- 1Faculty of Science, University of Alberta, Edmonton, AB, Canada
- 2Centre for the Studies of Asphyxia and Resuscitation, Royal Alexandra Hospital, Edmonton, AB, Canada
- 3Department of Pediatrics, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada
Background: In 2015, the neonatal resuscitation guidelines incorporated the use of electrocardiography (ECG) to monitor heart rate of newborns. However, previous studies have indicated that cardiac arrest with pulseless electrical activity rhythm (PEA) may occur in the delivery room, rendering this method problematic.
Objective: To evaluate the accuracy of ECG and auscultation to assess heart rate during PEA.
Methods: A total of 45 piglets (age 1–3 days, weight 1.7–2.3 kg) were exposed to 30 min normocapnic alveolar hypoxia followed by asphyxia until asystole, achieved by disconnecting the ventilator and clamping the endotracheal tube. During asphyxia, heart rate (HR) was assess using auscultation, ECG, and carotid blood flow (CBF). At the time of asystole (defined as zero CBF) HR auscultated using a neonatal/infant stethoscope was compared to ECG traces.
Results: The median (IQR) duration of asphyxia was 325 (200–491) s. In 8 (18%) piglets, CBF, ECG, and auscultation identified asystole. In 22 (49%) piglets no CBF and no audible heart sounds, were observed, while ECG displayed a HR ranging from 17 to 75/min. Fifteen (33%) piglets remained bradycardic (defined as HR of < 100/min) after 10 min of asphyxia, which was identified by CBF, ECG, and auscultation. The overall accuracy of ECG and auscultation in the detection of HR were 51 and 80%, respectively (p = 0.004).
Conclusion: In cases with PEA ECG is not superior in correctly identifying HR in newborn piglets.
Introduction
Immediately after birth, a newborns heart rate (HR) is assessed to determine the effectiveness of spontaneous respiratory effort and the need for subsequent interventions (1, 2). Changes in a newborn's HR are considered the most sensitive indicator of effectiveness for each intervention. Therefore, identifying a rapid, reliable, and accurate method to measure the newborn's HR is critically important (1, 2). Until 2015, auscultation of the precordium was recommended as the preferred physical examination method, and pulse oximetry was recommended as an adjunct to provide a non-invasive, rapid, and continuous assessment of HR during resuscitation (1, 2). Studies comparing clinical assessment with pulse oximetry reported that auscultation or palpation underestimates HR by −14 and −21 beats per minute, respectively, suggesting they are both unreliable and inaccurate (3). Also, several studies reported that a ECG displayed a reliable HR faster than pulse oximetry, and pulse oximetry tended to underestimate the newborn's HR and might have led to potentially unnecessary interventions (4–7). However, most of the included newborns did not require resuscitation, and very few required chest compressions. Therefore, ECG to assess HR in newborns who require chest compression should be approached with caution.
In this of particular importance as we recently described that cardiac arrest with pulseless electrical activity (PEA) was present in ~50% of neonatal piglets exposed to hypoxia and asphyxia (8). Cardiac arrest with PEA, defined as the presence of electrical activity without any associated mechanical activity is causes by hypoxia, hyper-/hypokalemia, hypovolemia, hypothermia, hydrogen ions (acidosis), tension pneumothorax, cardiac tamponade, thrombosis (coronary and pulmonary), and toxins (9–11). Although cardiac arrest with PEA rhythm can consist of both wide and narrow QRS-complexes, the causes of these patterns vary. PEA with narrow QRS-complexes stem from mechanical conditions whilst wide complex PEA results from metabolic problems (9–11). In adults, cardiac arrest with PEA rhythm occurs in 35–40% of in-hospital arrests, and 22–30% of out-of-hospital arrests (9–11). However, information regarding PEA in newborns is lacking. A case series of two preterm infants reported cardiac arrest with PEA due to hypocalcaemia after administration of fresh frozen plasma (12). In addition, a study in newborn piglets reported 43% of asphyxiated piglets experienced cardiac arrest with PEA, with ECG indicating a HR between 15 and 80 beats per minute (8, 13, 14).
