- 1Division of General and Thoracic Surgery, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 2Neonatal Surgery Unit, Department of Medical and Surgical Neonatology, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy
- 3Department of Pediatric Surgery, Spirito Santo Hospital and G. d'Annunzio University of Chieti and Pescara, Chieti, Italy
Purpose: Surgical site infections (SSI) contribute to postoperative morbidity and mortality in children. Our aim was to evaluate the prevalence and identify risk factors for SSI in neonates.
Methods: Using a defined strategy, three investigators searched articles on neonatal SSI published since 2000. Studies on neonates and/or patients admitted to neonatal intensive care unit following cervical/thoracic/abdominal surgery were included. Risk factors were identified from comparative studies. Meta-analysis was conducted according to PRISMA guidelines using RevMan 5.3. Data are (mean ± SD) prevalence.
Results: Systematic review—of 885 abstracts screened, 48 studies (27,760 neonates) were included. The incidence of SSI was 5.6% (1,564 patients). SSI was more frequent in males (61.8%), premature babies (77.4%), and following gastrointestinal surgery (95.4%). Meta-analysis—10 comparative studies (16,442 neonates; 946 SSI 5.7%) showed that predictive factors for SSI development were gestational age, birth weight, age at surgery, length of surgical procedure, number of procedure per patient, length of preoperative hospital stay, and preoperative sepsis. Conversely, preoperative antibiotic use was not significantly associated with development of SSI.
Conclusions: Younger neonates and those undergoing abdominal procedures are at higher risk for SSI. Given the lack of evidence-based literature, prospective studies may help determine the risk factors for SSI in neonates.
Introduction
Surgical site infections (SSI) are infections that occur postoperatively in the area of the body where the surgery took place. SSI can be superficial and involve the skin only, or more serious and involve other tissues, organs, or implanted material. SSI are among the most common hospital acquired diseases and are an important cause of morbidity and mortality in all patients, including neonates and infants (1, 2).
Whilst the incidence and risk factors for SSI in adults and more recently in children have been defined and management guidelines have been established (3, 4), yet little is known about SSI in neonates and infants.
The incidence of SSI is 2–5% in adult patients undergoing inpatient surgery (3). Risk factors associated with SSI included co-morbidities, advanced age, risk indices, patient frailty, and surgery complexity (5).
In children the rate of SSI ranged from 2.5 to 5.4% and dirty wounds, inpatient status, increased duration of surgery, or certain surgical disciplines (cardiovascular, general surgery, neurosurgery, and orthopedics) were associated with increased risk of developing an SSI (4).
Previous studies have shown that the incidence of SSI in neonates and infants can be as high as 17% (1, 2). In this population of patients, several conditions have been reported to be associated with an increased risk of SSI, including admission to the neonatal intensive care unit (NICU), history of prematurity, low birth weight, mechanical ventilation, central venous access, associated co-morbidities, prolonged antibiotic administration, postsurgical hyperglycemia, and neutropenia (1, 2, 6, 7).
In the present study, we aimed to establish the incidence of SSI in neonates and to identifyprognostic factors that may help stratify which neonates are at increased risk to develop this complication. A better understanding of the causes leading to SSI could reduce their incidence, help define guidelines, and eventually improve outcome.
Methods
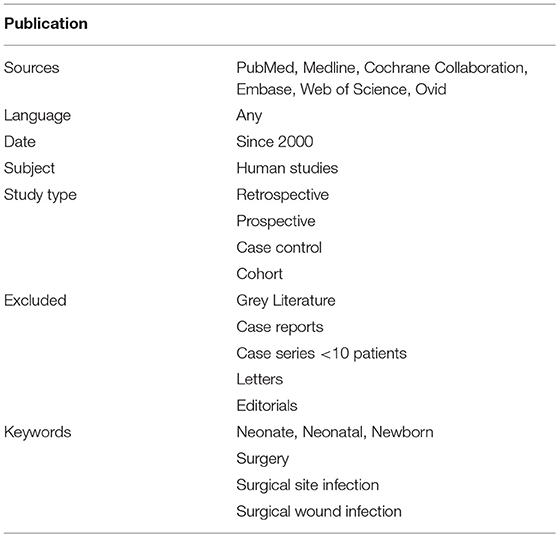
To investigate the incidence and risk factors of SSI in neonates, we conducted a systematic review of the literature and complemented it with a meta-analysis of comparative studies. Both the systematic review and the meta-analysis were drafted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (8). The protocol for this systematic review was registered on PROSPERO—international prospective register of systematic reviews (registration number: CRD42017069003) (9). Using a defined search strategy, three investigators (VDC, AB, and GL) independently searched scientific databases (PubMed, Medline, Cochrane Collaboration, Embase, Web of Science, Ovid) using a combination of keywords (Table 1). MeSH headings and terms used were “neonate OR newborn” AND “surgery OR surgical” AND “wound infection OR surgical site infection” (Supplementary File 1).
All gray literature publications (i.e., reports, theses, conference proceedings, bibliographies, commercial documentations, and official documents not published commercially) were excluded. Only studies on neonates (<44 wks gestational age) and/or neonates admitted to the NICU following cervical/thoracic/abdominal surgery published since 2,000 were included. Case reports, case series with <10 patients, animal studies, and opinion articles were excluded. The full text of the potentially eligible studies was retrieved and independently assessed for eligibility by the same three investigators. Any disagreement over the eligibility of particular studies was resolved through discussion with the other two authors (FM and AZ). Outcome measures included demographic data, type and district of surgery, SSI development, preoperative systemic infection, preoperative antibiotic prophylaxis, length of procedure, and number of procedure per patient. Risk factors of SSI were identified from comparative studies.
Statistical Analysis
Data were analyzed using GraphPad Prism 6.2 Macintosh Version (10). Data were compared using Fisher's exact test and are expressed as mean ± SD. When median and range were reported, mean ± SD were estimated, as previously reported (11).
The meta-analysis was conducted with RevMan 5.3 (12), using the fixed-effects model to produce risk ratio (RR) for categorical variables and mean differences (MD) for continuous variables, along with 95% confidence intervals (CI). We produced I2 values to assess homogeneity and quantify the dispersion of effect sizes.
Quality Assessment
Risk of bias for individual studies was assessed in duplicate (VDC and GL) using the methodological index for non-randomized studies (MINORS) (13). Differences between the two reviewers were resolved through consensus and discussion with another author (AZ). The total score for this 12-item instrument ranges 0–24 points with a validated “gold standard” cut-off of 19.8.
Two authors (FM and AZ) independently evaluated the present systematic reviews and meta-analysis using A Measurement Toll to Assess Systematic Reviews (AMSTAR) (14). The PRISMA checklist of our study was then completed (8).
