- 1Laboratory for Urologic Tissue Engineering and Stem Cell Therapy, Department of Urology, University Hospital, Zurich, Switzerland
- 2Division of Pediatric Urology, Department of Pediatric Surgery, University Children‘s Hospital, Zurich, Switzerland
Several congenital disorders can cause end stage bladder disease and possibly renal damage in children. The current gold standard therapy is enterocystoplasty, a bladder augmentation using an intestinal segment. However, the use of bowel tissue is associated with numerous complications such as metabolic disturbance, stone formation, urine leakage, chronic infections, and malignancy. Urinary diversions using engineered bladder tissue would obviate the need for bowel for bladder reconstruction. Despite impressive progress in the field of bladder tissue engineering over the past decades, the successful transfer of the approach into clinical routine still represents a major challenge. In this review, we discuss major achievements and challenges in bladder tissue regeneration with a focus on different strategies to overcome the obstacles and to meet the need for living functional tissue replacements with a good growth potential and a long life span matching the pediatric population.
Introduction
Congenital disorders such as posterior urethral valves, bladder extrophy, and neurogenic bladder result in reduced bladder capacity, impaired compliance, incontinence, and possibly renal damage. Despite decades of experience in the management of end stage bladder disease, current therapy options are not curative. Enterocystoplasty—bladder augmentation using an intestinal segment—is the gold standard therapy if medical management fails. However, it is associated with severe complications, including metabolic disturbances, stone formation, urine leakage, and chronic infections owing to the inherent absorptive and secretory properties of the gastrointestinal segments (1–3). Given the limited success and high morbidity with current treatment options, tissue engineering (TE) has been considered as a novel treatment approach. The regeneration of bladder tissue derived from the patient's own cells may represent an attractive option particularly for patients of pediatric urology. The pediatric population presents several opportunities for the application of TE, as the regenerative capacity is significantly greater in infants and children than in adults. However, the specific needs of the pediatric population, primarily the need for living functional tissue replacements with a good growth potential and a long life span need to be addressed.
First attempts to replace bladder tissue by synthetic materials were performed in the 1950s, where plastic urinary bladder substitutes were implanted into patients (4, 5). The first biomaterials used for reconstruction of the urinary bladder for clinical applications were gelatin sponge (6, 7), cellular collagen biomatrix (8) resin sprayed paper (9), bovine pericardium (10), and dura (11). However, due to unsatisfactory postoperative results this technique was suspended.
The urinary bladder possesses a unique anatomy, allowing for repetitive expansion, and contraction and withstanding the urine pressure. Furthermore, the bladder is lined with a highly specialized multilayer epithelium, the urothelium, which acts as a tight urine barrier (12). The complexity of this structure poses a challenge for regenerative medicine. During the last two decades, TE has become a rapidly growing field of research in biotechnology and medicine. It is driven by the fascinating idea of generating autologous tissue substitutes for the treatment of tissue defects and organ failure. Several animal studies have shown promising results in bladder TE. The concept of TE involves the integration of various interacting components: the applied cells need to be held together by a tridimensional scaffold which provides the shape and initial mechanical strength, and molecular signals need to induce tissue regeneration in vivo. There are two common approaches in bladder engineering. The acellular approach includes the use of natural or synthetic biomaterials to enhance the body's natural growth to regenerate and repair itself (13). In the cellular approach, the removed donor tissue is dissociated into individual cells, either mechanically and by enzymatic digestion. Subsequently, the functional cells are either directly implanted into the host or seeded on a suitable biomaterial after expansion in culture and thereafter implanted into a non-functional site of the bladder (14).
Several cell based approaches (15–17) in different animal models (18–20) were successful with the formation of native tissue-like epithelialization and progressive muscle and blood vessel formation (21). Atala's group created engineered bladder tissue in a canine model using autologous cells and showed functional and anatomical characteristics of a normal bladder (3, 22). In a first clinical study human bladder was engineered for patients (aged 4–19 years) with end-stage bladder disease by isolating patient's autologous bladder urothelial and muscle cells, expanding the cells in vitro, and seeding them on a biodegradable collagen-polyglycolic acid scaffolds. The implanted composite engineered bladders were reported to show sustainably improved functional parameters (23). However, the majority of treated patients did not achieve good bladder capacity and compliance, but developed fibrosis of the artificial bladder wall. A recent phase II study (24), using an autologous cell seeded biodegradable composite scaffold for augmentation cystoplasty in children with spina bifida did not provide improved bladder compliance or capacity. Even though these two clinical trials were similar in design and used the same cell types, the differences in cell number, type of biomaterial, or surface area grafted and type of surgery might have influenced the outcome. To date, clinical translation has failed to establish a reliably effective treatment. Various hurdles such as early tissue fibrosis, lack of vascularization, insufficient urine barrier and inadequate contractibility are challenges encountered in regenerative medicine (25, 26). In this review, we discuss the major achievements and challenges in bladder tissue regeneration and focus on different strategies to overcome obstacles.
Cellular Application in Tissue Engineering
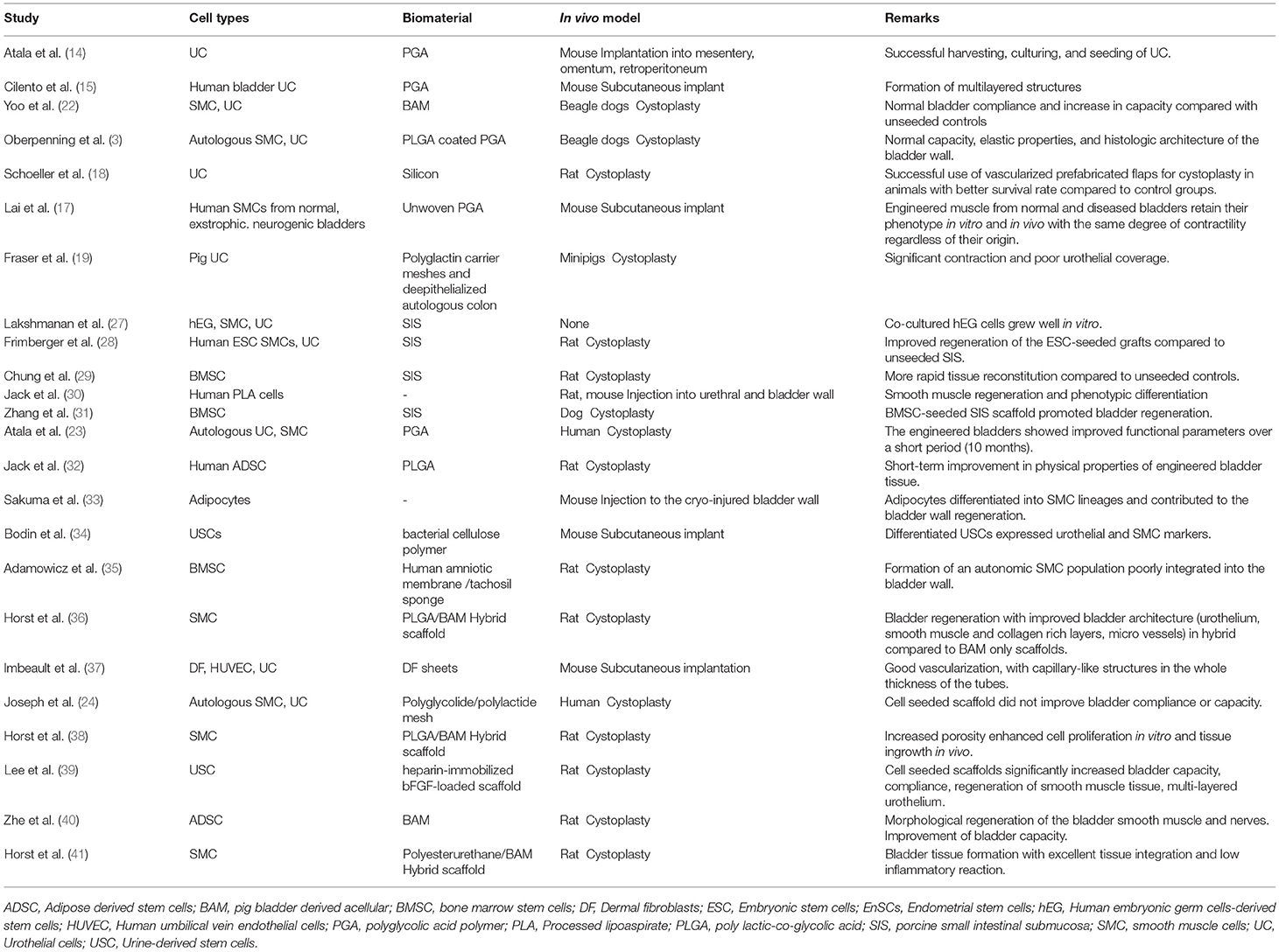
Autologous cells are a perfect match for bladder engineering as they don't provoke inflammation and immune rejection, which are adverse effects of non-self donor cells. They can be derived from bladder tissue or from stem cells of another origin, such as the bone marrow or adipose tissue, however research in this regard is still less advanced (Table 1). Urinary bladder is a hollow organ composed of smooth muscle, urothelium, lamina propia, extracellular matrix, nerves, and vessels. Since detrusor muscle and urothelium represent the main properties of the bladder, the main focus of attention for bladder engineering was directed toward cells originating from these two tissues. Normal human bladder urothelium and muscle cells have been isolated from biopsies, expanded in culture, and characterized regarding differentiation characteristics and other biological functions (15, 42–44). The successful use of autologous cells for human bladder engineering derived from patients with end-stage bladder disease was shown by Atala et al. (23) and Joseph et al. (24). However, the use of autologous cells presupposes the availability of viable cells devoid of any genetic defect within the damaged or diseased tissue. In case of infection, altered tissue composition or malignancy, the adult autologous cells may be abnormal. Moreover, biopsies can lead to several problems including donor site morbidity, limited sample size, restricted proliferation ability, and loss of contractile phenotype of the cells during in vitro culture and expansion (45).
