- 1Division of Critical Care Medicine, Department of Pediatrics, Alfred I. DuPont Hospital for Children, Wilmington, DE, United States
- 2Division of Critical Care Medicine, Department of Pediatrics, Cooper University Hospital, Camden, NJ, United States
- 3Department of Pediatrics, Children's Hospital, Western University, London, ON, Canada
- 4Cooper University Hospital, Cooper Research Institute, Cooper Medical School of Rowan University, Camden, NJ, United States
- 5Department of Anaesthesia/Intensive Care Unit, Aminu Kano Teaching Hospital, Kano, Nigeria
- 6Department of Paediatrics, Lagos University Teaching Hospital/College of Medicine, University of Lagos, Lagos, Nigeria
- 7Department of Paediatrics, University of Nigeria Teaching Hospital, Enugu, Nigeria
- 8Department of Pediatrics and Emergency Medicine, University of British Columbia and BC Children's Hospital, Vancouver, BC, Canada
Background: Infections leading to sepsis are major contributors to mortality and morbidity in children world-wide. Determining the capacity of pediatric hospitals in Nigeria to manage sepsis establishes an important baseline for quality-improvement interventions and resource allocations.
Objectives: To assess the availability and functionality of resources and manpower for early detection and prompt management of sepsis in children at tertiary pediatric centers in Nigeria.
Methods: This was an online survey of tertiary pediatric hospitals in Nigeria using a modified survey tool designed by the World Federation of Pediatric Intensive and Critical Care Societies (WFPICCS). The survey addressed all aspects of pediatric sepsis identification, management, barriers and readiness.
Results: While majority of the hospitals 97% (28/29) reported having adequate triage systems, only 60% (16/27) follow some form of guideline for sepsis management. There was no consensus national guideline for management of pediatric sepsis. Over 50% of the respondents identified deficit in parental education, poor access to healthcare services, failure to diagnose sepsis at referring institutions, lack of medical equipment and lack of a definitive protocol for managing pediatric sepsis, as significant barriers.
Conclusions: Certain sepsis-related interventions were reportedly widespread, however, there is no standardized sepsis protocol, and majority of the hospitals do not have pediatric intensive care units (PICU). These findings could guide quality improvement measures at institutional level, and healthcare policy/spending at the national level.
Introduction
Infections leading to sepsis is a major cause of pediatric morbidity and mortality globally, however, 80% of infectious disease-related deaths occur in low and middle income countries (LMICs) (1). Children in LMICs are 18 times more likely to die under the age of five than children in higher income countries (HICs), a disparity caused by the interplay between economic, social and political factors and the inability to provide timely medical care partly due to resource limitations (2). The disparity in resources is starkly revealed by the findings of a continent wide survey showing that the Surviving Sepsis Campaign (SSC) guidelines can be fully implemented in only 1.5% (4/263) of African ICUs (3). Recognition of this resource gap led the “Global Intensive Care Working Group” of the European Society of Intensive Care Medicine to adapt the SSC guidelines to LIMCs (4–6).
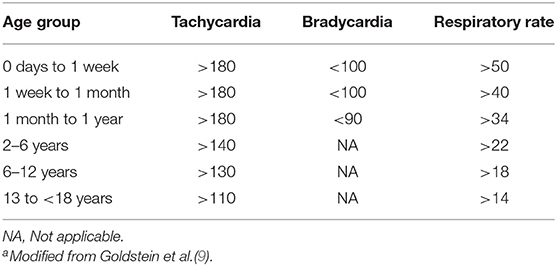
In addition, defining sepsis could be challenging, and any definition needs to be context-appropriate for proper identification of patients. In resource rich environment, the criteria for defining sepsis syndrome include the presence or suspicion of infection associated with a systemic inflammatory response syndrome (SIRS). The SIRS is characterized by the presence of at least two of the four criteria below, one of which must be either an abnormal temperature or leucocyte count;
• Core temperature (rectal, bladder, or oral) of >101.3°F (>38.5°C) or <96.8°F (<36°C)
• Tachycardia more than 2 standard deviations (SD) above the normal or bradycardia (a mean heart rate <10th percentile for age) in children >1 year in the absence of external stimulus or drugs.
• Respiratory rate >2 SD above normal for age
• Leucocyte count elevated or depressed for the patient age
In LMICs, due to the limitations of SIRS, criteria for sepsis definition is based on clinical syndrome, clinical presentations and age of the patient. The suggested criteria for providers to make diagnosis of suspected sepsis in children include (7–9) proven or highly suspected infection and the presence of any two of the following;
• Abnormal temperature (axillary >38°C or <36°C) documented or by history1
• Abnormal heart rate for age: tachycardia or bradycardia (<1 year)2
• Respiratory insufficiency: tachypnea for age3
• Malaise and/or apathy4
See Table 1 for age-specific vital signs.
