- Laboratory of Immunology, Division of Pediatrics, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
Objective: To show the different physician's approaches and the difficulties in the diagnosis and management of the Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis (PFAPA) syndrome, and to quantify the impact of the disease on the families and on the healthcare system.
Study Design: Retrospective analysis on 40 patients diagnosed with PFAPA, focusing on the clinical phenotype, the process of diagnosis, and the management of the febrile episodes. The direct and indirect annual economic cost related to PFAPA in the period preceding the diagnosis were also investigated.
Results: The median age of patients at disease onset was 1.75 years and the median time to diagnosis was 14.5 months. During the diagnostic process, only 45% of our patients was firstly addressed to rheumatologic consultation, 32.5% to otorinolaryngologist (ORL), and 22.5% to immunologic consultation. Genetic investigations were performed in the 20% of the cohort. Overall population experienced a median of 60 annual days of fever and, during the critical phase, 40% of patients received more than 5 cycles of antibiotic/year. Seventy five percent required laboratory investigations, 18 (45%) needed to access to emergency department and 15 (37.5%) have been hospitalized. The annual mean direct cost was 1659.5 € for each patient, and the estimated mean indirect cost was 5811.6 € for each parent.
Conclusion: Despite a benign clinical course, PFAPA syndrome is associated with a significant impact on the patients, their families and the national healthcare system. PFAPA patients require a large number of medical examinations and laboratory or instrumental investigations during the diagnostic approach and often receive inappropriate treatments. Therefore, we suggest the necessity of a greater awareness and knowledge of the disease among primary care physicians and, finally, of the adoption of more specific diagnostic criteria.
Introduction
The Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis syndrome (PFAPA), firstly described in 1987 by Marshall et al. (1), is the most common among the autoinflammatory diseases of childhood (2), and is featured by recurrent episodes of fever accompanied by at least one of the PFAPA-associated symptoms (aphtosis, pharyngitis, and adenitis). The episodes have a mean duration of 3–6 days in the absence of appropriate treatment, and recur every 2–7 weeks (3–6). Although several works have focused on the definition of new diagnostic criteria (7, 8), the diagnosis of PFAPA syndrome is currently defined according to the modified Marshall's criteria proposed by Thomas et al. (9), which require the presence of recurrent typical febrile episodes in children younger than 5 years old in the absence of evidence of infections or cyclic neutropenia. The recommended treatment of the disease flare is a single dose of prednisone at the dosage of 0.5–2 mg/kg or betamethasone at the dosage of 0.1–0.2 mg/kg, although in some patients a second dose of corticosteroid could be necessary. Colchicine represents the main medical therapeutic option for the reduction of the frequency of the flares, while the indications for tonsillectomy and adenotonsillectomy are still not completely defined: the surgical strategy, however, has showed a beneficial effect on PFAPA syndrome, often permitting the resolution of symptoms (10–12), and therefore has to be considered a favorable option in selected patients.
Despite the increase of scientific contributions to improve the understanding of the clinical features of PFAPA syndrome, the disease is not yet sufficiently recognized (13). Therefore, the diagnostic process and the management of the febrile episodes are often heterogeneous, and require several medical examinations and diagnostic investigations.
The aim of this study is to evidence the main difficulties in the diagnostic process and clinical management of PFAPA patients for both primary care physicians and specialists, and to analyze the impact of the disease, classically defined as “benign,” on the patients, their families and the national healthcare system.
Methods
The study includes 40 Italian patients (form Tuscany region) diagnosed with PFAPA syndrome between 2016 and 2018, according to the modified Marshall's criteria, followed at the Immuno-Rheumatologic Unit of the University of Pisa. The investigation was retrospectively performed. The clinical phenotype, the disease management by the physicians, the annual number of days of fever and the annual direct and indirect economic costs of the disease, focusing on the period before the formulation of the definitive diagnosis, were analyzed for each patient.
Clinical Phenotype
We pointed out the clinical manifestations of the febrile episodes, in order to put in evidence the eventual presence of non-specific signs.
Disease Diagnosis and Management
Concerning the formulation of diagnosis, we focused on the first consultation requested with a medical specialist (rheumatologist, immunologist, and otorinolaryngologist), and the need of a second-level analysis (i.e., molecular analysis for autoinflammatory diseases). The time to diagnosis, since first symptoms, was also reported.