Smooth muscle cells (SMCs) play an important role in the functionality of the bladder and both good proliferation potential and contractile function are essential for successful tissue regeneration. Unfortunately, even mature SMCs isolated from healthy sources have shown limited proliferation capacity and loss of the contractile phenotype followed by a change to a synthetic form during in vitro expansion (45). The phenotypic switch between a synthetic (proliferative) or contractile (quiescent) but active phenotype can occur reversibly and transiently in vitro and in vivo (46, 47). SMCs derived from neuropathic bladders have been shown to retain their pathological characteristics in vitro (48). Therefore, to overcome these limitations, embryonic (27), adult, and induced pluripotent stem cells (49) have been considered for bladder engineering.
In order to create clinically applicable engineered bladder tissue using stem cells, distinct selection criteria such as accessibility with minimal invasiveness, the ability to yield large number of cells in a limited time frame, only minor changes during in vitro culturing, reproducibility with a high differentiation potential are mandatory. Therefore, the type and quality of stem cells for bladder engineering are critical factors.
Embryonic stem cells (ESCs) can be isolated from the blastocyst inner cell mass. They are pluripotent cells with the ability to differentiate into any cell type and with an unlimited expansion potential in vitro (50, 51). Recently, ESC were differentiated to mesenchymal like stem cells (MSCs) by differentiation with growth factor cocktails and supporting feeder cells (OP9) (52). ESC can be induced to become SMCs under retinoic acid treatment, expressing SMC gene markers (53–55). Therefore, they are a valuable tool to study the differentiated SMC and to test their response to therapeutic agents. In a recent study using a rat model, MSCs derived from human ESCs were shown to more effectively improve the contractile function and the potential to repair the histological injury in interstitial cystitis/bladder pain syndrome than adult bone-marrow derived cells (56). The co-culture of human ESCs with bladder SMCs and urothelium seeded on porcine small intestinal submucosa (SIS) generated viable grafts in vitro (27). In a follow up study, the same construct was used to augment a previously injured rat bladder, resulting in an improved regeneration of the ESC-seeded graft compared to unseeded SIS (28). However, several safety issues such as the formation of teratoma, potential immune reactions, and the risk of differentiating into unwanted cell types limit their applicability for bladder engineering.
The ability of adult stem cells to differentiate and self-renew makes them a suitable source for bladder engineering. The adult stem cells can be isolated from virtually every tissue and organ type in mammals (57). Several adult stem cell types with different availabilities are currently used for bladder bioengineering, including adipose derived stem cells (ADSCs) (58), bone marrow stem cells (29), endometrial cells, menstrual blood cells and urine derived stem cells (UDSCs).
Human ADSCs have several advantages in TE applications due to their mutipotency, ease of access and high proliferative potential. They can be isolated either from subcutaneous fat tissue biopsies or by liposuction; both procedures are less invasive and painful than bone marrow aspiration. Human ADSC have surface antigens similar to MSCs derived from human bone marrow stromal cells (58). Several studies have shown efficient differentiation of ADSCs to SMCs and urothelial cells when placed in specific induction media (59–61). In a rat model, Jack et al. (30) delivered human processed lipoaspirate cells into the bladder and urethra. The cells remained viable for up to 12 weeks, showed evidence of incorporation into the recipient smooth muscle and differentiated with time (30). Enhanced bladder architecture and function was observed in small animal models upon ADSC injection (62) or in combination with an acellular scaffold (63). Moreover, in another study on a rat model, bladder acellular matrix (BAM) seeded with ADSCs showed enhanced detrusor muscle and neuronal regeneration, as well as improved bladder capacity (40). Furthermore, human ADSCs were differentiated into SMCs with smooth muscle inductive media and grown on PLGA scaffolds in a athymic rat model for bladder regeneration. The organ bath results demonstrated smooth muscle contraction of the seeded implants but not the acellular implants after 12 weeks in vivo (32). Moreover, human mature adipocyte derived cells could be differentiated into SMCs and contribute to the regeneration of the bladder wall (33).
Bone marrow derived MSCs (BM-MSCs) or stromal cells possess a self-renewal capacity and a potential to differentiate into the myogenic lineage. They are easily isolated due to their tendency to readily adhere to plastic culture dishes (64). Upon induction with TGF-beta1, they can differentiate to SMC, characterized by the expression of specific contractile proteins including alpha-SMA, calponin and SM-MHC (65). In some studies, BM-MSCs were evaluated as an alternative to bladder SMCs when healthy bladder tissue was unavailable (31). An in vivo study in a rat model showed that amniotic membranes seeded with BM-MSC could regenerate detrusor muscle and urothelium in the bladder wall but with no proper urinary bladder function (35). In a similar study performed by Chung et al., BM-MSCs seeded on SIS showed rapid cellular regeneration of bladder constituents morphologically, presenting a possible solution to overcome the fibrosis occurring in unseeded SIS bladder augmentations (29). A similar study in a canine hemicystectomy model using BM-MSC seeded SIS for augmentation demonstrated effective bladder regeneration with solid smooth muscle bundles throughout the graft (31). Although a few studies showed formation of smooth muscle using BM-MSCs in preclinical studies, its clinical application in bladder engineering is limited, due to the low isolation yield, difficulties in harvesting and expansion, and last but not least the painful collection procedure.
Biomaterials
The complex anatomy and function of the urinary bladder pose unique challenges for the selection of scaffolds, cell types and cell sources for its bioengineering. The scaffold plays a key role in tissue regeneration and in re-establishing the biological function of the bladder tissue. Among the characteristics of the biomaterial determining the success of tissue regeneration are biocompatibility, biodegradability and scaffold architecture (66). In addition, an ideal scaffold for bladder TE should provide a microenvironment that promotes cell adhesion and a tissue organization similar to the native tissue (3, 67–71). Furthermore, the construct should serve as a barrier to urine, to protect the underlying tissue from the cytotoxic urine (72–74). It should display appropriate mechanical properties to sustain the mechanical forces necessary for bladder filling and emptying. Furthermore, the scaffold must be biodegradable at the proper rate to optimize integration into the bladder without triggering inflammation and foreign body reaction (70, 75). A special challenge ensuring regeneration and long term survival of the tissue in vivo is an adequate vascularization allowing for adequate oxygenation and nutrition of the regenerating tissue (76, 77). Scaffold materials for urologic tissue regeneration that are currently being investigated and have shown promise in clinical applications are mainly naturally or artificially derived biodegradable materials.
Acellular matrices are chemically and mechanically decellularized matrices such as BAM (78) and porcine SIS (79, 80). These collagen-based scaffolds have the advantage to maintain inherent bioactivity and feature the tridimensional architecture of the native tissue (81). The acellular matrices have been applied both preclinically (22, 79, 82, 83) and clinically (8, 84) with different outcomes (8, 23, 85). Major disadvantages of natural acellular matrices are the variability in physical and biochemical properties among batches (86, 87), the alterations of the physiological environment due to decellularization and sterilization processes (71, 88) and the possible triggering of an immune response (83, 89).
Similar to acellular matrices, naturally-derived polymers like collagen or silk, produced in a number of configurations and densities, provide distinct properties mimicking the structural and mechanical properties of native tissue extracellular matrix (ECM) while being biodegradable. They have shown great promise in a number of models of TE for regenerative medicine in numerous medical applications (90–96).
