- Department of Epidemiology and Health Statistics, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, China
Objectives: We aim to assess the correlation of multidrug-resistant Staphylococcus aureus (MDR S. aureus) carriage between mothers and their newborn infants.
Materials and Methods: We conducted a prospective cohort study of mothers and their newborn infants in two hospitals in Shenzhen, China, from August to November 2015. We collected demographic and clinical information from mothers and newborn infants by face-to-face questionnaires and medical datasets. Serial swabs were collected from mothers and their newborn infants for further experiments. Maternal-infant correlation was assessed using the Poisson regression model.
Results: The prevalence of MDR S. aureus vaginal carriage in mothers was 4.7% (86/1834). The incidence of MDR S. aureus carriage in newborn infants was 1.3% (23/1834). The adjusted relative risk and 95% confidence interval of maternal-infant MDR S. aureus carriage was 7.63 (2.99–19.49). Six MDR S. aureus maternal-infant pairs were concordant. The phenotypic and molecular characteristics of MDR S. aureus isolates were similar between mothers and their newborn infants.
Conclusion: MDR S. aureus vaginal carriage in mothers was associated with an increased risk for MDR S. aureus carriage in their newborn infants.
Introduction
Staphylococcus aureus (S. aureus) is one of the most important human bacterial pathogens that cause nosocomial and community infections, and results in substantial morbidity and mortality (1). Multidrug-resistant S. aureus (MDR S. aureus) is of greater significance because of its more severe clinical outcomes (2).
Those persons who are colonized with MDR S. aureus have a higher risk for subsequent infections than non-colonized individuals (3, 4). Most infections are caused by the same MDR S. aureus strain that previously colonized the person (5). In response to these findings, there has been increasing attention to the detection of MDR S. aureus carriage, with subsequent decolonization of carriers as a potential method for the prevention of MDR S. aureus infection.
Most of the available data on the detection of MDR S. aureus carriage have been in hospitalized non-pregnant adults (6, 7). Despite the fact that MDR S. aureus outbreaks in infants have been linked to the infected or colonized mother, there are limited data on MDR S. aureus carriage rates among mothers or on the risk of transmission of MDR S. aureus from pregnant MDR S. aureus carriers to their newborn infants (8, 9). The maternal-infant relatedness of MDR S. aureus carriage is of particular interest.
Accordingly, we determined whether vaginal carriage of MDR S. aureus in the mothers is independently correlated with MDR S. aureus carriage in their newborn infants. Antimicrobial susceptibility, virulence factors, and clonality of isolates were also evaluated. The hypothesis of this study is that there is maternal-infant vertical MDR S. aureus carriage.
Materials and Methods
This study was approved by the Ethics Committee of Guangdong Pharmaceutical University and was performed in accordance with the approved guidelines. Written informed consent was obtained from the mothers and their newborn infants involved in the study before enrollment.
This prospective cohort study was conducted in two hospitals in Shenzhen, China, between August and November 2015. The two hospitals, Longhua Central Hospital and Guanlan People's Hospital, are large hospitals whose obstetric services deliver between 10,000 and 15,000 neonates per year; these are two of the largest delivery hospitals in China. The target populations were mothers and their newborn infants. Chinese mothers with gestation between 35 and 40 weeks were voluntarily included. Mothers with twin or multiple gestations, cesarean section, or acute diseases were excluded. Newborn infants without sample collection were also excluded. The sample size was calculated using the power two proportions method with 4.00% proportion (approximate prevalence of S. aureus in the newborns), 4.00 ratio, 0.05 alpha, and 0.80 power. Taking a 10% loss of follow-up into consideration, the estimated minimum sample size of this study was 107 for each group.
A face-to-face questionnaire was administered by trained personnel that aimed to collect demographic information and information regarding potential factors influencing MDR S. aureus carriage during pregnancy. Medical records of the mothers and their newborn infants were reviewed by two members of the study team who were blinded to the maternal and infant MDR S. aureus carriage status.
