- 1Department of Pediatric Perioperative Medicine and Intensive Care, Extracorporeal Membrane Oxygenation Centre Karolinska, Karolinska University Hospital, Stockholm, Sweden
- 2Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden
In recent years the number of extracorporeal membrane oxygenation (ECMO) cases in neonates has been relatively constant. Future expansion lays in new indications for treatment. Regionalization to high-volume ECMO centers allows for optimal utilization of resources, reduction in costs, morbidity, and mortality. Mobile ECMO services available “24-7” are needed to provide effective logistics and reliable infrastructure for patient safety. ECMO transports are usually high-risk and complex. To reduce complications during ECMO transport communication using time-out, checklists, and ECMO A-B-C are paramount in any size mobile program. Team members' education, clinical training, and experience are important. For continuing education, regular wet-lab training, and simulation practices in teams increase performance and confidence. In the future the artificial placenta for the extremely premature infant (23–28 gestational weeks) will be introduced. This will enforce the development and adaptation of ECMO devices and materials for increased biocompatibility to manage the high-risk prem-ECMO (28–34 weeks) patients. These methods will likely first be introduced at a few high-volume neonatal ECMO centers. The ECMO team brings bedside competence for assessment, cannulation, and commencement of therapy, followed by a safe transport to an experienced ECMO center. How transport algorithms for the artificial placentae will affect mobile ECMO is unclear. ECMO transport services in the newborn should firstly be an out-reach service led and provided by ELSO member centers that continuously report transport data to an expansion of the ELSO Registry to include transport quality follow-up and research. For future development and improvement follow-up and sharing of data are important.
Introduction
At the dawn of extracorporeal membrane oxygenation (ECMO) in the 1970s the neonatal population was the first group acknowledged to benefit from this new organ support (1). The number of hospitals which offered ECMO treatment was limited and the risk of transporting neonates on conventional respiratory support was considered high (2, 3). Thus, in 1975 the alternative to transport the patient on conventional critical care support, i.e., to initiate ECMO at the referring hospital before transporting the patient was performed in a neonate (4). Subsequently, the feasibility of ECMO transports has been repeatedly confirmed (5–7).
Concerning most aspects of ECMO outcome (patient safety, resource utilization, quality, morbidity, mortality), there is concensus that ECMO is best provided at high-volume ECMO centers (8–10). However, case mix may influence survival data (11), and Bailly et al. found no association between center size and outcome (12). As recently as a decade ago, only a small number of centers worldwide provided mobile ECMO services for bedside assessment and cannulae insertion. After stabilization, the patient was transported on ECMO for continued support at an ECMO center (13, 14). A transport preceded by bedside assessment, decision, and cannulation for ECMO by direct involvement of the transport team is defined as a primary transport (14, 15). A secondary transport is a transfer of a patient already on ECMO, often for a day or more, i.e., the mobile team was not directly involved in the cannulation procedure.
In the last decades the numbers of neonatal and pediatric ECMO cases have leveled off or tended to decrease for certain diagnoses (16, 17). In adults, the volume of respiratory and cardiac ECMO treatments and number of ECMO centers are increasing (18). These “young” units gain experience over time albeit the annual treatment volume is unlikely to qualify them as high-volume centers (>20–30 respiratory runs per year) (10). In the future, however, these units may serve as support centers in larger clinical ECMO networks. One example of this, the Hub-and-Spoke model, has already been implemented in various health care systems (14, 19, 20). For these to be effective, a “24-7” on-call transport service is needed that provides both primary and secondary transports. Note, a network need not be restrained by national borders for certain diagnoses, i.e., congenital diaphragmatic hernia (CDH), or by a limited population too low to support a low-incidence high-cost therapy. Transports of neonates and children have been described in both national networks (14, 21, 22) and in networks transcending national borders (13, 23, 24).