Collagen Type I, a ubiquitous structural protein, has been studied extensively as a possible scaffold in soft tissue TE applications (97). Collagen has already been approved by the FDA (Food and Drug Administration). It is bioactive, does not provoke immune responses and can easily be extracted from animal and human tissues (90, 92). However, hydrogel scaffolds from collagen offer only limited mechanical strength and different methods such as crosslinking (98), ultracentrifugation (99), or evaporation methods (100) are needed to produce high concentration collagen scaffolds. More recently plastic compression of the collagen hydrogel has been applied in different fields of TE and regenerative medicine e.g., for urinary bladder regeneration (92, 93). In this cell-independent technique developed by Brown et al. (101) and (102), excess water of the collagen hydrogel is removed via mechanical compression. This enables the fabrication of denser and stronger 3D nano- and micro-scale structures as compared to conventional gels (101). Promising preclinical results with compressed collagen scaffolds in TE suggest a potential for these constructs to be used as scaffolds for bladder tissue regeneration (92).
Silk fibroin (SF), another naturally derived material, has been proposed as biomaterial for soft tissue engineering owing to its versatility and biocompatibility (91, 103). Derived from Bombyx mori cocoons, this protein based polymer addresses many of the mechanical characteristics required for urologic TE applications (91, 96). Preclinical research involving SF scaffolds in urinary tissue regeneration has been encouraging as robust regeneration of smooth muscle and urothelium have been demonstrated (94, 95). SF scaffolds in combination with seeded bladder or mucosal cell populations are a promising strategy for engineering of functional urethral tissues (96).
Synthetic polymers are rapidly gaining ground as scaffold materials. In urologic TE, biodegradable synthetic biomaterials with appropriate mechanical properties for soft tissue regeneration such as poly (lactic-co-glycolic acid) (PLGA), polyurethane (104, 105), and poly(ε-caprolactone)/poly (L-lactic acid) (PCL/PLLA) (106) have found their application. Their main advantage is the manufacturing process which allows for suitable features of micro-nanostructure, strength and degradation in a constant quality and even on a large scale. Problems related to tissue harvesting are avoided with the use of these materials. However, none of these materials convinced in vivo. Disadvantages of synthetic polymers are their biological inertness and the lack of the molecular signals that are relevant for directing cell activity and fate. Furthermore, they can induce foreign body reaction, and degradation may produce acidic byproducts that may affect the local microenvironment of the regenerating tissue, causing inflammation and cell death (107, 108). Unfortunately, no single biomaterial or cell source provides all the desirable properties for successful urological tissue regeneration. Current technologies in bladder TE have been hampered by an inability to efficiently initiate blood supply to the graft, ultimately leading to complications that include graft contraction, ischemia, and perforation. These deficiencies therefore necessitate the evaluation of new strategies combining the gained knowledge to closer fulfill these requirements.
Strategies to Succeed
The reason for tissue engineering a whole or partial bladder is to be able to deliver a functional substitute. Since the bladder wall is subjected to mechanical forces during filling and emptying cycles (109) selection criteria for cells and biomaterials are specific. Furthermore, in the pediatric population, a living functional tissue replacement with a good growth potential and a long life span is of main concern. To this end, the use of adult stem cells is given the most attention. Ideal stem cells need to be accessible with minimal invasiveness, have the ability to expand in a short period, and maintain a stable phenotype, while not changing during in vitro culturing but having a high differentiation potential. Therefore, the type, quality and quantity of stem cells for bladder engineering are critical factors.
Besides the already mentioned ESC (110), ADSC (58), and BM-MSCs (29) an other suitable stem cell candidate for urological tissue reconstruction are USCs. They can be isolated from voided urine within 24 h after urine collection (34, 111). USCs show MSCs characteristics and can be differentiated to SMCs, expressing all SMC lineage specific markers (111) with contractile function comparable to native SMCs (112). Originating in the urinary tract system, USCs are suggested as a good stem cell source for bladder TE with the benefits of simple, safe, low-cost and non-invasive collection technique (34). It was demonstrated that a USCs can differentiate in porous bacterial cellulose scaffolds, which may assist in the development of an engineered urinary conduit (34). Furthermore, it was shown that human USCs seeded scaffold-heparin-bFGF grafts improved biocompatibility, increased bladder capacity and compliance, as indicated by smooth muscle and urothelium layer in a partial cystectomy rat model (39).
In addition, a few studies showed that endometrial stem cells, which are of mesenchymal origin, can differentiate to SMCs and are suitable for bladder engineering (113). These cells can be harvested from the endometrium by two methods: either by an endometrial biopsy from the uterus or by collection of menstrual blood. In contrast to bone marrow and adipose tissue cells, for which at least a local anesthesia is required, these cells can be harvested without any anesthetic procedure (114). Furthermore, endometrial stem cells could also differentiate into urothelium using keratinocyte and epithelial growth factors, and in combination with 3D-silk-collagen they could serve as a suitable scaffold for building urinary bladder wall in females (115). However, endometrial-derived stem cells have not yet been used in any in vivo study.
Recent reports have shown that induced pluripotent stem cells (iPSCs) may answer the need for alternative cell sources for bladder regeneration. The iPSCs are reprogrammed, terminally differentiated somatic cells which have developed ESC-like cells characteristics following expression of various pluripotency transcription factors (116). Theoretically, iPSCs can produce an unlimited number of differentiated cells for autologous cell therapies (117). With this approach a patient's cells may be directed to become iPSCs and subsequently to differentiate and repair tissue. However, safety and efficiency is still under investigation. SMCs generated from iPSCs were shown to acquire contractile features and express contractile proteins (118, 119). Moad et al., for the first time, generated iPSCs derived from human urinary tract cells (bladder and ureter) which offers a potential for bladder engineering and in vitro studies (49). However, there are continuing concerns regarding induction of tumors by iPSCs. Currently discussed solutions include modifications in induction methods toward virus-free, transgene-free reprogramming and xeno-free systems (120, 121). In addition, the use of iPSCs requires an appropriate protocol for efficient in vitro differentiation, and in order to address safety issues its effect should be tested in vivo.
Current studies have shown that autologous cells in combination with biomaterials are the best options for bladder engineering. In addition, the construction of a three-dimensional scaffold in vitro before in vivo implantation would facilitate the terminal differentiation of the cells in vivo. The optimization techniques such as co-culture of different cell types and predifferentiation before implantation showed improved cells survival in vivo (122). Son et al. demonstrated that human dental pulp stem cells co-cultured with bladder derived SMCs or in a SMCs-conditioned medium with the addition of the transforming growth factor beta 1 (TGF-β1) can differentiate efficiently into bladder specific SMCs. This approach can be used as a less invasive alternative to harvest stem cells for smooth muscle regeneration and for bladder engineering (123). De-differentiation of SMCs from a contractile phenotype to a synthetic phenotype, which is characterized by SMCs hypertrophy and fibrosis is a known problem in bladder engineering. Methods to maintain the cell phenotype include cell culture microenvironment, the use of growth factors, the optimization of biomechanical and surface properties of the biomaterials and mechanical stimulation (47, 124).
The fabrication of hybrid or composite scaffolds consisting of at least two different biomaterials ideally allows the combination of the positive characteristics of the different compounds and even to develop new biomaterials with a wider range of physicochemical properties (36, 71). The use of hybrids of BAM and synthetic polymers has been described by our own group among others. We developed a bilayered scaffold by direct electrospinning of PLGA (36, 38) or Polyurethan microfibers (41) onto the luminal side of a BAM and demonstrated, that these scaffolds seeded with bladder SMCs supported the regeneration of a multi-layered bladder wall consisting of urothelium, lamina propria, and detrusor muscle resembling native control bladder in rats. Ajalloueian et al. combined CC hydrogels with electrospun PLGA sheets and studied the effect of different fibrillary densities on fibroblast performance (125). They showed that by decreasing the collagen content of CC hydrogel, not only a better cell environment and optimal mechanical properties are achieved, but also the application costs of this biopolymer are reduced. Another method to combine the physical properties of synthetic polymers with the biochemical as well as molecular characteristics of naturally derived scaffolds is the blending of natural and synthetic polymers as for example described by Moshfeghian et al. (126). They evaluated the formation of chitosan–PLGA blend matrices using controlled-rate freezing and lyophilization technique. By altering the freezing conditions they were able to control pore morphology and degradation kinetics of the scaffold with a positive influence on SMC spreading and colonization in vitro. Franck et al. produced a silk-based biomaterial coated with ECM (collagens or fibronectin), blending more than one naturally derived polymer to synthesize scaffolds for bladder tissue engineering (119). This composite scaffold was shown to be biocompatible and to support primary cultures of bladder UC, SMC, and pluripotent stem cell adhesion, proliferation, and differentiation. Such approaches can be adapted to a number of characteristics that are appropriate for bladder augmentation including mechanical properties, permeability, pore size, degradation characteristics, and biological activity (71).
