- 1Department of Anesthesiology and Critical Care Medicine, The Children's Hospital of Philadelphia, Philadelphia, PA, United States
- 2Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
Numerous studies have examined the strategy of tight glucose control (TGC) with intensive insulin therapy (IIT) to improve clinical outcomes in critically ill adults and children. Although early studies of TGC with IIT demonstrated improved outcomes at the cost of elevated hypoglycemia rates, subsequent studies in both adults and children have not demonstrated any benefit from such a strategy. Differences in patient populations, variable glycemic targets, and glucose control protocols, inconsistency in attaining these targets, heterogeneous intermittent sampling, and measurement techniques, and variable expertise in protocol implementation are possible reasons for the contrasting results from these studies. Notably, differences in modes of nutrition support may have also contributed to these disparate results. In particular, combined use of early parenteral nutrition (PN) and a strategy of TGC with IIT may be associated with improved outcomes, while combined use of enteral nutrition (EN) and a strategy of TGC with IIT may be associated with equivocal or worse outcomes. This article critically examines published clinical trials that have employed a strategy of TGC with IIT in critically ill children to highlight the role of EN vs. PN in influencing clinical outcomes including efficacy of TGC, and adverse effects such as occurrence of hypoglycemia and hospital acquired infections. The perspective afforded by this article should help practitioners consider the potential importance of mode of nutrition support in impacting key clinical outcomes if they should choose to employ a strategy of TGC with IIT in critically ill children with hyperglycemia.
Introduction
Clinical trials of tight glucose control (TGC) with intensive insulin therapy (IIT) to improve outcomes in critically ill adults and children have promised much, but delivered little. While the first studies in critically ill adults in surgical intensive care units (ICUs) demonstrated improvements in mortality and morbidity from TGC with IIT (1, 2), later studies in medical and mixed medical/surgical ICUs observed worse outcomes (3–6). Disappointingly, TGC with IIT resulted in substantial increases in hypoglycemia in all these studies with corresponding poor clinical and neurological outcomes (7–9). Similarly, in critically ill children, the first study of TGC with IIT was notable for significant decreases in length of stay and inflammation, but came at a cost of substantial increase in hypoglycemia rates (10). Subsequent studies in critically ill children were unable to demonstrate any benefits from TGC with IIT, and continued to report elevated hypoglycemia rates in spite of measures such as continuous glucose monitoring (CGM) and computer guided decision making to reduce hypoglycemia (11–13). Follow-up neurodevelopmental studies have observed worse clinical outcomes from hypoglycemia due to TGC with IIT in critically ill children (14, 15). Differences in patient populations, variable glycemic targets and glucose control protocols, inconsistency in attaining these targets, heterogeneous intermittent sampling and measurement techniques, and variable expertise in protocol implementation are possible reasons for the contrasting results from these studies (16–18). Notably, differences in modes of nutrition support may have also contributed to these disparate results. In particular, combined use of early parenteral nutrition (PN) and TGC with IIT may be associated with improved outcomes, while combined use of enteral nutrition (EN) and TGC with IIT may be associated with equivocal or worse outcomes. This article will examine how mode of nutrition support may influence blood glucose (BG) concentrations, and provide perspectives on how nutrition support may influence the efficacy of TGC with IIT and occurrence of adverse events.
Stress Hyperglycemia in Pediatric Critical Illness
Stress hyperglycemia commonly occurs in critically ill children, even in those with previously normal glucose homeostasis (19–25). Over two-thirds of critically ill children experience moderate hyperglycemia [BG concentrations > 150 mg/dL (> 8.3 mmol/L)], while severe hyperglycemia [BG concentrations > 200 mg/dL (> 11 mmol/L)] occurs in as many as one-third of critically ill children (19–25). Stress hyperglycemia develops via a combination of increase in gluconeogenesis (relative to glucose uptake and turnover) and development of insulin resistance (26). Both of these mechanisms are mediated by increases in inflammatory cytokines as well as elevated levels of counter-regulatory hormones (catecholamines, cortisol, glucagon and growth hormone) (27, 28). Additional mechanisms for stress hyperglycemia include impairments in pancreatic beta-cell function with corresponding reduction in insulin secretion (29).
The mode of nutrition support in the ICU can also exacerbate stress hyperglycemia (30). Critically ill children are often prescribed PN due to inability to tolerate EN in critical illness states. The provision of excess carbohydrate calories in PN can result in elevated BG concentrations. While normal infants and children may have substantially higher glucose turnover rates than adults (31), limited data from critically ill children suggest that glucose infusion rates (GIR) < 5 mg/kg/min may be optimal for glucose utilization from PN (32, 33). The practice of cycling PN may also be associated with stress hyperglycemia, most likely due to impaired insulin secretion (34). Commonly used predictive equations to calculate energy expenditure needs in critical illness states are inferior to targeted indirect calorimetry, and often result in over prescription of calories (35–37). In contrast, nutrition strategies such as supplementation of PN with glutamine and the administration of low calorie PN may reduce the development of stress hyperglycemia during critical illness (38, 39).