Assessing baseline prevalence of sepsis and septic shock in Nigeria is challenging due to dearth of epidemiological data on sepsis and septic shock in Nigeria and other LMICs. Literature reviews between 1980 and 2008 did not show any studies on the incidence, prevalence, mortality or case-fatality from sepsis in developing countries (10). However, the Sepsis Prevalence, Outcomes, and Therapy (SPROUT) Study found a point prevalence of severe sepsis of 23.1% across Africa, though only three sites from Africa were included in the study (11). More recently, a systemic review of the global burden of pediatric and neonatal sepsis found an aggregate estimate of 48 sepsis cases and 22 severe sepsis cases in children per 100,000 person-years globally but emphasized that due to lack of population-based data from low-resource settings, combined with lack of standardization of diagnostic criteria and sepsis definitions, it would be difficult to get an accurate estimation of global burden of pediatric sepsis (12).
Ultimately, Nigeria is the second highest contributor to under-five mortality (much due to sepsis) in the world (13). Determining the capacity of pediatric hospitals to manage sepsis establishes an important baseline from which to advocate for resources and for any quality-improvement interventions aimed at improving sepsis-related hospital outcomes. We therefore surveyed the heads of department of pediatrics at teaching hospitals and federal medical centers (tertiary pediatric centers) across the country to determine the resources available to manage children with sepsis. Our primary objective was to assess the availability and functionality of supplies and medications for the management of sepsis. Our secondary objectives included assessing the availability of emergency triage systems, skilled healthcare providers, use of guidelines and the barriers to early detection and prompt management of sepsis.
Materials and Methods
This study was approved by the Institutional Review Board (IRB) of Nemours Office of Human Subject Protection/ Alfred I duPont Hospital for Children, Wilmington, Delaware USA. The institutions in Nigeria did not require IRB approval for the survey. The online survey instrument designed by the World Federation of Pediatric Intensive and Critical Care Societies (WFPICCS) was modified and used to address all aspects of pediatric sepsis identification, management, barriers, and readiness (14). We modified the survey to reflect the local health practices and environment in Nigeria, such as replacing the Pediatric Specialist designation with Consultant Pediatrician, and the types of health facility as Teaching Hospitals, Federal Medical Centers (FMCs) and Other (Specialist Hospitals). The hospitals are divided into teaching and non-teaching hospitals. Out of 31 hospitals, 26 are teaching hospitals, and 5 are non-teaching hospitals. All the Federal Medical Centers (five in number) are non-teaching hospitals. Of the 26 teaching hospitals, 21 are owned and operated by the federal government, while the remaining 5 are state owned and operated.
The contacts of the Heads of Department of Pediatrics of the teaching hospitals and FMCs were obtained from the secretariat of the Pediatric Association of Nigeria (PAN). However, PAN did not have a comprehensive list of all the heads of departments, so we were unable to obtain this information for all the health institutions especially the Federal Medical Centers and the state owned teaching hospitals.
Data Collection
The survey was distributed by e-mail with a cover letter explaining the purpose of the survey and a link to the online survey. A portable document format (PDF) attachment of a sample of the survey was also available online to provide respondents an opportunity to review the questions and to obtain the data needed in answering the actual online survey. One survey per institution was sent to be completed by the Heads of Department of Pediatrics or their designee. While the survey did not contain any questions that would identify either the respondent or the institution, we included questions to identify the type of institution. The survey data was collected and managed using Survey Monkey tools housed at Cooper Medical School of Rowan University, Camden New Jersey, USA.
We assessed the availability and functionality of the medical supplies, equipment and medications at the healthcare institutions (see Supplementary Material for a copy of the survey tool).
Statistical Analysis
We included all the returned surveys in our analysis. N value represented the total responses for a particular question in the survey. The data was summarized by providing a descriptive analysis where frequencies and percents were reported.
Results
Basic Characteristics of the Hospitals
Thirty-one surveys representing 31 of about 59 tertiary hospitals and Federal Medical Centers (FMCs) in Nigeria were completed and included in the analysis. Twenty-six (84%) of these were teaching hospitals while five (16%) were Federal Medical Centers. The federal teaching hospitals are well-established academic centers while some of the state-owned teaching hospitals and FMCs are either newly established or have smaller pediatric departments. The median age of children seen at these hospitals was 14 years (25.5 days-16.3 years), Over a period of 12 months, the respondents reported a total of 44,337 hospital visits, 12,912 emergency room visits, and an average of 881 patient were evaluated for sepsis in the emergency rooms, and 553 had a primary diagnosis of sepsis. They reported a median of 77 pediatric in-patient beds (range 30–190). All the surveys were completed by the Heads of Department of Pediatrics at these institutions.
Triage and Emergency Care
All the hospitals reported having separate emergency rooms or acute care areas that were open 24 hours a day, and majority of them 97% (28/29) have systems in place to screen and prioritize very sick children. The resident doctors and nurse were primarily responsible for the triage process, and were trained on the use of The Emergency Triage Assessment and Treatment (ETAT) and the Integrated Management of Childhood Illnesses (IMCI) for screening and prioritizing severely ill children (15, 16). The Advanced Pediatric Life Support (APLS) or the American College of Critical Care Medicine/Pediatric Advanced Life Support (ACCM/PALS) guidelines were not commonly used (17, 18) (Figure 1). Children with suspected sepsis were reported to receive priority emergency attention. The median time reported from arrival to prioritization was 12.5 minutes, while the median time from prioritization to being seen by a physician was 20 minutes.