The concept of natural self-assembly of cells differs from all other TE techniques that use pre-formed synthetic scaffolds. This innovative scaffold-free technique relies on the ability of cells to produce and assemble their own ECM (127). Initially introduced for skin TE (128), this approach also enabled the reconstruction of other tissues such as blood vessel, heart valve, cornea, adipose tissue, vaginal mucosa, and urinary tissues (129). In a recent study, Orabi et al. were able to produce a multi-layered construct with histological and molecular properties similar to native tissue in vitro. For this approach, they used bladder-specific stromal cells from the lamina propria co-cultured with UCs or SMCs (130). However, the self-assembly technique still needs to be investigated for urologic tissue regeneration in vivo.
To improve the outcome of bladder regeneration, scaffolds can be functionalized with growth factors, creating a microenvironment that simulates the integration of the tissue engineered constructs (131, 132). Physiologically, growth factors are components of the ECM, which are actively released after injury. They play a crucial role in tissue repair and the prevention of fibrosis. The therapeutic use of recombinant growth factors is based on the hypothesis that through appropriate signaling they induce and/or accelerate the healing process. Several growth factors have been identified as important in the development of functional urological tissue (133), mainly vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) (134, 135). The use of VEGF alone or in combination with NGF resulted in improved bladder wall regeneration and angiogenesis (134–136). When using stem cells, the presence of appropriate growth factors is essential for cell differentiation (137, 138). In most cases, incorporation of biologically active molecules into the scaffold material has been aimed at rapid restoration of vascular networks to maintain tissue viability and long-term survival. Growth factors are considered to be critically important modulators during all phases of tissue regeneration (71). To provide an effective delivery of growth factors, some challenges must be overcome. Because of the high instability of growth factors in vivo, various slow-release devices of natural, synthetic and composite materials have been designed (139, 140). Biomaterials with degradable porous reservoir structures or pre-encapsulated microspheres have been used to control effective targeting (141). For an effective long-term delivery, growth factors can be encapsulated in biodegradable polymers, such as poly(lactic-co-glycolic acid) (PLGA) or poly-L-lysine (PLL) (142, 143). Those systems are designed to release the loaded protein in a sustained manner following the degradation of the polymer. To improve the controlled delivery, Layman et al. developed ionic-albumin microspheres that allow the time-controlled release of two growth factors (144).
Nanoparticles have not only been used for controlled delivery of bioactive molecules and growth factors, in TE they have been used in order to improve the mechanical and biological performance of the regenerated tissue (145). For example nanoparticles can play a vital role in enhancing the mechanical properties of the scaffold as shown in skin TE, where the tensile strength of collagen or silk fibroin was improved by using TiO2 (146) or hydroxyapatite nanoparticles, respectively (147). Furthermore, nanoparticles can mimic the natural nanostructure of ECM components of tissues, and therewith influence cellular activities such as adhesion, growth and differentiation of stem cells (148, 149). Although the use of nanoparticles recently made an enormous progress, in vivo experimentation to verify the successful results from in vitro studies (150) are still needed.
The establishment of a functional vascularization represents one of the major challenges for the implementation of TE applications in clinical practice. The survival of larger and complex tissue substitutes after implantation depends on the rapid development of an adequate vascularization. Furthermore, vascularization is a major prerequisite for a complete restoration of organ structure and functionality. Classical approaches to promote vascularity in tissue substitutes focus on the stimulation of vascular ingrowth into tissue constructs by optimizing the material properties of scaffolds (38, 151, 152) or by enriching implants with proangiogenetic factors (153–155). A promising approach is the incorporation of growth factors which can be released in a time-dependent manner at the implantation site. Therefore, various slow-release devices of natural, synthetic, and composite materials have been designed (156, 157). An additional approach to supply growth factors is the use of transfected cells, which overexpress angiogenic factors (158).
Prevascularization of tissue constructs with networks of capillaries aims to accelerate functional anastomosis with host tissue upon implantation. In vitro prevascularization of thicker constructs and the connection to the host vasculature in vivo is essential to guarantee immediate supply to the cells within the construct. These requirements determine the success of the applied transplant (158, 159). However, angiogenesis in a large avascular graft in vivo does not occur fast enough to avoid hypoxic conditions (160). This innovative approach basically aims at the generation of preformed microvascular networks in tissue constructs prior to their implantation by co-culturing endothelial cells (ECs) with supporting cells (161). The co-culture approach is the most biomimetic option, which can be achieved by growing ECs with mural cells, such as fibroblasts (162) or SMCs (163) or MSCs (164). Also the self-assembly technique showed promising results with endothelialized substitutes for skin (165) and urethral reconstruction (37). After implantation, these networks can then be rapidly perfused with blood by inosculation with the surrounding host microvasculature (166) or by surgical anastomosis of feeding and draining blood vessels (167, 168). This enhances earlier vascularization of the graft, thus potentially decreases the risk of ischemia, necrosis and fibrosis and enhances graft regeneration and thereby long term function. As the feasibility of engineering blood vessels in bladder grafts becomes reality, inosculation and prompt nourishment of grafts upon transplantation will further potentiate the clinical use of bioengineered bladder tissue (73). However, mimicking natural vascular architecture and rebuilding microvascular networks in vitro is still challenging and limits clinical applications. These promising achievements lead to further advancement of these prevascularization concepts and their adaptation to individual therapeutic interventions will markedly contribute to a broad implementation of TE applications in clinical practice.
Outlook and Clinical Translation
Urinary diversions made from engineered bladder tissue would remove the need for bowel tissue for bladder reconstruction. An off-the-shelf bladder tissue would revolutionize reconstructive urology and would allow a substantial reduction in morbidity and improve the long term outcome of bladder augmentation, especially in the pediatric patient. With recent advances in isolating, growing, and differentiating host stem cells, an increased understanding of the cell niche required to maintain the artificial tissue, and novel techniques for the generation of an intact blood supply, it appears that the major elements for the engineering of a functional bladder wall are achievable. Despite impressive progress in the field of bladder TE over the past decades, the successful transfer of these approaches into clinical routine still represents a major challenge. Large animal trials are necessary to confirm the applicability of the approaches in a model similar to the growing human organism to meet the special needs of this patient group.
As discussed in this review there are several strategies to overcome the hurdles of TE which lead to new approaches in bladder regeneration. In order to improve the bladder engineering for clinical application we suggest further unifications of the strategies and approaches including a collaborative effort of experts of different fields. With its complex nature and distinct mechanical properties, the development of a next generation bioengineered bladder tissue requires the combined knowledge and techniques of material science and cell biology to be successful in future clinical application.
Data Availability
The datasets for this manuscript are not publicly available because its a review. Requests to access the datasets should be directed to bWF5YS5ob3JzdEBrc2lwaS51emguY2g=.
Author Contributions
MH and SS wrote the manuscript. DE and RG authors contributed to the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks to Damina Balmer for the critical assessment of this manuscript.
References
1. Mcdougal WS. Metabolic complications of urinary intestinal diversion. J Urol. (1992) 147:1199–208. doi: 10.1016/S0022-5347(17)37517-1
2. Kaefer M, Hendren WH, Bauer SB, Goldenblatt P, Peters CA, Atala A, et al. Reservoir calculi: a comparison of reservoirs constructed from stomach and other enteric segments. J Urol. (1998) 160:2187–90. doi: 10.1016/S0022-5347(01)62290-0
3. Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. (1999) 17:149–55. doi: 10.1038/6146
4. Bohne AW, Urwiller KL. Experience with urinary bladder regeneration. J Urol. (1957) 77:725–32. doi: 10.1016/S0022-5347(17)66624-2
5. Portilla Sanchez R, Blanco FL, Santamarina A, Casals Roa J, Mata J, Kaufman A. Vesical regeneration in the human after total cystectomy and implantation of a plastic mould. Br J Urol. (1958) 30:180–8. doi: 10.1111/j.1464-410X.1958.tb06231.x
6. Tsuji I, Kuroda K, Fujieda J, Shiraishi Y, Kunishima K. Clinical experiences of bladder reconstruction using preserved bladder and gelatin sponge bladder in the case of bladder cancer. J Urol. (1967) 98:91–2. doi: 10.1016/S0022-5347(17)62828-3
7. Orikasa S, Tsuji I. Enlargement of contracted bladder by use of gelatin sponge bladder. J Urol. (1970) 104:107–10. doi: 10.1016/S0022-5347(17)61680-X
8. Caione P, Boldrini R, Salerno A, Nappo SG. Bladder augmentation using acellular collagen biomatrix: a pilot experience in exstrophic patients. Pediatr Surg Int. (2012) 28:421–8. doi: 10.1007/s00383-012-3063-0
