied, and 3.5% dissatisfied.
Conclusions: Endoscopic injection with Dx/HA for primary VUR appears to have good long-term clinical success rates and high parental satisfaction, mirroring our previously reported short-term results. Post-operative ureteral obstruction is rare but may occur years post-operatively, justifying initial sonographic surveillance, and repeat imaging in symptomatic patients.
Introduction
Optimal VUR management remains controversial. While open ureteral reimplantation has a reported success of 96–98% in older studies (1, 2), the most recent larger series reported a 93.5% radiographic success rate defined as no post-operative VUR and a 95.9% clinical success rate defined as absence of post-operative fUTI (3).
Endoscopic injection with Dx/HA has variable reported radiographic cure rates of 67–93% (4–8), likely dependent on technique, surgeon experience, and patient factors. Lower cure rates are associated with high-grade reflux, duplicated systems, and neurogenic bladder dysfunction; higher resolution rates are seen in the absence of anatomic abnormalities or bladder/bowel dysfunction (BBD) (5). The hydrodistention implantation technique (HIT) injection method is associated with better outcomes than the older subureteric transurethral injection (STING) procedure, with several authors reporting radiographic success rates ≥80% (9–11). The Double HIT affords the highest success rates (8, 12), and has emerged as the most common injection technique in the United States (13).
Despite encouraging short-term results with Dx/HA (8), few studies with at least 5-year outcomes have been published, and these have used inconsistent measures of success (6, 14). Among patients who initially had no VUR after Dx/HA injection, 13–26% were seen to have recurrence on subsequent voiding cystourethrogram (VCUG) 1–5 years after surgery (9, 14). Recent reports of delayed ureteral obstruction after Dx/HA have raised questions about the need for long-term monitoring of patients following endoscopic VUR treatment (15–17).
In this study, we aim to characterize clinical outcomes and long-term satisfaction after Dx/HA injection for primary VUR at a high-volume pediatric hospital with ≥5-year follow-up. We hypothesize that the majority of patients will continue to be free of fUTIs, and their parents will be satisfied with the surgical outcome.
Materials and Methods
Patient Selection
After approval by the Institutional Review Board, patients between 0 and 18 years of age who underwent endoscopic Dx/HA injection by one of four experienced pediatric urologists at our institution between 2008 and 2012 were identified.
In order to characterize the outcomes of children with primary VUR, patients with secondary VUR or inadequately treated BBD were excluded. BBD was defined clinically as presence of prolonged urinary holding, urinary urgency/frequency, incontinence inappropriate for patient age/developmental stage, constipation, or fecal soiling. BBD has been shown to predispose a child to recurrent UTIs and affect surgical cure rates for VUR (18, 19). We attempted to contact the families using the last recorded phone number. Verbal consent for participation was obtained from a parent/legal guardian of minor children or from patients ≥18 years old at time of the survey.
Parental Survey
Survey questions included number and type of UTIs pre- and post-operatively, including fUTIs and whether fUTI treatment included hospitalization. Parents were asked whether the child ever had a “high fever” during any of their UTIs, as they often did not recall the exact maximum temperature after a post-operative interval of >5 years. Our primary outcome was clinical success, defined as no post-operative fUTIs in patients with ≥1 fUTI pre-operatively. Secondary outcomes included parental satisfaction and need for secondary surgery.
Parents were queried regarding presence of BBD symptoms before or after surgery. They were also asked about the total number of operations for VUR or post-operative obstruction. Parental satisfaction with surgical outcome was assessed on a 3-point scale as “satisfied,” “partially satisfied,” or “dissatisfied.”
Chart Review
Chart review of patient characteristics, surgical details, and post-operative care was performed. A fUTI was defined as symptomatic UTI with positive urine culture and fever ≥38°C (20). When this information was unavailable, the pediatric urologist's documentation of a fUTI was considered sufficient. A VCUG that was performed within 1 year after surgery was considered a “post-operative screening VCUG.” Radiographic cure was defined as the absence of VUR post-operatively. Radiographic improvement was defined as a decrease in maximum VUR by at least 2 grades or conversion from bilateral to unilateral VUR.
To assess whether families who completed the phone survey were representative of the entire cohort undergoing Dx/HA injection, an additional 121 patients who had surgery during the same time period but were unable to be contacted for the phone survey (comparison group) were randomly selected and compared to the survey group.
All patients were observed for at least 1 year prior to surgical intervention. All surgeons utilized the double HIT as previously described (7, 8, 13). Although only 61.9% of the patients had bilateral VUR demonstrated on pre-operative VCUG, 92.9% of patients were treated bilaterally. Prior studies have reported de novo contralateral reflux in 4.5–10.1% of patients treated unilaterally (21, 22). In our practice, orifices with hydrodistention grades 2 or 3 contralateral to a radiographically refluxing orifice are typically injected concurrently in order to minimize this risk. Post-operative studies were at the discretion of the surgeon, and practice patterns have changed over the study time period. Early in our Dx/HA experience, VCUGs were routinely obtained post-operatively; however, this is no longer the case. Currently, renal/bladder ultrasounds are routinely obtained at ~1 month post-operatively.
