
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 04 February 2019
Sec. Pediatric Immunology
Volume 7 - 2019 | https://doi.org/10.3389/fped.2019.00007
This article is part of the Research TopicChildhood VasculitisView all 19 articles
Kawasaki disease (KD) is anacute febrile coronary vasculitis disease in children. In general, this disease can be treated with a single dose of 2 g/kg intravenous immunoglobulin (IVIG). However, the best timing for administering steroid treatment in acute-stage KD is still under debate. In this study, we recruited 174 participants to survey the transcript levels of steroid hormone receptors in KD patients. The chip studies consisted of 18 KD patients that were analyzed before IVIG treatment and at least 3 weeks after IVIG administration, as well as 36 control subjects, using GeneChip® HTA 2.0. Another cohort consisting of 120 subjects was analyzed to validate qRT-PCR. Our microarray study demonstrated significant downregulated expressions of the mRNA levels of NR1A2, RORA, NR4A1-3, THRA, and PPARD in KD patients in comparision to the controls. However, these genes increased considerably in KD patients after IVIG administration. After PCR validation, our data only revealed decreased NR4A2 mRNA expression in the KD patients compared to those of the controls, which increased after they received IVIG treatment. Our study is the first to report the potential effective utilization of steroid treatment in KD. Prior to IVIG treatment, decreased steroid receptors allowed for the reduced treatment role of steroids. However, after IVIG treatment, increased steroid receptors indicate that steroids are effective as a supplementary treatment for KD.
Kawasaki disease (KD), an acute inflammatory conronary vasculitis, has an unknown etiology but involves numerous organs and influences children <5 years old. In 1974, Dr. Tomisaki Kawasaki first published in English his observations on 50 cases of KD (1). KD presents with the following symptoms: sustained fever for more than 5 days, conjunctivitis without discharge, oral, and throat mucosal inflammatory change, non-suppurative lymphadenopathy, macular-papular erythematous skin rashes, and desquamation of the fingertips t (2). Furthermore, KD mainly affects the coronary arteries (2). The most important known complication of KD is coronary artery lesions (CAL) and coronary artery aneurysms (CAA) (3–5). Of all the untreated children, 20% develop sequelae of vasculitis with coronary artery aneurysm (6).
The generally accepted standard treatment of KD consists of high-dose intravenous immunoglobulin (IVIG) with a combination of high-dose aspirin, which can decrease CAA complications from 20–25% to 3–5% (3). Our research team has reported that, like other autoimmune diseases, KD was related to higher levels of interleukin (IL)-17A and IL-6 cytokine profiles (7). Although intravenous prednisolone has been extensively used to treat other autoimmune diseases suffered by children, the use of steroids for treating KD is still being debated. As reported by randomized trial studies of KD, when using corticosteroids in the beginning of treatment, the coronary dimensions and development of CAL did not differ (8–10). However, in addition to IVIG therapy, corticosteroids can quickly reduce serum cytokine levels and systemic inflammation in KD (9, 11). The combination of intravenous prednisolone plus a second course of IVIG therapy has been effective in patients of KD who were initially resistant to IVIG treatment alone (12, 13). In the current consensus, clinicians have postponed using steroid therapy in primary treatment but will often use it in the second course of IVIG administration or in IVIG-resistant KD patients. In fact, glucocorticoids belong to steroid hormones that interact with the DNA binding domain and initiate signal transduction after binding to steroid hormones (14). Although steroid hormones play a crucial role in inhibiting inflammation, the gene expressions of steroid hormones have not been thoroughly studied in KD patients. Therefore, in this study, we have comprehensively studied the mRNA expressions of steroid hormone receptors in different stages of KD patients and control subjects, as well as the outcome of the disease.
