- 1Asian Institute of Tele-surgery (IRCAD-Taiwan), Chang Bing Show Chwan Memorial Hospital, Lukang, Taiwan
- 2Division of Family Medicine, Chang Bing Show Chwan Memorial Hospital, Lukang, Taiwan
- 3Research Assistant Center, Show Chwan Memorial Hospital, Changhua, Taiwan
- 4Department of Medical Research, Chang Bing Show Chwan Memorial Hospital, Lukang, Taiwan
- 5Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 6Division of Pediatric Allergy, Immunology and Rheumatology, Department of Pediatrics, Show Chwan Memorial Hospital, Changhua, Taiwan
- 7Department of Nursing, Meiho University, Pingtung, Taiwan
Yolk sac tumor (YST), which most frequently arises in the gonads as a type of germ cell tumor, is rare in children but is highly malignant. It has been suggested that alpha-fetoprotein (AFP) can be applied as a feasible tumor marker because its level was elevated in >90% of YST. The treatment generally involves debulking surgery of tumors followed by systemic chemotherapy. Metastasis process of YST in children is different from that in adults and thus the treatment option is required. In this study, we described a rare case of YST in terms of the clinical manifestation, imaging, and histopathology findings, diagnosis and treatment in an 8-year-old girl. Furthermore, it is important to investigate more thoroughly a patient with history of intermittent abdominal pain and fever with previously multiple accesses, because these might be the critical signs for YST that should be alarmed for early treatment. Although YST is rare in children, pediatric physicians should be aware of this and prompt treatment should be addressed.
Introduction
Ovarian germ cell tumors (OGCTs) in which neoplasms form in the germ cells of the ovary, account for about 15~20% of all ovarian neoplasms (1). Only 1~2% of OGCTs are malignant called malignant ovarian germ cell tumors (MOGCTs) and constitute around 3–5% of all malignant ovarian neoplasms (2). Yolk sac tumor (YST) or endodermal primitive tumor accounting the second most common tumor in MOGCTs (3) is rare and typically occurs in gonads (4, 5). YST often presents in young women or adolescent girls with the ages between 18 and 30 years old; ~33% of YST patients are premenarchal (1, 6). In comparison with epithelial ovarian tumors, YST is highly malignant growing rapidly with a very brief duration of symptoms which metastasizes fast and intrudes all intra-abdominal structures and retroperitoneal lymph nodes (5, 7). YST was universally life-threatening before the development of combination chemotherapy. With the introduction of novel chemotherapeutic regimens in the end of 1970s, the 5-year survival rates of YST significantly improved