Results
Systematic Review
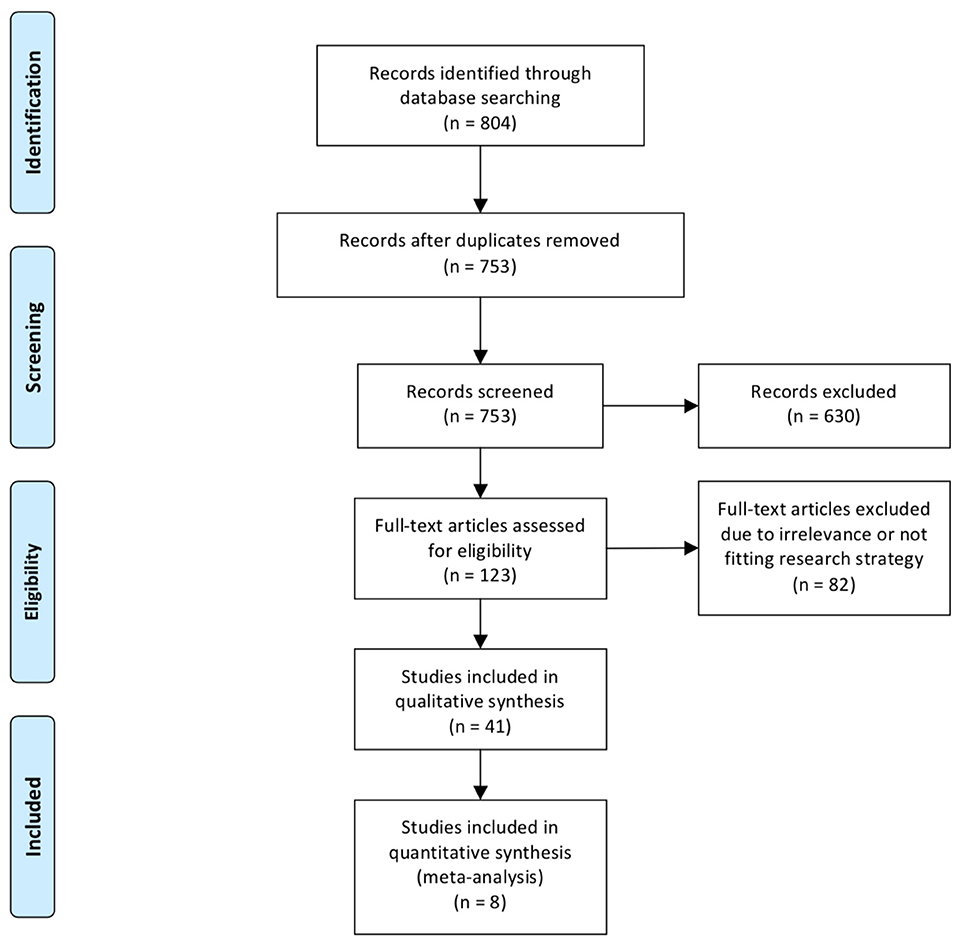
Of the 885 abstracts analyzed, 48 full articles, for a total of 27,760 patients (16,517 males, 59.5%) met our inclusion criteria (2, 15–61) (Figure 1). The overall incidence of SSI was 5.6% (n = 1,564) with a slight prevalence of male gender (61.8%) and premature babies (77.4%, gestational age at birth: 33 ± 7 weeks). The majority of neonates with SSI had gastrointestinal and/or colorectal surgery (95.4%), followed by thoracic (3.0%), and other (1.6%) procedures.
Comparative Studies
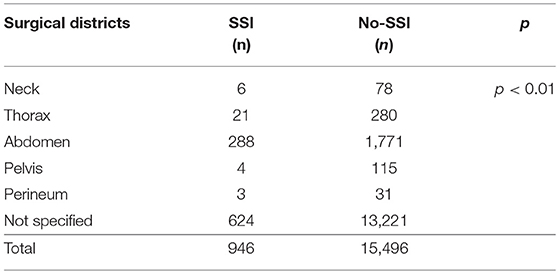
We analyzed 10 comparative studies (Tables 2a,b) (16–18, 22, 24, 34, 45, 47, 51, 60). Among these articles, there were only two prospective cohort studies (45, 60) and one national prospective database (16). No randomized studies were included. The papers included reported 946 cases of SSI out of 16,442 patients (5.7%). The distribution of surgical districts is significantly different between patients who developed SSI and those who did not (Table 3). SSI development was significantly associated with abdominal surgery (288/2,059 cases, 13.9%) in comparison with other surgical districts. In 13,845 patients the surgical district was not specified. When reported, the most common type of abdominal surgery was laparotomy for congenital abdominal wall defect (601 cases), necrotizing enterocolitis (133), malrotation (33), small bowel atresia (34), volvulus (17), or not specified congenital bowel obstruction (418).