Statistical Analysis
For comparisons between groups, chi-square tests were used for categorical variables, and Wilcoxon rank-sum tests were used for continuous variables. All analyses were performed using SAS v. 9.4 (Cary, NC). P-values ≤ 0.05 were considered statistically significant.
Results
Demographics
Between 2008 and 2012, 701 patients underwent Dx/HA injection for VUR; 126 were excluded for non-primary VUR. Attempts were made to contact the remaining 575. In this group, 439 (76.3%) could not be contacted, 6 (1.0%) had a language barrier, 23 (4.0%) did not consent to participate, and 8 (1.4%) did not participate for other reasons. Families of 99 patients (83 females, 16 males) participated in the phone survey.
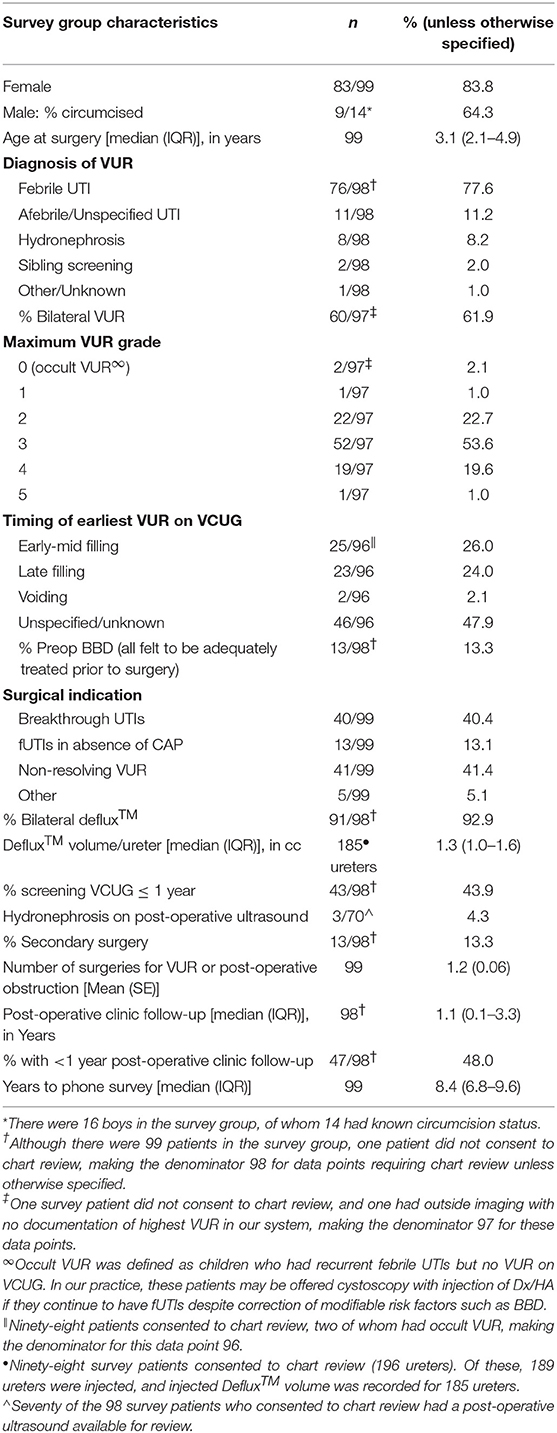
Survey patient characteristics are shown in Table 1. Median time from initial surgery to completion of the phone survey was 8.4 years (IQR 6.8–9.6). No differences were found between survey and comparison patients (Supplemental Table).
Compared to chart review findings, more parents reported during the phone survey that their child experienced pre-operative BBD (31.6 vs. 13.3%, p = 0.002). It is unclear whether this is due to recall bias, poor documentation in the medical record, or both. Of those who reported pre-operative BBD in their children, 17/31 (54.8%) reported recurrent/persistent BBD in the first post-operative year. Two of 68 (2.9%) had new-onset BBD in the first year after surgery. Ten patients with pre-operative BBD (32.2%) reported current BBD, and 1/68 (1.5%) without pre-operative BBD currently have BBD.
Thirteen patients (13.1%) underwent secondary surgery, with the majority undergoing repeat Dx/HA injection. Seven patients underwent secondary Dx/HA injection for asymptomatic radiographic failure at a median of 0.35 (IQR 0.33–0.77) years post-operatively, while five patients underwent Dx/HA injection (n = 3) or ureteral reimplantation (n = 2) for VUR with fUTIs at a median of 2.2 (IQR 1.3–5.1) years. One patient underwent ureteral reimplantation for symptomatic obstruction 2.8 years post-operatively. Only 3/99 (3.0%) underwent additional surgery other than repeat injection.
There was good concordance between the total number of surgeries reported on the phone survey and those recorded in the medical record. One parent stated that her child underwent additional surgeries at an outside institution and was considered concordant. Ninety four of 98 (95.9%) reported concordant number of surgeries, whereas five parents (5.1%) recalled one less surgery than recorded in the medical record.
There was also good concordance between types of UTIs reported before surgery. Of the 83 parents who reported ≥1 fUTI pre-operatively, 75 had ≥1 fUTI documented in the medical record. Of the remaining eight, five had a documented “non-febrile or unspecified UTI,” two had reflux nephropathy with scarring, and one did not consent for chart review. All six patients without pre-operative UTIs according to phone survey had been diagnosed with VUR upon workup for prenatal ultrasound or sibling screening.