During the H1N1 pandemic the need for mobile ECMO became evident. However, the medical community has lost control over the number of centers with transport capabilities and the quantity and quality of transports performed. Most importantly, we need to know more about transport related adverse events, how these should be best managed or avoided, and how they correlate to short- and long-term morbidity and mortality outcomes. ECMO transports are unregulated in most countries (14), and authorized transport programs are sparse. Only a handful of publications are based on large numbers of transports (13, 24–26). Even fewer have reported complications during transport. Despite the ELSO transport guidelines (15), an international standard concerning transport management, definitions on adverse events and follow-up are lacking (14). In mixed populations transports adverse events vary from “zero” to >30% (24, 25, 27, 28). In neonates, transport complications may reach as high as 40% as reported by Burgos et al. (23). This high number may coincide with case mix as well as a high frequency of venoarterial ECMO patients and fixed wing transport, both associated with an increased risk for transport complications (24). In a mixed group of both neonatal and pediatric patients five adverse events were reported in 20 ECMO transports (21). In an attempt to identify adverse events a four-level risk category scale was introduced by Ericsson et al. (29), and a revision recently published (24). Death during transport is reported to be rare, <0.5% (14, 24, 25). With no international registry and low published numbers, robust data on mortality is lacking. ECMO transport seems to be safe, at least in the hands of experienced teams. Besides the publications from a few high-volume mobile ECMO programs, additional data is published as cases series or case reports. In a review of 27 case series compared to all ELSO Registry patients, Bryner et al. (25) found no difference in survival for patients transported when stratified for age or ECMO indication. Similar survival results comparing ECMO retrievals and non-transported ECMO cases have been reported from single centers (13, 27).
The aim of this work is to elucidate different approaches to the future development and role for mobile ECMO in the neonatal patient population.
Discussion
Where Are We Now?
The decreasing number of ECMO cases in neonates (16) may be attributed to improved technologies and experience in invasive ventilation support, e.g., inhaled nitric oxide, high frequency ventilation, percussive ventilation, etc. It may also be influenced by subtle changes in antenatal care and intervention, e.g., intrauterine procedures such as bronchial blockers used in lung hypoplasia/CDH (30, 31).
The current expansion of adult respiratory and cardiac ECMO, which may see further growth if extracorporeal cardiopulmonary resuscitation becomes well established, not only brings resources but also spread knowledge and awareness concerning the utilization of extracorporeal support in all age groups. Thus, even though referrals for ECMO mainly occur in adult patients, this may also benefit neonates and pediatric patients. An adult center may, depending on local surgeons' training and skills, and hospitals' pediatric/neonatal critical care experiences, provide rescue for a rapidly deteriorating critically ill child. The child is secured, a mobile ECMO team retrieves the patient to an appropriate ECMO center. However, contemporary regional resource utilization may redirect such secondary transport to another region's neonatal or pediatric ECMO center. Adult and pediatric mobile ECMO programs may work in parallel. For example, in the United Kingdom (67 million population) one center, Glenfield Hospital, Leicester, performs all ECMO transports of children, whereas five centers perform regionalized adult transfers (14).
Safe transport—the following applies to any size ECMO transport program.
Training and Education
The basic training and experience required to become a member of a transport team varies between centers and countries (14). Familiarity with transport equipment used and what differs from the devices used in the ward (14, 24). Guidelines for basic ECMO training and the requirements for team members are published and updated by ELSO (15, 32). Regular training for team members should be arranged with scenarios led by a senior staff. Each scenario is followed by a short discussion. “Water-drills,” using a saline primed ECMO circuit are easily organized, may be performed in small groups and offer opportunities to become familiar with equipment and to train separate procedures (33, 34). Water-drills are excellent to mimic situations in narrow spaces (e.g., elevator, aircraft, etc.). Full high-fidelity simulator training is resource demanding and one complete team is taken from clinical duty for half to an entire day. If more time is allowed, such day may start with a few lectures. However, realistic scenarios are extremely valuable to all team members. Closed loop communication and clear leadership often prove to be what separates the well-performing from the less well-performing team. One to two simulation days per staff per year at centers with >10 treatments/year, and more often in centers of <5–10 treatments/year would be reasonable (33, 35, 36). Team composition, organization of transport program, funding, etc. are not the scope of this work but can be found in the literature (14, 15).