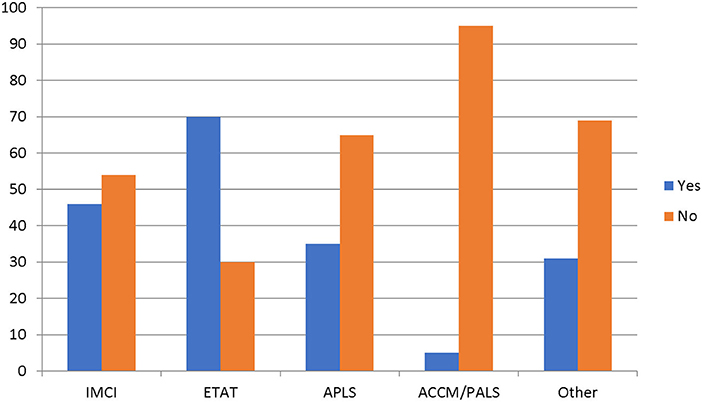
Figure 1. Specific guidelines used to prioritize sick children. IMCI, Integrated Management of Children Illnesses; ETAT, Emergency Triage, Assessment and Treatment; APLS, Advanced Pediatric Life Support; ACCM/PALS, American College of Critical Care Medicine/Pediatric Advanced Life Support.
Patient Care in the 1st Hour
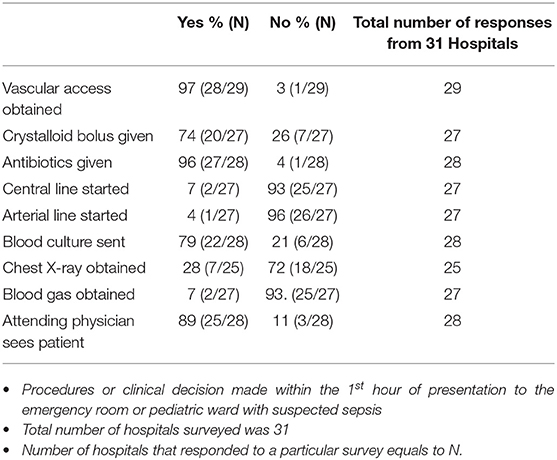
Patients with suspected sepsis were reported to receive fluid boluses, antibiotics, and were evaluated by the Consultant Pediatrician within the first hour of presentation to the hospital (Table 2).
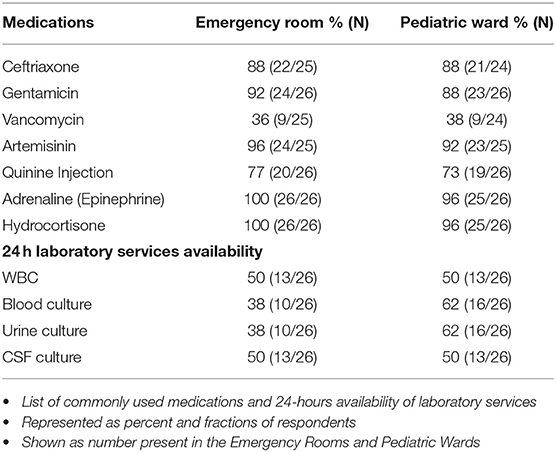
Intravenous Ceftriaxone and Gentamicin were the two commonly used antibiotics combination, and the resident doctors were reported to make this decision in 90% of cases. All the hospitals in this survey reported having resources to do complete blood count (CBC) and blood culture. Additionally, 86% (25/29) of the hospitals reported having the capacity to do blood smears for malaria parasites, while 83% (24/29) could do blood chemistry, and 72% (21/29) had resources to do urinalysis. However, they do not routinely do blood gases or coagulation profile. Fifty two percent of the hospitals reported that children who were severely ill in the emergency department were transferred to a designated special care area.
In-patient Care
All acutely ill children with sepsis who were transferred to the pediatric wards were seen first by the resident doctors who would evaluate and institute therapy. The pediatric wards were open 24 h a day, seven days a week at most hospitals. On the average, there were seventy-three pediatric beds on the ward, and majority of the hospitals had beds that were designated for acutely ill children, however, there were no Pediatric Intensive Care Units (PICU) in 75%, (15/20) of the tertiary pediatric hospitals.
Resources for Monitoring
Of all the hospitals that responded to this question, 100% (28/28) reported that they had pulse oximetry machine while 93% (25/27) had non-invasive blood pressure machines available at the hospitals, however, only 52% (13/25) had cardiac monitors. While 60% (16/27) of the hospitals stated that they follow some form of guidelines while treating sepsis, there was no consensus on any particular guideline. The guidelines listed ranged from departmental guideline, unit protocol and institutional guideline. The providers reportedly relied mainly on clinical examinations to guide sepsis management. Overall, 93% (26/28 and 25/27) used improvement in mental status and urine output, respectively, as their goal during sepsis management. Additionally, 88% (22/25), 85% (22/26), and 85% (23/27) of the respondents would use reduction in heart rate, improvement in blood pressure and improvement in peripheral perfusion, respectively as their goal directed targets in sepsis management. Only 13% (2/15) of institutions used serum markers such as lactate.