In turn, stress hyperglycemia can affect the delivery of nutrition during critical illness in a variety of ways. Stress hyperglycemia may influence the ability to provide consistent or adequate EN during critical illness due to delayed gastric emptying and slowing down of gut motility (40). Stress hyperglycemia can also impair the prokinetic action of erythromycin on gastric emptying (41). Altered gut motility and insensitivity to prokinetic agents may result in intolerance to EN. Studies in critically ill adults have demonstrated the association of intolerance to EN with stress hyperglycemia and BG variability (42). Stress hyperglycemia also results in altered nutrient utilization during critical illness. Stress hyperglycemia exacerbates protein catabolism in skeletal muscle in critically ill adults with severe burns (43). Stress hyperglycemia may also reduce the activity of lipoprotein lipase contributing to the development of hypertriglyceridemia through reduced clearance of circulating triglycerides (44).
Stress hyperglycemia during critical illness thus results in the rapid availability of glucose as a fuel for metabolic processes occurring in vital organs in the body. During the acute phase of critical illness coinciding with high metabolic demands, stress hyperglycemia may be adaptive to favor survival. However, during the chronic phases of critical illness, persistence of stress hyperglycemia may reflect impaired allostasis with potential for harm from increased oxidative damage due to propagation of the proinflammatory response, and impaired cellular repair and tissue healing (30). Though stress hyperglycemia is often justified as an adaptive response to critical illness (45, 46), numerous studies in critically ill children have observed the association of stress hyperglycemia with poor clinical outcomes across a variety of disease states (19–25, 47–53). Consequently, the strategy of TGC with IIT emerged as a viable and rational solution to improve outcomes in critically ill children experiencing stress hyperglycemia.
TGC With IIT in Critically Ill Children
Early studies of TGC with IIT in critically ill children focused on children with severe burns and very low birth weight neonates (54–56). Subsequently, this practice of TGC with IIT was studied in more general populations of critically ill children with varying results (10–13). The variability in observed outcomes across these studies are due to several important methodological and epidemiological differences that are summarized in Table 1. In the study by Vlasselaers et al, BG concentrations were controlled to age-specific fasting ranges in the intervention group that were substantially lower than the BG ranges in the intervention groups in the other three studies (Safe Pediatric Euglycemia after Cardiac Surgery—SPECS, Control of Hyperglycaemia in Pediatric Intensive Care—CHiP and Heart and Lung Failure—Pediatric Insulin Titration—HALF-PINT). The control group BG range also differed between these studies. Additionally, there was greater separation of BG concentrations in the study by Vlasselaers et al, compared to the SPECS, CHiP, and HALF-PINT studies. Another important difference between these studies was the striking variation in overall incidence of acquired infections in study subjects. The study by Vlasselaers et al. observed a much higher incidence of acquired infection in the control group than SPECS, CHIP, or HALF-PINT which could possibly reflect important definitional and epidemiological differences compared to the latter three studies. Consequently, the trial by Vlasselaers et al. was more likely than the other three trials to have identified a positive benefit from TGC with IIT.