Sterile swabs moistened with sterile saline water were used to get specimens from the vagina of the enrolled mothers prior to delivery by trained personnel. Infant specimens for MDR S. aureus were sampled from the nasal cavity, ear canal, oral cavity, and umbilicus by trained nurses immediately after birth. All specimens were then inoculated into enrichment broth tubes containing 1% tryptone, 7.5% sodium chloride, 1% mannitol, and 0.25% yeast extract.
All included mothers were informed of the research question and outcome measures by well-trained staff in person before participating. The trained personnel informed the mothers of the results of S. aureus carriage by telephone calls. Chinese mothers with gestation between 35 and 40 weeks were voluntarily included in this study. Included mothers were asked to complete face-to-face questionnaires and collected vaginal samples by trained personnel.
Swabs were inoculated into mannitol salt agar plates at 37 ± 1°C for 24 h of incubation. Isolates were confirmed to be S. aureus by a combination of gram staining, catalase reaction, hemolysis test, DNase test, and coagulase tests.
Antimicrobial susceptibility testing was performed by the Kirby-Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute guidelines of 2015. The following antimicrobial disks were used: cefoxitin, clindamycin, rifampicin, moxifloxacin, tobramycin, gentamicin, sulfamethoxazole-trimethoprim, linezolid, and erythromycin. Isolates were defined as resistant if they were resistant or intermediate to the antimicrobial disk. S. aureus isolates were classified as MDR if they were resistant to no less than three antibiotic classes (10).
Multilocus sequence typing (MLST) was used to type MDR S. aureus, which involved sequencing the seven housekeeping genes (11). Then, the sequence types (STs) and allelic profiles were confirmed by querying the MLST database (http://eburst.mlst.net). STs were clustered into a clonal complex (CC) by using the eBURST software program (Department of Infectious Disease Epidemiology, Imperial College London, London, UK; http://eburst.mlst.net). The presence of virulence genes including Panton-valentine leukocidin (Pvl), toxic shock syndrome toxin (Tst), exfoliative toxin A (Eta), and exfoliative toxin B (Etb) using polymerase chain reaction assays as in previous studies was determined as previously described (12, 13).
All data were entered in duplicate into the EpiData version 3.0 database (The EpiData Association, Odense Denmark). Missing data were excluded. Categorical variables were compared by Pearson's chi-squared test or the Fisher exact test when appropriate. To identify variables that might confound the correlation of MDR S. aureus carriage between mothers and newborn infants, influencing factors with a P-value < 0.2 were identified as adjustment variables using the Poisson regression model to estimate the correlations of isolates between mothers and newborn infants. Relative risks (RRs) and 95%confidence intervals (CIs) were used to assess the maternal-infant relatedness of MDR S. aureus carriage. The relationship between populations and main STs of MDR S. aureus isolates was illustrated by a minimum spanning tree (PHYLOVIZ software version 2.0; http://www.phyloviz.net). A two-sided P-value < 0.05 was considered as being of statistical significance. All analyses were performed using Stata version 14.2 (College Station, Texas, USA).
Results
Overall, 1968 mothers were preliminarily enrolled, but only 1,834 mothers and their newborn infants met the inclusion criteria and consented to participate in this study. We found that there were 133 S. aureus isolates and the prevalence of MDR S. aureus vaginal carriage in mothers was 4.7% (86/1834). There was no statistical difference of MDR S. aureus carriage in mothers from the two different hospitals (χ2 = 0.029, P-value = 0.865).
There were 60 S. aureus isolates and the incidence of MDR S. aureus carriage in newborn infants was 1.3% (23/1834). There was no statistical difference of MDR S. aureus carriage in newborn infants from the two different hospitals (χ2 = 0.846, P-value = 0.358).
MDR S. aureus vaginal carriage in mothers (χ2 = 69.163, P-value < 0.001) was significantly correlated with MDR S. aureus carriage in their newborn infants. However, there were no other significant variables correlated with MDR S. aureus carriage in infants. More details can be found in Table 1.