Meta-Analysis
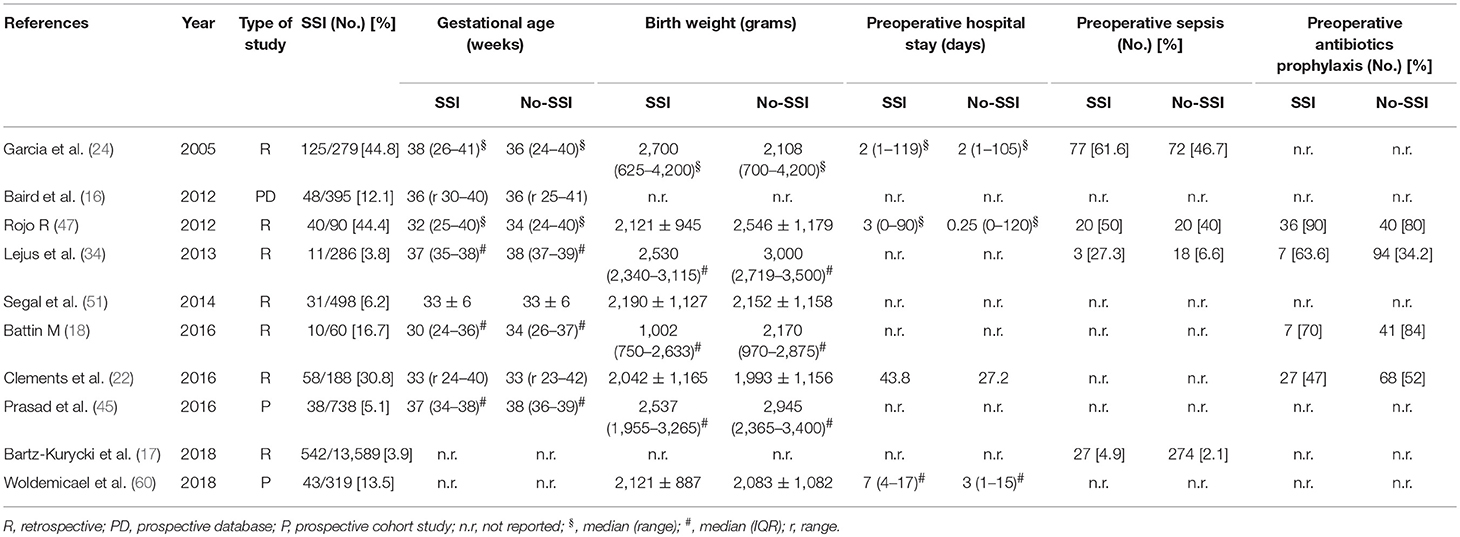
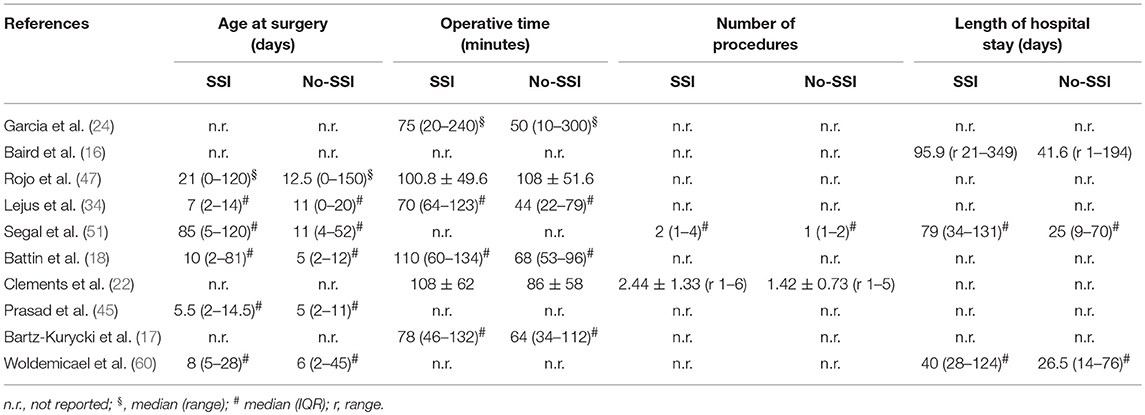
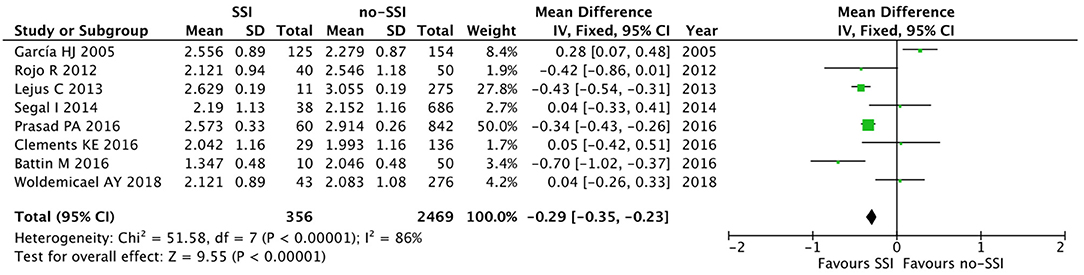
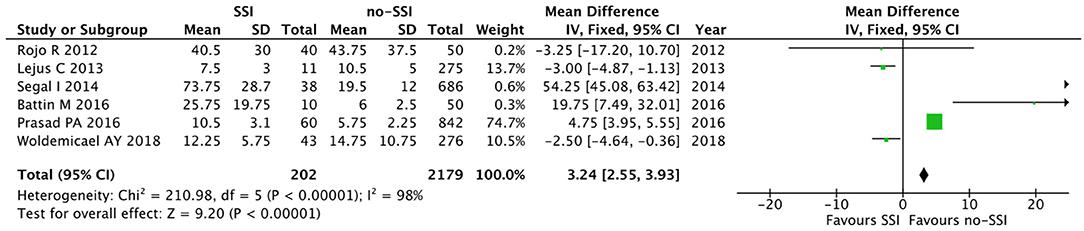
The meta-analysis of the 10 comparative studies (16–18, 22, 24, 34, 45, 47, 51, 60) showed that there was a slight albeit significant difference between neonates with and without SSI for gestational age (34.2 ± 2.4 weeks vs. 34.7 ± 2.3; p < 0.00001, MD −1.02, 95% CI [−1.22, −0.82], I2 = 86%; Figure 2) and birth weight (2,171 ± 479 grams vs. 2,384 ± 411; p < 0.00001, MD −0.29, 95% CI [−0.35, −0.23], I2 = 86%; Figure 3). Neonates with SSI were older at surgery compared to those without SSI (28.4 ± 24.4 days vs. 16.7 ± 14.3; p < 0.00001, MD 3.24, 95% CI [2.55, 3.93], I2 = 98%; Figure 4). The other main predictive factors for the development of an SSI were length of surgical procedure (SSI 96.7 ± 11.2 min vs. no SSI 71.2 ± 20.8; p < 0.00001, MD 15.82, 95% CI [14.06, 17.58], I2 = 85%; Figure 5), number of procedure per patient (SSI 2.3 ± 0.1 procedures vs. no SSI 1.3 ± 0.1; p < 0.00001, MD 1.00, 95% CI [0.79, 1.22], I2 = 0%; Figure 6), length of preoperative hospital stay (SSI 21.3 ± 11.4 days vs. no SSI 21.0 ± 13.5; p < 0.00001, MD 3.17, 95% CI [2.13, 4.21], I2 = 29%; Figure 7), and preoperative systemic infection (SSI 127/718 neonates, 17.7% vs. no SSI 384/13,526, 2.8%; p < 0.00001, OR 2.07, 95% CI [1.54, 2.78], I2 = 7%; Figure 8). Conversely preoperative antibiotics prophylaxes were not significantly associated with a reduced development of SSI (SSI 77/119 neonates, 64.7% vs. no SSI 243/505, 48.1%; p = 0.63, OR 1.12, 95% CI [0.70, 1.80], I2 = 53%; Figure 9). As expected, neonates with SSI showed a significant lengthened hospital stay in comparison with those without SSI (SSI 93.1 ± 42.6 days vs. no SSI 45.8 ± 20.6; p < 0.00001, MD 36.45, 95% CI [31.21, 41.68], I2 = 94%; Figure 10).