Concerning the management of the single febrile episode, we investigated the execution of hematological routine exams and other investigations (for example, imaging). We documented the need of access to the emergency department and hospitalization. Finally, we reported the inappropriate prescription of antibiotics, individuating patients that received more than 5 cycles of antibiotic/year.
Impact of the Disease and Economic Costs
The number of annual days of fever and the annual number of days spent for medical consultations out of the febrile episodes were calculated.
Direct costs related to the disease were calculated according to the Diagnosis Related Group (DRG) system (for the episodes of hospitalization) and to the specific schedules published by the Italian Ministry of Health in 2017 (for the other investigations)1. Concerning the cost of the antibiotic therapy, we used as a parameter the cost of amoxicillin-clavulanic acid, the most widely used drug in our cohort.
The indirect costs of a single day of work lost by each parent was estimated in 95€, derived from the equation: mean declared income in 2017 (20.940€)/220 days of work (14).
Disease Outcome
The response to conventional treatment of the flares with corticosteroids and the need of preventive therapy with colchicine or adenotonsillectomy were reported.
Statistical Analysis
The data on the categorical variables are reported as the percentage and absolute value. The data on the continuous variables with normal distribution (skewness between +1 and −1) are presented as the mean value and standard deviation. In case of asymmetric distribution, the data are presented as median value.
Results
Demographic Data and Clinical Phenotype
We included 40 patients (25 males, 15 females), with a median age at disease onset of 1.75 years (age range: 1–8 years). The clinical phenotype, the median duration of single episodes and the interval between episodes are reported in Table 1.
By the analysis of comorbidities emerged that 4 patients suffered from febrile seizures, 3 from atopic diseases, 3 from mild IgA deficiency, 1 from myoclonic seizure, 1 from hypothyroidism, and 1 from eosinophilic gastric disease. Concerning auxological parameters, 3 patients showed severe growth restriction (stature <3rd percentile for age and sex) and 1 patient was in the range of obesity.
In 14 patients a positive familial history for recurrent inflammatory episodes of the upper respiratory tract, suitable with PFAPA syndrome, was reported.
Disease Management
Formulation of Diagnosis
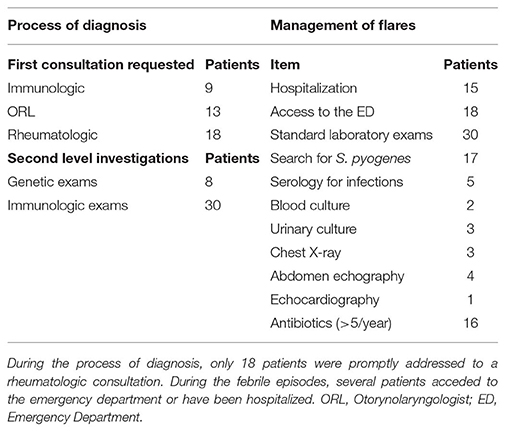
The median time to diagnosis, since first symptoms, was 14.5 months. In 18 cases (45%) the first consultation requested by the primary care physician was rheumatologic, with the clinical suspect of PFAPA syndrome, while 13 patients (32.5%) received an otorinolaryngologist (ORL) consultation and 9 (22.5%) an immunologic consultation, with the referral question of “recurrent respiratory infections,” before the rheumatologic assessment. A high number of patients (30 patients, 75%) required immunologic investigations, including the determination of immunoglobulin levels and lymphocyte subpopulations. Moreover, 8 patients (20%) have been investigated for periodic fever-associated genes (MEFV, MVK, TNFRSF1A), and in all the cases the analysis resulted negative.
Febrile Episodes
In the period preceding the diagnosis, during the febrile episodes, 30 patients (75%) received first level laboratory investigations (blood cell count, renal and hepatic function, markers of inflammation) with a mean of 1.6 ± 1.9 exams/patient/year; 17 patients (42.5%) received the rapid search for Streptococcus pyogenes in pharynx, with a mean of 2.2 ± 2.3 exams/patient/year. During the episodes, 8 patients (18%) required imaging investigations (chest X-ray, abdominal echography, cardiac echography); only 5 patients (12.5%) underwent investigations for specific infectious diseases (serology, blood and urinary culture). Eighteen patients (45%) acceded to emergency department during at least one episode, for the presence of fever or febrile seizures (mean of 1.5 ± 1.3/patient/year) and 15 patients (37.5%) have been hospitalized (median of 5 days of hospitalization).