Post-operative Urinary Tract Infections
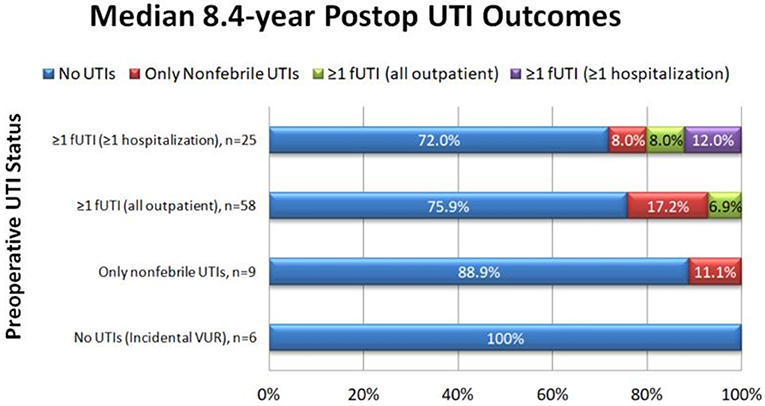
In the survey group, there was an 89.2% reduction in the number of patients with fUTIs pre-operatively compared to after Dx/HA injection (83/98 = 84.5% pre-operatively vs. 9/98 = 9.2% post-operatively, p < 0.001). Upon patient stratification by severity of pre-operative UTIs, similar results were seen (Figure 1). Only six of the nine patients who continued to have fUTIs after surgery had a post-operative VCUG, due to loss to follow-up or parental refusal of VCUG. Of Four of those patients (66.7%) had reflux on post-operative VCUG.
No patients without pre-operative fUTIs developed post-operative fUTIs. Of the patients with ≥1 fUTI who never required hospitalization for treatment before surgery, only 4/58 (6.9%) reported any fUTIs after surgery and none required hospitalization, for a 93.1% clinical success rate. Of patients who reported ≥1 hospitalization for treatment of fUTI pre-operatively, 5/25 (20.0%) reported any fUTIs after surgery, for an 80.0% clinical success rate. Three of these patients reported inpatient fUTI treatment after the initial surgery, two of whom went on to require multiple additional anti-reflux surgeries.
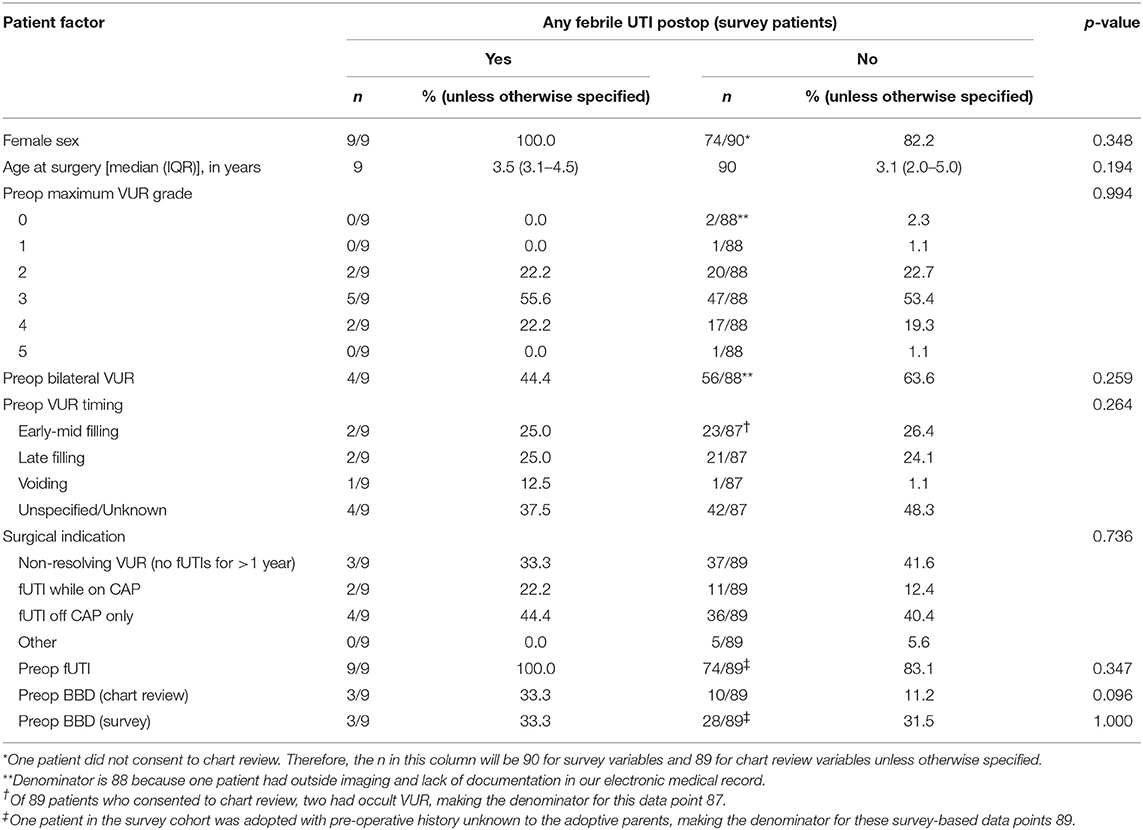
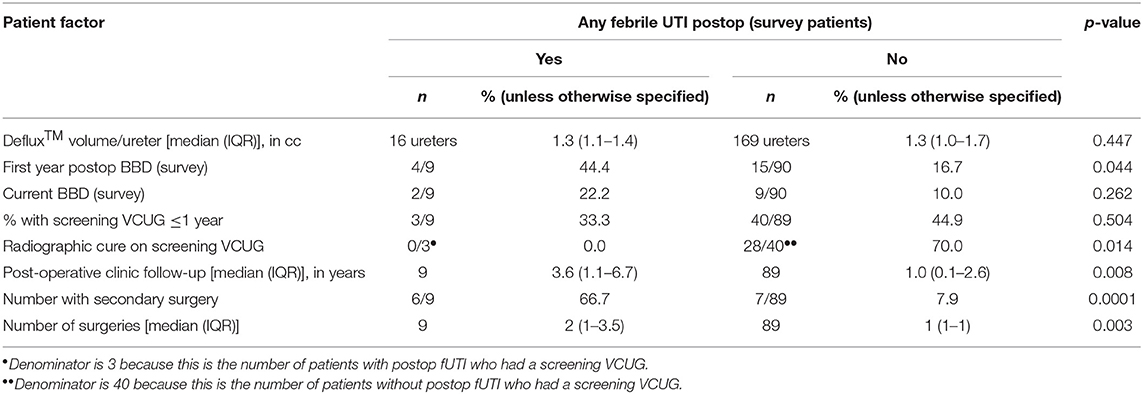
No pre-operative patient factors were associated with post-operative fUTIs (Table 2). Radiographic cure and presence of BBD in the first year after surgery were associated with clinical success (Table 3). Patients who reported ≥1 post-operative fUTI had longer follow-up (p = 0.008) and were more likely to undergo additional surgeries (p = 0.003).
Of the 83 survey patients who had ≥1 fUTI pre-operatively, 50 had a post-operative VCUG, with persistent VUR seen in 18 (36%). Seven patients underwent immediate reoperation for lack of radiographic cure, while 11 were observed. Of the 11 observed, 2 (18.2%) subsequently developed a fUTI and underwent repeat surgery, while 9 (81.8%) reported no UTIs (n = 7) or only non-febrile UTIs (n = 2) and were considered clinical successes.
Among the patients with radiographic cure, clinical success rate was 30/32 (93.8%). Nine patients demonstrated radiographic improvement, with a clinical success of 7/9 (77.8%). Nine patients with neither cure nor improvement on post-operative VCUG had a clinical success rate of 7/9 (77.8%). There was no significant difference in the clinical success rates between these three groups (p = 0.25), although interpretation is limited by small sample size.
Parental Satisfaction
Parental satisfaction data was available for 85 patients. Eighty (94.1%) survey respondents reported good satisfaction with the outcome. Parents of two patients (2.4%) with recurrent fUTIs after surgery reported partial satisfaction. Three families (3.5%) were dissatisfied with surgery due to persistent VUR requiring multiple operations (n = 1) or failure to cure VUR in patients with incidental diagnoses and no UTIs before or after surgery (n = 2).
Discussion