Preparing for Transport
(Given that the patient is stable enough for transport.) Infusion lines, ECMO and ventilator tubing, cables, oxygen bottles, etc. are checked, fastened, and secured accordingly. Emergency equipment, i.e., an emergency box (saline, antiseptics, sterile clamps, and scissors, connectors, syringes, 3-way stopcocks), rescue kit (dry oxygenator and centrifugal pump connected with tubing ready for priming with saline), console and drive-unit, as well as blood products are controlled according to a checklist. This checklist ensures confidence of availability of all emergency equipment at “arm's length reach” from the patient. Before un-plugging from the ward a timeout is performed. In this, Situation-Background-Assessment-Recommendation (SBAR) is a suitable structured format (37, 38). Information about “red flags” is important, e.g., circuit clots, earlier bleeding site, etc. Checklist and timeout should be utilized before leaving any location, e.g., ward, CT, operating room, or vehicle (15, 39, 40).
On Transport
The timeout is extremely important for safe management when personnel unfamiliar with ECMO are asked to contribute outside their usual comfort zone. An example is when airport staff assists in a patient transfer between transport vehicles. Every step should be explained, and a clear back-up plan has to be known by all participants. The SBAR would be prolonged.
Continuous re-evaluation of the patient follows the classic A-B-C. The ECMO A-B-C, displayed in Table 1, focuses on ECMO gear and performance in a structured way and may be used:
1) In emergencies for effective and fast problem solving.
2) For the continuous re-evaluation of patient treatment.
3) In everyday practice as part of ECMO circuit and patient survey at beginning of each shift.
4) After every device or patient related intervention, i.e., if the patient has been moved from one bed to another, if equipment has been changed, etc.
5) After change from one power and/or oxygen source to another, e.g., when the patient has been “un-plugged” on the ward and now relies on batteries and gas bottles, as well as after “plug-in” in ambulance/aircraft, etc.

Table 1. Shows an ECMO A-B-C to be used for problem solving in emergencies and for routine evaluation of device performance and function in extracorporeal membrane oxygenation support.
For all staff to use same robust algorithm, applicable to any occasion, increases confidence and safety for and around the patient.
To reduce complications in neonatal transports, data available today tell us to keep transport time short (24, 29) and to acknowledge that fixed wing (FW) aircraft transports carry a higher risk than ground ambulance. Concerning patient safety, it is important to get to the patient as fast as possible (15). For shorter distances <650–800 km, a rapid response concept would be to use helicopter (rotating wing, RW). The mobile ECMO team may dispatch and land at the referring hospital's roof or close nearby reaching the patient bedside much faster than in any ground ambulance and/or FW combination.
When ECMO has been commenced there is more time to consider transport options. The choice of transport vehicle has to be put in its full context as should associated complications. For transports >650–800 km the only feasible mode of transport to keep transport time down would be FW. The most likely contributors to the increased risk observed in FW are longer time on transport and two additional patient movements between transport vehicles. In these procedures, focus may be diverted from patient monitoring and thermoregulation to more practical issues. If staff is aware of which complications are to be expected in the different phases of a transport, numbers may be reduced.
Heat losses and lack of heat conservation are known problems during transports and awareness concerning these problems are important for safe transports. Experiences from transports of neonates show that these patients are at risk of accidental hypothermia (23, 24), and heaters should always be used. During movement of the patient between transport vehicles or from the ambulance to the ward the heater cannot be operated unless an uninterruptible power supply (UPS) is used. However, very few transport programs use UPS (14). In an ex-vivo simulation in a mock of a 3 kg newborn on body temperature presented by Ericsson and Westlund at the 35th Annual CNMS: ECMO & the Advanced Therapies for Cardiovascular and Respiratory Failure, 2019, Keystone, CO, USA, it was not only shown that hypothermia was a risk in out-door transport but also during in-hospital transports, Figure 1. In future designs of transport devices heat loss due to convection and conduction should be taken seriously and prioritized. Future mobile ECMO may expand into transporting smaller patients, and the smaller the patient, the higher the risk for hypothermia. The importance of an ECMO A-B-C (and checklist, SBAR) cannot be emphasized enough to promote a high level of safety.