Co-morbid Conditions
Protein-energy malnutrition was the commonest 92%, (24/26) co-morbid condition reported among children with sepsis in our study. Other co-morbid illnesses reported include malaria, Human Immunodeficiency Virus (HIV) infection, measles, sickle cell disease, anemia, malignancies and congenital heart diseases. Of these, malaria 100%, (25/25), Staphylococcus aureus 92%, (22/24), Streptococcus pneumonia 57%, (13/24) and Gram-negative rods 83%, (20/24) were reported as the most common causes of mortality among children who died from sepsis. Over a period of 1 year, respondents reported that an average of 15% (82/553) of children admitted with the diagnosis of sepsis died.
Barriers to Sepsis Management
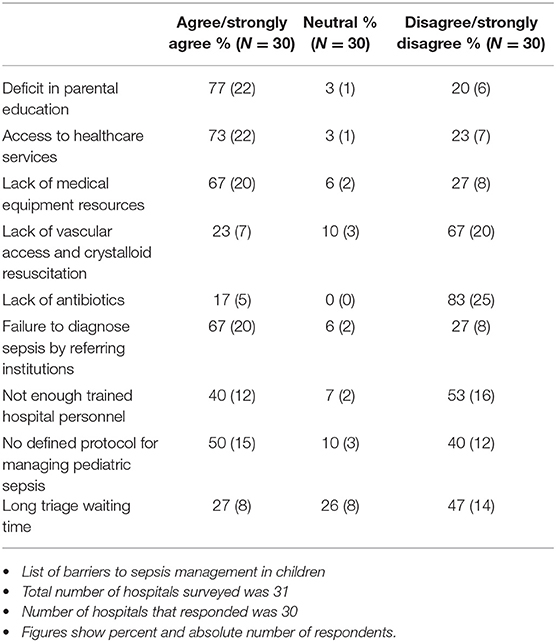
The common barriers to appropriate sepsis management in children reported were lack of parental awareness of early signs of sepsis 77%, (22/30), poor access to healthcare services 73%, (22/30), failure to diagnose sepsis at the referring health institution (67%, 20/30), lack of medical equipment 67%, (20/30), and lack of a definitive protocol for managing pediatric sepsis 50%, (15/30) (Table 3).
Triage waiting time, adequate number of trained personnel, vascular access, fluids for resuscitation and availability of antibiotics were not considered strong barriers to sepsis management. Other important barriers reported include financial constraints among families (poverty, high cost of medications, and the need for out of pocket payment), late or delayed presentation to the hospital, transportation system with inaccessible roads, lack of clean water, lack of rapid diagnostic tests and other laboratory resources, and cultural barriers (need for father's approval before the mother could bring child to the hospital).
Equipment and Supplies
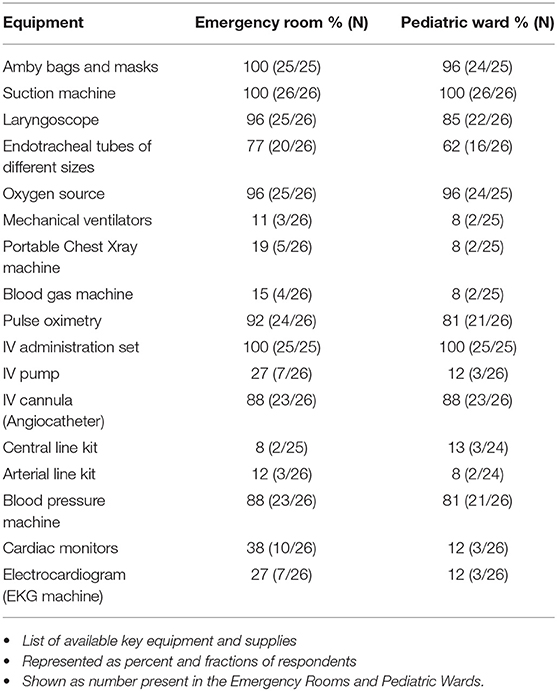
There was reported availability of respiratory supplies for simple oxygen supplementation and bag-valve mask ventilation in both the emergency rooms and pediatric wards for children with sepsis; however, 81% (21/26) of the hospitals surveyed reported that they did not have mechanical ventilators in the emergency rooms, while 88% (22/26) did not have ventilators on the pediatric wards (Table 4). Similarly, 58% (15/26) of the hospitals reportedly did not have cardiac monitors in the emergency rooms while 85% (22/26) did not have monitors on the pediatric ward. Along the same line, 88% (22/25) of the respondents reported that they did not have central line kit either in the emergency room or the pediatric ward 88% (21/24). Ceftriaxone, Gentamicin, and anti-malaria medication were available in most of the hospitals. Only 50% (13/26) of the hospitals had 24 h laboratory services for WBC count, while less than half of the hospitals could not do blood, urine or CSF cultures on a 24-h basis (Table 5).
Discussion
This study provides a survey of available resources for managing sepsis in children at tertiary pediatric centers in Nigeria. We surveyed only teaching hospitals and federal medical centers in Nigeria. These hospitals provide the highest level of care for children with varied disease conditions, and the resources available to them for managing pediatric sepsis would be considered the highest in the country, and the standard of practice that other healthcare facilities in the country aspire to. Our data showed that all the hospitals have emergency room and triage areas reserved for children, and they have systems in place to screen and prioritize very sick children. In this survey, the presence of systems at these hospitals that consistently screens and prioritizes very sick children were considered adequate triage systems, although we were unable to ascertain if this was indeed the case by direct observation. The emergency rooms and acute care areas are open for service 24 h a day, 7 days a week. Most of the hospitals used ETAT to triage patients, and this is consistent with the practice in other resource limited settings (19–21).