from 14% to nearly 90% (8). Especially adding cisplatin to combination therapies, prognosis of the patients reached excellent values, even for patients with advanced stages (5). Therefore, YST is rare in children and malignant; however, it could be cured usually. In this study, we described a rare case of YST in an 8-year-old girl in terms of the clinical presentation, imaging findings, diagnosis, and treatment.
Case Presentation
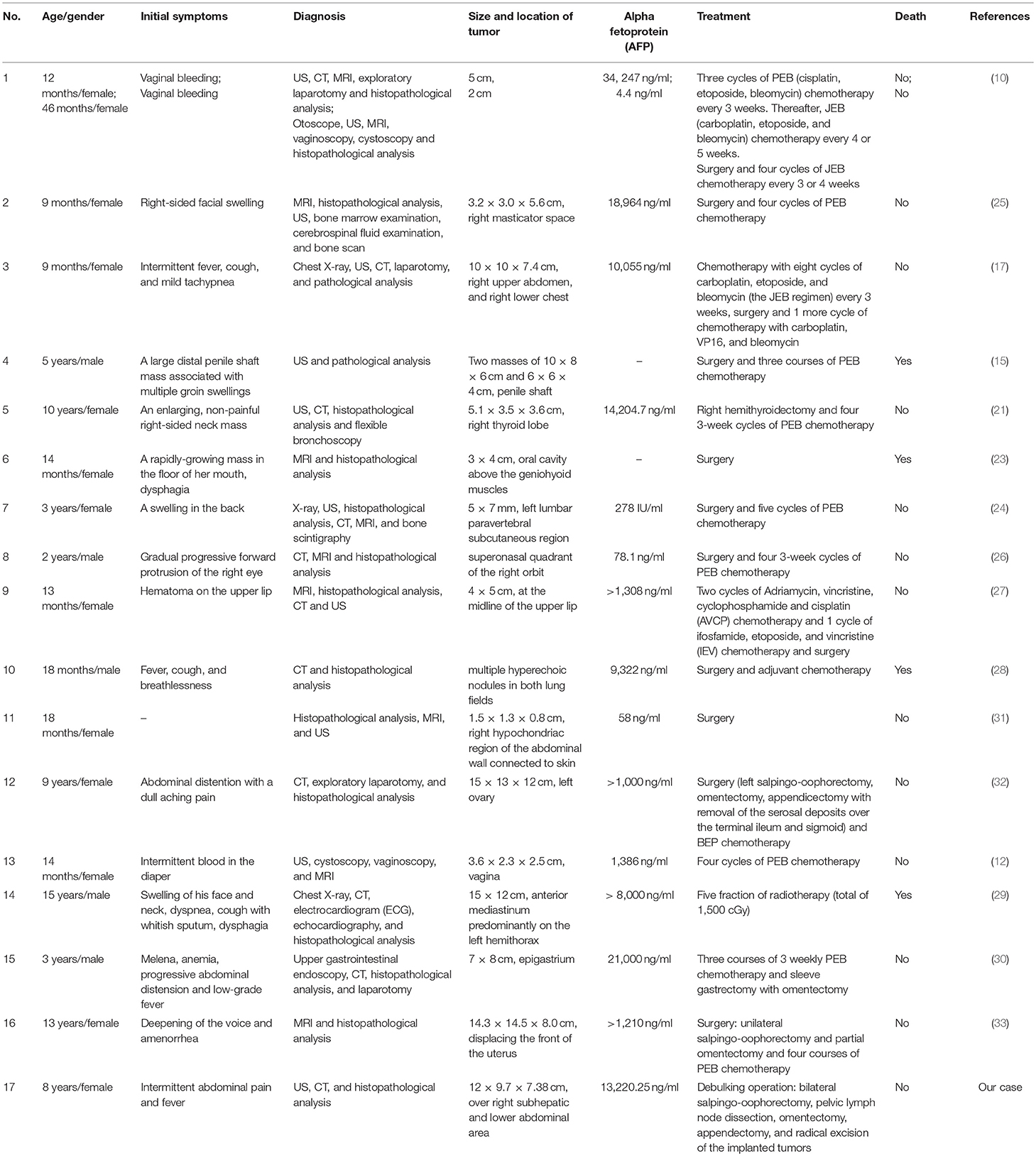
An 8-year-old girl suffered from intermittent abdominal pain and fever for 3 weeks. She had visited local clinics and regional hospital for several times. Oral medication was prescribed for pain and constipation, but her symptoms persisted. Then, she was brought to the Pediatrics outpatient department with nausea, vomiting and lower abdominal pain for 2 months. On physical examination, lower abdomen tenderness, and pale looking were noted. The ultrasonography (US) showed a huge pelvic tumor measuring 11.1 × 9.4 cm in size with cystic mass (3.6 × 2.8 cm) and bloody ascites over right subhepatic and lower abdominal area. There was no family history of cancer. The clinical diagnosis was pelvic mass, possibly neoplastic in nature. Computed tomography (CT) revealed a large pelvic mass, measuring 12 × 9.7 × 7.38 cm in size with internal heterogeneity and gynecological origin (ovary or urterus), enlarged paraaortic and mesenteric lymph nodes and fluid in Morrison pouch (Figure 1A). A three-dimensional (3D) virtual model was created from 1.25-mm thin-slice CT images and the green area illustrates the tumor (Figure 1B). The personal 3D model of patient's pelvic part may further improve the understanding of complex anatomy of the uterus, bladder and blood vessels. The values of tumor markers, alpha-fetoprotein (AFP), CA125 and beta human chorionic gonadotropin (hCG) were 13220.25 ng/ml, 536.7 U/ml, and <1.2 mIU/ml, respectively. The tumor was at the central pelvic cavity with direct invasion to the surrounding organs including the rectal and sigmoid colon walls, small bowel walls, the bladder wall and the cul-de-sac. In addition, it also involved the left para-adnexal tissues and the tip of appendix. Thus, the patient underwent debulking operation in which bilateral salpingo-oophorectomy, pelvic lymph node dissection, omentectomy, appendectomy, and radical excision of the implanted tumors were performed. Although the left ovary per se was grossly not involved by tumor, metastasis occurred as tumor invasion to the left periadnexal tissues and the left infundibulopelvic ligament were noted, thus left salpingo-oophorectomy was also done in order to prevent residual tumors and possible secondary surgery.