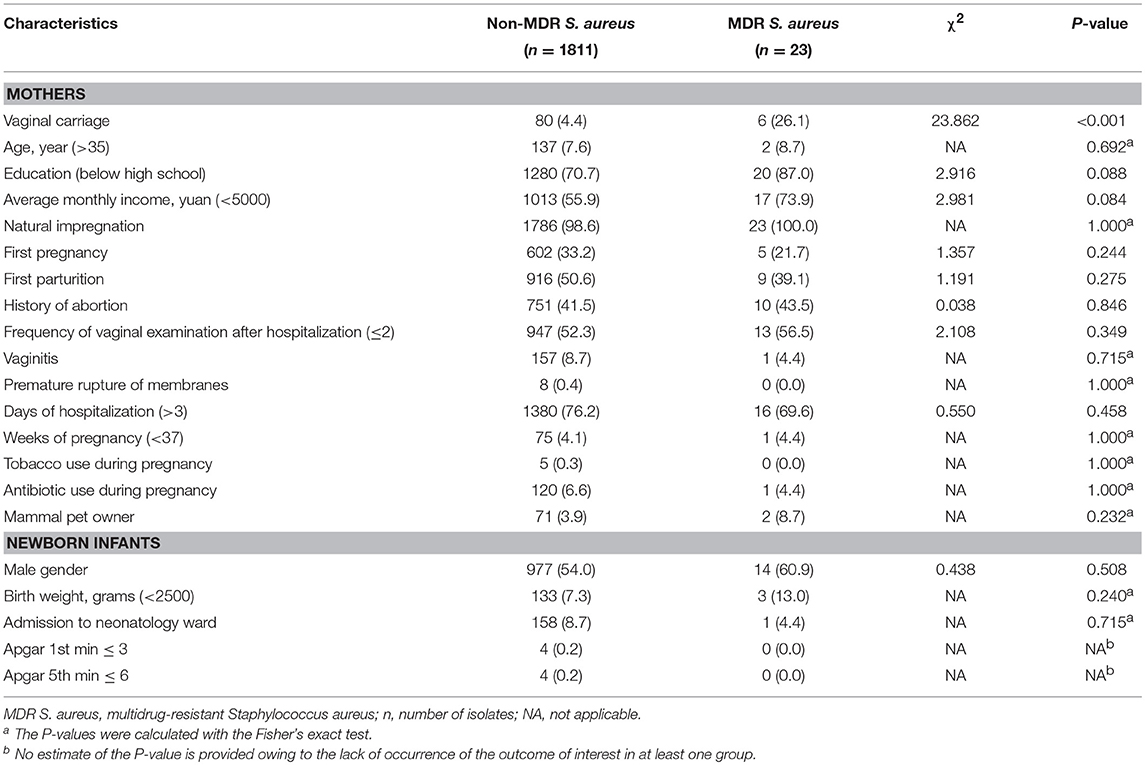
Table 1. Characteristics of multidrug-resistant Staphylococcus aureus carriage among newborn infants in Shenzhen, 2015 [n (%)].
After adjusting for the age of mothers, education level of mothers, average monthly income, and gender of newborn infants, MDR S. aureus vaginal carriage in mothers was still a risk factor for MDR S. aureus carriage in newborn infants [adjusted RR (aRR) = 7.63; 95%CI, 2.99–19.49; P-value < 0.001]. More details can be found in Table 2.
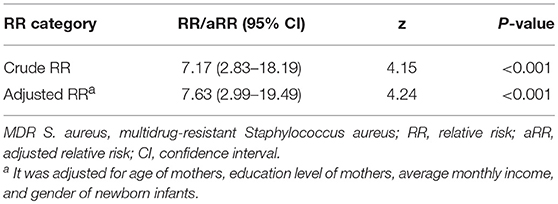
Table 2. Correlations of multidrug-resistant Staphylococcus aureus carriage between mothers and their newborn infants in Shenzhen, 2015.