KD patients were required to meet the KD diagnosis criteria of the American Heart Association (15, 16) and had to be treated with a single dosage of IVIG treatment (2 g/kg) over 12 h in our hospital. In this study, we adopted Affymetrix GeneChip® Human Transcriptome Array 2.0 to quantify and compare the gene expressions of steroid hormone receptors (Supplementary Table 3) in 18 KD patients (both before and at least 3 weeks after IVIG administration), as well as in 18 healthy and 18 febrile controls. Then, we validated the mRNA levels of genes in a separate cohort (Supplementary Table 1) of 48 KD patients, 48 healthy controls, and 24 febrile subjects using real-time quantitative PCR. The subjects in the febrile controls had been diagnosed with acute pharyngitis, tonsillitis, bronchopneumonia, pneumonia, or urinary tract infection. We further collected peripheral blood samples from patients of KD both before receiving IVIG treatment (pre-IVIG) and then again at least 3 days after completing IVIG treatment, as previously described in another study (17, 18). CAL was identified through echocardiography and defined as previously described (19, 20). This study was ratified by the Institutional Review Board of Chang Gung Memorial Hospital, and we obtained written informed consent from the parents or guardians of all participants. The enrolled children could withdraw from the study at any time during the study period, and we anonymized all experimental results prior to analysis.
First, we collected whole blood samples from the participants, which were then subjected to white blood cell (WBC) enrichment, as described in one of our previous studies (21). We used an isolation kit (mirVana™ miRNA Isolation Kit, Catalog number: AM1560, Life Technologies, Carlsbad, CA) as the manufacturer's instructions, as previously described (22).
To obtain strong and unbiased results, we developed pooled RNA libraries by evenly pooling six RNA samples to create three pooled healthy controls, three fever controls, three pre-IVIG, and three post-IVIG libraries, as previously described (18). We executed a microarray assay study to establish gene expression profiles with GeneChip® Human Transcriptome Array 2.0 (HTA 2.0, Affymetrix, Santa Clara). Following the Affymetrix instruction manual, the HTA 2.0 chips' raw data were subjected to quality control, as previously described (18).
To quantify mRNA levels of interest, we adopted the LightCycler® 480 Real-Time PCR System (Roche Molecular Systems, Inc. Indiana, USA) to perform qRT- PCR according to the manufacturer's instructions and performed PCR using a SYBR Green PCR Master Mix containing 10 μM with specific forward and reverse primers (Supplementary Table 2). The relative quantification of gene expressions was based on the comparative threshold cycle (CT) method, thus enabling us to determine the target amount as 2−(ΔCTtarget−ΔCTcalibrator) or 2−ΔΔCT (23). We carried out all experiments twice to verify the efficiency of amplification.
All data are presented as mean ± standard error. Chips were evaluated with Partek (Partek, St. Louis), which is commercial software specifically designed to analyze microarray data. Either student's t-test, paired sample t-test or one-way ANOVA, as appropriate, was adopted to assess the quantitative data. We used SPSS version 12.0 for Windows XP (SPSS, Inc., Chicago, USA) for all statistical analyses. Two-sided p < 0.05 was considered statistically significant.
We surveyed 57 steroid hormone receptors in 174 cases, including 66 KD patients, 42 fever controls, and 66 non-fever controls. At the start of this study, we employed the GeneChip® Human Transcriptome Array 2.0 (Data Sheet 1) to determine the gene expression profiling of steroid hormone receptors in both KD patients and control subjects and all data from the microarray was submitted to NCBI GEO (please refer to accession number GSE109351). We further conducted GO analysis on the genes that were downregulated in KD and found that the steroid hormone receptor pathway was significant. (Enrichment Score = 12.1597, p = 0.00000523719). As shown in Supplementary Table 3 and Figure 1, we observed differential expressions of NR1A2, RORA, and NR4A1-3 in KD patients in comparison to both the febrile and healthy controls. The mRNA levels of NR1A2, RORA, NR4A1-3, THRA, and PPARD were significantly lower in acute-stage KD patients than in the healthy controls (p < 0.05). These gene expression levels significantly increased in KD patients after they underwent IVIG treatment (KD3 vs. KD1 in Supplementary Table 3). Nevertheless, we found no significant differences in these genes between KD patients and the febrile controls.