9. Fujita K. The use of resin-sprayed thin paper for urinary bladder regeneration. Invest Urol. (1978) 15:355–7.
10. Moon SJ, Kim DH, Jo JK, Chung JH, Lee JY, Park SY, et al. Bladder reconstruction using bovine pericardium in a case of enterovesical fistula. Korean J Urol. (2011) 52:150–3. doi: 10.4111/kju.2011.52.2.150
11. Arikan N, Ozdiler E, Yaman O, Gogus O. Augmentation duracystoplasty in neurogenic bladder dysfunction. Int J Urol. (1995) 2:172–5. doi: 10.1111/j.1442-2042.1995.tb00448.x
12. Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol. (2009) 6:596–611. doi: 10.1038/nrurol.2009.201
13. Kim BS, Mooney DJ. Engineering smooth muscle tissue with a predefined structure. J Biomed Mater Res. (1998) 41:322–32. doi: 10.1002/(SICI)1097-4636(199808)41:2<322::AID-JBM18>3.0.CO;2-M
14. Atala A, Vacanti JP, Peters CA, Mandell J, Retik AB, Freeman MR. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J Urol. (1992) 148:658–62. doi: 10.1016/S0022-5347(17)36685-5
15. Cilento BG, Freeman MR, Schneck FX, Retik AB, Atala A. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J Urol. (1994) 152:665–70. doi: 10.1016/S0022-5347(17)32676-9
16. Pariente JL, Kim BS, Atala A. In vitro biocompatibility assessment of naturally derived and synthetic biomaterials using normal human urothelial cells. J Biomed Mater Res. (2001) 55:33–9. doi: 10.1002/1097-4636(200104)55:1<33::AID-JBM50>3.0.CO;2-7
17. Lai JY, Yoon CY, Yoo JJ, Wulf T, Atala A. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J Urol. (2002) 168:1853–7. Discussion 1858. doi: 10.1097/01.ju.0000030040.76258.5a
18. Schoeller T, Lille S, Stenzl A, Ninkovic M, Piza H, Otto A, et al. Bladder reconstruction using a prevascularized capsular tissue seeded with urothelial cells. J Urol. (2001) 165:980–5. doi: 10.1016/S0022-5347(05)66588-3
19. Fraser M, Thomas DF, Pitt E, Harnden P, Trejdosiewicz LK, Southgate J. A surgical model of composite cystoplasty with cultured urothelial cells: a controlled study of gross outcome and urothelial phenotype. BJU Int. (2004) 93:609–16. doi: 10.1111/j.1464-410X.2003.04675.x
20. Nuininga JE, Van Moerkerk H, Hanssen A, Hulsbergen CA, Oosterwijk-Wakka J, Oosterwijk E, et al. A rabbit model to tissue engineer the bladder. Biomaterials. (2004) 25:1657–61. doi: 10.1016/S0142-9612(03)00519-2
21. Burmeister D, Aboushwareb T, Tan J, Link K, Andersson KE, Christ G. Early stages of in situ bladder regeneration in a rodent model. Tissue Eng Part A. (2010) 16:2541–51. doi: 10.1089/ten.tea.2009.0697
22. Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. (1998) 51:221–5. doi: 10.1016/S0090-4295(97)00644-4
23. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. (2006) 367:1241–6. doi: 10.1016/S0140-6736(06)68438-9
24. Joseph DB, Borer JG, De Filippo RE, Hodges SJ, Mclorie GA. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J Urol. (2014) 191:1389–95. doi: 10.1016/j.juro.2013.10.103
25. Hautmann RE. Urinary diversion: ileal conduit to neobladder. J Urol. (2003) 169:834–42. doi: 10.1097/01.ju.0000029010.97686.eb
26. Kates M, Singh A, Matsui H, Steinberg GD, Smith ND, Schoenberg MP, et al. Tissue-engineered urinary conduits. Curr Urol Rep. (2015) 16:8. doi: 10.1007/s11934-015-0480-3
27. Lakshmanan Y, Frimberger D, Gearhart JD, Gearhart JP. Human embryoid body-derived stem cells in co-culture with bladder smooth muscle and urothelium. Urology. (2005) 65:821–6. doi: 10.1016/j.urology.2004.11.022
28. Frimberger D, Morales N, Gearhart JD, Gearhart JP, Lakshmanan Y. Human embryoid body-derived stem cells in tissue engineering-enhanced migration in co-culture with bladder smooth muscle and urothelium. Urology. (2006) 67:1298–303. doi: 10.1016/j.urology.2005.12.005
29. Chung SY, Krivorov NP, Rausei V, Thomas L, Frantzen M, Landsittel D, et al. Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol. (2005) 174:353–9. doi: 10.1097/01.ju.0000161592.00434.c1
30. Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA, Rodriguez LV. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. (2005) 174:2041–5. doi: 10.1097/01.ju.0000176489.96993.84
31. Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. (2005) 96:1120–5. doi: 10.1111/j.1464-410X.2005.05741.x
32. Jack GS, Zhang R, Lee M, Xu Y, Wu BM, Rodriguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. (2009) 30:3259–70. doi: 10.1016/j.biomaterials.2009.02.035
33. Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, et al. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. (2009) 182:355–65. doi: 10.1016/j.juro.2009.02.103
34. Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. (2010) 31:8889–901. doi: 10.1016/j.biomaterials.2010.07.108
35. Adamowicz J, Juszczak K, Bajek A, Tworkiewicz J, Nowacki M, Marszalek A, et al. Morphological and urodynamic evaluation of urinary bladder wall regeneration: muscles guarantee contraction but not proper function–a rat model research study. Transplant Proc. (2012) 44:1429–34. doi: 10.1016/j.transproceed.2012.01.144
36. Horst M, Madduri S, Milleret V, Sulser T, Gobet R, Eberli D. A bilayered hybrid microfibrous PLGA–acellular matrix scaffold for hollow organ tissue engineering. Biomaterials. (2013) 34:1537–45. doi: 10.1016/j.biomaterials.2012.10.075
37. Imbeault A, Bernard G, Rousseau A, Morissette A, Chabaud S, Bouhout S, et al. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can Urol Assoc J. (2013) 7:E4–9. doi: 10.5489/cuaj.187
38. Horst M, Milleret V, Notzli S, Madduri S, Sulser T, Gobet R, et al. Increased porosity of electrospun hybrid scaffolds improved bladder tissue regeneration. J Biomed Mater Res A. (2014) 102:2116–24. doi: 10.1002/jbm.a.34889
39. Lee JN, Chun SY, Lee HJ, Jang YJ, Choi SH, Kim DH, et al. Human urine-derived stem cells seeded surface modified composite scaffold grafts for bladder reconstruction in a rat model. J Korean Med Sci. (2015) 30:1754–63. doi: 10.3346/jkms.2015.30.12.1754
40. Zhe Z, Jun D, Yang Z, Mingxi X, Ke Z, Ming Z, et al. Bladder acellular matrix grafts seeded with adipose-derived stem cells and incubated intraperitoneally promote the regeneration of bladder smooth muscle and nerve in a rat model of bladder augmentation. Stem Cells Dev. (2016) 25:405–14. doi: 10.1089/scd.2015.0246
41. Horst M, Milleret V, Noetzli S, Gobet R, Sulser T, Eberli D. Polyesterurethane and acellular matrix based hybrid biomaterial for bladder engineering. J Biomed Mater Res B Appl Biomater. (2017) 105:658–67. doi: 10.1002/jbm.b.33591
42. Liebert M, Hubbel A, Chung M, Wedemeyer G, Lomax MI, Hegeman A, et al. Expression of mal is associated with urothelial differentiation in vitro: identification by differential display reverse-transcriptase polymerase chain reaction. Differentiation. (1997) 61:177–85. doi: 10.1046/j.1432-0436.1997.6130177.x
43. Rackley RR, Bandyopadhyay SK, Fazeli-Matin S, Shin MS, Appell R. Immunoregulatory potential of urothelium: characterization of NF-kappaB signal transduction. J Urol. (1999) 162:1812–6. doi: 10.1016/S0022-5347(05)68243-2
44. Ma F, Higashira H, Ukai Y, Hanai T, Kiwamoto H, Park YC, et al. A new enzymic method for the isolation and culture of human bladder body smooth muscle cells. Neurourol Urodyn. (2002) 21:71–9. doi: 10.1002/nau.2034
45. Huber A, Badylak SF. Phenotypic changes in cultured smooth muscle cells: limitation or opportunity for tissue engineering of hollow organs? J Tissue Eng Regen Med. (2012) 6:505–11. doi: 10.1002/term.451
46. Aikawa M, Sakomura Y, Ueda M, Kimura K, Manabe I, Ishiwata S, et al. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. (1997) 96:82–90. doi: 10.1161/01.CIR.96.1.82
47. Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. (2010) 16:467–91. doi: 10.1089/ten.teb.2009.0630
48. Lin HK, Cowan R, Moore P, Zhang Y, Yang Q, Peterson JA, et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol. (2004) 171:1348–52. doi: 10.1097/01.ju.0000108800.47594.8b