Children with sepsis were reported to be triaged and evaluated by a physician in a median time of 12.5 and 20 min, respectively, and they reportedly received fluid boluses and antibiotics within the first hour. This practice follows some of the international pediatric guidelines (the Society for Critical Medicine Medicine/Pediatric Advanced Life Support SCCM/PALS guidelines and the Surviving Sepsis Campaign, SSC guideline) that recommend administration of 20 ml/kg of crystalloids over 5 min, and the administration of 60 ml/kg in the first hour. However, in resource-limited settings, more cautious fluid boluses have been recommended for febrile children with poor perfusion and those that meet the WHO shock criteria, while blood transfusion is reserved for children with severe anemia (22–24).
Children with sepsis were reported to receive the 1st dose of antibiotics within the 1st hour of presentation to the hospital in this survey. This practice is in agreement with the guidelines from resource-rich countries, which recommends antibiotics administration within the first hour of recognizing sepsis or septic shock in resource limited settings (5). In our setting, the choice of antibiotics was a challenge due to lack of epidemiological data, poor microbiological laboratory capacity and reduced availability of antibiotics. In Nigeria, ceftriaxone and gentamicin were the combined antibiotics of choice, though WHO guidelines recommend gentamicin and penicillin for hospitalized neonates and children in resource-limited settings (25). The reason for the choice of ceftriaxone over penicillin was not elucidated in this study
Our findings were consistent with other studies in Sub-Saharan Africa and other resource-limited settings, which showed widespread lack of diagnostic facilities and capacity for sepsis management, making international guidelines irrelevant for sepsis definitions, diagnosis and treatment (7, 26, 27). We found that only about fifty percent of the hospitals have laboratory services for 24 h for basic laboratory tests as full blood count, basic metabolic profile, blood, and urine cultures. This is a perennial issue in African countries, where the availability, accessibility, and affordability of blood culture is a common problem (28). Only 2% of the hospitals surveyed in Nigeria do have the capacity to measure any serum markers like serum lactate. This could potentially lead to missed opportunities for early detection of sepsis, and loss of an important guide to sepsis treatment (29, 30). Additionally, physical barriers including long distances to formal healthcare facilities, poor road networks and inadequate transport systems adversely affect access to health care and consequently, delay in care delivery. Studies have shown that both infant and childhood mortality rates increased as the distance to health facilities increased, whereas in a village, merely increasing the number of maternity clinics by one was associated with a decrease in infant mortality (31, 32). In Nigeria, there is an established significant association between physical barrier (including distance to healthcare facility and transport cost) to accessing health care and under-five mortality (33).
We found inadequate systems and structures to support the delivery of adequate care to children with severe sepsis. Seventy-five percent of hospitals surveyed do not have pediatric intensive care units, and most do not have the capacity to provide mechanical ventilation to support children with sepsis who are in respiratory failure. This is worrisome because studies from LMICs showed that large volumes of fluid during resuscitation could lead to pulmonary edema, and up to half of these children might need intubation and mechanical ventilation (34, 35). Safe delivery of invasive positive pressure ventilation and monitoring require the availability of functional Pediatric Intensive Care Unit (PICU).
Establishing PICUs in LMICs require assembling the necessary equipment/supplies, training of healthcare providers, and commitment from the hospital leadership (36, 37). In Nigeria, poor healthcare financing poses a threat to the provision of adequate medical equipment, supplies and medications that are essential for the delivery of critical care services for children. In 2015, the health expenditure to Gross Domestic Product (GDP) in Nigeria was 3.56%, despite the fact that in 2001, Nigeria signed the Abuja declaration where 53 African countries pledged to devote 15% of their national budget to health (38).
It was quite revealing in our study that almost all (92%) patients with sepsis were malnourished, and 15% of these patients died in the year preceding our study. This is consistent with other studies that showed high case fatality rate in children with septic shock and severe acute malnutrition, with mortality rate as high as 40% (39, 40). To date, issues related to fluid resuscitation in the malnourished child remain unsettled. Obonyo et al. reported in a prospective observational study that malnourished children showed no compromised cardiac function in response to fluid loading, and this could support the adoption of a more liberal approach to fluid resuscitation (41, 42). However, based on of constellation of evidence cautious approach and frequent re-evaluation is recommended (43). Acute malnutrition was defined here as children who were−2 Z-score in weight for height or bilateral pitting edema (44). The hospitals all kept data on the nutritional status of all children attending their institutions.
Another area in our study that negatively impacts sepsis management in children is the scanty data on the resistance pattern of the bacteria causing diseases in most Sub-Saharan African countries. The unregulated sale of antibiotics in shops and drug stores in most communities, the use in animal husbandry, and the widespread use in clinics and hospitals based on clinical syndromes rather than on laboratory sensitivity tests promote emergence of resistance (45, 46). In Nigeria, the key factors promoting antimicrobial resistance include unregulated antibiotics sale, proliferation of unlicensed medicine stores, shortage of licensed prescribers, poor antimicrobial resistance (AMR) awareness and use of antibiotics in animals without prescription (47).