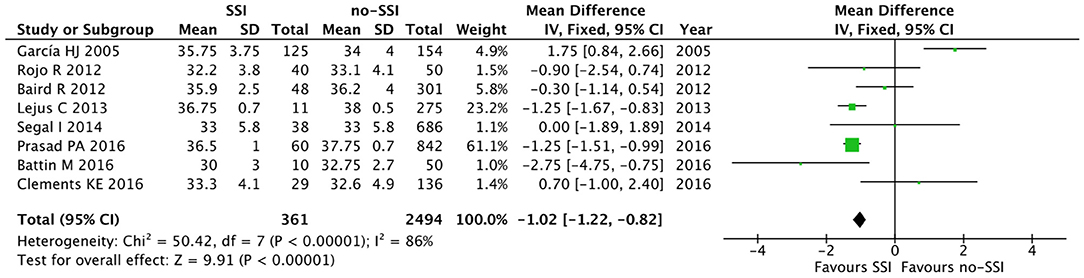
Figure 2. Forest plot comparison of gestational age at birth of neonates with or without postoperative SSI.
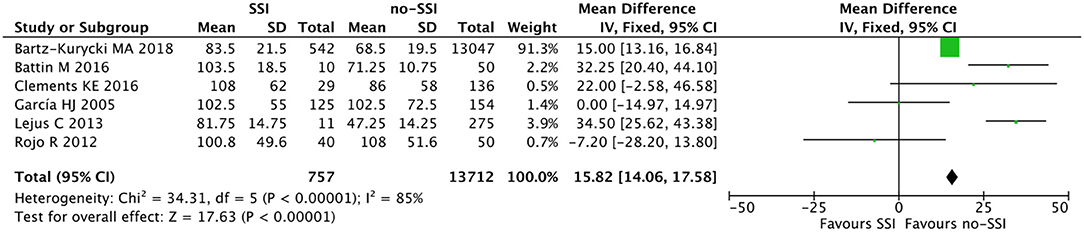
Figure 5. Forest plot comparison of length of procedure of neonates with or without postoperative SSI.
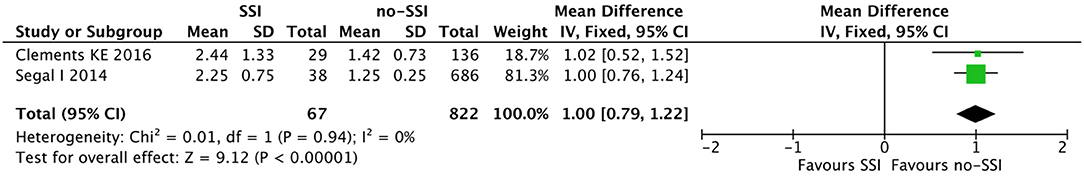
Figure 6. Forest plot comparison of number of procedure per patient in neonates with or without postoperative SSI.
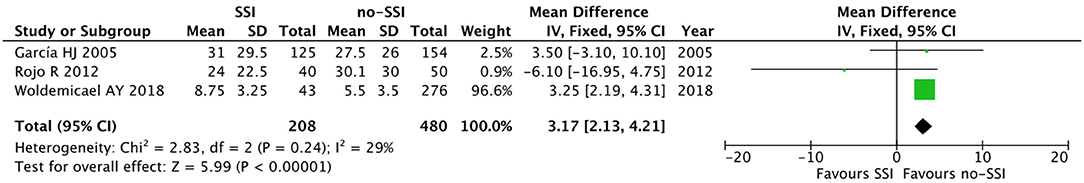
Figure 7. Forest plot comparison of preoperative hospital stay in neonates with or without postoperative SSI.
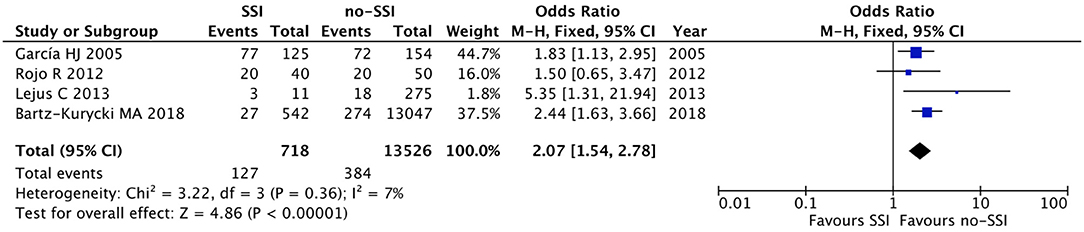
Figure 8. Forest plot comparison of preoperative systemic infection in neonates with or without postoperative SSI.
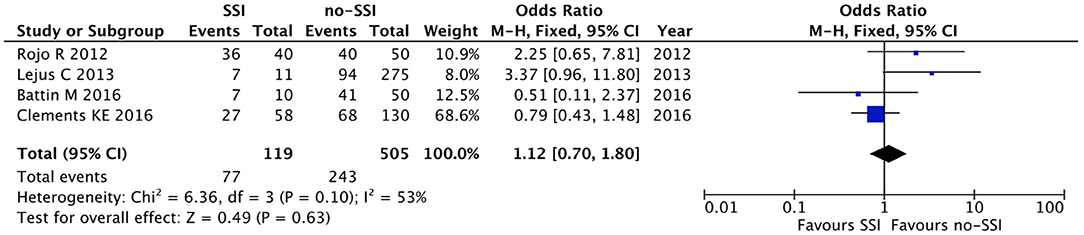
Figure 9. Forest plot comparison of preoperative antibiotic prophylaxis in neonates with or without postoperative SSI.
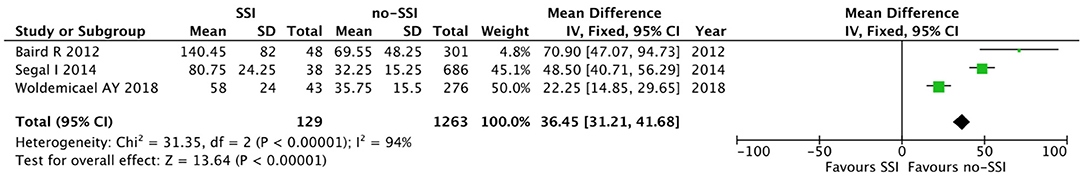
Figure 10. Forest plot comparison of length of hospital stay in neonates with or without postoperative SSI.
Discussion
The overall rate of SSI in adult and pediatric patients is ~ 2–5% and SSI are associated with increased morbidity, mortality, healthcare costs, and length of hospital stay (3, 62). Risk factors for SSI have been identified in predominantly adult populations and include advanced age, hyperglycemia, malnutrition, co-morbidities, risk indices, patient frailty, prior infections, complexity of surgery, increased operative time, and increased blood loss during surgery (5, 45). With regard to children, certain surgical disciplines (cardiovascular, general surgery, neurosurgery, and orthopedics) were associated with increased risk of developing an SSI (4). SSI development substantially increases the clinical and economic burden of surgery, because of prolonged hospitalization of the patient, diagnostic tests, and treatment. Moreover, SSI negatively impact on patient physical and mental health as well as on loss of earnings during recovery (63, 64).