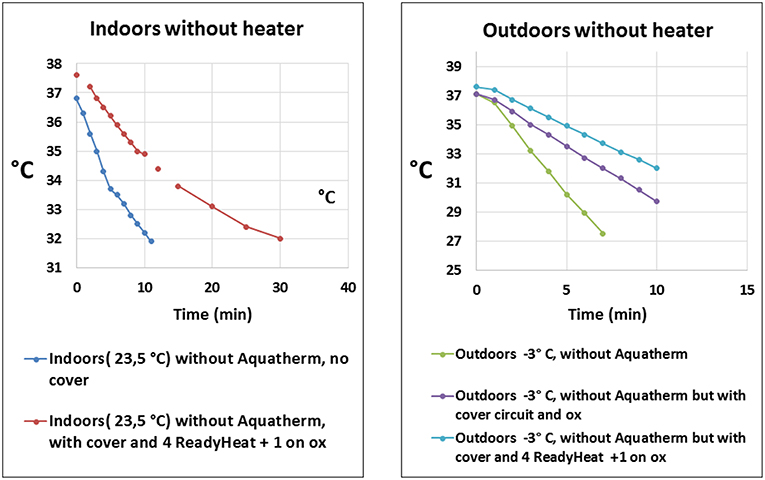
Figure 1. Displays the temperature drop vs. time when simulating a transport of a 3 kg newborn on extracorporeal membrane oxygenation without activated heater (blood warmer). Different means of passive or active protection against hypothermia was used. The left panel shows patient core temperature during movement indoors at an ambient temperature of 23.5°C without activated heater. The right panel shows patient core temperature during transport outdoors at an air temperature of −3°C. With permission from A. Ericsson and C.J. Westlund, ECMO Center Karolinska, Karolinska University Hospital, Stockholm, Sweden (2019). ox, membrane oxygenator. Aquatherm: heater, HICO-AQUATHERM 660; Hirtz & Co., Cologne, Germany. ReadyHeat: Ready-Heat™, disposable self-warming blanket, TechTrade LLC, Jersey City, NJ,USA.
Even though ECMO in its early development centered on neonates, devices available today are in many cases developed for adults. Centrifugal pumps, for example, are with few exceptions developed for full-flow ECMO in the adult. When used in neonates these pumps may be too coarse in their flow dynamics for safe use. A centrifugal pump running at low flow speed may induce hemolysis and platelet/coagulation activation due to long residence for platelets and red blood cells inside the pump (and other circuit components) (41). Proper sizing of pump devices will reduce the risk of hemolysis and coagulation activation, thus also bleeding complications. Effective integrated heaters are important for safe transport of the newborn. With miniaturized implantable gas-exchangers the need for heaters may decrease in the future. These products, however, are not likely to be seen in neonates initially but rather in chronic adult patients bridged for transplant, etc.
Where Will We Go?
Today the number of neonatal ECMO treatments has become rather constant in most of the developed world. Thus, the likelihood of seeing an increase in the number of neonatal ECMO transports with conventional diagnoses and established criteria for ECMO support is low. Socioeconomic and other factors slow or inhibit the extension of major ECMO programs. New methods for extracorporeal life support for the premature are in development. The artificial placenta (AP) focuses on support in the extremely premature (23–28 weeks gestational age, GA) (42–44). To predict the volume of extremely prematurely born infants to be offered AP, or the spread of this life support mode and the extent of engagement by mobile ECMO teams is impossible. First clinical trials will likely start within 5 years (45). However, this may occur sooner as single center studies in humans are planned in the near future to be followed by multicenter approaches, and the method may be commercially available in the not too distant future (personal communication: Professor Alan W. Flake, Center for Fetal Research, Department of Surgery, Children's Hospital of Philadelphia, Philadelphia, PA, USA). A major and perhaps unforeseen impact of the AP is that it will elicit an ethical debate where public opinion and media pressure will enforce research concerning prem-ECMO. Prem-ECMO is support in the prematurely born GA 28–34 weeks who are “too old for the AP,” but still “too young for conventional ECMO” (46, 47). Today these infants are denied ECMO due to the high risk of cerebral bleeding complications. Improvements in design are centered on coating/lining materials, pumps, gas exchangers, and cannulae. However, insights in the management of anticoagulation as well as ventilation strategies are important. The ventilated lung (and inotropes) may be part of the pathophysiology of cerebral bleedings in the preterm (48, 49). Concerning prem-ECMO transport, it could start tomorrow—the infrastructure is already available by caring for the GA 34+ weeks children.