The most predominant proportion of antibiotic resistance in the 86 MDR S. aureus isolates from mothers was penicillin (97.7%), followed by erythromycin (73.3%), clindamycin (64.0%), tobramycin (38.4%), cefoxitin (26.7%), trimethoprim-sulfamethoxazole (20.9%), moxifloxacin (19.8%), gentamicin (12.8%), linezolid (5.8%), and rifampicin (2.3%). The most predominant proportion of antibiotic resistance in 23 MDR S. aureus isolates from the newborn infants was penicillin (95.7%), followed by erythromycin (91.3%), clindamycin (69.6%), cefoxitin (47.8%), trimethoprim-sulfamethoxazole (30.4%), gentamicin (21.7%), tobramycin (21.7%), rifampicin (21.7%), moxifloxacin (13.0%), and linezolid (8.7%). The proportion of resistant rifampicin (P-value = 0.004) in MDR S. aureus isolates was significantly different between mothers and their newborn infants. More details can be found in Table 3.
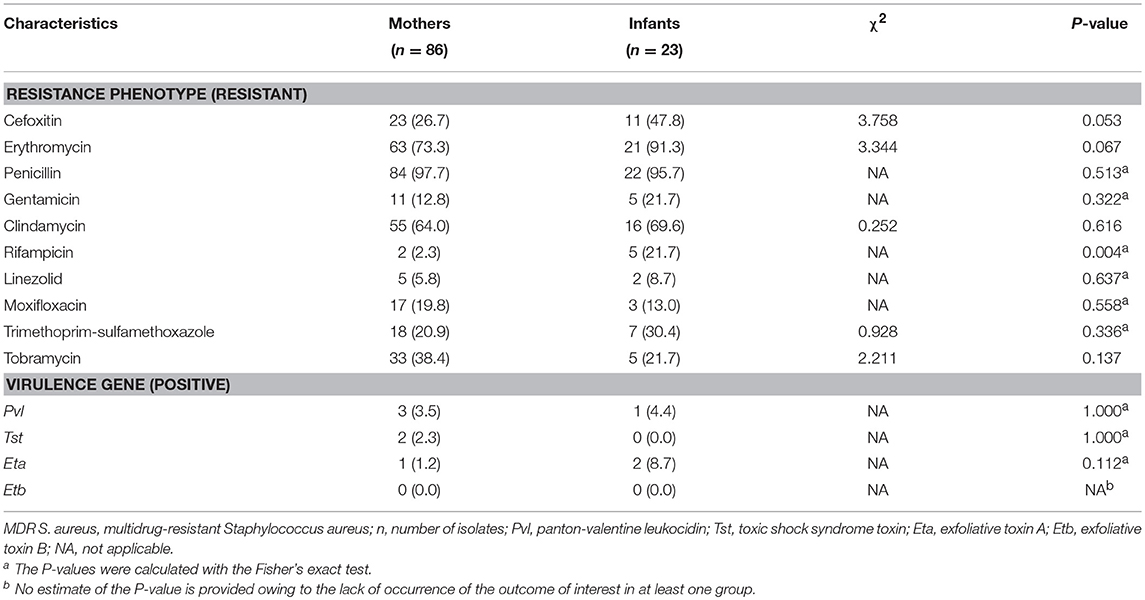
Table 3. Phenotypic and genetic characteristics of multidrug-resistant Staphylococcus aureus isolates between mothers and their newborn infants in Shenzhen, 2015 [n (%)].
The results indicated that the proportions of virulence genes of isolates in both mothers and their newborns were low. The proportion of the positive Pvl gene in MDR S. aureus isolates was significantly different between mothers and newborn infants (Fisher's exact test, P-value = 0.041). There were no significant differences of virulence genes in MDR S. aureus isolates between mothers and their newborn infants. More details can be found in Table 3.
The most common CC of 86 MDR S. aureus isolates in mothers was CC5 (51.2%), followed by CC7 (19.8%), CC59 (14.0%), CC88 (5.8%), CC398 (3.5%), CC20 (2.3%), CC45 (2.3%), and CC121 (1.2%). The most common CC of 23 MDR S. aureus isolates in newborn infants was CC5 (38.1%), followed by CC59 (28.6%), CC45 (9.5%), CC88 (9.5%), CC7 (4.8%), CC22 (4.8%), and CC398 (4.8%).