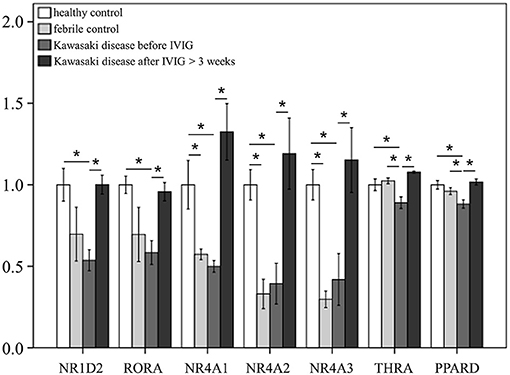
Figure 1. mRNA expressions of steroid hormone receptors by GeneChip® Human Transcriptome Array 2.0 between patients of Kawasaki disease (KD) and control subjects. Data are expressed as mean ± standard error for the three replications. *Significance (p < 0.05).
Through qRTPCR, we examined the mRNA levels of NR1A2, RORA, NR4A1-3, THRA, and PPARD in a separate cohort of 48 KD patients and 48 healthy controls. Our data showed decreased NR4A2 mRNA levels (p < 0.001) in KD patients in comparison to those of the healthy control subjects (Figure 2). The mRNA level of NR4A2 also increased following IVIG treatment (p = 0.018) (Figure 3). However, we observed no remarkable difference in the NR4A2 mRNA levels between patients of KD with IVIG resistance or CAL and those who did not (p = 0.73 and p = 0.29, respectively) (Figure 4).
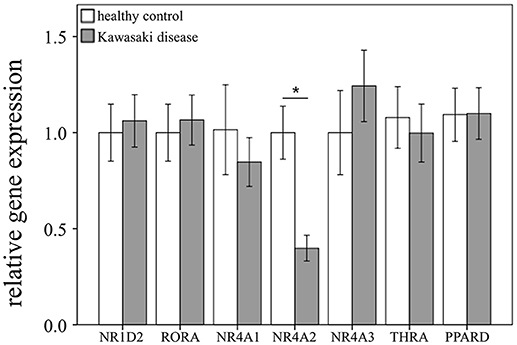
Figure 2. Analyses of mRNA expression of steroid hormone receptors in the peripheral white blood cells of 48 patients with KD and 48 healthy controls using qRT-PCR. Data are expressed as mean ± standard error. *Significance (p < 0.05) between the groups.
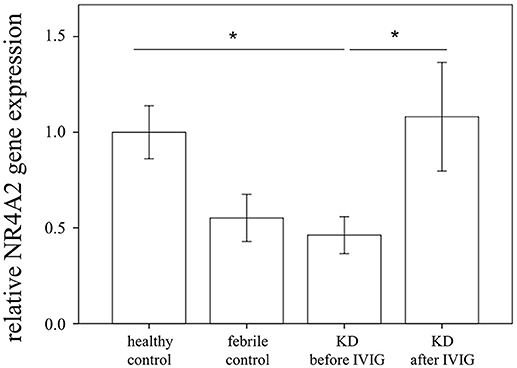
Figure 3. Analyses of NR4A2 mRNA in the peripheral white blood cells of 48 patients with KD before and after intravenous immunoglobin administration, as well as 48 healthy and 24 febrile controls using qRT-PCR. Data are expressed as mean ± standard error. *Significance (p < 0.05) between the groups.
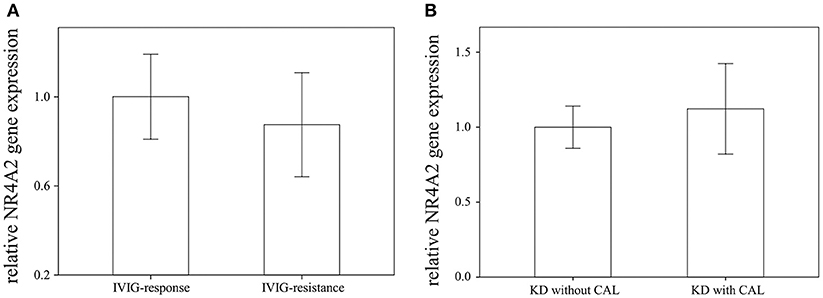
Figure 4. Comparison of NR4A2 mRNA in KD patients (A) with intravenous immunoglobulin (IVIG) (n = 6) resistance and without (n = 42), as (B) well as with (n = 28) without (n = 20) coronary artery lesion (CAL). Data are presented as mean ± standard error.