Since approval by the Food and Drug Administration in 2001, Dx/HA has become a widely used VUR treatment approach with short-term clinical success rates >90% in some series (4, 7, 8). From 2002 to 2004, the number of subureteral injections performed in the United States increased 288% while open ureteral reimplantation rates remained stable (23). Its minimally invasive nature and low incidence of post-operative bladder spasms, hematuria, emergency room visits, and readmissions favor Dx/HA, although open ureteral reimplantation is associated with higher initial success and fewer reoperations (24).
The American Academy of Pediatrics published clinical practice guidelines in 2011 for the diagnosis and management of initial febrile UTIs among children aged 2–24 months recommending VCUG only for children with an abnormal renal ultrasound or recurrent febrile UTIs (20, 25). The guidelines were reaffirmed in 2016 (21). Since 2011, the number of VCUGs and subsequent surgical interventions (open reimplantation and endoscopic injection) for VUR have decreased nationally (26). A recent survey of pediatric urologists revealed that more surgeons would perform Dx/HA injection (23.7%) rather than open surgery (19.8%) for primary VUR grade 3 or higher, although most respondents indicated “neither” or would take other factors such as renal scarring and parental preference into account before recommending a treatment modality (27).
When evaluating surgical outcomes, defining “success” is paramount, as the same data subjected to different outcome measures may yield widely divergent results (7, 28). Following Dx/HA injection, “success” has been variably defined as no VUR on post-operative VCUG (per patient or per ureter), ≥2-grade improvement, absence of dilating reflux, no need for additional surgery, no need for open surgery, or no fUTI, among others (7, 12, 14, 28, 29). We defined success clinically as no post-operative fUTIs. We found that parental recall of number of UTIs, fUTIs, and hospitalizations was not exact, but nearly all parents could remember whether the child had at least one occurrence of each outcome. Defining success radiographically is problematic in our cohort, since we do not routinely obtain post-operative VCUGs in patients who are doing well clinically. Only 60.2% of patients eligible for evaluation for clinical success (50/83) had a post-operative VCUG. Of those patients with a post-operative VCUG, 32 had no VUR, and an additional nine demonstrated radiographic improvement, while the remaining nine had neither cure nor improvement. A true radiographic success rate cannot be calculated in our cohort because we do not routinely obtain post-operative VCUGs on all patients. Yet the radiographic cure rate would be expected to be lower than the clinical success rate. Garcia-Aparicio et al. previously reported their clinical success rate (91.7%) to be higher than a strictly defined delayed radiological success rate (79.8%) in 215 ureters (28). We chose a clinical definition of success for our primary outcome because we believe that this is the most relevant definition to patients and families.