Our study shows that neonates undergoing abdominal surgery are at high risk of SSI and that age at surgery and length of procedure are the main predictors of SSI in those admitted to NICU. Similarly, male gender and gestational age may be associated to the development of SSI, but the present data are not conclusive.
Although there is an abundance of literature on SSI in adults, there is a lack of studies having examined risk factors for SSI in neonates undergoing surgical interventions.
In the present systematic review of the literature on more than 27,000 patients, we found an incidence of SSI of 5.5%. Interestingly, the overall rate of SSI in the neonatal age group in our review is comparable to the rates reported in the older pediatric age group (51, 65, 66). This finding suggests that neonates may be less prone to SSI than it might be expected based on their alleged fragility, as they represent a special population that is thought to be at higher risk for infection due to their immature immune systems (6, 67, 68).
According to our study, premature infants represent a significant proportion of infants who require surgical interventions (51), and this is confirmed by the overall prevalence of SSI in this cohort (77%). When examining the type of surgical intervention, the vast majority of infants underwent gastrointestinal surgery (96%). In particular, abdominal surgery was significantly associated with an increased risk of SSI (23, 54). The most common type of surgical intervention described in the included articles was laparotomy for congenital abdominal wall defects, necrotizing enterocolitis, or congenital bowel obstruction. This surgical procedure is known to compromise the integrity of the gastrointestinal tract and to potentially result in bacterial translocation. Surgical wounds following a neonatal laparotomy are classified, at best, as clean-contaminated wounds, which justify the highest prevalence of SSI in this sub-group (22). The Canadian Pediatric Surgery Network recently reported an overall 15% incidence of SSI in infants with gastroschisis who underwent immediate (<6 h after birth) or delayed closure (55). For this reason, to reduce time of visceral exposure, the authors have proposed the gastroschisis sutureless closure, as it is also associated with a reduced risk of SSI (54).
To define the risk factors that are to be considered by the surgeon to estimate the risk of SSI at the time of neonatal surgery, we selected comparative studies that analyzed neonates with or without SSI. Interestingly, we observed that neonates who developed SSI had a younger GA compared to those who did not. This was a validation of the results from the systematic review that showed a high prevalence of prematurity among patients who developed SSI. Likewise, also a lower birth weight was associated with an increased risk of SSI in the included studies, even if the SSI group had an older age at the time of their procedures compared to the group who neonates who did not develop SSI. Moreover, this group also had a longer preoperative length of stay and it likely required a greater number of invasive diagnostic and therapeutic procedures per patient, as already reported by Garcia and Lejus (24, 34). In fact, this study confirmed a significant difference in number of surgical procedures between neonates with SSI and those without. This figure may be related to the severity of illness, with sicker patients being more likely to require additional procedures, although one third of SSI did occur after a single or first procedure. A long preoperative admission is a risk factor for SSI, as it is proportional to the severity of the underlying clinical conditions, the need for invasive devices and treatments (included prolonged antibiotics), and it promotes nosocomial flora colonization. Furthermore, neonates admitted to NICU are highly susceptible to nosocomial infections. As an expected consequence, the presence of a systemic infection significantly influenced the SSI incidence in our study.
Our present study also highlighted that the length of surgery is another risk factor for SSI. Length of surgery was not identified by all studies as a risk factor for SSI, and our meta-analysis confirmed the findings of Clements and coworkers who found a longer operative time in patients developing SSI (22). Prolonged visceral exposure may negatively impact on surgical outcome as consequence of skin contamination. Furthermore, the longer the operative time the deeper the surgical stress response, as the invasiveness of surgery and the length of procedure significantly correlated to oxidative stress activation and cortisol response (69–71). The latter may have an impact on postoperative outcome, such as the development of infectious complications, including SSI.
This study did not show any difference in preoperative antibiotic administration between neonates who developed SSI and those who did not. Whilst standardized preoperative antibiotic protocols in the adult population have shown to reduce the rate of SSI, a consensus is lacking among pediatric surgeons regarding preoperative antibiotic prophylaxis especially in neonates (1, 4). The different definitions of antibiotic prophylaxis and antibiotic regimen used in the analyzed studies may have led to our findings. The isolation of skin flora from a large number of wound cultures suggests that standardization of preoperative prophylaxis could potentially have an impact on the rate of SSI as has previously been demonstrated in pediatric patients (72, 73).
Limitations of the Study
Our study has some limitations. The first relates to the relative small number of studies available for the meta-analysis, with only two prospective cohort study and one national prospective database included. Nonetheless, the population of neonates reported in the studies was not small, with more than 27,000 neonates included.
The second limitation is that we could not analyze important variables, such as the use of adequate antibiotic prophylaxis, as they were not reported in the few studies selected in our analysis. Finally, the third limitation is the relative heterogeneity of the patient population: although we tried to limit the study to neonates, we included both patients with a post-conceptional age below 44 weeks and infants admitted in the NICU following abdominal, cervical, and thoracic surgery.
As a consequence, in our meta-analysis, none of the studies reached the gold standard cut-off on MINORS of 19.8 out of 24 (Table 4). However, when independently assessed by two authors using AMSTAR, the present systematic reviews and meta-analysis received a relevant score (Supplementary File 2) and the PRISMA checklist was completed (Supplementary File 3).
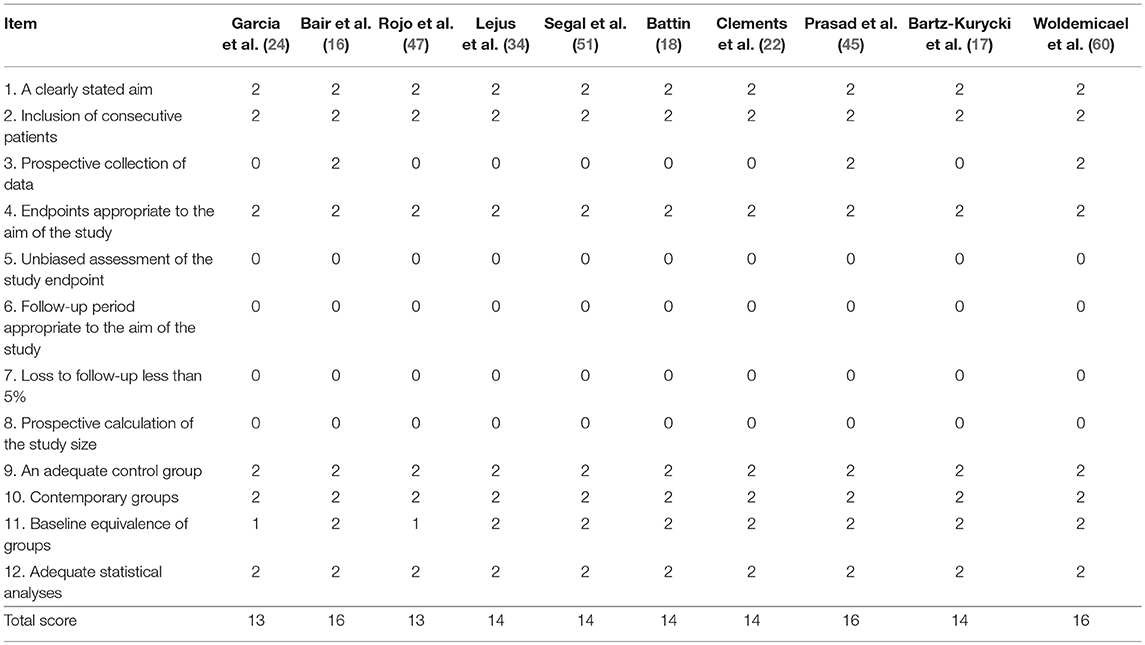
Table 4. Risk of bias assessment for individual studies using methodological index for nonrandomized studies (MINORS) (13).