The minimum spanning tree demonstrated a good concordance of certain STs between mothers and their newborn infants; for example, MDR S. aureus isolates belonging to ST1, ST6, ST7, ST59, ST88, ST398, and ST965 were found in both mothers and newborn infants. More details can be found in Figure 1.
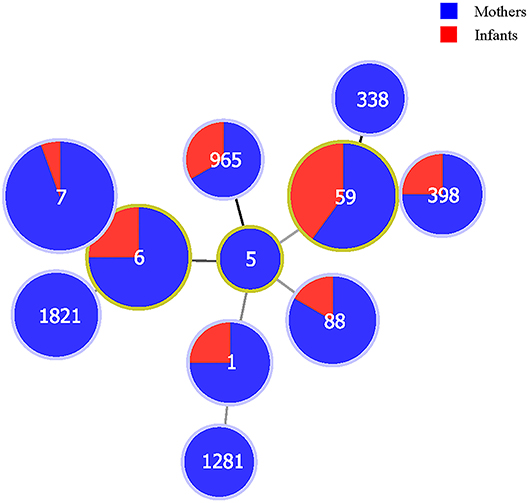
Figure 1. The correlations between populations (mothers and newborn infants) and sequence types of multidrug-resistant Staphylococcus aureus isolates in Shenzhen, 2015. The numbers in the circles are the sequence types. The size of the circles represents the number of isolates. The distance of lines between each circle represents the degree of the relationship.
Overall, there were six MDR S. aureus maternal-infant pairs. These six MDR S. aureus maternal-infant pairs were concordant, with the same phenotypic and molecular characteristics. More details can be found in Table 4.
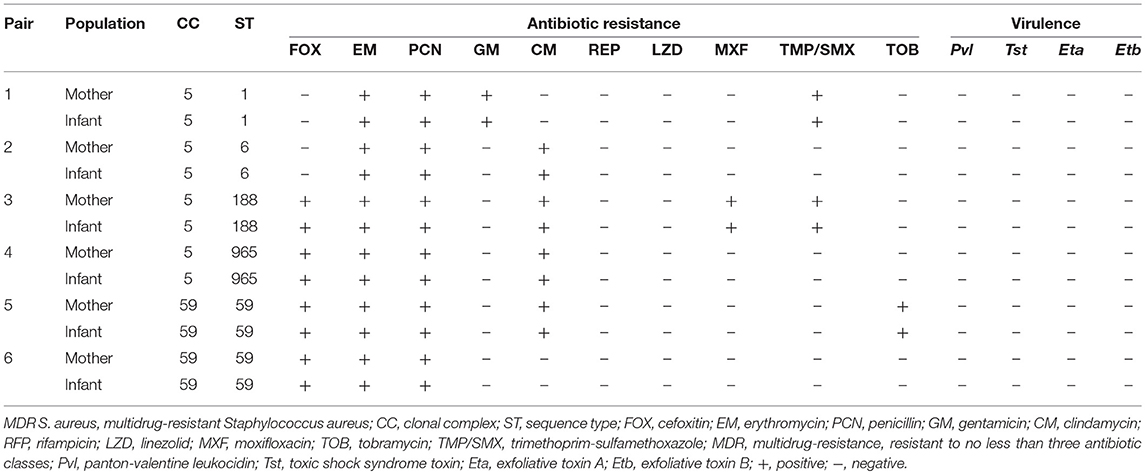
Table 4. Characteristics of six maternal-infant pairs with multidrug-resistant Staphylococcus aureus isolates in Shenzhen, 2015.
