Epstein-Barr virus (EBV) infects >90% of adults worldwide and is closely linked to multiple B-cell malignancies, including Burkitt lymphoma, diffuse large B-cell lymphoma, Hodgkin lymphoma, and post-transplant lymphoproliferative disorder (PTLD) (1). Epstein-Barr virus also infects T-cells and natural killer (NK) cells causing EBV-associated T- and NK-cell (EBV-T/NK) malignancies, including extranodal NK/T-cell lymphomas, nasal type (ENKL), aggressive NK-cell leukemia, and lymphoproliferative diseases (LPDs). These EBV-associated T/NK-cell tumors have basically neoplastic properties with clonal proliferation and organ infiltration (2).
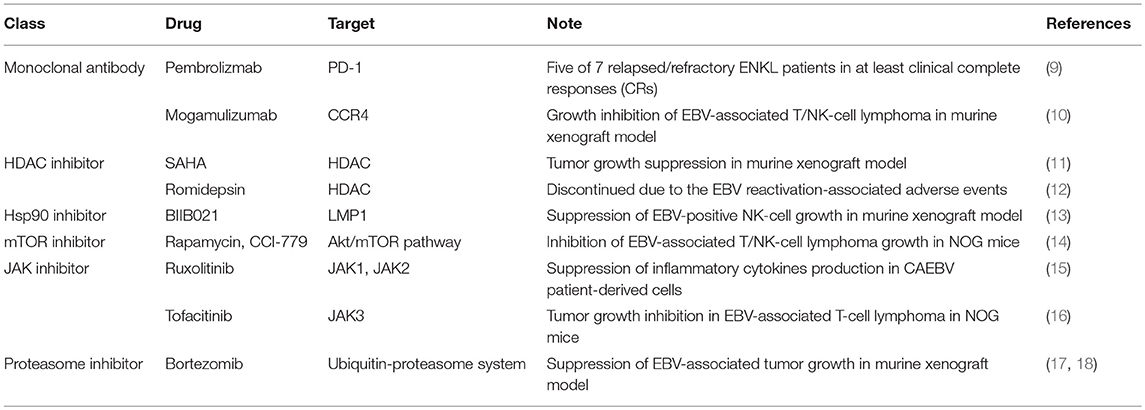
Chronic active EBV infection (CAEBV), an EBV-T/NK LPD, is a potential life-threatening illness in children and young adults, characterized by the clonal proliferation of EBV-infected lymphocytes (3, 4). The T/NK-cell type of this disease is more frequent in East Asians and some Native American populations in Western countries. CAEBV patients from the United States more often have EBV in B- or T-cells (3, 5). Patients with CAEBV often progress to overt lymphoma or leukemia. Although concurrent chemoradiotherapy along with non-anthracycline-based chemotherapy has improved the survival of patients with these EBV-T/NK malignancies, the survival outcome remains poor because of relapse or treatment-related mortality (6). The only curative treatment is stem-cell transplantation, albeit the incidence of transplantation-related complications is high (7, 8). To improve the treatment of EBV-T/NK malignancies, novel approaches using molecular targets have been attempted (Table 1).
Immune checkpoint blockade with monoclonal antibodies directed at the inhibitory immune receptors, programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1), has emerged as a successful treatment approach for patients with advanced cancers. Since EBV-infected lymphoma cells upregulate PD-L1 (19), these molecules are, therefore, the target of the antitumor effect. Pembrolizumab, the humanized anti-PD-1 monoclonal antibody, is effective for relapsed/refractory ENKL (9), suggesting that checkpoint inhibitors have a promising effect in the treatment of relapsed disease.
In addition to checkpoint inhibitors, some antibodies and inhibitors are also treated as potential molecular therapeutic targets in the developmental and preclinical stages. Kanazawa et al. showed that CC chemokine receptor 4 (CCR4) was expressed on most EBV-infected T/NK-cell lines and a humanized anti-CCR4 monoclonal antibody, mogamulizumab, inhibited the growth of EBV-positive NK-cell lymphomas in a murine xenograft model (10). Another challenge is targeting histone deacetylase (HDAC). The HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA) and romidepsin, have been approved by the United States Food and Drug Administration and their efficacies in non-Hodgkin lymphoma, acute myeloid leukemia, cutaneous T-cell lymphoma, and relapsed and refractory peripheral T-cell lymphoma have been confirmed by clinical trials (20–22). SAHA suppressed tumor progression and metastasis in a murine xenograft model, although there were no significant differences observed between EBV- positive and EBV-negative cell lines (11). However, a pilot study using romidepsin for the treatment of relapsed/refractory ENKL patients in Korea was discontinued due to serious adverse events. As romidepsin treatment caused EBV reactivation, patients developed fever and elevated liver enzyme and bilirubin levels immediately after their first dose of romidepsin (12). These results suggest that the further accumulation of evidence in the preclinical stage is required for safer application of drug candidates in clinical trials.