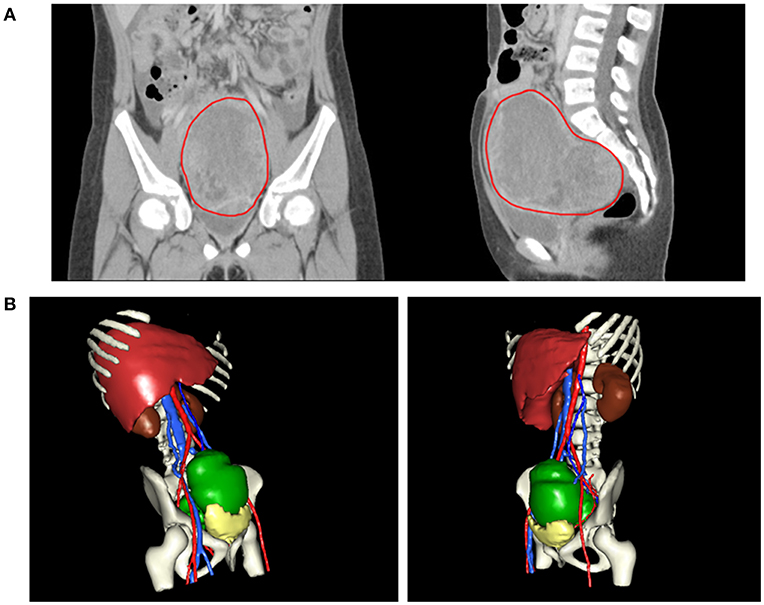
Figure 1. (A) CT revealing a large pelvic mass measuring 12 × 9.7 × 7.38 cm in size (circled by red line). (B) A three-dimensional virtual model created from 1.25-mm thin-slice CT images showing the tumor of 12 × 9.7 × 7.38 cm in size (green).
The patient was discharged 5 days after operation and recovered well. Gross examination of the specimen submitted for pathology showed a piece of necrotic tissue, measuring 3.5 × 2.0 × 0.3 cm in size. H&E stained sections of the specimen revealed tumor necrosis with small focus of atypical cells with hyperchromatic nuclei mainly in glandular or reticular pattern. Immunohistochemical staining revealed the residual cells were immunoreactive to AFP and glypican-3 (GPC-3). The findings were consistent with YST. The patient was then referred to the oncologist for further treatment and under stringent follow-up.
Discussion
YST is a rare tumor of childhood, which account for ~3.5% of all childhood cancers (<15 years) and usually arises in gonads, testis or ovary, thus a type of germ cell tumor. The incidence between the ages 15 and 19 rises to 16% (9). Approximately one-third are extra-gonadal origins, such as vagina, mediastinum, pineal gland, cervix, endometrium, and sacrococcygeal area (9–12). It has also been reported that YST occurred in penile shaft, urachus, stomach, liver, lungs, heart, thyroid, nasal region, cranial base, vulva, retroperitoneum, prostate, pericardium, diaphragm, mesentery, mouth, ears, omentum, eyes, and subcutaneous region (13–30). Also, the recent YST case reports in children are summarized in Table 1. There are two types of MOGCTs, germinomatous and non-germinomatous (2, 34). YST are the commonest non-germinomatous MOGCTs (34). In children, the vast majority of YST (85%) presented as clinical stage I in comparison with 35% in adults (35).
There are a number of diagnostic tools applied for YST, such as US, CT, magnetic resonance imaging (MRI) and histopathological analysis (Table 1). US characterizes the adnexal mass and shows ascites or hepatic metastasis. CT scan detects carcinosis and adenopathy, MRI reveals the hyper-vascularized and hemorrhagic feature of the mass (36). Moreover, exploratory laparotomy is emerging as a tool to detect the details of the tumor and surrounding involvement and also for biopsy (10, 32). YST are heterogeneous with a number of different histolopathological subtypes. In newborns and younger children, YST are predominant variant, whereas there are a wide variety of subtypes in adolescent. The typical histopathological features of YST are solid, tubular and focal papillary patterns with Schiller-Duval bodies and sinusoidal structures with fibrovascular cores lining formed by tumor cells, frequent mitotic figures and are cytokeratinpositive (14, 31).