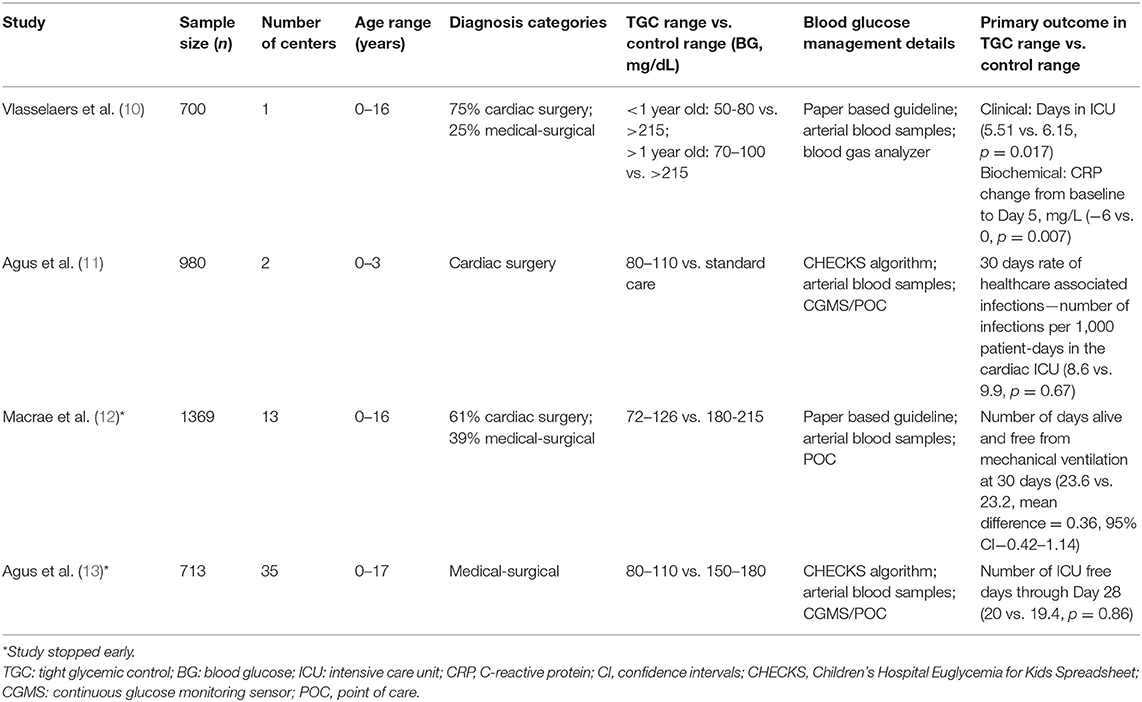
Table 1. Studies of tight glycemic control with intensive insulin therapy in critically ill children—demographics and methodology.
Published meta-analyses of studies of TGC with IIT in critically ill children have not observed any benefits from this strategy (57–59). In 2014, Srinivasan et al. carried out the first systematic review and quantitative meta-analysis of the 4 studies till date that had examined the efficacy and safety of TGC with IIT in critically ill children (excluding neonates) (57). This meta-analysis observed that TGC with IIT did not reduce 30-day mortality, but did appear to reduce acquired infection in critically ill children at the expense of higher incidence of hypoglycemia. Subsequently, Zhao et al. published an updated meta-analysis in 2018 including the results from the recent HALF-PINT trial and observed that TGC with IIT did not reduce 30-day mortality or acquired infection in critically ill children, but resulted in substantial increases in hypoglycemia rates (58). Recently, Chen et al. carried out a meta-analysis of 6 studies of TGC with IIT in critically ill children and preterm neonates, and concluded that the practice of TGC with IIT did not confer any benefits but did result in significant increases in hypoglycemia (59). Notably, none of these above meta-analyses took into account mode of nutrition support when evaluating the safety and efficacy of TGC with IIT in critically ill patients.
Modes of Nutrition Support: Implications for Efficacy and Safety of TGC With IIT
Data From Adult Studies
In the single-center surgical and medical adult ICU studies of TGC with IIT from Leuven, early use of PN for nutrition support was heavily favored during ICU admission (1, 3). Per existing European Society of Parenteral and Enteral Nutrition (ESPEN) guidelines at the time of these studies, the use of early PN to reach goal energy needs within 3 days of admission was emphasized if they were not expected to tolerate EN or had a contraindication to use of EN (60). In the original Leuven surgical ICU study from Leuven, subjects received on an average 1,100 kcal/day. The majority of energy delivery was via PN started on the first day of ICU admission (768 kcal/day in the form of 20% intravenous dextrose solution from the first day onwards) (1). Similarly, in the subsequent Leuven medical ICU study, the majority of energy delivery was from PN started on the first day of ICU admission (ranging between 750 and 800 kcal/day) (3). In contrast, the NICE-SUGAR study, in accordance with customary practice in Australia, New Zealand and Canada at the time of the study, almost exclusively relied on the use of EN for nutrition support during ICU admission to provide on average 880 kcal/day with goal energy needs reached by 7–10 days (7). In the NICE-SUGAR study, PN was used to supplement EN delivery and energy from PN never exceeded 300 kcal/day.
Data From Pediatric Studies
In the pediatric Leuven study (similar to the adult Leuven studies), PN was started at ICU admission (in the form of 20% intravenous dextrose solutions) while attempts were made to start EN as soon as feasible based on the underlying condition (10). On average, infants received 42 kcal/kg/day and children received 28 kcal/kg/day during their ICU admission with substantial energy delivery via exclusive PN in over 40% of the study population (and PN with partial EN in the remaining 60%). To a lesser extent than the pediatric Leuven study, the SPECS trial also relied predominantly on PN for energy delivery in over 50% of the study population (11). During the period of study enrollment in the SPECS trial, subjects in the TGC group received a median of 41% of their total caloric intake via EN, while 38% of subjects in the control group received a median of 38% of their total caloric intake via EN. In contrast, the CHiP and HALF-PINT trials largely relied on EN for energy delivery based on current clinical practice guidelines at the time of the studies (12, 13). In the CHiP trial, by Day 7 of enrollment, the median enteral caloric intake was approximately 20 kcal/kg/day and the median parenteral caloric intake was approximately 5 kcal/kg/day in enrolled subjects (12). In the HALF-PINT trial, by Day 7 of enrollment, median EN delivery was approximately 25 kcal/kg/day which accounted for approximately 60% of total energy delivered in enrolled subjects (13). Table 2 summarizes key differences in nutrition support, measures of glycemia, and primary outcomes with effect size across these pediatric studies.