49. Moad M, Pal D, Hepburn AC, Williamson SC, Wilson L, Lako M, et al. A novel model of urinary tract differentiation, tissue regeneration, and disease: reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur Urol. (2013) 64:753–61. doi: 10.1016/j.eururo.2013.03.054
50. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. (1998) 282:1145–7. doi: 10.1126/science.282.5391.1145
51. Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, et al. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell. (2016) 19:544–57. doi: 10.1016/j.stem.2016.08.020
52. Kim JM, Hong KS, Song WK, Bae D, Hwang IK, Kim JS, et al. Perivascular progenitor cells derived from human embryonic stem cells exhibit functional characteristics of pericytes and improve the retinal vasculature in a rodent model of diabetic retinopathy. Stem Cells Transl Med. (2016) 5:1268–76. doi: 10.5966/sctm.2015-0342
53. Blank RS, Swartz EA, Thompson MM, Olson EN, Owens GK. A retinoic acid-induced clonal cell line derived from multipotential P19 embryonal carcinoma cells expresses smooth muscle characteristics. Circ Res. (1995) 76:742–9. doi: 10.1161/01.RES.76.5.742
54. Drab M, Haller H, Bychkov R, Erdmann B, Lindschau C, Haase H, et al. From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J. (1997) 11:905–15. doi: 10.1096/fasebj.11.11.9285489
55. Xie CQ, Zhang J, Villacorta L, Cui T, Huang H, Chen YE. A highly efficient method to differentiate smooth muscle cells from human embryonic stem cells. Arterioscler Thromb Vasc Biol. (2007) 27:e311–312. doi: 10.1161/ATVBAHA.107.154260
56. Kim A, Yu HY, Lim J, Ryu CM, Kim YH, Heo J, et al. Improved efficacy and in vivo cellular properties of human embryonic stem cell derivative in a preclinical model of bladder pain syndrome. Sci Rep. (2017) 7:8872. doi: 10.1038/s41598-017-09330-x
57. Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. (2004) 116:639–48. doi: 10.1016/S0092-8674(04)00208-9
58. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. (2001) 7:211–28. doi: 10.1089/107632701300062859
59. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. (2002) 13:4279–95. doi: 10.1091/mbc.e02-02-0105
60. Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong BF, et al. Transdifferentiation of human adipose-derived stem cells into urothelial cells: potential for urinary tract tissue engineering. Cell Tissue Res. (2012) 347:737–46. doi: 10.1007/s00441-011-1317-0
61. Salemi S, Tremp M, Plock JA, Andersson KE, Gobet R, Sulser T, et al. Differentiated adipose-derived stem cells for bladder bioengineering. Scand J Urol. 49:407–14. doi: 10.3109/21681805.2015.1004642
62. Tremp M, Salemi S, Largo R, Andersson KE, Plock JE, Aboushwareb T, et al. Adipose-derived stem cells (ADSCs) and muscle precursor cells (MPCs) for the treatment of bladder voiding dysfunction. World J Urol. (2014) 32:1241–8. doi: 10.1007/s00345-013-1200-6
63. Zhu WD, Xu YM, Feng C, Fu Q, Song LJ, Cui L. Bladder reconstruction with adipose-derived stem cell-seeded bladder acellular matrix grafts improve morphology composition. World J Urol. (2010) 28:493–8. doi: 10.1007/s00345-010-0508-8
64. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. (1999) 284:143–7. doi: 10.1126/science.284.5411.143
65. Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. (2002) 278:72–83. doi: 10.1006/excr.2002.5561
66. Perez JR, Kouroupis D, Li DJ, Best TM, Kaplan L, Correa D. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol. (2018) 6:105. doi: 10.3389/fbioe.2018.00105
67. Eberli D, Soker S, Atala A, Yoo JJ. Optimization of human skeletal muscle precursor cell culture and myofiber formation in vitro. Methods. (2009) 47:98–103. doi: 10.1016/j.ymeth.2008.10.016
68. O'brien FJ. Biomaterials and scaffolds for tissue engineering. Materials Today. (2011) 14:88–95. doi: 10.1016/S1369-7021(11)70058-X
69. Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys. (2011) 49:832–64. doi: 10.1002/polb.22259
70. Gill BC, Damaser MS, Chermansky CJ. Future perspectives in bladder tissue engineering. Curr Bladder Dysfunct Rep. (2014) 10:443–8. doi: 10.1007/s11884-015-0327-2
71. Lin HK, Madihally SV, Palmer B, Frimberger D, Fung KM, Kropp BP. Biomatrices for bladder reconstruction. Adv Drug Deliv Rev. (2015) 82–3:47–63. doi: 10.1016/j.addr.2014.11.020
72. Song L, Murphy SV, Yang B, Xu Y, Zhang Y, Atala A. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng Part B Rev. (2014) 20:163–72. doi: 10.1089/ten.teb.2013.0103
73. Osborn SL, Kurzrock EA. Bioengineered bladder tissue–close but yet so far! J Urol. (2015) 194:619–20. doi: 10.1016/j.juro.2015.06.020
74. Vaegler M, Maurer S, Toomey P, Amend B, Sievert KD. Tissue engineering in urothelium regeneration. Adv Drug Deliv Rev. (2015) 82–3:64–8. doi: 10.1016/j.addr.2014.11.021
75. Sharma AK. Is a functional urinary bladder attainable through current regenerative medicine strategies? Cent European J Urol. (2013) 66:207–8. doi: 10.5173/ceju.2013.02.art24
76. Levenberg S, Langer R. Advances in tissue engineering. Curr Top Dev Biol. (2004) 61:113–34. doi: 10.1016/S0070-2153(04)61005-2
77. Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. (2005) 26:1857–75. doi: 10.1016/j.biomaterials.2004.07.006
78. Rosario DJ, Reilly GC, Ali Salah E, Glover M, Bullock AJ, Macneil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med. (2008) 3:145–56. doi: 10.2217/17460751.3.2.145
79. Kropp BP, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, et al. Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol. (1996) 155:2098–104. doi: 10.1016/S0022-5347(01)66117-2
80. Wang Y, Liao L. Histologic and functional outcomes of small intestine submucosa-regenerated bladder tissue. BMC Urol. (2014) 14:69. doi: 10.1186/1471-2490-14-69
81. Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. (2004) 12:367–77. doi: 10.1016/j.trim.2003.12.016
82. Vaught JD, Kropp BP, Sawyer BD, Rippy MK, Badylak SF, Shannon HE, et al. Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: functional innervation and receptor expression. J Urol. (1996) 155:374–8. doi: 10.1016/S0022-5347(01)66663-1
83. Brown AL, Farhat W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. (2002) 23:2179–90. doi: 10.1016/S0142-9612(01)00350-7
84. Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol. (2013) 9:878–83. doi: 10.1016/j.jpurol.2012.12.001
85. Ribeiro-Filho LA, Sievert KD. Acellular matrix in urethral reconstruction. Adv Drug Deliv Rev. (2015) 82–3:38–46. doi: 10.1016/j.addr.2014.11.019
86. Farhat W, Chen J, Erdeljan P, Shemtov O, Courtman D, Khoury A, et al. Porosity of porcine bladder acellular matrix: impact of ACM thickness. J Biomed Mater Res A. (2003) 67:970–4. doi: 10.1002/jbm.a.10171
87. Lin HK, Godiwalla SY, Palmer B, Frimberger D, Yang Q, Madihally SV, et al. Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa. Tissue Eng Part B Rev. (2014) 20:73–83. doi: 10.1089/ten.teb.2013.0126
88. Davis NF, Mcguire BB, Callanan A, Flood HD, Mcgloughlin TM. Xenogenic extracellular matrices as potential biomaterials for interposition grafting in urological surgery. J Urol. (2010) 184:2246–53. doi: 10.1016/j.juro.2010.07.038
89. Roth CC, Mondalek FG, Kibar Y, Ashley RA, Bell CH, Califano JA, et al. Bladder regeneration in a canine model using hyaluronic acid-poly(lactic-co-glycolic-acid) nanoparticle modified porcine small intestinal submucosa. BJU Int. (2011) 108:148–55. doi: 10.1111/j.1464-410X.2010.09757.x
90. Furthmayr H, Timpl R. Immunochemistry of collagens and procollagens. Int Rev Connect Tissue Res. (1976) 7:61–99. doi: 10.1016/B978-0-12-363707-9.50008-3
91. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. (2003) 24:401–16. doi: 10.1016/S0142-9612(02)00353-8
92. Engelhardt EM, Stegberg E, Brown RA, Hubbell JA, Wurm FM, Adam M, et al. Compressed collagen gel: a novel scaffold for human bladder cells. J Tissue Eng Regen Med. (2010) 4:123–30. doi: 10.1002/term.222