If, or rather when, prem-ECMO transport is launched, it seems clear that these transports will include high risk patients providing new challenges. Even given that we have proper devices the risk of hypothermia remains. Smaller patient not only requires thinner cannulae, but the margin for error in placement will be small and risk of dislodgement considerable. The implementation of prem-transport has to be guided by adequate protocols and be evaluated. Today we are far beyond the time when anecdotes can mark the path to be followed.
In this article, the impact of stem cell/gene therapy in the neonate will not be discussed and what the future holds is yet to be seen (50). However, AP patients have been suggested as one group for gene therapy (44).
In ECMO transports a lowest acceptable number, or minimum of total accumulated annual transport hours required to ensure patient safety has not been published. However, it may be assumed that the larger the patient volume the better the outcome with reduced morbidity and mortality. The first step needed for us as a community would be to agree on standards, acknowledge that adverse event do occur in any mobile ECMO program and from this create a platform to improve and develop our programs. Resources should be allocated to expand the ELSO Registry with a transport module for reporting but also for extraction of own in- and processed out-put data. ELSO Centers of Excellence with recognized transport programs could be encouraged to take the lead in the development and support of interhospital mobile ECMO and act as “role-models” for safe and reliable mobile ECMO.
Conclusions
The expansion of neonatal ECMO into new geographical regions is limited. Hence, future volume increases in mobile ECMO in neonates depend on the introduction of new methods for the (extremely) premature: the artificial placenta (GA 23–28 weeks) and what comes thereafter, prem-ECMO (GA 28–34 weeks).
For safe transport of any age patient and in any size program, basic requirements for education, clinical training and experience are needed. Regular wet-lab training and high-fidelity team simulations using clinical scenarios increase performance. Time-outs, checklists and ECMO A-B-C are paramount for safety in-hospital and on transport. For future development and improvement follow-up and sharing of data is important.
ECMO transport services in the newborn should include an out-reach service provided by ELSO member centers that report transport related data to an expansion of the ELSO Registry for transport quality follow-up and research.
Data Availability
No datasets were generated or analyzed for this study.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
LB is a Medical Advisory Board member of Eurosets (Mirandola, Modena, Italy), and of Xenios (Heilbronn, Germany).
Acknowledgments
The author was grateful to Associate Professor Jan Hultman and Associate Professor Peter Radell for editing the language, and to ECMO specialist physician Anders Ericsson and ECMO specialist nurse C. Jerker Westlund for the permission to use data on hypothermia. JH, PR, AE, and CJW are affiliated with Department of Pediatric Perioperative Medicine and Intensive Care, Karolinska University Hospital, Stockholm, Sweden.
References
1. Bartlett RH, Gazzaniga AB, Toomasian J, Coran AG, Roloff D, Rucker R. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure: 100 cases. Ann Surg. (1986) 204:236–45. doi: 10.1097/00000658-198609000-00003
2. Butt W, McDougall, Shann F. Is it safe to transport infants for extracorporeal membrane oxygenation. J Paediatr Child Health. (1990) 26:286. doi: 10.1111/j.1440-1754.1990.tb01074.x
3. Boedy RF, Howell CG, Kanto WP. Hidden mortality rate associated with extracorporeal membrane oxygenation. J Pediatr Surg. (1991) 117:1762–4. doi: 10.1016/S0022-3476(05)81098-4
4. Bartlett RH, Gazzaniga AB, Fong SW, Jefferies MR, Roohk HV, Haiduc N. Extracorporeal membrane oxygenator support for cardiopulmonary failure. Experience in 28 cases. J Thorac Cardiovasc Surg. (1977) 73:375–86.
5. Cornish JD, Carter JM, Gerstmann DR, Null DM Jr. Extracorporeal membrane oxygenation as a means of stabilizing and transporting high risk neonates. ASAIO Trans. (1991) 37:564–8.