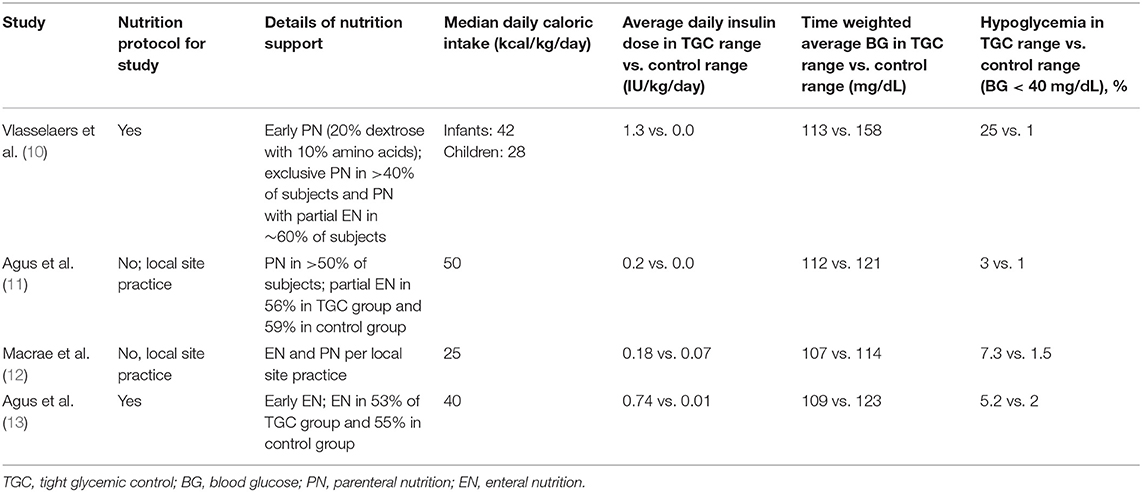
Table 2. Studies of tight glycemic control with intensive insulin therapy in critically ill children—nutrition support and glycemia.
Interaction of TGC With IIT and Mode of Nutrition Support (EN vs. PN)
The mode of nutrition support (EN or PN) is a key variable that has important implications for the efficacy and safety of TGC with IIT in both adults and children. In the single center Leuven adult studies, the aggressive early initiation of PN to rapidly reach goal energy needs by 48–72 h following admission may have aggravated the problem of stress hyperglycemia and risk of worse outcomes in a population of critically ill adults with high prevalence of pre-existing diabetes mellitus. Consequently, the strategy of TGC with IIT may have proven beneficial in this setting (61). In contrast, use of TGC with IIT in the relatively nutrition restricted setting of NICE-SUGAR may have been harmful by evoking a global substrate deficit via insulin-induced suppression of proteolysis, lipolysis, glycogenolysis, and gluconeogenesis. By suppressing these important compensatory mechanisms which play a vital role in states of energy deprivation and starvation, TGC with IIT likely resulted in worse outcomes in this large study that relied predominantly on EN (61).
A published meta-analysis by Marik et al. in 2010 took into account mode of nutrition support (EN vs. PN) using meta-regression techniques to control for proportion of intravenously delivered calories from PN (62). This meta-analysis demonstrated reduced mortality from TGC with IIT when PN was utilized as the predominant mode of nutrition support. After excluding the two Leuven trials that predominantly employed PN as the mode of nutrition support, the authors observed that mortality was lower with control patients receiving usual care with EN suggesting that TGC with IIT may be harmful in patients receiving exclusive or predominant EN (62).
In published studies of TGC with IIT in critically ill children till date, less granular information is available regarding timing, intensity, duration and mode of nutrition support. This limits our ability to provide detailed analyses of interaction of mode of nutrition support and TGC with IIT on clinical outcomes similar to adult studies. Similar to the adult studies, the pediatric studies also vary in nutrition support practice which makes direct comparisons challenging. The pediatric Leuven study demonstrated benefits of TGC with IIT coupled with a nutrition support strategy that relied mainly on early PN (10). In contrast, the HALF-PINT study predominantly emphasized EN delivery and observed trends to better outcomes with usual care targeting BG of 150–180 mg/dL (8.3–10 mmol/L) (13). The schematic comparison of differences in mode of nutrition support (EN vs. PN) and potential impact on TGC with IIT to influence outcomes is depicted in Figure 1 using the example of the pediatric Leuven and HALF-PINT studies.