To our knowledge, we were the first to perform a comprehensive survey of transcripts of 57 steroid hormone receptors between KD patients and control subjects. Our important findings include that KD patients demonstrated a differential expression of NR1A2, RORA, NR4A1-3, THRA, and PPARD when compared to control subjects. The mRNA levels of NR1A2, RORA, NR4A1-3, THRA, and PPARD were significantly decreased in acute-stage KD than in the healthy controls. These gene expression values significantly increased in KD patients who were administered IVIG. However, we found no remarkable differences in these genes between acute-stage KD patients and febrile controls. In addition, the expression of steroid hormone receptors demonstrated no difference between KD patients and fever controls, thus indicating that steroids may have no treatment role in children with fever from infectious diseases and acute-stage KD prior to IVIG treatment. While the expression of relative steroid hormone receptors was lower in the fever controls than in the healthy controls, it did not reach statistical significance (Figure 3).
Performing qRT-PCR, we also found decreased NR4A2 mRNA levels in the KD patients compared to the controls, which increased following IVIG treatment, thus agreeing with the results of Transcriptome Array 2.0. Regarding nuclear steroid receptors, the orphan NR4A subfamily, which includes NR4A1, NR4A2, and NR4A3, has recently been investigated as a therapeutic target for treating inflammatory diseases (24). Although the underlying mechanisms remain unclear, a negative feedback mechanism that maintains inflammatory homeostasis is involved (24). In one recent study, the NR4A subfamily was shown to regulate the development of T-regulatory cells by activating Foxp3 (25). Interestingly, the overexpression of NR4A2 inhibited the proliferation of vascular smooth muscle cells, while NR4A2 silencing enhanced their growth (26).
Even though administering a single high dose of IVIG plus aspirin is standard for treating children with KD, 10–30% of patients continue to have persistent or recurrent fever after the first course of IVIG treatment (4). Such IVIG non-responders are at a higher risk for the development of CAL and may require supplemental or alternative anti-inflammation therapy (27). In the comparison of intravenous methylprednisolone (IVMP) combined with IVIG and IVIG therapy alone, it was found to not significantly improve KD outcome in primary treatment for patients (28). Although IVMP seems not to have beneficial value in primary treatment and the mechanism remains unknown, IVMP therapy appears to benefit IVIG-resistant KD patients (29). A study from Japan, known as the Randomized controlled trial to Assess Immunoglobulin plus Steroid Efficacy for KD (RAISE), found that prednisolone with initial IVIG can reduce the prevalence of CAL formation (13, 27). Moderate evidence has indicated that using steroids in primary treatment for KD may be associated with a decreased duration of clinical symptoms, length of hospital stay, and improvement regarding the development of CAL (30).
Discovered in 1967 in Japan, the first report on KD was published in English in 1974. IVIG has been used to treat KD since 1983 (31). Prior to establishing IVIG treatment, many other therapies, including steroids, antibiotics, and anti-inflammation medications, were prescribed for KD patients but did not demonstrate significant effectiveness in reducing the formation of aneurysm. Only high-dose IVIG treatment can significantly reduce the aneurysm formation rate from 25 to 3–5%. Overall, steroids only have a treatment role in KD when combined with IVIG or after IVIG treatment but not when administered without IVIG. Our study suggests that this finding might due to decreased steroid receptor expression in KD prior to IVIG treatment. This study has some limitations. First, all of our KD patients belonged to the Asian population, so this result would need to be investigated in other populations with KD. Secondly, the febrile controls were children with infectious diseases, and the febrile control results need further investigation besides infectious disease. Finally, this study still needs more cases before a final conclusion can be reached about steroid receptors in KD or fever controls.
This report is the first to explore the effectiveness of steroid treatment in KD. Prior to IVIG treatment, decreased steroid receptors reduced the treatment role of steroids. Following IVIG treatment, increased steroid receptors make steroids effective as a supplementary treatment for KD in regard to preventing coronary artery lesions.