Lack of parenteral awareness of sepsis and inability of referring healthcare providers to identify sepsis were among the significant barriers to sepsis management in over 50% of the respondents. Similarly, an international survey in Europe and United States showed that a mean of 88% of the interviewee have never heard the word “sepsis” (48). Even among healthcare workers, there was knowledge deficit with regards to sepsis awareness (49–51). In this study, the definitions of deficit in parental education and lack of parental awareness were discerned by the providers of care and was their judgement based on history taking.
Since information-seeking behaviors related to sepsis usually follow awareness campaigns (52), we recommend increased public awareness about sepsis. This could be in the form of public service announcements in print and electronic media (including radio and television), in major Nigerian languages. The Ministries of Health and Information could leverage the wide use of social media in Nigeria, especially WhatsApp to push information on sepsis The Medical and Dental Council of Nigeria (MDCN), the apex regulatory agency for medical practitioners in Nigeria, could require Sepsis Awareness CME credits for healthcare providers for their license renewals. Health policy makers in Nigeria should consider honoring the 2001 Abuja Declaration that pledged 15% of the National Budget to healthcare expenditures. Further studies could explore drafting of a national pediatric sepsis guideline, and assessing the acceptability and use of the new guideline.
Limitations of our Study
We surveyed only tertiary pediatric centers in Nigeria, which might not reflect all the pediatric care in Nigeria, and we had no way to verify the responses that were self-reported. In addition, we did not obtain data to determine the outcomes at these institutions.
Strength of our Study
This was a nation-wide survey with national and international collaborations. Our survey tool was a modification of a survey instrument designed by the World Federation of Pediatric Intensive and Critical Societies (WFPICCS). The findings and recommendations from this study could form a basis for the creation and adoption of Pediatric Sepsis Guideline in Nigeria.
Conclusion
In our survey of tertiary care pediatric hospitals in Nigeria we found that certain sepsis-related interventions were widespread (e.g., adequate triage systems, administration of antibiotics within an hour) while others were neither available nor accessible at most hospitals (e.g., PICUs, availability of laboratory services 24 h/day, sepsis protocol). This information can be used to guide policy, healthcare spending and quality improvement interventions aimed at improving sepsis related outcomes in children.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
ON and BM wrote the initial manuscript and revisions for the final draft. MF, HK, AO, and TO contributed in interpreting the results, reviewing, and writing the manuscript. KH created the online survey tool, housed the database and provided data analysis. NK drafted the modified survey and had overall oversight of proof reading and editing the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Pediatric Association of Nigeria (PAN) for providing us with the list of Heads of Department of Pediatrics of the tertiary pediatrics centers in Nigeria.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00234/full#supplementary-material
Footnotes
1. ^Modified from Kortz et al. (8).
2. ^Modified from Kortz et al. (8).
References
1. Schultz MJ, Dunser MW, Dondorp AM, Adhikari NK, Iyer S, Kwizera A, et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med. (2017) 43:612–24. doi: 10.1007/s00134-017-4750-z
2. Kissoon N, Carapetis J. Pediatric sepsis in the developing world. J Infect. (2015) 71:S21–6. doi: 10.1016/j.jinf.2015.04.016
3. Baelani I, Jochberger S, Laimer T, Otieno D, Kabutu J, Wilson I, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Critical Care. (2011) 15:R10. doi: 10.1186/cc9410
4. Mer M, Schultz MJ, Adhikari NK, European Society of Intensive Care Medicine. Core elements of general supportive care for patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. (2017) 43:1690–4. doi: 10.1007/s00134-017-4831-z
5. Thwaites CL, Lundeg G, Dondorp AM. Recommendations for infection management in patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. (2016) 42:2040–2. doi: 10.1007/s00134-016-4415-3
6. Musa N, Murthy S, Kissoon N. Pediatric sepsis and septic shock management in resource-limited settings. Intensive Care Med. (2016) 42:2037–9. doi: 10.1007/s00134-016-4382-8
7. Dunser MW, Festic E, Dondorp A, Kissoon N, Ganbat T, Kwizera A, et al. Recommendations for sepsis management in resource-limited settings. Intensive Care Med. (2012) 38:557–74. doi: 10.1007/s00134-012-2468-5
8. Kortz TB, Sawe HR, Murray B, Enanoria W, Matthay MA, Reynolds T. Clinical presentation and outcomes among children with sepsis presenting to a public tertiary Hospital in Tanzania. Front Pediatr. (2017) 5:278. doi: 10.3389/fped.2017.00278
9. Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. (2005) 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6
10. Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. (2012) 2:010404. doi: 10.7189/jogh.01.010404
11. Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. (2015) 191:1147–57. doi: 10.1164/rccm.201412-2323OC
12. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–30. doi: 10.1016/S2213-2600(18)30063-8
13. Wen LS, Oshiomogho JI, Eluwa GI, Steptoe AP, Sullivan AF, Camargo CA Jr. Characteristics and capabilities of emergency departments in Abuja, Nigeria. Emerg Med J. (2012) 29:798–801. doi: 10.1136/emermed-2011-200695
14. Kang KT, Chandler HK, Espinosa V, Kissoon N. Systems for paediatric sepsis: a global survey. West Indian Med J. (2014) 63:703–10. doi: 10.7727/wimj.2013.326
16. World Health Organization, Department of Child Adolescent Health, UNICEF. Handbook IMCI: Integrated Management of Childhood Illness. World Health Organization (2005).