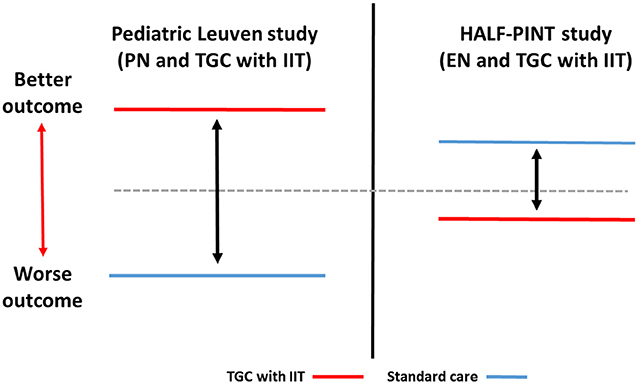
Figure 1. Schematic comparison of interaction of TGC with IIT and mode of nutrition support (EN vs. PN) with impact on outcomes in critically ill children. Pediatric Leuven study: Vlasselaers et al. (10); HALF-PINT study: Agus et al. (13). TGC with IIT, Tight glucose control with intensive insulin therapy; EN, enteral nutrition; PN, parenteral nutrition.
The important question for practitioners is whether any one mode of nutrition support—EN or PN—is superior to another to improve outcomes in critically ill adults and children. In critically ill adults, early EN initiation compared to early PN initiation did not reduce mortality or reduce secondary infections, but was associated with more digestive complications and hypoglycemia (63, 64). Based on current evidence, use of PN is feasible and appears to result in rapid increase in energy and protein delivery with the ability to reach target goals early on during critical illness (65). In contrast, use of EN is often delayed due to illness severity and frequently does not reach goal energy and protein needs due to recurrent interruptions to enteral feeding (66). Early initiation of EN (compared to delayed initiation of EN) appears to be associated with improved clinical outcomes in critically ill adults and children (67, 68). However, it was not clear until recently if early initiation of PN compared to delayed initiation of PN was beneficial or harmful in critically ill subjects. This vital question of timing of PN initiation (early vs. delayed) was addressed in critically ill adults and children by the Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients (EPaNIC) study and the Early vs. Late Parenteral Nutrition in the Pediatric Intensive Care Unit (PEPaNIC) study, respectively (69, 70). In both the adult and pediatric studies, delayed PN initiation compared to early PN initiation was associated with better clinical outcomes in the form of fewer acquired infections and lower ICU dependency. There were no mortality differences between the two groups in either study. In both studies, TGC with IIT was employed to maintain normoglycemia. Notably, the delayed PN initiation group experienced more hypoglycemia episodes in both studies, likely due to greater proportion of nutrition support in the form of EN provided to this group during study enrollment coupled with a strategy of TGC with IIT. This finding of more frequent hypoglycemia episodes in the delayed PN initiation group is similar to other studies of TGC with IIT in critically ill patients that have relied predominantly on EN for nutrition support (6, 12, 13). Interestingly, even though the delayed PN initiation group experienced fewer infections, there was more inflammation (as measured by C-reactive protein levels) in this group compared to the early PN initiation group in both studies (69, 70). This raises the intriguing possibility that a strategy that relies predominantly on EN might favor an uncoupling of the inflammatory state from processes involved in autophagy and maintenance of the gut function to improve clinical outcomes (71–73).