93. Engelhardt EM, Micol LA, Houis S, Wurm FM, Hilborn J, Hubbell JA, et al. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. (2011) 32:3969–76. doi: 10.1016/j.biomaterials.2011.02.012
94. Mauney JR, Cannon GM, Lovett ML, Gong EM, Di Vizio D, Gomez P, et al. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials. (2011) 32:808–18. doi: 10.1016/j.biomaterials.2010.09.051
95. Huang JW, Xu YM, Li ZB, Murphy SV, Zhao W, Liu QQ, et al. Tissue performance of bladder following stretched electrospun silk fibroin matrix and bladder acellular matrix implantation in a rabbit model. J Biomed Mater Res A. (2016) 104:9–16. doi: 10.1002/jbm.a.35535
96. Sack BS, Mauney JR, Estrada CR Jr. Silk Fibroin Scaffolds for Urologic Tissue Engineering. Curr Urol Rep. (2016) 17:16. doi: 10.1007/s11934-015-0567-x
97. Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. (2008) 89:338–44. doi: 10.1002/bip.20871
98. Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. (2001) 22:3145–54. doi: 10.1016/S0142-9612(01)00067-9
99. Ramanujan S, Pluen A, Mckee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J. (2002) 83:1650–60. doi: 10.1016/S0006-3495(02)73933-7
100. Helary C, Ovtracht L, Coulomb B, Godeau G, Giraud-Guille MM. Dense fibrillar collagen matrices: a model to study myofibroblast behaviour during wound healing. Biomaterials. (2006) 27:4443–52. doi: 10.1016/j.biomaterials.2006.04.005
101. Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. (2005) 15:1762–70. doi: 10.1002/adfm.200500042
102. Abou Neel EA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. (2006) 2:986. doi: 10.1039/b609784g
103. Panilaitis B, Altman GH, Chen J, Jin HJ, Karageorgiou V, Kaplan DL. Macrophage responses to silk. Biomaterials. (2003) 24:3079–85. doi: 10.1016/S0142-9612(03)00158-3
104. Yao C, Hedrick M, Pareek G, Renzulli J, Haleblian G, Webster TJ. Nanostructured polyurethane-poly-lactic-co-glycolic acid scaffolds increase bladder tissue regeneration: an in vivo study. Int J Nanomed. (2013) 8:3285–96. doi: 10.2147/IJN.S44901
105. El-Taji OM, Khattak AQ, Hussain SA. Bladder reconstruction: the past, present and future. Oncol Lett. (2015) 10:3–10. doi: 10.3892/ol.2015.3161
106. Shakhssalim N, Rasouli J, Moghadasali R, Aghdas FS, Naji M, Soleimani M. Bladder smooth muscle cells interaction and proliferation on PCL/PLLA electrospun nanofibrous scaffold. Int J Artif Organs. (2013) 36:113–20. doi: 10.5301/ijao.5000175
107. Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. (2000) 17:100–6. doi: 10.1023/A:1007582911958
108. Jeong SI, Kim BS, Kang SW, Kwon JH, Lee YM, Kim SH, et al. In vivo biocompatibilty and degradation behavior of elastic poly(L-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials. (2004) 25:5939–46. doi: 10.1016/j.biomaterials.2004.01.057
109. Baskin L, Meaney D, Landsman A, Zderic SA, Macarak E. Bovine bladder compliance increases with normal fetal development. J Urol. (1994) 152:692–5. Discussion 696–7. doi: 10.1016/S0022-5347(17)32682-4
110. Oottamasathien S, Wang Y, Williams K, Franco OE, Wills ML, Thomas JC, et al. Directed differentiation of embryonic stem cells into bladder tissue. Dev Biol. (2007) 304:556–66. doi: 10.1016/j.ydbio.2007.01.010
111. Zhang Y, Mcneill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. (2008) 180:2226–33. doi: 10.1016/j.juro.2008.07.023
112. Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, et al. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS ONE. (2013) 8:e53980. doi: 10.1371/journal.pone.0053980
113. Shoae-Hassani A, Sharif S, Seifalian AM, Mortazavi-Tabatabaei SA, Rezaie S, Verdi J. Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women. BJU Int. (2013) 112:854–63. doi: 10.1111/bju.12195
114. Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. (2013) 13:1387–400. doi: 10.1517/14712598.2013.826187
115. Shoae-Hassani A, Mortazavi-Tabatabaei SA, Sharif S, Seifalian AM, Azimi A, Samadikuchaksaraei A, et al. Differentiation of human endometrial stem cells into urothelial cells on a three-dimensional nanofibrous silk-collagen scaffold: an autologous cell resource for reconstruction of the urinary bladder wall. J Tissue Eng Regen Med. (2015) 9:1268–76. doi: 10.1002/term.1632
116. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. (2006) 126:663–76. doi: 10.1016/j.cell.2006.07.024
117. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. doi: 10.1016/j.cell.2007.11.019
118. Xie CQ, Huang H, Wei S, Song LS, Zhang J, Ritchie RP, et al. A comparison of murine smooth muscle cells generated from embryonic versus induced pluripotent stem cells. Stem Cells Dev. (2009) 18:741–8. doi: 10.1089/scd.2008.0179
119. Franck D, Gil ES, Adam RM, Kaplan DL, Chung YG, Estrada CR, et al. Evaluation of silk biomaterials in combination with extracellular matrix coatings for bladder tissue engineering with primary and pluripotent cells. PLoS ONE. (2013) 8:e56237. doi: 10.1371/journal.pone.0056237
120. Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. (2010) 7:618–30. doi: 10.1016/j.stem.2010.08.012
121. Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. (2011) 8:424–9. doi: 10.1038/nmeth.1593
122. Zupancic D, Mrak Poljsak K, Kreft ME. Co-culturing porcine normal urothelial cells, urinary bladder fibroblasts and smooth muscle cells for tissue engineering research. Cell Biol Int. (2018) 42:411–24. doi: 10.1002/cbin.10910
123. Song B, Jiang W, Alraies A, Liu Q, Gudla V, Oni J, et al. Bladder smooth muscle cells differentiation from dental pulp stem cells: future potential for bladder tissue engineering. Stem Cells Int. (2016) 2016:6979368. doi: 10.1155/2016/6979368
124. Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol (1985). (2005) 98:2321–7. doi: 10.1152/japplphysiol.01114.2004
125. Ajalloueian F, Zeiai S, Fossum M, Hilborn JG. Constructs of electrospun PLGA, compressed collagen and minced urothelium for minimally manipulated autologous bladder tissue expansion. Biomaterials. (2014) 35:5741–8. doi: 10.1016/j.biomaterials.2014.04.002
126. Moshfeghian A, Tillman J, Madihally SV. Characterization of emulsified chitosan-PLGA matrices formed using controlled-rate freezing and lyophilization technique. J Biomed Mater Res A. (2006) 79:418–30. doi: 10.1002/jbm.a.30849
127. Auger FA, Remy-Zolghadri M, Grenier G, Germain L. The self-assembly approach for organ reconstruction by tissue engineering. e-biomed. (2000) 1:75–86. doi: 10.1089/152489000414642
128. Pouliot R, Larouche D, Auger FA, Juhasz J, Xu W, Li H, et al. Reconstructed human skin produced in vitro and grafted on athymic mice. Transplantation. (2002) 73:1751–7. doi: 10.1097/00007890-200206150-00010
129. Saba I, Jakubowska W, Bolduc S, Chabaud S. Engineering tissues without the use of a synthetic scaffold: a twenty-year history of the self-assembly method. Biomed Res Int. (2018) 2018:5684679. doi: 10.1155/2018/5684679
130. Orabi H, Rousseau A, Laterreur V, Bolduc S. Optimization of the current self-assembled urinary bladder model: organ-specific stroma and smooth muscle inclusion. Can Urol Assoc J. (2015) 9:E599–607. doi: 10.5489/cuaj.2953
131. Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol. (2003) 170:1633–8. doi: 10.1097/01.ju.0000084021.51099.8a
132. Mondalek FG, Ashley RA, Roth CC, Kibar Y, Shakir N, Ihnat MA, et al. Enhanced angiogenesis of modified porcine small intestinal submucosa with hyaluronic acid-poly(lactide-co-glycolide) nanoparticles: from fabrication to preclinical validation. J Biomed Mater Res A. (2010) 94:712–9. doi: 10.1002/jbm.a.32748
133. Johnson SC, Smith ZL, Sack BS, Steinberg GD. Tissue Engineering and Conduit Substitution. Urol Clin North Am. (2018) 45:133–41. doi: 10.1016/j.ucl.2017.09.014
134. Youssif M, Shiina H, Urakami S, Gleason C, Nunes L, Igawa M, et al. Effect of vascular endothelial growth factor on regeneration of bladder acellular matrix graft: histologic and functional evaluation. Urology. (2005) 66:201–7. doi: 10.1016/j.urology.2005.01.054