6. Heulitt MJ, Taylor BJ, Faulkner SC, Baker LL, Chipman CW, Harrell JH, et al. Inter-hospital transport of neonatal patients on extracorporeal membrane oxygenation: mobile-ECMO. Pediatrics. (1995) 95:562–6.
7. Prodhan P, Fiser RT, Cenac S, Bhutta AT, Fontenot E, Moss M, et al. Intrahospital transport of children on extracorporeal membrane oxygenation: indications, process, interventions, and effectiveness. Pediatr Crit Care Med. (2010) 11:227–33. doi: 10.1097/PCC.0b013e3181b063b2
8. Karamlou T, Vafaeezadeh M, Parrish AM, Cohen GA, Welke KF, Permut L, et al. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. (2013) 145:470–5. doi: 10.1016/j.jtcvs.2012.11.037
9. Freeman C, Bennett T, Casper TC, Larsen G, Hubbard A, Wilkes J, et al. Pediatric and neonatal extracorporeal membrane oxygenation; does center volume impact mortality? Crit Care Med. (2014) 42:512–9. doi: 10.1097/01.ccm.0000435674.83682.96
10. Barbaro R, Odetalo F, Kidwell K, Bartlett R, Davis MM, Annich G. Association between hospital extracorporeal membrane oxygenation (ECMO) volume and mortality. Am J Resp Crit Care Med. (2015) 191:894–901. doi: 10.1164/rccm.201409-1634OC
11. Mascio CE, Austin EH III, Jacobs JP, Jacobs ML, Wallace AS, He X, et al. Perioperative mechanical circulatory support in children: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. (2014) 147:658–64: discussion 664–5. doi: 10.1016/j.jtcvs.2013.09.075
12. Bailly DK, Reeder RW, Winder M, Barbaro RP, Pollack MM, Moler FW, et al. Development of the pediatric extracorporeal membrane oxygenation prediction model for risk-adjusting mortality. Pediatr Crit Care Med. (2019) 20:426–34. doi: 10.1097/PCC.0000000000001882
13. Broman LM, Holzgraefe B, Palmér K, Frenckner B: The Stockholm experience: interhospital transports on extracorporeal membrane oxygenation. Crit Care. (2015) 19:278. doi: 10.1186/s13054-015-0994-6
14. Broman LM, Dirnberger D, Malfertheiner MV, Aokage T, Morberg P, Næsheim T, et al. International survey on extracorporeal membrane oxygenation transport. ASAIO J. (2019). doi: 10.1097/MAT.0000000000000997 [Epub ahead of print].
15. Guidelines for ECMO Transport Extracorporeal Life Support Organization (ELSO), Ann Arbor MI. Available online at: https://www.elso.org/Portals/0/Files/ELSO%20GUIDELINES%20FOR%20ECMO%20TRANSPORT_May2015.pdf (accessed June 20, 2019).
16. Ford JW. Neonatal ECMO: current controversies and trends. Neonatal Netw. (2006) 25:229–38. doi: 10.1891/0730-0832.25.4.229
17. Butt W, MacLaren G. Extracorporeal membrane oxygenation 2016: an update. F1000Res. (2016) 5:F1000 Faculty Rev-750. doi: 10.12688/f1000research.8320.1
18. ELSO Registry Extracorporeal Life Support Organization (ELSO), Ann Arbor MI. Available online at: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx (accessed April 22, 2019).
19. Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. (2014) 190:488–96. doi: 10.1164/rccm.201404-0630CP
20. Broman LM. Inter-hospital transports on extracorporeal membrane oxygenation in different health-care systems. J Thorac Dis. (2017) 9:3425–9. doi: 10.21037/jtd.2017.07.93
21. Di Nardo M, Lonero M, Pasotti E, Cancani F, Perrotta D, Cecchetti C, et al. The first five years of neonatal and pediatric transports on extracorporeal membrane oxygenation in the center and south of Italy: the pediatric branch of the Italian “Rete Respira” network. Perfusion. (2018) 33:24–30. doi: 10.1177/0267659118766829
22. Fouilloux V, Gran C, Ghez O, Chenu C, El Louali F, Kreitmann B, et al. Mobile extracorporeal membrane oxygenation for children: single-center 10 years' experience. Perfusion. (2019) 34:384–91. doi: 10.1177/0267659118824006
23. Burgos CM, Fletcher-Sandersjöö A, Frenckner B, Broman LM. Transport on extracorporeal membrane oxygenation for congenital diaphragmatic hernia: a unique center experience. J Ped Surg. (2019) S0022-3468(18)30817-0. doi: 10.1016/j.jpedsurg.2018.11.022 [Epub ahead of print].