17. European Resuscitation Council 2000 Pediatric Life Support Working Group. Guidelines of the European Resuscitation Council 2000 for advanced pediatric life support. A statement of the Pediatric Life Support Working Group following approval by the executive committee of the European Resuscitation Council. Anaesthesist. (2002) 51:406–8.
18. Kissoon N, Orr RA, Carcillo JA. Updated American College of Critical Care Medicine–pediatric advanced life support guidelines for management of pediatric and neonatal septic shock: relevance to the emergency care clinician. Pediatr Emerg Care. (2010) 26:867–9. doi: 10.1097/PEC.0b013e3181fb0dc0
19. Ralston ME, Day LT, Slusher TM, Musa NL, Doss HS. Global paediatric advanced life support: improving child survival in limited-resource settings. Lancet. (2013) 381:256–65. doi: 10.1016/S0140-6736(12)61191-X
20. Hategeka C, Mwai L, Tuyisenge L. Implementing the Emergency Triage, Assessment and Treatment plus admission care (ETAT+) clinical practice guidelines to improve quality of hospital care in Rwandan district hospitals: healthcare workers' perspectives on relevance and challenges. BMC Health Serv Res. (2017) 17:256. doi: 10.1186/s12913-017-2193-4
21. Kapoor R, Sandoval MA, Avendano L, Cruz AT, Soto MA, Camp EA, et al. Regional scale-up of an Emergency Triage Assessment and Treatment (ETAT) training programme from a referral hospital to primary care health centres in Guatemala. Emerg Med J. (2016) 33:611–7. doi: 10.1136/emermed-2015-205057
23. World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. World Health Organization (2013).
24. World Health Organization. Recommendations for Management of Common Childhood Conditions: Newborn Conditions, Dystentery, Pneumonia, Oxygen Use and Delivery, Common Causes of Fever, Sever Acute Malnutrition, and Supportive Care: Evidence for Technical Update of Pocket Book Recommendations. World Health Organization (2012).
25. Fuchs A, Bielicki J, Mathur S, Sharland M, Van Den Anker JN. Reviewing the WHO guidelines for antibiotic use for sepsis in neonates and children. Paediatr Int Child Health. (2018) 38(sup1):S3–S15. doi: 10.1080/20469047.2017.1408738
26. Wiens MO, Kumbakumba E, Kissoon N, Ansermino JM, Ndamira A, Larson CP. Pediatric sepsis in the developing world: challenges in defining sepsis and issues in post-discharge mortality. Clin Epidemiol. (2012) 4:319–25. doi: 10.2147/CLEP.S35693
27. Williams PCM, Isaacs D, Berkley JA. Antimicrobial resistance among children in sub-Saharan Africa. Lancet Infect Dis. (2018) 18:e33–44. doi: 10.1016/S1473-3099(17)30467-X
28. Otu A, Elston J, Nsutebu E. Sepsis in Africa: practical steps to stem the tide. Pan Afr Med J. (2015) 21:323. doi: 10.11604/pamj.2015.21.323.6462
29. Tupchong K, Koyfman A, Foran M. Sepsis, severe sepsis, and septic shock: a review of the literature. Afr J Emerg Med. (2015) 5:127–35. doi: 10.1016/j.afjem.2014.05.004
30. Kwizera A, Festic E, Dunser MW. What's new in sepsis recognition in resource-limited settings? Intensive Care Med. (2016) 42:2030–3. doi: 10.1007/s00134-016-4222-x
31. Kadobera D, Sartorius B, Masanja H, Mathew A, Waiswa P. The effect of distance to formal health facility on childhood mortality in rural Tanzania, 2005–2007. Glob Health Action. (2012) 5:1–9. doi: 10.3402/gha.v5i0.19099
32. Frankenberg E. The effects of access to health care on infant mortality in Indonesia. Health Transit Rev. (1995) 5:143–63.
33. Adedini SA, Odimegwu C, Bamiwuye O, Fadeyibi O, De Wet N. Barriers to accessing health care in Nigeria: implications for child survival. Glob Health Action. (2014) 7:23499. doi: 10.3402/gha.v7.23499
34. Sankar J, Ismail J, Sankar MJ, C PS, Meena RS. Fluid Bolus Over 15–20 Versus 5–10 minutes each in the first hour of resuscitation in children with septic shock: a randomized controlled trial. Pediatr Crit Care Med. (2017) 18:e435–45. doi: 10.1097/PCC.0000000000001269
35. Santhanam I, Sangareddi S, Venkataraman S, Kissoon N, Thiruvengadamudayan V, Kasthuri RK. A prospective randomized controlled study of two fluid regimens in the initial management of septic shock in the emergency department. Pediatr Emerg Care. (2008) 24:647–55. doi: 10.1097/PEC.0b013e31818844cf
36. Slusher TM, Kiragu AW, Day LT, Bjorklund AR, Shirk A, Johannsen C, et al. Pediatric critical care in resource-limited settings-overview and lessons learned. Front Pediatr. (2018) 6:49. doi: 10.3389/fped.2018.00049
37. Turner EL, Nielsen KR, Jamal SM, von Saint André-von Arnim, Amelie, Musa NL. A review of pediatric critical care in resource-limited settings: a look at past, present, and future directions. Front Pediatr. (2016) 4:5. doi: 10.3389/fped.2016.00005