135. Loai Y, Yeger H, Coz C, Antoon R, Islam SS, Moore K, et al. Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A. (2010) 94:1205–15. doi: 10.1002/jbm.a.32777
136. Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T, et al. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. (2009) 103:1424–8. doi: 10.1111/j.1464-410X.2008.08129.x
137. Gordeladze JO, Reseland JE, Duroux-Richard I, Apparailly F, Jorgensen C. From stem cells to bone: phenotype acquisition, stabilization, and tissue engineering in animal models. ILAR J. (2009) 51:42–61. doi: 10.1093/ilar.51.1.42
138. Tian H, Bharadwaj S, Liu Y, Ma H, Ma PX, Atala A, et al. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. (2010) 31:870–7. doi: 10.1016/j.biomaterials.2009.10.001
139. Prokop A, Kozlov E, Nun Non S, Dikov MM, Sephel GC, Whitsitt JS, et al. Towards retrievable vascularized bioartificial pancreas: induction and long-lasting stability of polymeric mesh implant vascularized with the help of acidic and basic fibroblast growth factors and hydrogel coating. Diabetes Technol Ther. (2001) 3:245–61. doi: 10.1089/152091501300209624
140. Borselli C, Ungaro F, Oliviero O, D'angelo I, Quaglia F, La Rotonda MI, et al. Bioactivation of collagen matrices through sustained VEGF release from PLGA microspheres. J Biomed Mater Res A. (2010) 92:94–102. doi: 10.1002/jbm.a.32332
141. Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. (1991) 12:619–26. doi: 10.1016/0142-9612(91)90107-L
142. De Boer R, Knight AM, Spinner RJ, Malessy MJ, Yaszemski MJ, Windebank AJ. In vitro and in vivo release of nerve growth factor from biodegradable poly-lactic-co-glycolic-acid microspheres. J Biomed Mater Res A. (2010) 95:1067–73. doi: 10.1002/jbm.a.32900
143. Karal-Yilmaz O, Serhatli M, Baysal K, Baysal BM. Preparation and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(D,L-lactic-co-glycolic acid) microspheres using a double emulsion/solvent evaporation technique. J Microencapsul. (2011) 28:46–54. doi: 10.3109/02652048.2010.523795
144. Layman H, Li X, Nagar E, Vial X, Pham SM, Andreopoulos FM. Enhanced angiogenic efficacy through controlled and sustained delivery of FGF-2 and G-CSF from fibrin hydrogels containing ionic-albumin microspheres. J Biomater Sci Polym Ed. (2012) 23:185–206. doi: 10.1163/092050610X546417
145. Sensenig R, Sapir Y, Macdonald C, Cohen S, Polyak B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine. (2012) 7:1425–42. doi: 10.2217/nnm.12.109
146. Li N, Fan X, Tang K, Zheng X, Liu J, Wang B. Nanocomposite scaffold with enhanced stability by hydrogen bonds between collagen, polyvinyl pyrrolidone and titanium dioxide. Colloids Surf B Biointerfaces. (2016) 140:287–96. doi: 10.1016/j.colsurfb.2015.12.005
147. Kim H, Che L, Ha Y, Ryu W. Mechanically-reinforced electrospun composite silk fibroin nanofibers containing hydroxyapatite nanoparticles. Mater Sci Eng C Mater Biol Appl. (2014) 40:324–35. doi: 10.1016/j.msec.2014.04.012
148. Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. (2011) 5:7383–90. doi: 10.1021/nn2023057
149. Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, et al. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. (2013) 7:4785–98. doi: 10.1021/nn304966z
150. Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomed. (2018) 13:5637–55. doi: 10.2147/IJN.S153758
151. Rucker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mulhaupt R, et al. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. (2006) 27:5027–38. doi: 10.1016/j.biomaterials.2006.05.033
152. Choi SW, Zhang Y, Macewan MR, Xia Y. Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv Healthc Mater. (2013) 2:145–54. doi: 10.1002/adhm.201200106
153. Laschke MW, Rucker M, Jensen G, Carvalho C, Mulhaupt R, Gellrich NC, et al. Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds. J Biomed Mater Res A. (2008) 85:397–407. doi: 10.1002/jbm.a.31503
154. Rui J, Dadsetan M, Runge MB, Spinner RJ, Yaszemski MJ, Windebank AJ, et al. Controlled release of vascular endothelial growth factor using poly-lactic-co-glycolic acid microspheres: in vitro characterization and application in polycaprolactone fumarate nerve conduits. Acta Biomater. (2012) 8:511–8. doi: 10.1016/j.actbio.2011.10.001
155. Singh S, Wu BM, Dunn JC. Delivery of VEGF using collagen-coated polycaprolactone scaffolds stimulates angiogenesis. J Biomed Mater Res A. (2012) 100:720–7. doi: 10.1002/jbm.a.34010
156. Sharma AK, Donovan JL, Hagerty JA, Sullivan RR, Edassery SL, Harrington DA, et al. Do current bladder smooth muscle cell isolation procedures result in a homogeneous cell population? Implications for bladder tissue engineering. World J Urol. (2009) 27:687–94. doi: 10.1007/s00345-009-0391-3
157. Barati M, Mohammadi Samani S, Pourtalebi Jahromi L, Ashrafi H, Azadi A. Controlled-release in-situ gel forming formulation of tramadol containing chitosan-based pro-nanogels. Int J Biol Macromol. (2018) 118:1449–54. doi: 10.1016/j.ijbiomac.2018.06.152
158. Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. (2011) 63:300–11. doi: 10.1016/j.addr.2011.03.004
159. Rivron NC, Liu JJ, Rouwkema J, De Boer J, Van Blitterswijk CA. Engineering vascularised tissues in vitro. Eur Cell Mater. (2008) 15:27–40. doi: 10.22203/eCM.v015a03
160. Utzinger U, Baggett B, Weiss JA, Hoying JB, Edgar LT. Large-scale time series microscopy of neovessel growth during angiogenesis. Angiogenesis. (2015) 18:219–32. doi: 10.1007/s10456-015-9461-x
161. Wenger A, Stahl A, Weber H, Finkenzeller G, Augustin HG, Stark GB, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. (2004) 10:1536–47. doi: 10.1089/ten.2004.10.1536
162. Czajka CA, Drake CJ. Self-assembly of prevascular tissues from endothelial and fibroblast cells under scaffold-free, nonadherent conditions. Tissue Eng Part A. (2015) 21:277–87. doi: 10.1089/ten.tea.2014.0183
163. Sakamoto N, Kiuchi T, Sato M. Development of an endothelial-smooth muscle cell coculture model using phenotype-controlled smooth muscle cells. Ann Biomed Eng. (2011) 39:2750–8. doi: 10.1007/s10439-011-0372-8
164. Zhang L, Qian Z, Tahtinen M, Qi S, Zhao F. Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications. J Tissue Eng Regen Med. (2018) 12:e1325–36. doi: 10.1002/term.2512
165. Gibot L, Galbraith T, Huot J, Auger FA. A preexisting microvascular network benefits in vivo revascularization of a microvascularized tissue-engineered skin substitute. Tissue Eng Part A. (2010) 16:3199–206. doi: 10.1089/ten.tea.2010.0189
166. Laschke MW, Mussawy H, Schuler S, Kazakov A, Rucker M, Eglin D, et al. Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue. Tissue Eng Part A. (2011) 17:841–53. doi: 10.1089/ten.tea.2010.0329
167. Beier JP, Hess A, Loew J, Heinrich J, Boos AM, Arkudas A, et al. De novo generation of an axially vascularized processed bovine cancellous-bone substitute in the sheep arteriovenous-loop model. Eur Surg Res. (2011) 46:148–55. doi: 10.1159/000324408
Keywords: myelomeningocele, neurogenic bladder, bladder augmentation, tissue engineering, stem cells, pediatric
Citation: Horst M, Eberli D, Gobet R and Salemi S (2019) Tissue Engineering in Pediatric Bladder Reconstruction—The Road to Success. Front. Pediatr. 7:91. doi: 10.3389/fped.2019.00091
Received: 15 December 2018; Accepted: 01 March 2019;
Published: 29 March 2019.
Edited by:
Ricardo González, Kinder- und Jugendkrankenhaus AUF DER BULT, GermanyReviewed by:
Stephane Bolduc, Centre Hospitalier Universitaire de Québec, CanadaGermán Fernando Falke, Hospital Universitario Austral, Argentina
Copyright © 2019 Horst, Eberli, Gobet and Salemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maya Horst, bWF5YS5ob3JzdEBraXNwaS51emguY2g=
 Maya Horst
Maya Horst Daniel Eberli2
Daniel Eberli2 Rita Gobet
Rita Gobet