It has been suggested that AFP can be applied as a feasible tumor marker because its level was elevated in >90% of YST (37). In our case, the level of AFP was also increased (13220.25 ng/ml). Thus, the prognosis of patients can be monitored by the AFP level after operation. It has been reported that the lowering level of post-operative serum AFP could be an useful marker for determining if residual cancer cells still exists after surgery (38). The national comprehensive cancer network (2016) recommends that patients who completed clinical course are monitored by AFP every 2~4 months for 2 years after treatment (36). Also, response to chemotherapy could be assessed by the AFP level (34). In particular, a postoperative AFP level of >1,000 ng/ml could serve as a prognostic indicator for the ovarian YST patients (39). However, the studies suggest a slight increase in AFP should not be applied as the sole criterion for chemotherapy decision (40).
The exact pathogenesis of YST remains obscure. However, some studies propose that it occurs from malignant transformation of misplaced germ cells. During the 4–6th week of embryogenesis, germ cells migrate laterally between the embryonic ectoderm and endoderm; germ cell tumor can arise anywhere along the migration from the mesoderm to the future cranial area (4). Although the pathogenesis of extragonadal germ cell tumors is unclear, two possible explanations were proposed: (1) misintegration of germ cells during development of embryo and (2) distribution of germs cells to other organs (41). Therefore, more research is required for investigating the mechanism of pathogenesis in order to develop for effective treatment for YST.
The general treatment for YST is surgery for eliminating the primary tumor without severe morbidity (34) (Table 1). In patients with stage I YST, previous studies proved that treatment of adnexectomy showed equivalent results to extensive surgery (8, 42). The treatment of OGCTs in the advanced stage generally involves debulking surgery of tumors followed by adjuvant chemotherapy (7) (Table 1). Several studies support the regimen of BEP (bleomycin, etoposide, and cisplatin) for primary treatment of the OGCT patients (36). They demonstrated a significantly high 5-year survival rate of 94%, even for the patients with residual cancers (42–44). The national comprehensive cancer network recommends 3–4 BEP cycles after surgical resection (36). Furthermore, it was reported that platinum-based chemotherapy should be used for the more malignant tumors such as endodermal sinus tumor and mixed germ cell tumor (45, 46). Neoadjuvant chemotherapy could be considered for the patients having extensive intra-abdominal disorders when initial surgical debulking is not preferred (47). Chemotherapy is suggested for treating recurrence (37). BEP chemotherapy is considered as a gold-standard first-line treatment for germ cell tumors at all stages (48). An important issue to consider for treating young patients is to reserve fertility by using fertility sparing strategy. Fertility-sparing surgery is possible to achieve because most tumors are unilateral (34). Furthermore, minimally invasive surgery has been proved to have better prognosis (34). Rudaitis et al. (47) applied neoadjuvant chemotherapy of four BEP cycles to decrease the tumor size in order to minimize the extent of surgery and thus the impact on the patient's fertility. In spite of the risks of damaging the reproductive function of female patients involved in chemotherapy, previous investigations have revealed that most of the women could recover their normal menstrual and reproductive functions post treatment (49, 50). After treatment, follow-ups are required such as abdominal and pelvic examination, CT, chest X-ray and AFP levels (51). Furthermore, YST metastasizes through the hematogenous route in >50% of the pediatric patients in comparison with only 4–6% of the adult patients (52). This fact modifies the therapeutic strategy. Thus, Retroperitoneal Lymph Node Dissection (RPLND) would not be the appropriate treatment for pediatric patients and complications such as wound infection, atelectasis, pulmonary insufficiency secondary to bleomycin-induced interstitial fibrosis, chylous ascities, small bowel obstruction, and subsequently ejaculatory dysfunction could occur (37). Herein, we also provided our experience in managing YST in children.
Conclusion
Our case highlights the importance of YST in children, and we provided our valuable experiences in the approaches of diagnosis and treatment for YST in children. Furthermore, it is important to investigate more thoroughly a patient with history of intermittent abdominal pain and fever with previously multiple accesses, because these might be the critical signs for YST that should not be neglected in order to treat the patient earlier. Although YST is rare in children, pediatric physicians should be still aware of this as it can be fatal and prompt treatment should be addressed.
Ethics Statement
We have obtained verbal and written informed consent from the patient's guardian for the publication of this case report.