We aimed to determine the accuracy of auscultation and ECG compared to carotid blood flow during cardiac arrest with PEA in asphyxiated piglets. We hypothesized that auscultation is superior to ECG to assess HR during cardiac arrest with PEA.
Methods
This is a secondary analysis of two unpublished randomized controlled animal studies (These studies examined 18, 21, or 100% oxygen during either 3:1 Compression: Ventilation ratio or CC+SI (Chest compression + Sustained Inflation) during cardiopulmonary resuscitation (both studies are currently under peer review). The original studies were conducted in accordance with Animal Research Reporting of In vivo Experiments (ARRIVE) guidelines (13), and approved by the Animal Care and Use Committee (Health Sciences) University of Alberta (AUP00001764, AUP00002151). For this secondary analysis, we included 45 newborn mixed breed piglets (1–3 days of age, weighing 1.7–2.3 kg) from the original studies.
Piglets were instrumented following the induction of anesthesia using isoflurane, piglets were then intubated via a tracheotomy, and pressure-controlled ventilation (Acutronic Fabian HFO; Hirzel, Switzerland) was initiated at a respiratory rate of 16–20 breaths/min and pressure of 20/5 cm H2O. Oxygen saturation was kept within 90–100%, glucose levels and hydration were maintained with an intravenous infusion of 5% dextrose at 10 mL/kg/hr. The piglet's body temperature was maintained in the normal range of 38.5–39.5°C using an overhead warmer and water heated pads. During the experiment anesthesia was maintained with intravenous propofol 5–10 mg/kg/h and morphine 0.1 mg/kg/hr. Additional doses of propofol (1–2 mg/kg) and morphine (0.05–0.1 mg/kg) were administered as needed (14).
Study Protocol, Data Collection, and Analysis
All piglets had the right common carotid artery exposed and enclosed with a real-time ultrasonic flow probe (2 mm; Transonic Systems Inc., Ithaca, NY), and HR was continuously measured and recorded using ECG (Hewlett Packard 78833B monitor, Hewlett Packard Co., Palo Alto, CA). This setup allowed us to simultaneously monitor HR via ECG and carotid blood flow during the experiments (14). Hypoxia was induced by exposing piglets to 30 min of normocapnic alveolar hypoxia at a fractional inspired oxygen concentration of 0.10. Hypoxia was then followed by asphyxia until asystole, achieved by disconnecting the ventilator and clamping the endotracheal tube. The study protocol further specified that either after 10 min of asphyxia or after asystole chest compression will be initiated. Cardiac arrest was defined as zero carotid blood flow (CBF). TO assess cardiac arrest one investigator used a neonatal/infant stethoscope (3M™ Littmann® Classic II Infant Stethoscope, U.S.; GMS (n = 28) or PYC (n = 17). The investigator was blinded to the ECG or the CBF flow display. Once the investigator confirmed cardiac arrest (= unable to hear a heartbeat) a marker was placed within the LabChart program (ADInstruments, Dunedin, New Zealand) to indicate time of cardiac arrest. The marker was then compared to waveforms from the ECG and CBF to determine HR at the time of cardiac arrest by auscultation. The data are presented as mean ± SD for normally distributed continuous variables and median (IQR) when the distribution is skewed. The data were tested for normality and analyzed using Stata (Intercooled 10, Statacorp, Texas, USA). The rates of accuracy and predictive values in the detection of HR by ECG and auscultation were compared by z-test.
Results
We studied 45 piglets; the median (IQR) duration of asphyxia was 325 (200–491) s. In total, the piglets were asystolic in 30 cases. Eight asystolic piglets (18%) were lacking heart sounds, CBF, and an ECG HR. In 22 (49%) cases, CBF and auscultation indicated asystole but were accompanied by an ECG HR of 17–75 beats per minute. In the other words, 22 of 30 (73%) piglets had PEA as demonstrated by the presence of ECG HR but zero CBF and inaudible HR. Fifteen (33%) piglets remained bradycardic and had a HR > 100 beats per minute after 10 min of asphyxia, as indicated by auscultation, ECG and CBF. The overall accuracy of ECG and auscultation in the correct detection of HR (asystole and bradycardia) were 51 and 80%, respectively (z = −2.8937; p = 0.004). Predictive values, sensitivity and specificity are presented in Table 1. In the detection of asystole as indicated by no carotid blood flow, the sensitivity of auscultation was significantly higher than that by ECG (100 vs. 27%; z = −7.1925, p < 0.0001).