Sixteen patients (40%) received more than 5 cycles of antibiotic/year during episodes suitable for PFAPA. For 5 out of these patients, the primary care physicians continued to prescribe antibiotics for the treatment of febrile episodes even after the formulation of diagnosis by the rheumatologist.
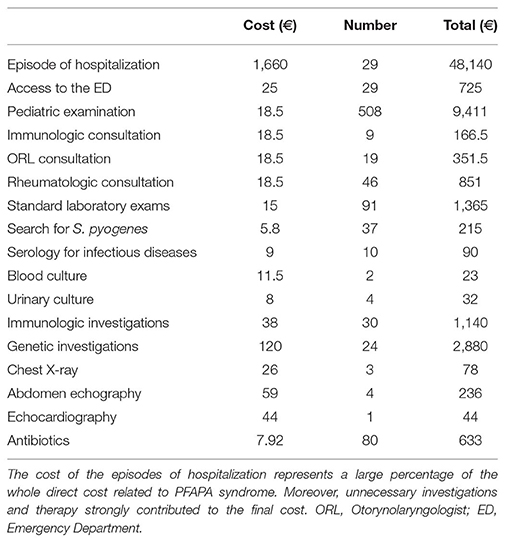
Table 2 summarizes the investigations performed during the process of diagnosis and the data about the management of the disease flares.
Impact of the Disease and Economic Costs
The median annual number of days of fever was 60, and we reported a median of 2 days spent for medical consultations with specialists out of the febrile episodes. The number of medical consultations in the afebrile period was 74, while the total number of febrile days was 2,373 (95 days spent in hospitalization). Consequently, considering the total of 2,447 days spent because of fever or of medical examinations (mean number of 61,195 days/patient), the estimated maximum indirect cost was 5811.6 € for each parent.
The annual direct cost of healthcare related to PFAPA syndrome, deriving from the previously described medical examinations, diagnostic analyses, hospitalizations and treatments, was 663,818€ in the whole cohort (mean of 1659.5 € for each patient), as showed in Table 3.
Disease Outcome
Out of our cohort, most of the patients obtained a satisfactory control of the disease flares with corticosteroids. Three patients required colchicine to reduce the frequency of the disease flares, obtaining in two cases an optimal response. Five patients underwent tonsillectomy: in 3 cases a complete remission was observed, while one patient showed only partial response, and one did not benefit from the surgical treatment. The patients with partial or absent response to tonsillectomy are currently receiving corticosteroids during the febrile episodes.
Discussion
Despite the existence of approved diagnostic criteria (the modified Marshall's criteria, as discussed in the background section), the diagnosis of PFAPA syndrome still faces considerable difficulties. Therefore, it is often delayed, and its symptoms are frequently misinterpreted as upper respiratory infections, leading to an inappropriate therapeutic strategy. This work evidences the most relevant differences in the approach to the diagnostic process and in the management of the febrile episodes, which involve both the primary care physician and the specialist. The diagnosis of PFAPA disease remains one of exclusion, and consequently patients often undergo several investigations. From the analysis of our cohort emerged that the time to diagnosis has been consistent, and that, in more than half of our patients (55%), the clinical phenotype was firstly interpreted as determined by recurrent respiratory infections, which resulted in ORL or immunologic consultations. On the contrary, only <50% of our patients was referred to a rheumatologic consultation for the presence of periodic fever, remarking that, especially in the first phase of the disease, the clinical phenotype of PFAPA syndrome may not be eloquent. In particular, the periodic pattern of fever is usually recognized tardily, leading to an avoidable diagnostic delay, highlighting the utility of a fever diary in patients with recurrent episodes of fever (15). In absence of a precise diagnosis, almost each febrile episode requires a medical examination, and, as resulted from our analysis, a large part of the patients needed to access to the emergency department (45%) or to be hospitalized (37.5%). This resulted in inappropriate investigations and therapies, which also strongly contributed to the economic costs of the disease on the healthcare system. During the febrile episodes, most of the patients received laboratory investigations, and a considerable percentage received an inappropriate antibiotic treatment. Surprisingly, for some of our patients, the primary care physicians continued to prescribe antibiotic therapy during the disease flares even after the formulation of the diagnosis of PFAPA syndrome by the rheumatologist. Apart from the consideration about the cost of a useless therapy, the use of antibiotics does not help in the resolution of the episode, and contributes to the development of drug-resistance. Even when the patient is addressed to the specialist, the formulation of a definitive diagnosis may require a significant time: in fact, despite the clinical phenotype of PFAPA is sufficiently suggestive, in most of the cases, to differentiate the syndrome from monogenic periodic fever syndromes, particularly Familial Mediterranean fever (FMF), tumor necrosis factor receptor-associated periodic syndrome (TRAPS), and mevalonate kinase deficiency (MKD), the process of exclusion of the monogenic syndromes often leads to an overuse of genetic examinations during the diagnostic process (16). Moreover, in certain circumstances, the use of genetic testing can be justified for differential diagnosis also in the cases of PFAPA displaying a classical picture, if the patient belongs to geographic areas where the rate of FMF carrier is considerable or in the case of incomplete response to adenotonsillectomy (17).