Conclusion
In summary, studies of TGC with IIT have demonstrated varying results due to numerous methodological and target population differences, but an important factor that may often go underappreciated is the mode of nutrition support in the form of either EN or PN, or a combination of the two. Specifically, when PN is the favored mode of nutrition support, TGC with IIT may be associated with improved outcomes compared to usual care, as usual care in this setting of early PN may be associated with more harm from uncontrolled hyperglycemia. In contrast, when EN (or predominantly EN) is the favored mode of nutrition support, TGC with IIT may be associated with equivocal or worse outcomes compared to usual care, as usual care in this setting of early EN may be more beneficial with maintenance of gut function and processes involved in autophagy. While pediatric data surrounding the interaction of mode of nutrition support and TGC with IIT is limited, the practitioner should consider the potential importance of mode of nutrition support in impacting key clinical outcomes, if they should choose to employ a strategy of TGC with IIT to manage critically ill children. Future studies of TGC with IIT should use targeted indirect calorimetry for more accurate estimation of energy needs, and ensure targeted protein delivery to meet minimum threshold goals. Such studies should also incorporate better decision making support for nutrition delivery and glucose control in the form of protocol-driven algorithms and continuous glucose monitoring technologies to ensure consistency in key variables that affect outcomes in this critically ill population.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Van den Berghe G, Wouters P, Weekers F, Weekers F, Verwaest C, Bruyninckx F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. (2001) 345:1359–67. doi: 10.1056/NEJMoa011300
2. Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. (2004) 79:992–1000. doi: 10.4065/79.8.992
3. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. (2006) 354:449–61. doi: 10.1056/NEJMoa052521
4. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. (2008) 358:125–39. doi: 10.1056/NEJMoa070716
5. Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. (2009) 35:1738–48. doi: 10.1007/s00134-009-1585-2
6. NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. (2009) 360:1283–97. doi: 10.1056/NEJMoa0810625
7. NICE-SUGAR Study Investigators, Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. (2012) 367:1108–18. doi: 10.1056/NEJMoa1204942
8. Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ (2009) 180:821–7. doi: 10.1503/cmaj.090206
9. Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. (2010) 85:217–24. doi: 10.4065/mcp.2009.0394
10. Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet (2009) 373:547–56. doi: 10.1016/S0140-6736(09)60044-1
11. Agus MS, Steil GM, Wypij D, Costello JM, Laussen PC, Langer M, et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med. (2012) 367:1208–19. doi: 10.1056/NEJMoa1206044
12. Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J, et al. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med. (2014) 370:107–18. doi: 10.1056/NEJMoa1302564
13. Agus MS, Wypij D, Hirshberg EL, Srinivasan V, Faustino EV, Luckett PM, et al. Tight Glycemic Control in Critically Ill Children. N Engl J Med (2017) 376:729–741. doi: 10.1056/NEJMoa1612348
14. Mesotten D, Gielen M, Sterken C, Claessens K, Hermans G, Vlasselaers D, et al. Neurocognitive development of children 4 years after critical illness and treatment with tight glucose control: a randomized controlled trial. JAMA (2012) 308:1641–50. doi: 10.1001/jama.2012.12424
15. Sadhwani A, Asaro LA, Goldberg C, Ware J, Butcher J, Gaies M, et al. Impact of tight glycemic control on neurodevelopmental outcomes at 1 year of age for children with congenital heart disease: a randomized controlled trial. J Pediatr. (2016) 174:193–198.e2. doi: 10.1016/j.jpeds.2016.03.048
16. Gunst J, Van den Berghe G. Blood glucose control in the intensive care unit: benefits and risks. Semin Dial (2010) 23:157–62. doi: 10.1111/j.1525-139X.2010.00702.x
17. Van den Berghe G, Mesotten D. Paediatric endocrinology: tight glycaemic control in critically ill children. Nat Rev Endocrinol. (2014) 10:196–7. doi: 10.1038/nrendo.2014.16
18. Gunst J, Van den Berghe G. Paediatric endocrinology: Critical illness - another trial, but are we any wiser? Nat Rev Endocrinol. (2017) 13:254–256. doi: 10.1038/nrendo.2017.29
19. Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. (2004) 5:329–36. doi: 10.1097/01.PCC.0000128607.68261.7C
20. Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. (2005) 146:30–4. doi: 10.1016/j.jpeds.2004.08.076
21. Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics (2006) 118:173–9. doi: 10.1542/peds.2005-1819
22. Yung M, Wilkins B, Norton L, Slater A, Paediatric Study Group; Australian and New Zealand intensive care society. glucose control, organ failure, and mortality in pediatric intensive care. Pediatr Crit Care Med. (2008) 9:147–52. doi: 10.1097/PCC.0b013e3181668c22
23. Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. (2008) 9:361–6. doi: 10.1097/PCC.0b013e318172d401
24. Preissig CM, Rigby MR. Pediatric critical illness hyperglycemia: risk factors associated with development and severity of hyperglycemia in critically ill children. J Pediatr. (2009) 155:734–9. doi: 10.1016/j.jpeds.2009.05.007