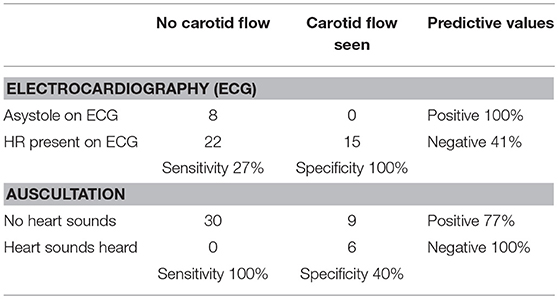
Table 1. Accuracy of ECG and auscultation when compared to the gold standard carotid blood flow during cardiac arrest with pulseless electrical activity.
Discussion
In this translational study using a newborn piglet model equivalent to a human infant at 36–38 weeks' gestation (15, 16), we found that ECG was in agreement with CBF in only 27% of cases. In 49% of the piglets, ECG displayed a heart rate of 17–75 beats per minute whilst CBF was absent and the HR inaudible. These results are similar to those found in a previous study involving asphyxiated piglets during which auscultation was 100% accurate and ECG falsely displayed a HR of 15 to 80 bpm in 43% of piglets (8). This presence of ECG activity without a detectable pulse is called cardiac arrest with PEA (9–11). The results from this study suggest that compromised infants might have a higher risk of developing PEA over other rhythms such as bradycardia or asystole. We believe that hypoxia and hypovolemia, are the most common causes of PEA in newborn infants (8). The Adult Advanced Cardiovascular Life Support defines cardiac arrest with PEA rhythm as the occurrence of cardiac electrical activity with no associated mechanical activity. Cardiac arrest with PEA rhythm might be sinus, atrial, junctional, or ventricular in origin, and is generally categorized as narrow QRS-complex (70% of cases) or wide QRS-complex PEA (10). Narrow QRS-complex PEA occurs when a significant pathophysiologic event has impaired the ability of the cardiovascular system to perfuse the body. Typically, this may be due to cardiac tamponade, pulmonary embolism, tension pneumothorax, or mechanical lung hyperinflation. In contrast, wide QRS-complex PEA represents primary electromechanical uncoupling of the myocytes and is more likely to be due to a metabolic condition (e.g., hyperkalaemia), left ventricular failure (due to ischemia), or agonal rhythm (clinically regarded as asystole with equivalent treatment approach) (9). PEA may also be caused by hypovolemia, tachydysrhythmias, and cardiomyopathy, and only a very small percentage are caused by asphyxia (17). In adults, cardiac arrest with PEA rhythm occurs in approximately 35–40% of in-hospital arrest and 22–30% of out-of-hospital cardiac arrest (11). Furthermore, cardiac arrest with PEA rhythm is associated with a poor prognosis, with a survival to discharge rate between 2 and 5% for out-of-hospital cardiac (11).
If neonatal healthcare providers observe an ECG HR but the infant remains unresponsive, they should suspect cardiac arrest with PEA and proceed with the appropriate resuscitation steps (1, 2). The current neonatal resuscitation algorithm states that chest compressions should be initiated once HR falls/remains below 60 beats per minute. However, during cardiac arrest with PEA ECG might display a HR < 60 beats per min and therefore might delay resuscitation efforts. Therefore, ECG should be used in a combination with other assessment methods including auscultation or palpation and pulse oximetry. In addition, novel methods including Doppler ultrasound or digital stethoscope have similar set-up times an accuracy compared to an ECG (18, 19). However, these technologies have only be studied in healthy newborn infants and not in compromised infants.