Discussion
Methicillin-Resistant Staphylococcus aureus (MRSA) carriage between mothers and their infants has been reported, but no previous studies reported the proportions of MDR S. aureus in both mothers and infants. The prevalence of MRSA in MDR S. aureus among mothers and their newborns was 26.7 and 47.8%, respectively. On the contrary, the prevalence of MDR S. aureus in MRSA among mothers and their newborns were 88.5 and 91.7%, respectively. These results were similar to current observed studies that MRSA is always MDR S. aureus, but MDR S. aureus is not necessarily MRSA (7, 11). Therefore, it is significant to assess the mother-infant relationship of MDR S. aureus. An American study reported that the proportion of MDR S. aureus carriage in community residents was 6.9% (14). Other previous studies have reported that the prevalence of S. aureus vaginal carriage in mothers (pregnant women) and infants ranged from 0.96–12.6% (15–18) to 5.4–17.7% (15, 16, 18–21), respectively. Therefore, the results suggested that MDR S. aureus carriage in our target populations were moderate when compared with other countries and regions.
This prospective cohort study identified risk factors for MDR S. aureus carriage in newborn infants and assessed the risk of MDR S. aureus vaginal carriage of mothers for MDR S. aureus carriage in their newborn infants. The gender of the newborn infants was not associated with MDR S. aureus isolates in this study, which was different to previous studies (22, 23). The mechanism between gender and MDR S. aureus carriage requires further exploration. No previous studies have particularly explored the maternal-infant relatedness of MDR S. aureus carriage. Therefore, we compared our results with other previous maternal-infant Staphylococcus aureus carriage. The aRR of maternal-infant MDR S. aureus carriage in our study was 7.63. We found that it was much higher than other previous studies, which reported that the RRs of maternal-infant Staphylococcus aureus carriage ranged from 2.04 to 5.70 (21, 24–26). The results demonstrated that maternal-infant MDR S. aureus transmission is much more hazardous.
There were few significant differences on phenotypic and virulence genetic characterizations in MDR S. aureus isolates between mothers and their newborn infants. We observed good consistency on certain STs of MDR S. aureus isolates between mothers and their newborn infants, such as ST1, ST6, ST7, ST59, ST88, ST398, and ST965. These STs were also reported in other previous studies (20, 27). Furthermore, we found that six concordant maternal-infant MDR S. aureus pairs had the same phenotypic and molecular characteristics. These results could further verify the homology of maternal-infant MDR S. aureus carriage.
In the current study, we found that more than 5% of MDR S. aureus isolates were resistant to linezolid and the rate was higher in isolates from newborns. This was not usual, and it could raise major issues in the case of infection with these strains (28). Healthcare workers should pay greater attention to mothers and newborns with linezolid resistance.
This study has some limitations. First, the correlation of MDR S. aureus carriage between mothers and infants needs to be explored further because of the limited sample size in this study. Second, whole-genome sequencing would further strengthen this study. Third, the potential importance of environmental contamination in MDR S. aureus carriage would need to be further explored. Fourth, participants in this study were volunteers, recruited using a convenient sampling approach. It is possible that selection bias may have occurred. Last, we did not follow up with the newborns because of insufficient financial and human support.
In summary, we identified vertical maternal-infant transmission of MDR S. aureus carriage in newborn infants. Newborn infants born with maternal MDR S. aureus carriage appear to be at higher risk of MDR S. aureus carriage. Accordingly, routine surveillance for MDR S. aureus carriage in mothers may be indicated. Prevention measures focused on controlling the spread of MDR S. aureus in mothers could be a more effective strategy when outbreaks of MDR S. aureus in newborn infants occur. Further work should seek to elucidate the potential role of maternally derived antibodies in modifying MDR S. aureus carriage risk in newborn infants.
Author Contributions
ZY designed the study, performed the data analysis, and revised the manuscript. JL collected the information, conducted the experiments, performed the data analysis, and wrote the manuscript.
Funding
This work was funded by the Innovation Fund of Guangdong Science and Technology Planning Project (grant number 2014A020213013) and Guangdong Pharmaceutical University Innovation and Strength School Funding Project (No.2016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We greatly thank Qianting Ou and Dongxin Lin for helping us with collecting samples and conducting the experiments.