25. Ognibene KL, Vawdrey DK, Biagas KV. The association of age, illness severity, and glycemic status in a pediatric intensive care unit. Pediatr Crit Care Med. (2011) 12:e386–90. doi: 10.1097/PCC.0b013e3182192c53
26. Mechanick JI. Metabolic mechanisms of stress hyperglycemia. J Parenter Enteral Nutr. (2006) 30:157–63. doi: 10.1177/0148607106030002157
27. Dufour S, Lebon V, Shulman GI, Petersen KF. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Physiol Endocrinol Metab. (2009) 297:E231–5. doi: 10.1152/ajpendo.00222.2009
28. Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock (2010) 33:369–74. doi: 10.1097/SHK.0b013e3181b92340
29. Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. (2001) 15:533–51. doi: 10.1053/beem.2001.0168
30. Srinivasan V. Stress hyperglycemia in pediatric critical illness: the intensive care unit adds to the stress! J Diabetes Sci Technol. (2012) 6:37–47. doi: 10.1177/193229681200600106
31. Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA, et al. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes (1977) 26:1016–23. doi: 10.2337/diab.26.11.1016
32. Sheridan RL, Yu YM, Prelack K, Young VR, Burke JF, Tompkins RG. Maximal parenteral glucose oxidation in hypermetabolic young children: a stable isotope study. J Parenter Enteral Nutr. (1998) 22:212–6. doi: 10.1177/0148607198022004212
33. Verbruggen SC, de Betue CT, Schierbeek H, Chacko S, van Adrichem LN, Verhoeven J, et al. Reducing glucose infusion safely prevents hyperglycemia in post-surgical children. Clin Nutr. (2011) 30:786–92. doi: 10.1016/j.clnu.2011.05.011
34. Lienhardt A, Rakotoambinina B, Colomb V, Souissi S, Sadoun E, Goulet O, et al. Insulin secretion and sensitivity in children on cyclic total parenteral nutrition. J Parenter Enteral Nutr. (1998) 22:382–6. doi: 10.1177/0148607198022006382
35. Coss-Bu JA, Jefferson LS, Walding D, David Y, Smith EO, Klish WJ. Resting energy expenditure in children in a pediatric intensive care unit: comparison of Harris-Benedict and Talbot predictions with indirect calorimetry values. Am J Clin Nutr. (1998) 67:74–80. doi: 10.1093/ajcn/67.1.74
36. Framson CM, LeLeiko NS, Dallal GE, Roubenoff R, Snelling LK, Dwyer JT. Energy expenditure in critically ill children. Pediatr Crit Care Med. (2007) 8:264–7. doi: 10.1097/01.PCC.0000262802.81164.03
37. Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med. (2011) 12:398–405. doi: 10.1097/PCC.0b013e3181fe279c
38. Grau T, Boñet A, Miñambres E, Pineiro L, Irles JA, Robles A, et al. The effect of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit Care Med. (2011) 39:1263–8. doi: 10.1097/CCM.0b013e31820eb774
39. Ahrens CL, Barletta JF, Kanji S, Tyburski JG, Wilson RF, Janisse JJ, et al. Effect of low-calorie parenteral nutrition on the incidence and severity of hyperglycemia in surgical patients: a randomized, controlled trial. Crit Care Med. (2005) 33:2507–12. doi: 10.1097/01.CCM.0000186746.64572.8A
40. Hebbard GS, Sun WM, Dent J, Horowitz M. Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol. (1996) 8:211–7. doi: 10.1097/00042737-199603000-00005
41. Petrakis IE, Kogerakis N, Prokopakis G, Zacharioudakis G, Antonakakis S, Vrachassotakis N, et al. Hyperglycemia attenuates erythromycin-induced acceleration of liquid-phase gastric emptying of hypertonic liquids in healthy subjects. Dig Dis Sci. (2002) 47:67–72. doi: 10.1023/A:1013211419605
42. Nguyen N, Ching K, Fraser R, Chapman M, Holloway R. The relationship between blood glucose control and intolerance to enteral feeding during critical illness. Intensive Care Med. (2007) 33:2085–92. doi: 10.1007/s00134-007-0869-7
43. Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. (2002) 30:2438–42. doi: 10.1097/00003246-200211000-00006
44. Kovár J, Fejfarová V, Pelikánová T, Poledne R. Hyperglycemia downregulates total lipoprotein lipase activity in humans. Physiol Res. (2004) 53:61–8.
45. Weise K, Zaritsky A. Endocrine manifestations of critical illness in the child. Pediatr Clin North Am. (1987) 34:119–30. doi: 10.1016/S0031-3955(16)36185-5
46. Gupta P, Natarajan G, Agarwal KN. Transient hyperglycemia in acute childhood illnesses: to attend or ignore? Indian J Pediatr. (1997) 64:205–10. doi: 10.1007/BF02752447
47. Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma (2001) 51:540–4. doi: 10.1097/00005373-200109000-00021
48. Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma (2003) 55:1035–8. doi: 10.1097/01.TA.0000031175.96507.48
49. Michaud LJ, Rivara FP, Longstreth WT Jr, Grady MS. Elevated initial blood glucose levels and poor outcome following severe brain injuries in children. J Trauma (1991) 31:1356–62. doi: 10.1097/00005373-199110000-00007
50. Branco RG, Garcia PC, Piva JP, Casartelli CH, Seibel V, Tasker RC. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med. (2005) 6:470–2. doi: 10.1097/01.PCC.0000161284.96739.3A
51. Yates AR, Dyke PC II, Taeed R, Hoffman TM, Hayes J, Feltes TF, et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. (2006) 7:351–5. doi: 10.1097/01.PCC.0000227755.96700.98
52. Day KM, Haub N, Betts H, Inwald DP. Hyperglycemia is associated with morbidity in critically ill children with meningococcal sepsis. Pediatr Crit Care Med. (2008) 9:636–40. doi: 10.1097/PCC.0b013e31818d350b
53. Tuggle DW, Kuhn MA, Jones SK, Garza JJ, Skinner S. Hyperglycemia and infections in pediatric trauma patients. Am Surg. (2008) 74:195–8.