Regardless of the definition of success, Dx/HA outcomes have improved with evolution of injection technique from STING to HIT to Double HIT in the early 2000s. At our institution, patient success rates >90% had been achieved after 200 cases and were reported in 2008 (12). There is also an individual learning curve as the surgeon learns proper needle placement and injection technique in combination with visual and tactile cues (30).
Other studies have evaluated UTI outcomes with continuous antibiotic prophylaxis (CAP), endoscopic injection, and ureteral reimplantation. Since VUR resolves spontaneously in many children, CAP has been a mainstay of initial treatment to prevent fUTIs. A large meta-analysis of CAP in over 1,500 patients demonstrated a reduction in the risk of recurrent fUTIs among both high-grade (20.8 vs. 29.0%, p = 0.008) and low-grade (6.4 vs. 12.9%, p = 0.002) reflux compared to observation (31). This analysis included data from the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial, which showed a 50% relative risk reduction in UTIs with CAP over a 2-year period (32). Among children with BBD, the treatment effect was even higher (79% relative risk reduction). Within this well-designed randomized controlled trial with regular study coordinator contact, 76.9% of families reported adherence with CAP administration ≥75% of the time. A “real-world” analysis of VUR management in >35,000 patients revealed that while CAP was the initial therapy in 76.5%, only 17% were adherent, and 58% had a new UTI diagnosis within 1 year of starting prophylaxis (33). CAP decreased the incidence of UTIs; however, breakthrough UTIs were more likely to be resistant to the prophylactic antibiotic (34). Given these concerns, it is not surprising that Dx/HA injection has replaced CAP in many patients (23).
Endoscopic injection for VUR has been shown to reduce UTI rate even when not radiographically curative. Baek et al. reported an 80% reduction of fUTIs in patients with resolution of VUR and 74% reduction in patients with persistent VUR after Dx/HA (29). Dwyer et al. found a higher radiographic cure rate for reimplantation compared to Dx/HA, but no difference in post-operative fUTIs (8% after reimplantation, 4% after Dx/HA, p = 0.24) (35). Elmore et al. found a significantly lower incidence of fUTI after Dx/HA compared to open surgery (5 vs. 24%, p = 0.02) (36). In the current study, although persistent VUR on post-operative screening VCUG was associated with post-operative fUTIs, the majority (81.8%) of patients observed with persistent VUR after surgery reported no fUTIs post-operatively. Moreover, 4/41 patients deemed radiographically cured or improved developed fUTIs post-operatively (9.8%). There was no difference in fUTI rates between groups of radiographic cure, improvement, and failure.
Kaye et al. similarly reported that some radiographic failures were clinical successes and vice versa (7). In their study, 19 of 302 radiographic successes (6.3%) developed fUTIs, and conversely, six of 18 patients who developed an fUTI post-operatively (33.3%) showed no VUR on post-operative VCUG. Chi et al. found that following endoscopic treatment with Dx/HA, 22% of patients with a negative post-operative VCUG had a UTI, including a 10.5% incidence of fUTIs (37). Of the patients who underwent repeat VCUG, half showed recurrent VUR.
Rates of fUTI after endoscopic injection vary from 5 to 19% (6, 29, 36, 38). A recent long-term study by Friedmacher et al. reported a fUTI rate of 5.1% in patients with grades IV and V VUR, with most infections occurring in the first year after surgery (6). Post-operative fUTIs were more common in girls (7.5 vs. 1.2%, p < 0.001) and in patients with new or persistent BBD (36.1 vs. 3.7%, p < 0.001). Although gender did not reach statistical significance in our cohort, all patients with reported fUTIs after Dx/HA injection were female. Pre-operatively treated BBD did not appear to be a risk factor for post-operative fUTIs, and nearly half of children with BBD before surgery reported resolution of their symptoms after surgery. However, children with new or persistent BBD in the first year after surgery were more likely to experience a post-operative fUTI. Symptoms of BBD should therefore be assessed post-operatively, with ongoing behavioral management as appropriate.