Author Contributions
LC, K-CY, and S-BY wrote the manuscript. H-JW reviewed and revised the manuscript. All authors approved the final version of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kojimahara T, Nakahara K, Takano T, Yaegashi N, Nishiyama H, Fujimori K, et al. Yolk sac tumor of the ovary: a retrospective multicenter study of 33 Japanese women by Tohoku Gynecologic Cancer Unit (TGCU). Tohoku J Exp Med. (2013) 230:211–7. doi: 10.1620/tjem.230.211
2. Dallenbach P, Bonnefoi H, Pelte MF, Vlastos G. Yolk sac tumours of the ovary: an update. Eur J Surg Oncol. (2006) 32:1063–75. doi: 10.1016/j.ejso.2006.07.010
3. Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992-1999. Gynecol Oncol. (2005) 97:519–23. doi: 10.1016/j.ygyno.2005.02.007
4. Dede M, Pabuccu R, Yagci G, Yenen MC, Goktolga U, Gunhan O. Extragonadal yolk sac tumor in pelvic localization. a case report and literature review. Gynecol Oncol. (2004) 92:989–91. doi: 10.1016/j.ygyno.2003.12.026
5. Umezu T, Kajiyama H, Terauchi M, Shibata K, Ino K, Nawa A, et al. Long-term outcome and prognostic factors for yolk sac tumor of the ovary. Nagoya J Med Sci. (2008) 70:29–34.
6. Fujita M, Inoue M, Tanizawa O, Minagawa J, Yamada T, Tani T. Retrospective review of 41 patients with endodermal sinus tumor of the ovary. Int J Gynecol Cancer. (1993) 3:329–35.
7. McBee WC Jr, Brainard J, Sawady J, Rose PG. Yolk sac tumor of the ovary associated with endometrioid carcinoma with metastasis to the vagina: a case report. Gynecol Oncol. (2007) 105:244–7. doi: 10.1016/j.ygyno.2006.07.042
8. Nawa A, Obata N, Kikkawa F, Kawai M, Nagasaka T, Goto S, et al. Prognostic factors of patients with yolk sac tumors of the ovary. Am J Obstet Gynecol. (2001) 184:1182–8. doi: 10.1067/mob.2001.113323
9. Bernstein L, Smith ML. Germ cell trophoblastic and other gonadal neoplasms ICCC X 1975–2004. In: Ries L, Melbert D, Krapcho M, editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute (2007). p. 125–37.
10. Watanabe N, Okita H, Matsuoka K, Kiyotani C, Fujii E, Kumagai M, et al. Vaginal yolk sac (endodermal sinus) tumors in infancy presenting persistent vaginal bleeding. J Obstet Gynaecol Res. (2010) 36:213–6. doi: 10.1111/j.1447-0756.2009.01083.x
11. Sudour-Bonnange H, Orbach D, Kalfa N, Fasola S, Patte C. Germ cell tumors in atypical locations: experience of the TGM 95 SFCE trial. J Pediatr Hematol Oncol. (2014) 36:646–8. doi: 10.1097/MPH.0000000000000083
12. Lightfoot MA, Bilgutay AN, Kirsch AJ. A rare case of pediatric vaginal yolk sac tumor. Urology. (2018) 119:137–9. doi: 10.1016/j.urology.2018.01.007
13. Liang TC, Lu MY, Chen SJ, Lu FL, Lin KH. Cardiac tamponade caused by intrapericardial yolk sac tumor in a boy. J Formos Med Assoc. (2002) 101:355–8.