24. Fletcher-Sandersjöö A, Frenckner B, Broman LM. A single-center experience of 900 interhospital transports on extracorporeal membrane oxygenation. Ann Thorac Surg. (2019) 107:119–27. doi: 10.1016/j.athoracsur.2018.07.040
25. Bryner B, Cooley E, Copenhaver W, Brierley K, Teman N, Landis D, et al. Two decades' experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg. (2014) 98:1363–70. doi: 10.1016/j.athoracsur.2014.06.025
26. Clement KC, Fiser RT, Fiser WP, Chipman CW, Taylor BJ, Heulitt MJ, et al. Single-institution experience with interhospital extracorporeal membrane oxygenation transport: a descriptive study. Ped Crit Care Med. (2010) 11:509–13. doi: 10.1097/PCC.0b013e3181c515ca
27. Foley DS, Pranikoff T, Younger JG, Swaniker F, Hemmila MR, Remenapp RA, et al. A review of 100 patients transported on extracorporeal life support. ASAIO J. (2002) 48:612–9. doi: 10.1097/00002480-200211000-00007
28. Sherren PB, Shepherd SJ, Glover GW, Meadows CI, Langrish C, Ioannou N, et al. Capabilities of a mobile extracorporeal membrane oxygenation service for severe respiratory failure delivered by intensive care specialists. Anaesthesia. (2015) 70:707–14. doi: 10.1111/anae.13014
29. Ericsson A, Frenckner B, Broman LM. Adverse events during inter-hospital transports on Extracorporeal membrane oxygenation. Prehospital Emergency Care. (2017) 21:448–55. doi: 10.1080/10903127.2017.1282561
30. Laberge JM, Flageole H. Fetal tracheal occlusion for the treatment of congenital diaphragmatic hernia. World J Surg. (2007) 31:1577–86. doi: 10.1007/s00268-007-9074-7
31. Shue EH, Miniati D, Lee H. in prenatal diagnosis and treatment of congenital diaphragmatic hernia. Clin Perinatol. (2012) 39:289–300. doi: 10.1016/j.clp.2012.04.005
32. ELSO Guidelines for Training and Continuing Education of ECMO Specialists Extracorporeal Life Support Organization (ELSO), Ann Arbor MI. Available online at: https://www.elso.org/Portals/0/IGD/Archive/FileManager/97000963d6cusersshyerdocumentselsoguidelinesfortrainingandcontinuingeducationofecmospecialists.pdf (accessed June 20, 2019).