References
1. Lowy FD. Staphylococcus aureus infections. N Engl J Med. (1998) 339:520–32. doi: 10.1056/NEJM199808203390806
2. Matteo B, Elda R. Multidrug-resistant bacteria: what is the threat? Hematology Am Soc Hematol Educ Program (2013) 2013:428–32. doi: 10.1182/asheducation-2013.1.428
3. Eells SJ, Chira S, David CG, Craft N, Miller LG. Non-suppurative cellulitis: risk factors and its association with Staphylococcus aureus colonization in an area of endemic community-associated methicillin-resistant S. aureus infections. Epidemiol Infect. (2011) 139:606–12. doi: 10.1017/S0950268810001408
4. Taormina DP, Konda SR, Liporace FA, Egol KA. Can preoperative nasal cultures of Staphylococcus aureus predict infectious complications or outcomes following repair of fracture nonunion? J Infect Public Health (2017) 839:1–5. doi: 10.1016/j.jiph.2017.10.007
5. Price JR, Cole K, Bexley A, Kostiou V, Eyre DW, Golubchik T, et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect. Dis. (2017) 17:207–14. doi: 10.1016/s1473–3099(16)30413–3
6. Ungureanu A, Zlatian O, Mitroi G, Drocas A, Tirca T, Calina D, et al. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol Med Rep. (2017) 16:8771–80. doi: 10.3892/mmr.2017.7746
7. Tfifha M, Ferjani A, Mallouli M, Mlika N, Abroug S, Boukadida J. Carriage of multidrug-resistant bacteria among pediatric patients before and during their hospitalization in a tertiary pediatric unit in Tunisia. Libyan J Med. (2018) 13:1419047. doi: 10.1080/19932820.2017.1419047
8. Williams K, Hopkins S, Turbitt D, Seng C, Cookson B, Patel BC, et al. Survey of neonatal unit outbreaks in North London: identifying causes and risk factors. J Hosp Infect. (2014) 88:149–55. doi: 10.1016/j.jhin.2014.06.012
9. Dramowski A, Aucamp M, Bekker A, Mehtar S. Infectious disease exposures and outbreaks at a South African neonatal unit with review of neonatal outbreak epidemiology in Africa. Int J Infect Dis. (2017) 57:79–85. doi: 10.1016/j.ijid.2017.01.026
10. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469–0691.2011.03570.x
11. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. (2000) 38:1008–15.
12. McClure JA, Conly JM, Lau V, Elsayed S, Louie T, Hutchins W, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. (2006) 44:1141–4. doi: 10.1128/JCM.44.3.1141–1144.2006
13. Jarraud S. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (Alleles), and human disease. Infect. Immunity (2002) 70:631–41. doi: 10.1128/iai.70.2.631–641.2002
14. Neyra RC, Frisancho JA, Rinsky JL, Resnick C, Carroll KC, Rule AM, et al. Multidrug-resistant and methicillin-resistant Staphylococcus aureus (MRSA) in hog slaughter and processing plant workers and their community in North Carolina (USA). Environ Health Perspect. (2014) 122:471–7. doi: 10.1289/ehp.1306741
15. Andrews JI, Fleener DK, Messer SA, Kroeger JS, Diekema DJ. Screening for Staphylococcus aureus carriage in pregnancy: usefulness of novel sampling and culture strategies. Am J Obstet Gynecol. (2009) 201:396 e1–5. doi: 10.1016/j.ajog.2009.06.062
16. Bourgeois-Nicolaos N, Lucet JC, Daubie C, Benchaba F, Rajguru M, Ruimy R, et al. Maternal vaginal colonisation by Staphylococcus aureus and newborn acquisition at delivery. Paediatr Perinat Epidemiol. (2010) 24:488–91. doi: 10.1111/j.1365–3016.2010.01139.x