14. Alkatan HM, Al-Kofide A, Al-Hussain H. Yolk sac tumor: histopathologic report of 2 cases. Can J Ophthalmol. (2008) 43:125–6. doi: 10.3129/i07-198
15. Samaila MO, Maitama HY, Abdullahi K, Mbibu H, Waziri GD. Yolk sac tumour of the penile shaft: a rare primary extra-gonadal presentation. Afr J Paediatr Surg. (2011) 8:241–3. doi: 10.4103/0189-6725.86074
16. Magni E, Sonzogni A, Zampino MG. Primary pure gastric yolk sac tumor. Rare Tumors. (2010) 2:e10. doi: 10.4081/rt.2010.e10
17. Choi YS, Liu HC, Yeh TC, Hou JY, Sheu JC, Chen BF, et al. Primary diaphragmatic yolk sac tumor and review of the literature. J Pediatr Hematol Oncol. (2011) 33:e77–9. doi: 10.1097/MPH.0b013e3181ff0e17
18. Haibin Z, Yue J, Yaxian X. Primary yolk sac tumor of the omentum: a case report and literature review. Eur J Gynaecol Oncol. (2010) 31:682–4. doi: 10.1159/000337281
19. Subrahmanya NB, Kapadi SN, Junaid TA. Primary yolk sac (endodermal sinus) tumour of the vulva: a case report. Med Princ Pract. (2011) 20:90–2. doi: 10.1159/000319917
20. Warren M, Thompson KS. Two cases of primary yolk sac tumor of the liver in childhood: case reports and literature review. Pediatr Dev Pathol. (2009) 12:410–6. doi: 10.2350/08-09-0521.1
21. Furtado LV, Leventaki V, Layfield LJ, Lowichik A, Muntz HR, Pysher TJ. Yolk sac tumor of the thyroid gland: a case report. Pediatr Dev Pathol. (2011) 14:475–9. doi: 10.2350/11-01-0975-CR.1
22. Filho BC, McHugh JB, Carrau RL, Kassam AB. Yolk sac tumor in the nasal cavity. Am J Otolaryngol. (2008) 29:250–4. doi: 10.1016/j.amjoto.2007.09.001
23. Zhang Q, Huang Y, Bao CY, Li LJ. Yolk sac tumour involving floor of mouth: case report. Br J Oral Maxillofac Surg. (2013) 51:e67–9. doi: 10.1016/j.bjoms.2012.04.074
24. Tekgunduz SA, Bozkurt C, Sahin G, Apaydin S, Oren AC, Balkaya E, et al. A subcutaneous paraspinal yolk sac tumor in a child. J Pediatr Hematol Oncol. (2014) 36:e115–7. doi: 10.1097/MPH.0b013e31829d140a
25. Pasricha S, Gupta A, Shah M, Vadodaria H. Extragonadal yolk sac tumor of face in a female infant: a case report. Indian J Pathol Microbiol. (2010) 53:592–3. doi: 10.4103/0377-4929.68269
26. Kamal S, Kaliki S, Sreedhar A, Mishra DK. Primary orbital yolk sac tumor: report of a case and review of literature. Int Ophthalmol. (2016) 36:435–44. doi: 10.1007/s10792-015-0142-y
27. Chen Z, Zheng P, Huang S, Zhang D. Yolk sac tumor of upper lip: a case report. Oncol Lett. (2017) 14:6238–42. doi: 10.3892/ol.2017.6918
28. Mondal K, Mandal R. Bilateral lung metastases unveils an asymptomatic sacrococcygeal yolk sac tumor. Indian J Pathol Microbiol. (2017) 60:565–7. doi: 10.4103/IJPM.IJPM_385_16
29. Imaniar R, Syahruddin E, Soepandi PZ, Putra AC, Nurwidya F. Mediastinal yolk sac tumor infiltrating the heart. Exp Oncol. (2018) 40:82–4. doi: 10.31768/2312-8852.2018
30. Mandelia A, Mutt N, Lal R, Prasad R. Yolk Sac tumor of stomach: case report and review of literature. J Indian Assoc Pediatr Surg. (2018) 23:232–3. doi: 10.4103/jiaps.JIAPS_17_18
31. van den Akker M, Vervloessem D, Huybrechs A, Declercq S, van der Werff Ten Bosch J. Yolk sac tumor in the abdominal wall of an 18-month-old girl: a case report. J Med Case Rep. (2017) 11:47. doi: 10.1186/s13256-017-1216-4
32. Sharma C, Shah H, Sisodiya Shenoy N, Makhija D, Waghmare M. Ovarian yolk sac tumour in a girl - case report. Dev Period Med. (2017) 21:101–3.
33. Yang M, Xin Y. Virilization in a girl caused by an ovarian yolk sac tumor: a case report. J Pediatr Adolesc Gynecol. (2019) S1083-3188(19)30001-4. doi: 10.1016/j.jpag.2019.01.001
34. Guida M, Pignata S, Palumbo AR, Miele G, Marra ML, Visconti F, et al. Laparoscopic treatment of a Yolk Sac Tumor: case report and literature review. Transl Med UniSa. (2013) 7:1–5.
35. Christopher SC. Prepubertal Testicular and Paratesticular Tumors: Medscape Reference. New York, NY: WebMD LLC (2011).