Our reoperative rate was 13/99 (13.1%), with the majority (10/13) undergoing repeat endoscopic injection. Most of these patients underwent reinjection shortly after the first injection due to asymptomatic radiographic failure, reflecting practice patterns of 5–10 years ago. Now, these patients would be more likely to be observed rather than immediately reoperated on, as we have found that most radiographic failures still represent durable clinical successes when observed. Only 3/99 (3%) underwent reimplantation, two for persistent VUR with fUTI, and one for obstruction. Ureterovesical junction obstruction may occur after ureteral reimplantation or endoscopic injection, sometimes long after initial treatment. Among our survey and comparison patients, 2/220 (0.9%) experienced symptomatic ureteral obstruction 2.8 and 6.9 years after surgery. Treatment involved temporary ureteral stenting in one patient and ureteral reimplantation in the other. This is consistent with other series reporting post-operative ureteral obstruction rates of 0.5–1.05% after endoscopic injection (15, 17, 39). Patients in these series presented between 1 day and 49 months after surgery and were treated with ureteral stenting or reimplantation. High-grade VUR, obstructed/refluxing megaureter, inflamed urothelium, and secondary VUR have been associated with ureteral obstruction (17, 39). As shown here and elsewhere (16, 17), ureteral obstruction is a rare complication of endoscopic injection that may present years after surgery. A high level of suspicion is needed to appropriately evaluate and manage post-operative obstruction.
Strengths of our study include long-term follow-up and direct contact with families. Although the survey participants had similar baseline characteristics to the total cohort, it is possible that those who participated in the survey may have had different outcomes than those who did not participate. Limitations of the study include low response rate and reliance on parental recall for certain outcomes rather than objective data. Since many patients do not return for recommended clinic visits or are released from follow-up 1–2 years post-operatively if doing well, an analysis of patients who continue to be seen in the pediatric urology clinic years later would likely be skewed toward those with ongoing clinical concerns. Some adverse outcomes, including “silent” ureteral obstruction, may not have been captured in this study. Due to the long-term nature of this research, contemporary VUR patients may differ from those in the study. For example, lower risk VUR may not be diagnosed or treated as frequently now due to changing practice patterns. Decision-making among the surgeons studied has also changed over the years, especially regarding use of routine post-operative VCUG and observing patients with radiographic failure in lieu of immediate reoperation. We no longer obtain VCUGs post-operatively in the majority of patients. While persistent non-clinical VUR is no longer being diagnosed/treated, this algorithm has been proven safe and effective (40).
Conclusion
Endoscopic Dx/HA for primary VUR appears to have a high and durable clinical success rate. Of patients with ≥1 fUTI pre-operatively, only 10.8% had recurrent fUTI at a median of 8.4 years post-operatively. Endoscopic treatment was associated with high parental satisfaction and reasonably low reoperation rate. Post-operative obstruction may present in a delayed fashion, justifying initial sonographic surveillance, and repeated work-up in symptomatic patients.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This studies involving human participants were reviewed and approved by Children's Healthcare of Atlanta Institutional Review Board. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors contributed to study design, interpretation of results, and writing/editing of manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00392/full#supplementary-material
Abbreviations
BBD, bladder and bowel dysfunction; CAP, continuous antibiotic prophylaxis; Dx/HA, dextranomer/hyaluronic acid; fUTI, febrile urinary tract infection; HIT, hydrodistention implantation technique; STING, subureteric transurethral injection; UTI, urinary tract infection; VCUG, voiding cystourethrogram; VUR, vesicoureteral reflux.
References
1. Elder JS, Peters CA, Arant BS Jr, Ewalt DH, Hawtrey CE, Hurwitz RS, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. (1997) 157:1846–51. doi: 10.1016/S0022-5347(01)64882-1
2. Barrieras D, Lapointe S, Reddy PP, Williot P, McLorie GA, Bigli D, et al. Are postoperative studies justified after extravescial ureteral reimplantation? J Urol. (2000) 164(3 Pt 2):1064–6. doi: 10.1016/S0022-5347(05)67251-5
3. Nelson CP, Hubert KC, Kokorowski PJ, Huang L, Prasad MM, Rosoklija I, et al. Long-term incidence of urinary tract infection after ureteral reimplantation for primary vesicoureteral reflux. J Pediatr Urol. (2013) 9:92–8. doi: 10.1016/j.jpurol.2011.12.009