Conclusion
In conclusion, SSI is a significant complication in neonates admitted to NICU that can negatively impact their outcome by prolonging hospital stay and increasing the risk for further complications, such as potentially fatal sepsis. Younger and smaller neonates at birth, those requiring longer or multiple operative procedures, and those with prolonged preoperative hospital stay and preoperative sepsis are at higher risk for SSI. These patients require special attention with close monitoring during their post-operative course. Given the lack of evidence in the literature, well-designed prospective studies on large cohorts of neonates may help setting up specific guidelines for the prevention and treatment of SSI in this particular population.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Author Contributions
VC, AB, GL, FM, and AZ: conception and design, analysis and interpretation, drafting, final approval. VC, AB, and GL: data acquisition. VC, GL, FM, and AZ: quality assessment. GL, FM, and AZ: revision.
Funding
This work was supported by the Canadian Institute of Health Research (CIHR) – SickKids Foundation New Investigator Research Grant (NI18-1270R).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00101/full#supplementary-material
Abbreviations
SSI, surgical site infection; NICU, neonatal intensive care unit.
References
1. Vu LT, Vittinghoff E, Nobuhara KK, Farmer DL, Lee H. Surgical site infections in neonates and infants: is antibiotic prophylaxis needed for longer than 24 h? Pediatr Surg Int. (2014) 30:587–92. doi: 10.1007/s00383–014-3506-x
2. Duque-Estrada EO, Duarte MR, Rodrigues DM, Raphael MD. Wound infections in pediatric surgery: a study of 575 patients in a university hospital. Pediatr Surg Int. (2003) 19:436–8. doi: 10.1007/s00383–002-0735–1
3. Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, et al. American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 Update. J Am Coll Surg. (2017) 224:59–74. doi: 10.1016/j.jamcollsurg.2016.10.029
4. Khoshbin A, So JP, Aleem IS, Stephens D, Matlow AG, Wright JG. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg. (2015) 262:397–402. doi: 10.1097/SLA.0000000000000938
5. Korol E, Johnston K, Waser N, Sifakis F, Jafri HS, Lo M, et al. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS ONE. (2013) 8:e83743. doi: 10.1371/journal.pone.0083743
6. Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol. (2008) 2008:628963. doi: 10.1155/2008/628963
7. Bhattacharyya N, Kosloske AM. Postoperative wound infection in pediatric surgical patients: a study of 676 infants and children. J Pediatr Surg. (1990) 25:125–9.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
9. 1111 PROSPERO International Prospective Register of Systematic Reviews. (2018). Available onlie at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017069003
10. 1111 GraphPad Software. San Diego, CA. Available online at: http://www.graphpad.com
11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 20:5:13. doi: 10.1186/1471-2288-5-13
12. Review Manager (RevMan). The Nordic Cochrane Centre. The Cochrane Collaboration, Copenhagen. (2014).
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
14. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. (2007) 7:10. doi: 10.1186/1471-2288-7-10
15. Adeniran JO, Abdur-Rahman L. One-stage correction of intermediate imperforate anus in males. Pediatr Surg Int. (2005) 21:88–90. doi: 10.1007/s00383-004-1211-x
16. Baird R, Gholoum S, Laberge JM, Puligandla P. Prematurity, not age at operation or incarceration, impacts complication rates of inguinal hernia repair. J Pediatr Surg. (2011) 46:908–11. doi: 10.1016/j.jpedsurg.2011.02.059
17. Bartz-Kurycki MA, Green C, Anderson KT, Alder AC, Bucher BT, Cina RA, et al. Enhanced neonatal surgical site infection prediction model utilizing statistically and clinically significant variables in combination with a machine learning algorithm. Am J Surg. (2018) 216:764–77. doi: 10.1016/j.amjsurg.2018.07.041
18. Battin M, Jamalpuri V, Bough G, Voss L. Antibiotic prophylaxis and neonatal surgical site infection. J Paediatr Child Health. (2016) 52:913–4. doi: 10.1111/jpc.13285
19. Beres A, Christison-Lagay ER, Romao RL, Langer JC. Evaluation of Surgisis for patch repair of abdominal wall defects in children. J Pediatr Surg. (2012) 47:917–9. doi: 10.1016/j.jpedsurg.2012.01.046
20. Bucher BT, Guth RM, Elward AM, Hamilton NA, Dillon PA, Warner BW, et al. Risk factors and outcomes of surgical site infection in children. J Am Coll Surg. (2011) 212:1033–8.e1. doi: 10.1016/j.jamcollsurg.2011.01.065
21. Chirdan LB, Uba FA, Ameh EA, Mshelbwala PM. Colostomy for high anorectal malformation: an evaluation of morbidity and mortality in a developing country. Pediatr Surg Int. (2008) 24:407–10. doi: 10.1007/s00383-008-2114-z
22. Clements KE, Fisher M, Quaye K, O'Donnell R, Whyte C, Horgan MJ. Surgical site infections in the NICU. J Pediatr Surg. (2016) 51:1405–8. doi: 10.1016/j.jpedsurg.2016.04.002
23. Eicher C, Seitz G, Bevot A, Moll M, Goelz R, Arand J, et al. Surgical management of extremely low birth weight infants with neonatal bowel perforation: a single-center experience and a review of the literature. Neonatology. (2012) 101:285–92. doi: 10.1159/000335325
24. García HJ, Rodríguez-Medina X, Franco-Gutiérrez M, Miranda-Novales G, Villegas-Silva R. Risk factors for surgical site infections in newborns in a neonatal intensive care unit. Rev Invest Clin. (2005) 57:425–33.