17. Top KA, Buet A, Whittier S, Ratner AJ, Saiman L. Predictors of Staphylococcus aureus rectovaginal colonization in pregnant women and risk for maternal and neonatal infections. J Pediatric Infect Dis Soc. (2012) 1:7–15. doi: 10.1093/jpids/pis001
18. Pinter DM, Mandel J, Hulten KG, Minkoff H, Tosi MF. Maternal-infant perinatal transmission of methicillin-resistant and methicillin-sensitive Staphylococcus aureus. Am J Perinatol. (2009) 26:145–51. doi: 10.1055/s-0028–1095179
19. Schaumburg F, Alabi AS, Mombo-Ngoma G, Kaba H, Zoleko RM, Diop DA, et al. Transmission of Staphylococcus aureus between mothers and infants in an African setting. Clin Microbiol Infect. (2014) 20:O390–6. doi: 10.1111/1469–0691.12417
20. Chatzakis E, Scoulica E, Papageorgiou N, Maraki S, Samonis G, Galanakis E. Infant colonization by Staphylococcus aureus: role of maternal carriage. Eur J Clin Microbiol Infect Dis. (2011) 30:1111–7. doi: 10.1007/s10096–011-1199–9
21. Lebon A, Moll HA, Tavakol M, van Wamel WJ, Jaddoe VW, Hofman A, et al. Correlation of bacterial colonization status between mother and child: the generation R study. J Clin Microbiol. (2010) 48:960–2. doi: 10.1128/JCM.01799–09
22. Yan X, Song Y, Yu X, Tao X, Yan J, Luo F, et al. Factors associated with Staphylococcus aureus nasal carriage among healthy people in Northern China. Clin Microbiol Infect. (2015) 21:157–62. doi: 10.1016/j.cmi.2014.08.023
23. Laub K, Tóthpál A, Kovács E, Sahin-Tóth J, Horváth A, Kardos S, et al. High prevalence of Staphylococcus aureus nasal carriage among children in Szolnok, Hungary. Acta Microbiol Immunol Hung. 65:59–72. doi: 10.1556/030.65.2018.001
24. Leshem E, Maayan-Metzger A, Rahav G, Dolitzki M, Kuint J, Roytman Y, et al. Transmission of Staphylococcus aureus from mothers to newborns. Pediatr Infect Dis J. (2012) 31:360–3. doi: 10.1097/INF.0b013e318244020e
25. Jimenez-Truque N, Tedeschi S, Saye EJ, McKenna BD, Langdon W, Wright JP, et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics (2012) 129:e1252–9. doi: 10.1542/peds.2011–2308
26. Roca A, Bojang A, Camara B, Oluwalana C, Lette K, West P, et al. Maternal colonization with Staphylococcus aureus and Group B streptococcus is associated with colonization in newborns. Clin Microbiol Infect. (2017) 23:974–9. doi: 10.1016/j.cmi.2017.04.020
27. Lamaro-Cardoso J, de Lencastre H, Kipnis A, Pimenta FC, Oliveira LS, Oliveira RM, et al. Molecular epidemiology and risk factors for nasal carriage of staphylococcus aureus and methicillin-resistant S. aureus in infants attending day care centers in Brazil. J Clin Microbiol. (2009) 47:3991–7. doi: 10.1128/JCM.01322–09
Keywords: Staphylococcus aureus, multidrug resistant, mothers, infants, cohort
Citation: Lin J and Yao Z (2018) Maternal-Infant Correlation of Multidrug-Resistant Staphylococcus aureus Carriage: A Prospective Cohort Study. Front. Pediatr. 6:384. doi: 10.3389/fped.2018.00384
Received: 21 September 2018; Accepted: 20 November 2018;
Published: 05 December 2018.
Edited by:
Hiromichi Hamada, Tokyo Women's Medical University Yachiyo Medical Center, JapanReviewed by:
Marine Butin, Centre Hospitalier Universitaire de Lyon, FranceGuillermo Soza, Universidad de La Frontera, Chile
Hiroyuki Shiro, Yokohama Rosai Hospital, Japan
Copyright © 2018 Lin and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenjiang Yao, emhqeWFvMjAwMUB5YWhvby5jb20=
 Jialing Lin
Jialing Lin Zhenjiang Yao
Zhenjiang Yao