4. Hacker FM, Frech-Dorfler M, von Rotz M, Rudin C. Endoscopic hyaluronic acid/dextranomer gel implantation is effective as first-line treatment of vesicoureteral reflux (VUR) in children: a single centre experience. Eur J Pediatr Surg. (2011) 21:299–303. doi: 10.1055/s-0031-1279700
5. Elder JS, Diaz M, Caldamone AA, Cendron M, Greenfield S, Hurwitz R, et al. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. J Urol. (2006) 175:716–22. doi: 10.1016/S0022-5347(05)00210-7
6. Friedmacher F, Colhoun E, Puri P. Endoscopic injection of dextranomer/hyaluronic acid as first line treatment in 851 consecutive children with high grade vesicoureteral reflux: efficacy and long-term results. J Urol. (2018) 200:650–5. doi: 10.1016/j.juro.2018.03.074
7. Kaye JD, Srinivasan AK, Delaney C, Cerwinka WH, Elmore JM, Scherz HC, et al. Clinical and radiographic results of endoscopic injection for vesicoureteral reflux: defining measures of success. J Pediatr Urol. (2012) 8:297–303. doi: 10.1016/j.jpurol.2011.02.006
8. Kalisvaart JF, Scherz HC, Cuda S, Kaye JD, Kirsch AJ. Intermediate to long-term follow-up indicates low risk of recurrence after Double HIT endoscopic treatment for primary vesico-ureteral reflux. J Pediatr Urol. (2012) 8:359–65. doi: 10.1016/j.jpurol.2011.07.006
9. Lee EK, Gatti JM, Demarco RT, Murphy JP. Long-term followup of dextranomer/hyaluronic acid injection for vesicoureteral reflux: late failure warrants continued followup. J Urol. (2009) 181:1869–74. doi: 10.1016/j.juro.2008.12.005
10. Gupta A, Snodgrass W. Intra-orifice versus hydrodistention implantation technique in dextranomer/hyaluronic acid injection for vesicoureteral reflux. J Urol. (2008) 180(Suppl. 4):1589–92. doi: 10.1016/j.juro.2008.04.073
11. Shim JS, Kim JW, Oh MM, Moon du G. Efficacy of hydrodistention implantation technique in treating high-grade vesicoureteral reflux. Korean J Urol. (2012) 53:194–9. doi: 10.4111/kju.2012.53.3.194
12. Molitierno JA, Scherz HC, Kirsch AJ. Endoscopic treatment of vesicoureteral reflux using dextranomer hyaluronic acid copolymer. J Pediatr Urol. (2008) 4:221–8. doi: 10.1016/j.jpurol.2007.11.015
13. Kirsch AJ, Arlen AM, Lackgren G. Current trends in dextranomer hyaluronic acid copolymer (deflux) injection technique for endoscopic treatment of vesicoureteral reflux. Urology. (2014) 84:462–8. doi: 10.1016/j.urology.2014.04.032
14. Lackgren G, Wahlin N, Skoldenberg E, Stenberg A. Long-term followup of children treated with dextranomer/hyaluronic acid copolymer for vesicoureteral reflux. J Urol. (2001) 166:1887–92. doi: 10.1016/S0022-5347(05)65713-8
15. García-Aparicio L, Rodo J, Palazon P, Martín O, Blázquez-Gómez E, Manzanares A, et al. Acute and delayed vesicoureteral obstruction after endoscopic treatment of primary vesicoureteral reflux with dextranomer/hyaluronic acid copolymer: why and how to manage. J Pediatr Urol. (2013) 9:493–7. doi: 10.1016/j.jpurol.2013.02.007
16. Christen S, Mendoza M, Gobet R, Bode P, Weber D. Late ureteral obstruction after injection of dextranomer/hyaluronic acid copolymer. Urology. (2014) 83:920–2. doi: 10.1016/j.urology.2013.10.053
17. Chertin B, Mele E, Kocherov S, Zilber S, Gerocarni Nappo S, Capozza N. What are the predictive factors leading to ureteral obstruction following endoscopic correction of VUR in the pediatric population? J Pediatr Urol. (2018) 14:538.e1–7. doi: 10.1016/j.jpurol.2018.04.021
18. Shaikh N, Hoberman A, Keren R, Gotman N, Docimo SG, Mathews R, et al. Recurrent urinary tract infections in children with bladder and bowel dysfunction. Pediatrics. (2016) 137:e20152982. doi: 10.1542/peds.2015-2982
19. Santos JD, Lopes RI, Koyle MA. Bladder and bowel dysfunction in children: an update on the diagnosis and treatment of a common, but underdiagnosed pediatric problem. Can Urol Assoc J. (2017) 11(1–2Suppl. 1):S64–72. doi: 10.5489/cuaj.4411
20. Subcommittee on Urinary Tract Infection SCoQI Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. (2011) 128:595–610. doi: 10.1542/peds.2011-1330
21. Kirsch AJ, Perez-Brayfield MR, Scherz HC. Minimally invasive treatment of vesicoureteral reflux with endoscopic injection of dextranomer/hyaluronic acid copolymer: the Children's Hospitals of Atlanta experience. J Urol. (2003) 170:211–5. doi: 10.1097/01.ju.0000072523.43060.a0
22. Menezes M, Mohanan N, Haroun J, Colhoun E, Puri P. New contralateral vesicoureteral reflux after endoscopic correction of unilateral reflux–is routine contralateral injection indicated at initial treatment? J Urol. (2007) 178(4 Pt 2):1711–3. doi: 10.1016/j.juro.2007.03.177
23. Lendvay TS, Sorensen M, Cowan CA, Joyner BD, Mitchell MM, Grady RW. The evolution of vesicoureteral reflux management in the era of dextranomer/hyaluronic acid copolymer: a pediatric health information system database study. J Urol. (2006) 176(4 Pt 2):1864–7. doi: 10.1016/j.juro.2006.04.088