25. Gilje EA, Hossain MJ, Vinocur CD, Berman L. Surgical site infections in neonates are independently associated with longer hospitalizations. J Perinatol. (2017) 37:1130–4. doi: 10.1038/jp.2017.107
26. Guelfand M, Santos M, Olivos M, Ovalle A. Primary anastomosis in necrotizing enterocolitis: the first option to consider. Pediatr Surg Int. (2012) 28:673–6. doi: 10.1007/s00383-012-3092-8
27. Gurien LA, Dassinger MS, Burford JM, Saylors ME, Smith SD. Does timing of gastroschisis repair matter? A comparison using the ACS NSQIP pediatric database. J Pediatr Surg. (2017) 52:1751–4. doi: 10.1016/j.jpedsurg.2017.02.008
28. Gupta S, Gupta R, Ghosh S, Gupta AK, Shukla A, Chaturvedi V, et al. Intestinal Atresia: Experience at a Busy Center of North-West India. J Neonatal Surg. (2016) 5:51. doi: 10.21699/jns.v5i4.405
29. Hagander L, Kabir M, Chowdhury MZ, Gunnarsdóttir A, Habib MG, Banu T. Major neonatal surgery under local anesthesia: a cohort study from Bangladesh. World J Surg. (2015) 39:953–60. doi: 10.1007/s00268-014-2895-2
30. Ichikawa S, Ishihara M, Okazaki T, Warabi K, Kato Y, Hori S, et al. Prospective study of antibiotic protocols for managing surgical site infections in children. J Pediatr Surg. (2007) 42:1002–7. doi: 10.1016/j.jpedsurg.2007.01.034
31. Inoue M, Uchida K, Ichikawa T, Nagano Y, Matsushita K, Koike Y, et al. Contaminated or dirty wound operations and methicillin-resistant Staphylococcus aureus (MRSA) colonization during hospitalization may be risk factors for surgical site infection in neonatal surgical patients. Pediatr Surg Int. (2018) 34:1209–14. doi: 10.1007/s00383-018-4338-x
32. Inoue M, Uchida K, Otake K, Koike Y, Okugawa Y, Kobayashi M, et al. Placement of prophylactic drains after laparotomy may increase infectious complications in neonates. Pediatr Surg Int. (2011) 27:975–9. doi: 10.1007/s00383-011-2905-5
33. Landisch RM, Massoumi RL, Christensen M, Wagner AJ. Infectious outcomes of gastroschisis patients with intraoperative hypothermia. J Surg Res. (2017) 215:93–7. doi: 10.1016/j.jss.2017.03.053
34. Lejus C, Dumont R, Gall CL, Guillaud C, Guen CG, Leclair MD, et al. A preoperative stay in an intensive care unit is associated with an increased risk of surgical site infection in neonates. J Pediatr Surg. (2013) 48:1503–8. doi: 10.1016/j.jpedsurg.2013.01.055
35. Lockhat A, Kernaleguen G, Dicken BJ, van Manen M. Factors associated with neonatal ostomy complications. J Pediatr Surg. (2016) 51:1135–7. doi: 10.1016/j.jpedsurg.2015.09.026
36. Mallick MS, Jado AM, Al-Bassam AR. Surgical procedures performed in the neonatal intensive care unit on critically ill neonates: feasibility and safety. Ann Saudi Med. (2008) 28:105–8. doi: 10.5144/0256-4947.2008.105
37. Martínez Castaño I, Aranda García MJ, Sánchez Morote JM, Ruiz Pruneda R, Fernández Ibieta M, Rojas Ticona J, et al. Prevention of the wound infection: little changes, huge results. Circ Pediatr. (2017) 30:138–41.
38. Murphy FJ, Mohee A, Khalil B, Lall A, Morabito A, Bianchi A. Versatility of the circumumbilical incision in neonatal surgery. Pediatr Surg Int. (2009) 25:145–7. doi: 10.1007/s00383-008-2303-9
39. Nagdeve NG, Bhingare PD, Naik HR. Neonatal posterior sagittal anorectoplasty for a subset of males with high anorectal malformations. J Indian Assoc Pediatr Surg. (2011) 16:126–8. doi: 10.4103/0971-9261.86863
40. Naji H, Foley J, Ehren H. Use of Surgisis for abdominal wall reconstruction in children with abdominal wall defects. Eur J Pediatr Surg. (2014) 24:94–6. doi: 10.1055/s-0033-1354587
41. Ndongo R, Tolefac PN, Tambo FFM, Abanda MH, Ngowe MN, Fola O, et al. Infantile hypertrophic pyloric stenosis: a 4-year experience from two tertiary care centres in Cameroon. BMC Res Notes. (2018) 11:33. doi: 10.1186/s13104-018-3131-1
42. Ohashi K, Koshinaga T, Uehara S, Furuya T, Kaneda H, Kawashima H, et al. Sutureless enterostomy for extremely low birth weight infants. J Pediatr Surg. (2017) 52:1873–7. doi: 10.1016/j.jpedsurg.2017.08.009
43. Osifo OD, Aghahowa SE. Audit of antibiotic therapy in surgical neonates in a tertiary hospital in Benin City, Nigeria. Afr J Paediatr Surg. (2011) 8:23–8. doi: 10.4103/0189-6725.78664
44. Palatnik A, Loichinger M, Wagner A, Peterson E. The association between gestational age at delivery, closure type and perinatal outcomes in neonates with isolated gastroschisis. J Matern Fetal Neonatal Med. (2018) 1:1–7. doi: 10.1080/14767058.2018.1519538
45. Prasad PA, Wong-McLoughlin J, Patel S, Coffin SE, Zaoutis TE, Perlman J, et al. Surgical site infections in a longitudinal cohort of neonatal intensive care unit patients. J Perinatol. (2016) 36:300–5. doi: 10.1038/jp.2015.191
46. Rijhwani A, Davenport M, Dawrant M, Dimitriou G, Patel S, Greenough A, et al. Definitive surgical management of antenatally diagnosed exomphalos. J Pediatr Surg. (2005) 40:516–22. doi: 10.1016/j.jpedsurg.2004.11.028
47. Rojo R, Fanjul M, Garcia-Casillas MA, Corona C, Tardáguila AR, Zornoza M, et al. Surgical wound infections in newborns: analysis of risk factors. Circ Pediatr. (2012) 25:129–34.