Y-HH and H-CK conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. X-YC, M-HL, and K-DC designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. H-CK conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
MOST 105-2314-B-182-050-MY3, which was provided by the Ministry of Science and Technology of Taiwan, as well as CMRPG8E0212 and CORPG8F0012, which were provided by Chang Gung Memorial Hospital in Taiwan. Although these institutes provided financial support, they had no influence on the collection, analysis, or interpretation of the data or the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00007/full#supplementary-material
1. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics (1974) 54:271–6.
2. Wang CL, Wu YT, Liu CA, Kuo HC, Yang KD. Kawasaki disease: infection, immunity and genetics. Pediatr Infect Dis J. (2005) 24:998–1004. doi: 10.1097/01.inf.0000183786.70519.fa
3. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. (1991) 324:1633–9. doi: 10.1056/NEJM199106063242305
4. Kuo HC. Preventing coronary artery lesions in Kawasaki disease. Biomed J. (2017) 40:141–6. doi: 10.1016/j.bj.2017.04.002
5. Kuo HC, Guo MM, Lo MH, Hsieh KS, Huang YH. Effectiveness of intravenous immunoglobulin alone and intravenous immunoglobulin combined with high-dose aspirin in the acute stage of Kawasaki disease: study protocol for a randomized controlled trial. BMC Pediatr. (2018) 18:200. doi: 10.1186/s12887-018-1180-1
6. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. (1986) 315:341–7. doi: 10.1056/NEJM198608073150601
7. Guo MM, Tseng WN, Ko CH, Pan HM, Hsieh KS, Kuo HC. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy (2015) 70:310–8. doi: 10.1111/all.12558
8. Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. (2003) 142:611–6. doi: 10.1067/mpd.2003.191
9. Jibiki T, Terai M, Kurosaki T, Nakajima H, Suzuki K, Inomata H, et al. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. Eur J Pediatr. (2004) 163:229–33. doi: 10.1007/s00431-003-1386-5
10. Inoue Y, Okada Y, Shinohara M, Kobayashi T, Kobayashi T, Tomomasa T, et al. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: clinical course and coronary artery outcome. J Pediatr. (2006) 149:336–41. doi: 10.1016/j.jpeds.2006.05.025
11. Okada Y, Shinohara M, Kobayashi T, Inoue Y, Tomomasa T, Kobayashi T, et al. Effect of corticosteroids in addition to intravenous gamma globulin therapy on serum cytokine levels in the acute phase of Kawasaki disease in children. J Pediatr. (2003) 143:363–7. doi: 10.1067/S0022-3476(03)00387-1
12. Ebato T, Ogata S, Ogihara Y, Fujimoto M, Kitagawa A, Takanashi M, et al. The clinical utility and safety of a new strategy for the treatment of refractory kawasaki disease. J Pediatr. (2017) 191:140–4. doi: 10.1016/j.jpeds.2017.08.076
13. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet (2012) 379:1613–20. doi: 10.1016/S0140-6736(11)61930-2
14. Straub RH, Cutolo M. Glucocorticoids and chronic inflammation. Rheumatology (2016) 55 (suppl. 2):ii6–14. doi: 10.1093/rheumatology/kew348
15. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on rheumatic fever, endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation (2004) 110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78
16. Kuo HC, Lo MH, Hsieh KS, Guo MM, Huang YH. High-dose aspirin is associated with anemia and does not confer benefit to disease outcomes in kawasaki disease. PLoS ONE (2015) 10:e0144603. doi: 10.1371/journal.pone.0144603
17. Kuo HC, Wang CL, Yang KD, Lo MH, Hsieh KS, Li SC, et al. Plasma prostaglandin E2 levels correlated with the prevention of intravenous immunoglobulin resistance and coronary artery lesions formation via CD40L in Kawasaki Disease. PLoS ONE (2016) 11:e0161265. doi: 10.1371/journal.pone.0161265