In our cohort of patients, the execution of inappropriate genetic testing was avoided, as patient at low risk of carrying specific mutations were identified according to the Gaslini diagnostic score (16, 18), preventing a relevant increase in the time to diagnosis and in the healthcare costs related to the disease. In addition to our findings, a study by Manthiram et al. (19) reported that, even among rheumatologists, the therapeutic management of the disease flare is heterogeneous, as physicians use different corticosteroid dosage (0.5, 1, and 2 mg/kg, in one or more doses) and timing (introduction at first, second, third day of fever). The same study reports also that the prevention of flares is differently managed among specialists, as rheumatologists are more likely to use colchicine, where infectious disease specialist are more likely to recommend tonsillectomy (19). These different behavioral habits highlight a diffuse variability in the adherence to the current recommendations for the management and the prevention of flares of PFAPA syndrome, only partly depending by the specialization of the physicians, and ascribable to the lack of a behavioral guiding protocol elaborated by the scientific community (20). Despite the benign clinical course of PFAPA syndrome, the disease has a considerable impact on the patients and their families. In fact, in absence of a correct treatment of the disease flare, the patients experience a high number of days of fever, having a potential impairment in their quality of life (21). Additionally, the large number of medical examinations, of laboratory and instrumental investigations, together with the days of work lost by the parents during the febrile episodes, leads a remarkable economic cost for the families and for the healthcare system, particularly in terms of indirect costs, derived by the days of work lost by the parents. Despite the limitations of a single center (Tuscany is recognized as a region with excellent patient care in the Italian scenario) retrospective analysis, our data evidence the difficulties in the recognition of PFAPA syndrome and in the appropriate management of PFAPA patients. Additionally, by comparing the costs of our cohort of patients with those ones derived from common diseases of childhood, emerged that PFAPA patients have a higher economic costs compared to conditions including asthma, atopic disease, recurrent respiratory infections, and celiac disease (22–25). On the contrary, the direct cost for a patient with juvenile idiopathic arthritis (JIA) is markedly higher, as expected (26–28).
The major reason for the delay in the diagnosis and inadequate treatment of PFAPA patients derives from the incomplete knowledge and awareness to this disease by primary care physicians. Therefore, the education of healthcare providers should be crucial to reduce the relevant impact of the disease on the patients, on the families and on the healthcare system, in term of avoidance of inappropriate medicalization and reduction of the number of annual days of fever. Moreover, since current diagnostic criteria have a low specificity (5), we postulate that the adoption of new, more specific criteria, will help to better “identify” the syndrome even among specialists, and to provide a correct, and possibly rapid, diagnosis, contributing in the avoidance of unnecessary examinations and treatments.