48. Saadia A, Prasad GR. Neonatal tracheostomy - issues and solutions. J Neonatal Surg. (2015) 4:13. doi: 10.21699/jns.v4i2.228
49. Sangkhathat S, Patrapinyokul S, Chiengkriwate P, Chanvitan P, Janjindamai W, Dissaneevate S. Infectious complications in infants with gastroschisis: an 11-year review from a referral hospital in southern Thailand. J Pediatr Surg. (2008) 43:473–8. doi: 10.1016/j.jpedsurg.2007.10.026
50. Schlueter RK, Azarow KS, Hines AG, Varman M, Abdessalam SF, Raynor SC, et al. Identifying strategies to decrease infectious complications of gastroschisis repair. J Pediatr Surg. (2015) 50:98–101. doi: 10.1016/j.jpedsurg.2014.10.001
51. Segal I, Kang C, Albersheim SG, Skarsgard ED, Lavoie PM. Surgical site infections in infants admitted to the neonatal intensive care unit. J Pediatr Surg. (2014) 49:381–4. doi: 10.1016/j.jpedsurg.2013.08.001
52. Singh V, Pathak M. Congenital neonatal intestinal obstruction: retrospective analysis at tertiary care hospital. J Neonatal Surg. (2016) 5:49. doi: 10.21699/jns.v5i4.393
53. Sinha S, Kumar Sarin YP, Deshpande V. Neonatal sacrococcygeal teratoma: our experience with 10 cases. J Neonatal Surg. (2013) 2:4. doi: 10.21699/jns.v2i1.5
54. Stanger J, Mohajerani N, Skarsgard ED. Canadian Pediatric Surgery Network (CAPSNet). Practice variation in gastroschisis: factors influencing closure technique. J Pediatr Surg. (2014) 49:720–3. doi: 10.1016/j.jpedsurg.2014.02.066
55. Suri M, Langer JC. A comparison of circumumbilical and transverse abdominal incisions for neonatal abdominal surgery. J Pediatr Surg. (2011) 46:1076–80. doi: 10.1016/j.jpedsurg.2011.03.032
56. Tanaka T, Okazaki T, Fukatsu Y, Okawada M, Koga H, Miyano G, et al. Surgical intervention for congenital diaphragmatic hernia: open versus thoracoscopic surgery. Pediatr Surg Int. (2013) 29:1183–6. doi: 10.1007/s00383-013-3382-9
57. Tsuji Y, Maeda K, Ono S, Yanagisawa S, Baba K, Usui Y. A new paradigm of scarless abdominal surgery in children: transumbilical minimal incision surgery. J Pediatr Surg. (2014) 49:1605–9. doi: 10.1016/j.jpedsurg.2014.06.009
58. Walker S, Datta A, Massoumi RL, Gross ER, Uhing M, Arca MJ. Antibiotic stewardship in the newborn surgical patient: a quality improvement project in the neonatal intensive care unit. Surgery. (2017) 162:1295–303. doi: 10.1016/j.surg.2017.07.021
59. Weil BR, Leys CM, Rescorla FJ. The jury is still out: changes in gastroschisis management over the last decade are associated with both benefits and shortcomings. J Pediatr Surg. (2012) 47:119–24. doi: 10.1016/j.jpedsurg.2011.10.029
60. Woldemicael AY, Bradley S, Pardy C, Richards J, Trerotoli P, Giuliani S. Surgical site infection in a tertiary neonatal surgery centre. Eur J Pediatr Surg. (2018). doi: 10.1055/s-0038-1636916. [Epub ahead of print].
61. Wu Y, Lai W, Pei J, Zhao Y, Wang Q, Xiang B. Hyperglycemia and its association with clinical outcomes in postsurgical neonates and small infants in the intensive care unit. J Pediatr Surg. (2016) 51:1142–5. doi: 10.1016/j.jpedsurg.2016.01.001
62. Kulaylat AN, Engbrecht BW, Rocourt DV, Rinaldi JM, Santos MC, Cilley RE, et al. Measuring surgical site infections in children: comparing clinical, electronic, and administrative data. J Am Coll Surg. (2016) 222:823–30. doi: 10.1016/j.jamcollsurg.2016.01.004
63. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. (2017) 96:1–15. doi: 10.1016/j.jhin.2017.03.004
64. Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. (2016) 16:e276–87. doi: 10.1016/S1473-3099(16)30398-X
65. Davenport M, Doig CM. Wound infection in pediatric surgery: a study in 1,094 neonates. J Pediatr Surg. (1993) 28:26–30. doi: 10.1016/s0022-3468(05)80348-3
67. Gilje EA, Hossain MJ, Vinocur CD, Berman L. Surgical site infections in neonates are independently associated with longer hospitalizations. J Perinatol. (2017) 37:1130–4. doi: 10.1038/jp.2017.107.
68. Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol. (2006) 121:251–9. doi: 10.1016/j.clim.2006.08.010
69. Gil-Gómez R, Blasco-Alonso J, Castillo-Martín R, Milano-Manso G. Prognostic indicators after cardiac surgery in children and their relationship with the oxidative stress response. Rev Esp Anestesiol Reanim. (2016) 63:3–12. doi: 10.1016/j.redar.2015.01.015
70. Khoo B, Boshier PR, Freethy A, Tharakan G, Saeed S, Hill N, et al. Redefining the stress cortisol response to surgery. Clin Endocrinol. (2017) 87:451–8. doi: 10.1111/cen.13439
71. Prete A, Yan Q, Al-Tarrah K, Akturk HK, Prokop LJ, Alahdab F, at al. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol. (2018) 89:554–67. doi: 10.1111/cen.13820
72. Porras-Hernandez J, Bracho-Blanchet E, Tovilla-Mercado J, Vilar-Compte D, Nieto-Zermeño J, Davila-Perez R, et al. A standardized perioperative surgical site infection care process among children with stoma closure: a before-after study. World J Surg. (2008) 32:2316–23. doi: 10.1007/s00268-008-9617-6
Keywords: newborn, wound infection, neonatal surgery, risk factors, systematic review, meta-analysis
Citation: Catania VD, Boscarelli A, Lauriti G, Morini F and Zani A (2019) Risk Factors for Surgical Site Infection in Neonates: A Systematic Review of the Literature and Meta-Analysis. Front. Pediatr. 7:101. doi: 10.3389/fped.2019.00101
Received: 06 January 2019; Accepted: 05 March 2019;
Published: 29 March 2019.
Edited by:
Oliver J. Muensterer, Johannes Gutenberg University Mainz, GermanyReviewed by:
Maximilian Weniger, Massachusetts General Hospital and Harvard Medical School, United StatesAna Catarina Fragoso, Universidade do Porto, Portugal
Copyright © 2019 Catania, Boscarelli, Lauriti, Morini and Zani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Augusto Zani, YXVndXN0by56YW5pQHNpY2traWRzLmNh
 Vincenzo Davide Catania
Vincenzo Davide Catania Alessandro Boscarelli
Alessandro Boscarelli Giuseppe Lauriti
Giuseppe Lauriti Francesco Morini
Francesco Morini Augusto Zani
Augusto Zani