24. Wang HS, Tejwani R, Wolf S, Wiener JS, Routh JC. Readmissions, unplanned emergency room visits, and surgical retreatment rates after anti-reflux procedures. J Pediatr Urol. (2017) 13:507.e501–7. doi: 10.1016/j.jpurol.2017.03.016
25. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: The Diagnosis and Management of the Initial Urinary Tract Infection in Febrile Infants and Young Children 2–24 Months of Age. Pediatrics. (2016) 138:e20163026. doi: 10.1542/peds.2016-3026
26. Garcia-Roig M, Travers C, McCracken CE, Kirsch AJ. National trends in the management of primary vesicoureteral reflux in children. J Urol. (2018) 199:287–93. doi: 10.1016/j.juro.2017.09.073
27. Cambareri GM, Hanna MK, Stock JA. Practice patterns among pediatric urologists in the use of Deflux(R) for vesicoureteral reflux: a survey. J Pediatr Urol. (2013) 9(6 Pt A):955–61. doi: 10.1016/j.jpurol.2013.01.016
28. García-Aparicio L, Blázquez-Gómez E, Vila Santandreu A, Camacho Diaz JA, Vila-Cots J, Ramos Cebrian M, et al. Routine delayed voiding cystourethography after initial successful endoscopic treatment with Dextranomer/Hialuronic Acid Copolimer (Dx/HA) of vesicoureteral reflux (VUR). Is it necessary? Actas Urol Esp. (2016) 40:635–9. doi: 10.1016/j.acuroe.2016.02.022
29. Baek M, Kang MY, Lee HE, Park K, Choi H. Clinical value of persistent but downgraded vesicoureteral reflux after dextranomer/hyaluronic acid injection in children. J Korean Med Sci. (2013) 28:1060–4. doi: 10.3346/jkms.2013.28.7.1060
30. Kim SW, Lee YS, Han SW. Endoscopic injection therapy. Investig Clin Urol. (2017) 58(Suppl. 1):S38–45. doi: 10.4111/icu.2017.58.S1.S38
31. de Bessa J Jr, de Carvalho Mrad FC, Mendes EF, Bessa MC, Paschoalin VP, Tiraboschi RB, et al. Antibiotic prophylaxis for prevention of febrile urinary tract infections in children with vesicoureteral reflux: a meta-analysis of randomized, controlled trials comparing dilated to nondilated vesicoureteral reflux. J Urol. (2015) 193(Suppl. 5):1772–7. doi: 10.1016/j.juro.2014.10.092
32. RIVUR Trial Investigators, Hoberman A, Greenfield SP, Mattoo TK, Keren R, Mathews R, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. (2014) 370:2367–76. doi: 10.1056/NEJMoa1401811
33. Hensle TW, Hyun G, Grogg AL, Eaddy M. Part 2: examining pediatric vesicoureteral reflux: a real-world evaluation of treatment patterns and outcomes. Curr Med Res Opin. (2007) 23(Suppl. 4):S7–13. doi: 10.1185/030079907X226221
34. Lee T, Park JM. Vesicoureteral reflux and continuous prophylactic antibiotics. Investig Clin Urol. (2017) 58(Suppl. 1):S32–7. doi: 10.4111/icu.2017.58.S1.S32
35. Dwyer ME, Husmann DA, Rathbun SR, Weight CJ, Kramer SA. Febrile urinary tract infections after ureteroneocystostomy and subureteral injection of dextranomer/hyaluronic acid for vesicoureteral reflux–do choice of procedure and success matter? J Urol. (2013) 189:275–82. doi: 10.1016/j.juro.2012.09.011
36. Elmore JM, Kirsch AJ, Heiss EA, Gilchrist A, Scherz HC. Incidence of urinary tract infections in children after successful ureteral reimplantation versus endoscopic dextranomer/hyaluronic acid implantation. J Urol. (2008) 179:2364–7. doi: 10.1016/j.juro.2008.01.149
37. Chi A, Gupta A, Snodgrass W. Urinary tract infection following successful dextranomer/hyaluronic acid injection for vesicoureteral reflux. J Urol. (2008) 179:1966–9. doi: 10.1016/j.juro.2008.01.054
38. Fotso Kamdem A, Galli G, Aubert D. Long-term incidence of febrile UTI after DxHA treatment of VUR. J Pediatr Urol. (2014) 10:56–61. doi: 10.1016/j.jpurol.2013.06.002
39. Vandersteen DR, Routh JC, Kirsch AJ, Scherz HC, Ritchey ML, Shapiro E, et al. Postoperative ureteral obstruction after subureteral injection of dextranomer/hyaluronic Acid copolymer. J Urol. (2006) 176(4 Pt 1):1593–5. doi: 10.1016/j.juro.2006.06.101
Keywords: vesicoureteral reflux, urinary tract infection, endoscopic surgery, long-term effect, patient outcome assessment
Citation: Lightfoot M, Bilgutay AN, Tollin N, Eisenberg S, Weiser J, Bryan L, Smith E, Elmore J, Scherz H and Kirsch AJ (2019) Long-Term Clinical Outcomes and Parental Satisfaction After Dextranomer/Hyaluronic Acid (Dx/HA) Injection for Primary Vesicoureteral Reflux. Front. Pediatr. 7:392. doi: 10.3389/fped.2019.00392
Received: 21 May 2019; Accepted: 12 September 2019;
Published: 27 September 2019.
Edited by:
Francesco Morini, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Hiromu Miyake, Shizuoka Children's Hospital, JapanLuis Garcia-Aparicio, Hospital Sant Joan de Déu Barcelona, Spain
Copyright © 2019 Lightfoot, Bilgutay, Tollin, Eisenberg, Weiser, Bryan, Smith, Elmore, Scherz and Kirsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aylin N. Bilgutay, aylin.bilgutay@gmail.com
 Michelle Lightfoot1
Michelle Lightfoot1 Aylin N. Bilgutay
Aylin N. Bilgutay Scott Eisenberg
Scott Eisenberg Jake Weiser
Jake Weiser Edwin Smith
Edwin Smith Andrew J. Kirsch
Andrew J. Kirsch