38. Chiedozie N. Sustainable health: the bedrock for sustainable African development. Health Sci J. (2016) 10:1–9.
39. Hossain MI, Chisti J, Yoshimatsu S, Yasmin R, Ahmed T. Features in septic children with or without severe acute malnutrition and the risk factors of mortality. Pediatrics. (2015) 135(Supplement 1):S10. doi: 10.1542/peds.2014-3330Q
40. Chisti MJ, Salam MA, Bardhan PK, Faruque AS, Shahid AS, Shahunja KM, et al. Severe sepsis in severely malnourished young bangladeshi children with pneumonia: a retrospective case control Study. PLoS ONE. (2015) 10:e0139966. doi: 10.1371/journal.pone.0139966
41. Obonyo N, Maitland K. Fluid management of shock in severe malnutrition: what is the evidence for current guidelines and what lessons have been learned from clinical studies and trials in other pediatric populations? Food Nutr Bull. (2014) 35(2 Suppl):S71–8. doi: 10.1177/15648265140352S111
42. Obonyo N, Brent B, Olupot-Olupot P, Boele van Hensbroek M, Kuipers I, Wong S, et al. Myocardial and haemodynamic responses to two fluid regimens in African children with severe malnutrition and hypovolaemic shock (AFRIM study). Crit Care. (2017) 21:103. doi: 10.1186/s13054-017-1679-0
43. World Health Organization. Paediatric Emergency Triage, Assessment and Treatment: Care of Critically-ill Children. Available online at: http://www.who.int/maternal_child_adolescent/doc uments/paediatric-emergency-triage-update/en/ (accessed June 21, 2016) (2016).
44. Leidman E, Tromble E, Yermina A, Johnston R, Isokpunwu C, Adeniran A, et al. Acute malnutrition among children, mortality, and humanitarian interventions in conflict-affected regions - Nigeria, October 2016-March 2017. Morb Mortal Wkly Rep. (2017) 66:1332–5. doi: 10.15585/mmwr.mm6648a4
45. Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DS, Jureen R, et al. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect Dis. (2007) 7:43. doi: 10.1186/1471-2334-7-43
46. Eagar H, Swan G, van Vuuren M. A survey of antimicrobial usage in animals in South Africa with specific reference to food animals. J S Afr Vet Assoc. (2012) 83:16. doi: 10.4102/jsava.v83i1.16
47. Egwuenu A, Obasanya J, Okeke I, Aboderin O, Olayinka A, Kwange D, et al. Antimicrobial use and resistance in Nigeria: situation analysis and recommendations, 2017. Pan Afr Med J. (2018) 2:2. doi: 10.11604/pamj.cp.2018.8.2.701
48. Rubulotta FM, Ramsay G, Parker MM, Dellinger RP, Levy MM, Poeze M. An international survey: public awareness and perception of sepsis. Crit Care Med. (2009) 37:167–70. doi: 10.1097/CCM.0b013e3181926883
49. Poeze M, Ramsay G, Gerlach H, Rubulotta F, Levy M. An international sepsis survey: a study of doctors' knowledge and perception about sepsis. Critical Care. (2004) 8:R409. doi: 10.1186/cc2959
50. Robson W, Beavis S, Spittle N. An audit of ward nurses' knowledge of sepsis. Nurs Crit Care. (2007) 12: 86–92. doi: 10.1111/j.1478-5153.2007.00210.x
51. Ziglam HM, Morales D, Webb K, Nathwani D. Knowledge about sepsis among training-grade doctors. J Antimicrob Chemother. (2006) 57:963–5. doi: 10.1093/jac/dkl042
Keywords: sepsis, children, infections, guidelines, outcomes, Nigeria
Citation: Nwankwor OC, McKelvie B, Frizzola M, Hunter K, Kabara HS, Oduwole A, Oguonu T and Kissoon N (2019) A National Survey of Resources to Address Sepsis in Children in Tertiary Care Centers in Nigeria. Front. Pediatr. 7:234. doi: 10.3389/fped.2019.00234
Received: 13 February 2019; Accepted: 22 May 2019;
Published: 11 June 2019.
Edited by:
Demet Demirkol, Istanbul University, TurkeyReviewed by:
Oguz Dursun, Akdeniz University, TurkeyJhuma Sankar, All India Institute of Medical Sciences, India
Copyright © 2019 Nwankwor, McKelvie, Frizzola, Hunter, Kabara, Oduwole, Oguonu and Kissoon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Odiraa C. Nwankwor, b2RpcmFhLm53YW5rd29yQG5lbW91cnMub3Jn
 Odiraa C. Nwankwor
Odiraa C. Nwankwor Brianna McKelvie
Brianna McKelvie Meg Frizzola
Meg Frizzola Krystal Hunter4
Krystal Hunter4 Halima S. Kabara
Halima S. Kabara Abiola Oduwole
Abiola Oduwole Tagbo Oguonu
Tagbo Oguonu Niranjan Kissoon
Niranjan Kissoon