18. Huang YH, Li SC, Huang LH, Chen PC, Lin YY, Lin CC, et al. Identifying genetic hypomethylation and upregulation of Toll-like receptors in Kawasaki disease. Oncotarget (2017) 8:11249–58. doi: 10.18632/oncotarget.14497
19. Kuo HC, Wang CL, Liang CD, Yu HR, Huang CF, Wang L, et al. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr Allergy Immunol. (2009) 20:266–72. doi: 10.1111/j.1399-3038.2008.00779.x
20. Kuo HC, Yang KD, Liang CD, Bong CN, Yu HR, Wang L, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol. (2007) 18:354–9. doi: 10.1111/j.1399-3038.2007.00516.x
21. Li SC, Chan WC, Huang YH, Guo MM, Yu HR, Huang FC, et al. Major methylation alterations on the CpG markers of inflammatory immune associated genes after IVIG treatment in Kawasaki disease. BMC Med Genom. (2016) 9 (Suppl. 1) 37. doi: 10.1186/s12920-016-0197-2
22. Kuo H C, Chang JC, Yu HR, Wang CL, Lee CP, Huang LT, et al. Identification of an association between genomic hypomethylation of FCGR2A and susceptibility to Kawasaki disease and intravenous immunoglobulin resistance by DNA methylation array. Arthritis Rheumatol. (2015) 67:828–36. doi: 10.1002/art.38976
23. Yang YL, Wang FS, Li SC, Tiao MM, Huang YH. MicroRNA-29a alleviates bile duct ligation exacerbation of hepatic fibrosis in mice through epigenetic control of methyltransferases. Int J Mol Sci. (2017) 18:192. doi: 10.3390/ijms18010192
24. Rodriguez-Calvo R, Tajes M, Vazquez-Carrera M. The NR4A subfamily of nuclear receptors: potential new therapeutic targets for the treatment of inflammatory diseases. Expert Opin Ther Targets (2017) 21:291–304. doi: 10.1080/14728222.2017.1279146
25. Won HY, Hwang ES. Transcriptional modulation of regulatory T cell development by novel regulators NR4As. Arch Pharm Res. (2016) 39:1530–6. doi: 10.1007/s12272-016-0803-z
26. Bonta PI, Pols TW, van Tiel CM, Vos M, Arkenbout EK, Rohlena J, et al. Nuclear receptor Nurr1 is expressed in and is associated with human restenosis and inhibits vascular lesion formation in mice involving inhibition of smooth muscle cell proliferation and inflammation. Circulation (2010) 121:2023–32. doi: 10.1161/CIRCULATIONAHA.109.885673
27. Miura M. Role of glucocorticoids in Kawasaki disease. Int J Rheum Dis. (2018) 21:70–75. doi: 10.1111/1756-185X.13209
28. Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VLet al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. (2007) 356:663–75. doi: 10.1056/NEJMoa061235
29. Chen HH, Liu PM, Bong CN, Wu YT, Yang KD, Wang CL. Methylprednisolone pulse therapy for massive lymphadenopathy in a child with intravenous immunoglobulin-resistant Kawasaki disease. J Microbiol Immunol Infect. (2005) 38:149–52.
30. Wardle AJ, Connolly GM, Seager MJ, Tulloh RM. Corticosteroids for the treatment of Kawasaki disease in children. Cochr Database Syst Rev. (2017) 1:CD011188. doi: 10.1002/14651858.CD011188.pub2
Keywords: Kawasaki disease (KD), steroid receptor, Nr4a2, immunology, IVIG = intravenous immunoglobulin
Citation: Huang Y-H, Chen K-D, Lo M-H, Cai X-Y and Kuo H-C (2019) Decreased Steroid Hormone Receptor NR4A2 Expression in Kawasaki Disease Before IVIG Treatment. Front. Pediatr. 7:7. doi: 10.3389/fped.2019.00007
Received: 08 November 2018; Accepted: 10 January 2019;
Published: 04 February 2019.
Edited by:
Christian Michael Hedrich, University of Liverpool, United KingdomReviewed by:
Klaus Tenbrock, RWTH Aachen Universität, GermanyCopyright © 2019 Huang, Chen, Lo, Cai and Kuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho-Chang Kuo, ZXJpY2t1bzQ4QHlhaG9vLmNvbS50dw==; ZHIuaGNrdW9AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.