Our use of a piglet asphyxia model is a great strength of this translational study, as this model closely simulates delivery room events, with the gradual onset of severe asphyxia leading to cardiac arrest (15, 16). However, several limitations should be considered: Our asphyxia model uses piglets that have already undergone the fetal to neonatal transition, and piglets were sedated/anesthetized. Furthermore, our model requires piglets to be intubated with a tightly sealed endotracheal tube to prevent any endotracheal tube leak; this may not occur in the delivery room as mask ventilation is frequently used (20, 21). All these factors may affect the occurrence of PEA in the model, in addition to species differences. Nevertheless, our findings are still clinically relevant as cardiac arrest with PEA most likely are caused by hypoxia/asphyxia. Regardless, PEA is an area of concern in the delivery room and the limitations of using ECG should be further investigated.
Conclusion
During cardiac arrest with PEA ECG overestimates HR compared to carotid blood flow and auscultation. Therefore, ECG should be used in combination with other assessment methods including auscultation, palpation, and/or pulse oximetry.
Author Contributions
GS, P-YC, T-FL, MO: Conception and design; GS, P-YC, T-FL, MO, DL: Data collection, data analysis and interpretation, drafting of the article, critical revision of the article for important intellectual content, final approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the public for donation of money to our funding agencies: GS is a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation, a National New Investigator of the Heart and Stroke Foundation Canada and an Alberta New Investigator of the Heart and Stroke Foundation Alberta. The study was supported by a Grant from the SickKids Foundation in partnership with the Canadian Institutes of Health Research (CIHR–Institute of Human Development, Child and Youth Health (IHDCYH)), New Investigator Research Grant Program (Grant number–No. NI17-033) and a Grant-in-Aid from the Heart and Stroke Foundation Canada (Grant Number: G-15-0009284). We would like to acknowledge the Women and Children's Health Research Institute, University of Alberta for supporting the study. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
References
1. Perlman J, Wyllie JP, Wyckoff MH, Aziz K, Kim HS, Liley HG, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation (2015) 132:S204–41. doi: 10.1161/CIR.0000000000000276
2. Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman J, et al. Part 13: neonatal resuscitation 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care (Reprint). Pediatrics (2015) 136:S196–218. doi: 10.1542/peds.2015-3373G
3. Kamlin COF, Everest NJ, Davis PG, Morley CJ. Accuracy of clinical assessment of infant heart rate in the delivery room. Resuscitation (2006) 71:319–21. doi: 10.1016/j.resuscitation.2006.04.015
4. Dawson JA, Saraswat A, Simionato L, Thio M, Kamlin COF, Owen LS, et al. Comparison of heart rate and oxygen saturation measurements from Masimo and Nellcor pulse oximeters in newly born term infants. Acta Paediatr. (2013) 102:955–60. doi: 10.1111/apa.12329
5. Dawson JA, O'Donnell CPF, Sekhon J, Davis PG. Accuracy of pulse oximetry measurement of heart rate of newborn infants in the delivery room. J Pediatr. (2008) 152:756–60. doi: 10.1016/j.jpeds.2008.01.002
6. Finer N. Electrocardiogram provides a continuous heart rate faster than oximetry during neonatal resuscitation. Pediatrics (2012) 130:e1177–81. doi: 10.1542/peds.2012-0784
7. Mizumoto H, Tomotaki S, Shibata H, Ueda K, Akashi R, Uchio H, et al. Electrocardiogram shows reliable heart rates much earlier than pulse oximetry during neonatal resuscitation. Pediatr Int. (2011) 54:205–7. doi: 10.1111/j.1442-200X.2011.03506.x