Conclusion
Our work shows that the diagnosis of PFAPA syndrome is often difficult and delayed, and requires several medical examinations and diagnostic investigations, numerous accesses to the emergency or to the hospital and a wide familial involvement. Additionally, the majority of the patients receive an inappropriate antibiotic treatment. Despite the highly benign characteristics of the disease, it has a relevant impact on patients, families and on the healthcare system, in terms of both direct and indirect costs. Therefore, our work suggests the urgent need of a more diffuse knowledge of the disease among primary care providers, and the potential utility of new, more specific criteria, in order to favor a prompter recognition of the syndrome and a consequent approach that limits the amplifications in term of investigations and inadequate treatment.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
GC and GM wrote the paper. RC critically revised the paper. All the authors contributed to the analysis, interpretation of clinical data, read, and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PFAPA, Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis; ORL, Otorynolaryngologist; FMF, Familial Mediterranean fever; TRAPS, Tumor Necrosis Factor receptor-associated periodic syndrome; MKD, Mevalonate Kinase deficiency.
Footnotes
1. ^Ministero della Salute-Home. Available online at: http://www.salute.gov.it/portale/home.html
References
1. Marshall GS, Edwards KM, Butler J, Lawton AR. Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. J Pediatrics. (1987) 110:43–6. doi: 10.1016/S0022-3476(87)80285-8
2. Harel L, Hashkes PJ, Lapidus S, Edwards KM, Padeh S, Gattorno M, et al. The first international conference on periodic fever, aphthous stomatitis, pharyngitis, adenitis syndrome. J Pediatrics. (2018) 193:265–74.e3. doi: 10.1016/j.jpeds.2017.10.034
3. Vanoni F, Theodoropoulou K, Hofer M. PFAPA syndrome: a review on treatment and outcome. Pediatr Rheumatol Online J. (2016) 14:38. doi: 10.1186/s12969-016-0101-9
4. Feder HM, Salazar JC. A clinical review of 105 patients with PFAPA (a periodic fever syndrome). Acta Paediatr. (2010) 99:178–84. doi: 10.1111/j.1651-2227.2009.01554.x
5. Hofer M, Pillet P, Cochard MM, Berg S, Krol P, Kone-Paut I, et al. International periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome cohort: description of distinct phenotypes in 301 patients. Rheumatology. (2014) 53:1125–9. doi: 10.1093/rheumatology/ket460
6. Wekell P, Karlsson A, Berg S, Fasth A. Review of autoinflammatory diseases, with a special focus on periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis syndrome. Acta Paediatr. (2016) 105:1140–51. doi: 10.1111/apa.13531
7. Vanoni F, Federici S, Anton J, Barron KS, Brogan P, De Benedetti F, et al. An international delphi survey for the definition of the variables for the development of new classification criteria for periodic fever aphtous stomatitis pharingitis cervical adenitis (PFAPA). Pediatr Rheumatol Online J. (2018) 16:27. doi: 10.1186/s12969-018-0246-9
8. Vanoni F, Caorsi R, Aeby S, Cochard M, Anton J, Berg S, et al. Towards a new set of classification criteria for PFAPA syndrome. Pediatr Rheumatol Online J. (2018) 16:60. doi: 10.1186/s12969-018-0277-2
9. Thomas KT, Feder HM Jr, Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr. (1999) 135:15–21. doi: 10.1016/S0022-3476(99)70321-5
10. Forsvoll J, Oymar K. The role of tonsillectomy in the periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis syndrome; a literature review. BMC Ear Nose Throat Disorders. (2018) 18:3. doi: 10.1186/s12901-017-0049-5
11. Peridis S, Pilgrim G, Koudoumnakis E, Athanasopoulos I, Houlakis M, Parpounas K. PFAPA syndrome in children: a meta-analysis on surgical versus medical treatment. Int J Pediatr Otorhinolaryngol. (2010) 74:1203–8. doi: 10.1016/j.ijporl.2010.08.014
12. Gaggiano C, Rigante D, Sota J, Grosso S, Cantarini L. Treatment options for periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome in children and adults: a narrative review. Clin Rheumatol. (2019) 38:11–7. doi: 10.1007/s10067-018-4361-2