54. Pham TN, Warren AJ, Phan HH, Molitor F, Greenhalgh DG, Palmieri TL. Impact of tight glycemic control in severely burned children. J Trauma (2005) 59:1148–54. doi: 10.1097/01.ta.0000188933.16637.68
55. Jeschke MG, Kulp GA, Kraft R, Finnerty CC, Mlcak R, Lee JO, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. (2010) 182:351–9. doi: 10.1164/rccm.201002-0190OC
56. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. (2008) 359:1873–84. doi: 10.1056/NEJMoa0803725
57. Srinivasan V, Agus MS. Tight glucose control in critically ill children–a systematic review and meta-analysis. Pediatr Diabetes (2014) 15:75–83. doi: 10.1111/pedi.12134
58. Zhao Y, Wu Y, Xiang B. Tight glycemic control in critically ill pediatric patients: a meta-analysis and systematic review of randomized controlled trials. Pediatr Res. (2018) 83:930–935. doi: 10.1038/pr.2017.310
59. Chen L, Li T, Fang F, Zhang Y, Faramand A. Tight glycemic control in critically ill pediatric patients: a systematic review and meta-analysis. Crit Care (2018) 22:57. doi: 10.1186/s13054-018-1976-2
60. Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN Guidelines on parenteral nutrition: intensive care. Clin Nutr. (2009) 28:387–400. doi: 10.1016/j.clnu.2009.04.024
61. Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, et al. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. (2009) 94:3163–70. doi: 10.1210/jc.2009-0663
62. Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest (2010) 137:544–51. doi: 10.1378/chest.09-1737
63. Reignier J, Boisramé-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet (2018) 391:133–43. doi: 10.1016/S0140-6736(17)32146-3
64. Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. (2014) 371:1673–84. doi: 10.1056/NEJMoa1409860
65. Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA (2013) 309:2130–8. doi: 10.1001/jama.2013.5124
66. Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children–an international multicenter cohort study. Crit Care Med. (2012) 40:2204–11. doi: 10.1097/CCM.0b013e31824e18a8
67. Tian F, Heighes PT, Allingstrup MJ, Doig GS. Early enteral nutrition provided within 24 hours of ICU admission: a meta-analysis of randomized controlled trials. Crit Care Med. (2018) 46:1049–1056. doi: 10.1097/CCM.0000000000003152
68. Mikhailov TA, Kuhn EM, Manzi J, Christensen M, Collins M, Brown AM, et al. Early enteral nutrition is associated with lower mortality in critically ill children. J Parenter Enteral Nutr. (2014) 38(4):459–66. doi: 10.1177/0148607113517903
69. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. (2011) 365:506–17. doi: 10.1056/NEJMoa1102662
70. Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, et al. Early versus late parenteral nutrition in critically Ill children. N Engl J Med. (2016) 374:1111–22. doi: 10.1056/NEJMoa1514762
71. Colgan SP, Campbell EL. Oxygen metabolism and innate immune responses in the gut. J Appl Physiol. (2017) 123:1321–1327. doi: 10.1152/japplphysiol.00113.2017
72. Elshaer D, Begun J. The role of barrier function, autophagy, and cytokines in maintaining intestinal homeostasis. Semin Cell Dev Biol. (2017) 61:51–59. doi: 10.1016/j.semcdb.2016.08.018
Keywords: tight glucose control, intensive insulin therapy, enteral nutrition, parenteral nutrition, children, critical illness, outcomes
Citation: Srinivasan V (2018) Nutrition Support and Tight Glucose Control in Critically Ill Children: Food for Thought! Front. Pediatr. 6:340. doi: 10.3389/fped.2018.00340
Received: 09 July 2018; Accepted: 22 October 2018;
Published: 06 November 2018.
Edited by:
Alejandro Floh, Hospital for Sick Children, CanadaReviewed by:
Angela S. Czaja, University of Colorado, United StatesAntonio Rodriguez-Nunez, Universidade de Santiago de Compostela, Spain
Copyright © 2018 Srinivasan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vijay Srinivasan, c3Jpbml2YXNhbkBlbWFpbC5jaG9wLmVkdQ==
 Vijay Srinivasan
Vijay Srinivasan