33. Guerguerian AM, Ogino MT, Dalton HJ, Shekerdemian LS. Setup and maintenance of extracorporeal life support programs. Ped Crit Care Med. (2013) 14(5 Suppl 1):S84–93. doi: 10.1097/PCC.0b013e318292e528
34. Kim GW, Koh Y, Lim CM, Huh JW, Jung SH, Kim JB, et al. The effect of an improvement of experience and training in extracorporeal membrane oxygenation management on clinical outcomes. Korean J Intern Med. (2018) 33:121–9. doi: 10.3904/kjim.2015.027
35. Zakhary BM, Kam LM, Kaufman BS, Felner KJ. The utility of high-fidelity simulation for training critical care fellows in the management of extracorporeal membrane oxygenation emergencies: a randomized controlled trial. Crit Care Med. (2017) 45:1367–73. doi: 10.1097/CCM.0000000000002437
36. Fehr JJ, Shepard M, McBride ME, Mehegan M, Reddy K, Murray DJ, et al. Simulation-based assessment of ECMO clinical specialists. Simul Healthc. (2016) 11:194–9. doi: 10.1097/SIH.0000000000000153
37. Randmaa M, Mårtensson G, Leo Svenne C, Engström M. SBAR improves communication and safety climate and decreases incident reports due to communication errors in an anaesthetic clinic: a prospective intervention study. BM Open. (2014) 4:e004268. doi: 10.1136/bmjopen-2013-004268
38. Raiten JM, Lane-Fall M, Gutsche JT, Kohl BA, Fabbro M, Sophocles A, et al. Transition of care in the cardiothoracic intensive care unit: a review of handoffs in perioperative cardiothoracic and vascular practice. J Cardiothorac Vasc Anesth. (2015) 29:1089–95. doi: 10.1053/j.jvca.2015.01.003
39. Brunsveld-Reinders AH, Arbous MS, Kuiper SG, de Jonge E. A comprehensive method to develop a checklist to increase safety of intra-hospital transport of critically ill patients. Crit Care. (2015) 19:214. doi: 10.1186/s13054-015-0938-1
40. Oszvald Á, Vatter H, Byhahn C, Seifert V, Güresir E. “Team time-out” and surgical safety—experiences in 12,390 neurosurgical patients. Neurosurg Focus. (2012) 33:E6. doi: 10.3171/2012.8.FOCUS12261
41. Fuchs G, Berg N, Broman LM, Prahl Wittberg L. Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Scient Rep. (2018) 8:13985. doi: 10.1038/s41598-018-32247-y
42. Bird SD. Artificial placenta: analysis of recent progress. Eur J Obstet Gynecol Reprod Biol. (2017) 208:61–70. doi: 10.1016/j.ejogrb.2016.11.005
43. Metelo-Coimbra C, Roncon-Albuquerque R Jr. Artificial placenta: recent advances and potential clinical applications. Pediatr Pulmonol. (2016) 51:643–9. doi: 10.1002/ppul.23401
44. Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. (2017) 8:15112. doi: 10.1038/ncomms15794
45. Mychaliska GB. The artificial placenta: is clinical translation next? Pediatr Pulmonol. (2016) 51:557–9. doi: 10.1002/ppul.23412
46. Van Ommen CH, Neunert CE, Chitlur MB. Neonatal ECMO. Front Med. (2018) 5:289. doi: 10.3389/fmed.2018.00289
47. Church JT, Kim AC, Erickson KM, Rana A, Drongowski R, Hirschl RB, et al. Pushing the boundaries of ECLS: outcomes in <34 week EGA neonates. J Pediatr Surg. (2017) 52:1810–5. doi: 10.1016/j.jpedsurg.2017.03.054
48. Hefti MM, Trachtenberg FL, Haynes RL, Hassett C, Volpe JJ, Kinney HC. A century of germinal matrix intraventricular hemorrhage in autopsied premature infants: a historical account. Pediatr Dev Pathol. (2016) 19:108–14. doi: 10.2350/15-06-1663-OA.1
49. Wong J, Shah PS, Yoon EW, Yee W, Lee S, Dow K. Inotrope use among extremely preterm infants in Canadian neonatal intensive care units: variation and outcomes. Am J Perinatol. (2015) 32:9–14. doi: 10.1055/s-0034-1371703
Keywords: extracorporeal membrane oxygenation, neonatal, neonate, children, transport, inter-hospital, interhospital, prem
Citation: Broman LM (2019) Interhospital Transport on Extracorporeal Membrane Oxygenation of Neonates—Perspective for the Future. Front. Pediatr. 7:329. doi: 10.3389/fped.2019.00329
Received: 26 April 2019; Accepted: 22 July 2019;
Published: 06 August 2019.
Edited by:
Giacomo Cavallaro, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Graeme MacLaren, National University Hospital, SingaporeSusan Lee Bratton, The University of Utah, United States
Thomas Vincent Brogan, Seattle Children's Hospital, United States
Copyright © 2019 Broman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lars Mikael Broman, bGFycy5icm9tYW5Ac2xsLnNl
 Lars Mikael Broman
Lars Mikael Broman