13. Federici L, Rittore-Domingo C, Kone-Paut I, Jorgensen C, Rodiere M, Le Quellec A, et al. A decision tree for genetic diagnosis of hereditary periodic fever in unselected patients. Ann Rheumat Dis. (2006) 65:1427–32. doi: 10.1136/ard.2006.054304
14. MEF-Ministero dell'Economia e delle Finanze. Home-Dati e Statistiche Fiscali. Dipartimento delle Finanze (2018). Available online at: http://www1.finanze.gov.it/finanze3/pagina_dichiarazioni/dichiarazioni.php
15. Wekell P. Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome—PFAPA syndrome. Presse Med. (2019) 48 (1 Pt 2):e77–87. doi: 10.1016/j.lpm.2018.08.016
16. Gattorno M, Sormani MP, D'Osualdo A, Pelagatti MA, Caroli F, Federici S, et al. A diagnostic score for molecular analysis of hereditary autoinflammatory syndromes with periodic fever in children. Arthritis Rheumat. (2008) 58:1823–32. doi: 10.1002/art.23474
17. Pehlivan E, Adrovic A, Sahin S, Barut K, Kul Cinar O, Kasapcopur O. PFAPA syndrome in a population with endemic familial mediterranean fever. J Pediatrics. (2018) 192:253–5. doi: 10.1016/j.jpeds.2017.08.078
18. Gattorno M, Caorsi R, Meini A, Cattalini M, Federici S, Zulian F, et al. Differentiating PFAPA syndrome from monogenic periodic fevers. Pediatrics. (2009) 124:e721–8. doi: 10.1542/peds.2009-0088
19. Manthiram K, Li SC, Hausmann JS, Amarilyo G, Barron K, Kim H, et al. Physicians' perspectives on the diagnosis and management of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Rheumatol Int. (2017) 37:883–9. doi: 10.1007/s00296-017-3688-3
20. Batu ED. Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: main features and an algorithm for clinical practice. Rheumatol Int. (2019) 39:957–70. doi: 10.1007/s00296-019-04257-0
21. Grimwood C, Kone-Paut I, Piram M, Rossi-Semerano L, Hentgen V. Health-related quality of life in children with PFAPA syndrome. Orphanet J Rare Dis. (2018) 13:132. doi: 10.1186/s13023-018-0878-3
22. Ricci G, Bendandi B, Pagliara L, Patrizi A, Masi M. Atopic dermatitis in Italian children: evaluation of its economic impact. J Pediatr Health Care. (2006) 20:311–5. doi: 10.1016/j.pedhc.2006.04.009
23. Antonicelli L, Bucca C, Neri M, De Benedetto F, Sabbatani P, Bonifazi F, et al. Asthma severity and medical resource utilisation. Eur Respir J. (2004) 23:723–9. doi: 10.1183/09031936.04.00004904
24. Violato M, Gray A, Papanicolas I, Ouellet M. Resource use and costs associated with coeliac disease before and after diagnosis in 3,646 cases: results of a UK primary care database analysis. PLoS ONE. (2012) 7:e41308. doi: 10.1371/journal.pone.0041308
25. Boonacker CW, van den Aardweg MT, Broos PH, Hoes AW, Schilder AG, Rovers MM. Immediate adenoidectomy vs initial watchful waiting strategy in children with recurrent upper respiratory tract infections: an economic evaluation. JAMA Otolaryngol Head Neck Surg. (2013) 139:129–33. doi: 10.1001/jamaoto.2013.1324
26. Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy. (2015) 119:964–79. doi: 10.1016/j.healthpol.2014.12.016
27. Kuhlmann A, Schmidt T, Treskova M, Lopez-Bastida J, Linertova R, Oliva-Moreno J, et al. Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econom. (2016) 17 (Suppl. 1):79–87. doi: 10.1007/s10198-016-0786-1
Keywords: PFAPA, autoinflammatory diseases, diagnostic criteria, children, differential diagnosis, diagnostic delay
Citation: Costagliola G, Maiorino G and Consolini R (2019) Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis Syndrome (PFAPA): A Clinical Challenge for Primary Care Physicians and Rheumatologists. Front. Pediatr. 7:277. doi: 10.3389/fped.2019.00277
Received: 20 April 2019; Accepted: 20 June 2019;
Published: 05 July 2019.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Antonio Condino-Neto, University of São Paulo, BrazilFatma Dedeoglu, Boston Children's Hospital and Harvard Medical School, United States
Copyright © 2019 Costagliola, Maiorino and Consolini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Costagliola, Z2lvcmdpby5jb3N0YWdsaW9sYUBob3RtYWlsLmNvbQ==
 Giorgio Costagliola
Giorgio Costagliola Giuseppe Maiorino
Giuseppe Maiorino Rita Consolini
Rita Consolini