8. Patel S, Cheung PY, Solevåg AL, Barrington KJ, Kamlin COF, Davis PG, et al. Pulseless electrical activity: a misdiagnosed entity during asphyxia in newborn infants? Arch Dis Child Fetal Neonatal Ed. (2018). [Epub ahead of print]. doi: 10.1136/archdischild-2018-314907
9. Littmann L, Bustin DJ, Haley MW. A simplified and structured teaching tool for the evaluation and management of pulseless electrical activity. Med Princ Pract. (2014) 23:1–6. doi: 10.1159/000354195
10. Hauck MH, Studnek JS, Heffner ACH, Pearson DAP. Cardiac arrest with initial arrest rhythm of pulseless electrical activity: do rhythm characteristics correlate with outcome? Am J Emerg Med. (2015) 33:891–4. doi: 10.1016/j.ajem.2015.03.050
11. Mehta C, Brady W. Pulseless electrical activity in cardiac arrest: electrocardiographic presentations and management considerations based on the electrocardiogram. Am J Emerg Med. (2012) 30:236–9. doi: 10.1016/j.ajem.2010.08.017
12. Hyde P, Puddy V. Pulseless electrical activity after rapid administration of fresh frozen plasma. J Paediatr Child Health (2008) 44:464–6. doi: 10.1111/j.1440-1754.2008.01345.x
13. Kilkenny C, Altman DG, Browne WJ, Cuthill IC, Emerson M. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. (2010) 8:e1000412. doi: 10.1371/journal.pbio.1000412
14. Schmölzer GM, OReilly M, Lee T-F, Cowan S, Bigam DL. Cardiopulmonary resuscitation with chest compressions during sustained inflations: a new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation (2013) 128:2495–503. doi: 10.1161/CIRCULATIONAHA.113.002289
15. Solevåg AL, Cheung PY, Lie H, OReilly M, Aziz K, Nakstad B, et al. Chest compressions in newborn animal models: a review. Resuscitation (2015) 96:151–5. doi: 10.1016/j.resuscitation.2015.08.001
16. Solevåg AL, Cheung PY, OReilly M, Schmölzer GM. A review of approaches to optimise chest compressions in the resuscitation of asphyxiated newborns. Arch Dis Child Fetal Neonatal (2016) 101:F272–6. doi: 10.1136/archdischild-2015-309761
17. Myerburg RJ, Halperin HR, Egan DA, Boineau R, Chugh SS, Gillis AM, et al. Pulseless electric activity: definition, causes, mechanisms, management, and research priorities for the next decade: report from a National Heart, Lung, and Blood Institute workshop. Circulation (2013) 128:2532–41. doi: 10.1161/CIRCULATIONAHA.113.004490
18. Kevat AC, Dawson JA, Dawson JA, Kamlin COF. Evaluation of a digital stethoscope and smart device technology for assessment of heart rate in the newborn infant. Arch Dis Child Fetal Neonatal (2015) 100:F562–3. doi: 10.1136/archdischild-2015-308639
19. Dyson A, Jeffrey M, Kluckow M. Measurement of neonatal heart rate using handheld doppler ultrasound. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F116–9. doi: 10.1136/archdischild-2016-310669
20. Kamlin COF, Dawson JA, Dawson JA, Pas te AB, Morley CJ, Davis PG. Respiratory monitoring of neonatal resuscitation. Arch Dis Child Fetal Neonatal (2010) 95:F295–303. doi: 10.1136/adc.2009.165878
Keywords: infants, newborn, neonatal resuscitation, asphyxia, heart rate, electrocardiography, auscultation
Citation: Luong DH, Cheung P-Y, O'Reilly M, Lee T-F and Schmolzer GM (2018) Electrocardiography vs. Auscultation to Assess Heart Rate During Cardiac Arrest With Pulseless Electrical Activity in Newborn Infants. Front. Pediatr. 6:366. doi: 10.3389/fped.2018.00366
Received: 19 September 2018; Accepted: 12 November 2018;
Published: 27 November 2018.
Edited by:
Eugene Dempsey, University College Cork, IrelandReviewed by:
Fernando Cabañas, Hospital Universitario Quirónsalud Madrid, SpainAnup C. Katheria, Sharp Mary Birch Hospital for Women and Newborns, United States
Copyright © 2018 Luong, Cheung, O'Reilly, Lee and Schmolzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georg M. Schmolzer, Z2Vvcmcuc2NobW9lbHplckBtZS5jb20=
 Deandra H. Luong
Deandra H. Luong Po-Yin Cheung
Po-Yin Cheung Megan O'Reilly2
Megan O'Reilly2 Tze-Fun Lee
Tze-Fun Lee Georg M. Schmolzer
Georg M. Schmolzer