36. Eddaoualline H, Sami H, Rais H, Belbaraka R, El Omrani A, Khouchani M. Ovarian Yolk sac tumor: a case report and literature review. Clin Case Rep Int. (2018) 2:1057.
37. Khan IU, Jose J, Fawazy T, Hadi WA, Sharma PK. Testicular yolk sac tumor in an eight-month old child: a case report. Gulf Med. J. (2012) 1:37–40.
38. Sell A, Sogaard H, Norgaard-Pedersen B. Serum alpha-fetoprotein as a marker for the effect of post-operative radiation therapy and/or chemotherapy in eight cases of ovarian endodermal sinus tumour. Int J Cancer. (1976) 18:574–80.
39. Guo YL, Zhang YL, Zhu JQ. Prognostic value of serum alpha-fetoprotein in ovarian yolk sac tumors: a systematic review and meta-analysis. Mol Clin Oncol. (2015) 3:125–32. doi: 10.3892/mco.2014.417
40. Albany C, Einhorn L. Pitfalls in management of patients with germ cell tumors and slight elevation of serum alpha-fetoprotein. J Clin Oncol. (2014) 32:2114–5. doi: 10.1200/JCO.2014.56.0607
41. Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, Gerl A, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol. (2002) 20:1864–73. doi: 10.1200/JCO.2002.07.062
42. Ayhan A, Taskiran C, Bozdag G, Altinbas S, Altinbas A, Yuce K. Endodermal sinus tumor of the ovary: the Hacettepe University experience. Eur J Obstet Gynecol Reprod Biol. (2005) 123:230–4. doi: 10.1016/j.ejogrb.2005.04.021
43. Curtin JP, Morrow CP, D'Ablaing G, Schlaerth JB. Malignant germ cell tumors of the ovary: 20-year report of LAC-USC Women's Hospital. Int J Gynecol Cancer. (1994) 4:29–35.
44. Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. (1987) 316:1435–40.
45. Chang FH, Lai CH, Chu KK, Chang TC, Hsueh S, Hung IJ. Treatment of malignant germ cell tumors of the ovary. J Formos Med Assoc. (1994) 93:411–6.
46. Gobel U, Calaminus G, Schneider DT, Schmidt P, Haas RJ, Makei, et al. Management of germ cell tumors in children: approaches to cure. Onkologie. (2002) 25:14–22. doi: 10.1159/000055197
47. Rudaitis V, Mickys U, Katinaite J, Dulko J. Successful treatment of advanced stage yolk sac tumour of extragonadal origin: a case report and review of literature. Acta Med Litu. (2016) 23:110–6. doi: 10.6001/actamedica.v23i2.3327
48. Williams S, Blessing JA, Liao SY, Ball H, Hanjani P. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. (1994) 12:701–6.
49. Zanetta G, Bonazzi C, Cantu M, Binidagger S, Locatelli A, Bratina G, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. (2001) 19:1015–20. doi: 10.1200/JCO.2001.19.4.1015
50. Byrne J, Mulvihill JJ, Myers MH, Connelly RR, Naughton MD, Krauss MR, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. (1987) 317:1315–21.
51. Kawai M, Kano T, Furuhashi Y, Mizuno K, Nakashima N, Hattori SE, et al. Prognostic factors in yolk sac tumors of the ovary. A clinicopathologic analysis of 29 cases. Cancer. (1991) 67:184–92.
Keywords: alpha-fetoprotein, chemotherapy, yolk sac tumor, pediatric, surgery
Citation: Chen LH, Yip K-C, Wu H-J and Yong S-B (2019) Yolk Sac Tumor in an Eight-Year-Old Girl: A Case Report and Literature Review. Front. Pediatr. 7:169. doi: 10.3389/fped.2019.00169
Received: 23 January 2019; Accepted: 12 April 2019;
Published: 30 April 2019.
Edited by:
Qiangsong Tong, Huazhong University of Science and Technology, ChinaReviewed by:
Alfredo Berrettini, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, ItalyMaría José Martínez-Urrutia, Hospital de Niños La Paz, Bolivia
Copyright © 2019 Chen, Yip, Wu and Yong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su-Boon Yong, eW9uZ3N1Ym9vbkBnbWFpbC5jb20=
†Co-first authors
 Li Hsun Chen
Li Hsun Chen Kui-Chuen Yip2†
Kui-Chuen Yip2† Hsing-Ju Wu
Hsing-Ju Wu Su-Boon Yong
Su-Boon Yong