The EBV-encoded latent membrane protein 1 (LMP1) is a major oncogene that activates the nuclear factor kappa B (NF-κB), c-Jun N-terminal kinase (JNK), and phosphatidylinositol 3-kinase (PI3K) signaling pathways, thereby, promoting the cell growth and inhibiting apoptosis (23). LMP1 is expressed in EBV-infected T/NK-cells. Screening a library of small-molecule inhibitors identified heat shock protein 90 (Hsp90) inhibitors as suppressors of LMP1 expression (24). In EBV-positive cells, the synthetic Hsp90 inhibitor BIIB021 suppressed the LMP1 expression and that of its downstream signaling proteins NF-κB, JNK, and Akt. The BIIB021 inhibited the growth of established EBV-positive NK-cells in NOD/Shi-scid/IL-2Rγnull (NOG) mice (13). Moreover, constitutive PI3K/Akt/mTOR activation is critically involved in EBV-associated B-cell lymphoma (25, 26). Kawada et al. demonstrated that intraperitoneal treatment with an mTOR inhibitor significantly inhibited the growth of EBV-associated NK-cell lymphomas in a murine xenograft model and decreased the EBV load in peripheral blood, while T-cell lines were more sensitive to the mTOR inhibitors, but there were no significant differences between EBV-positive and EBV-negative cell lines (14). A series of studies of the JAK-STAT axis in EBV-T/NK LPDs provided new insight into its development. The STAT3 was activated in T/NK-cells in six of seven patients with CAEBV, promoting survival and cytokine production (15). Indeed, the selective JAK3 inhibitor, tofacitinib, significantly inhibited the growth of established tumors in NOG mice (16). We have already demonstrated the antitumor activity of the proteasome inhibitor bortezomib on EBV-associated lymphoma cells (17, 18). Therefore, combining these agents is a promising strategy to improve the treatment of EBV-T/NK lymphomas.
A fundamental question regarding the etiology of EBV-T/NK LPDs remains. The precise mechanism of T/NK-cell tumorigenesis remains to be elucidated because EBV-T/NK tumors are rare, and the generation and handling of EBV-positive T/NK cells are more difficult than with B-cells. To elucidate the genetic background related to these rare tumors, next-generation sequencing (NGS), including whole-genome sequencing and whole-exome sequencing, is a powerful, unbiased approach. Mutations of DDX3X, TP53, BCOR1, and STAT3 have been found in Chinese (27) and Japanese (28) patients with ENKL, although the mutation rates differed between these cohorts. Li et al. showed that genetic variation at HLA-DPB1 is a strong contributor to extranodal NK/T-cell lymphoma (29). These findings highlight a pathogenic link between genetic variation and EBV-associated neoplastic proliferation. However, the possibility that specific EBV strains or variants have a higher tendency to develop T/NK-cell tumors cannot be eliminated now. Notably, Kimura's group also revealed that the EBV genome in CAEBV patients harbored frequent intragenic deletions (Dr. Kimura, personal communication). The genetic data generated from NGS-based approaches are required for their subsequent validation as definitively disease causing. Therefore, patient registries and biospecimen repositories are needed to accelerate bridging research from the developmental and preclinical stages to a clinical setting. In Japan, a nationwide registry of EBV-T/NK LPDs has been started (currently only Japanese, https://www.med.nagoya-u.ac.jp/virus/caebv/). We hope that this registry will grow and be linked to international registries to improve the efficacy and quality of the treatment of EBV-associated tumors.
As Abraham Lincoln, the 16th president of the United States, once said, “I will prepare and someday my chance will come.”
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author thanks Drs. Hiroshi Kimura (Nagoya University) and Takayuki Murata (Fujita Health University) for critical reading and providing unpublished data.
This work was supported in part by grants from the JSPS KAKENHI Grant Number JP16H06231, the Japan Agency for Medical Research and Development (AMED) 17fm0208016, the Takeda Science Foundation, the 24th General Assembly of the Japanese Association of Medical Sciences and the Kitamura Memorial Foundation for Research of Blood Diseases.
References
1. Cohen JI. Epstein-Barr virus infection. N Engl J Med. (2000) 343:481–92. doi: 10.1056/NEJM200008173430707
2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
3. Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood (2011) 117:5835–49. doi: 10.1182/blood-2010-11-316745
4. Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood (2012) 119:673–86. doi: 10.1182/blood-2011-10-381921
5. Quintanilla-Martinez L. The 2016 updated WHO classification of lymphoid neoplasias. Hematol Oncol. (2017) 35(Suppl. 1):37–45. doi: 10.1002/hon.2399
6. Suzuki R. NK/T cell lymphoma: updates in therapy. Curr Hematol Malig Rep. (2018) 13:7–12. doi: 10.1007/s11899-018-0430-5
7. Gotoh K, Ito Y, Shibata-Watanabe Y, Kawada J, Takahashi Y, Yagasaki H, et al. Clinical and virological characteristics of 15 patients with chronic active Epstein-Barr virus infection treated with hematopoietic stem cell transplantation. Clin Infect Dis. (2008) 46:1525–34. doi: 10.1086/587671
8. Kawa K, Sawada A, Sato M, Okamura T, Sakata N, Kondo O, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant. (2011) 46:77–83. doi: 10.1038/bmt.2010.122
9. Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood (2017) 129:2437–42. doi: 10.1182/blood-2016-12-756841
10. Kanazawa T, Hiramatsu Y, Iwata S, Siddiquey M, Sato Y, Suzuki M, et al. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin Cancer Res. (2014) 20:5075–84. doi: 10.1158/1078-0432.CCR-14-0580
11. Siddiquey MN, Nakagawa H, Iwata S, Kanazawa T, Suzuki M, Imadome K, et al. Anti-tumor effects of suberoylanilide hydroxamic acid on Epstein-Barr virus-associated T cell and natural killer cell lymphoma. Cancer Sci. (2014) 105:713–22. doi: 10.1111/cas.12418
12. Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. (2016) 27:508–13. doi: 10.1093/annonc/mdv596
13. Suzuki M, Takeda T, Nakagawa H, Iwata S, Watanabe T, Siddiquey MN, et al. The heat shock protein 90 inhibitor BIIB021 suppresses the growth of T and natural killer cell lymphomas. Front Microbiol. (2015) 6:280. doi: 10.3389/fmicb.2015.00280
14. Kawada J, Ito Y, Iwata S, Suzuki M, Kawano Y, Kanazawa T, et al. mTOR inhibitors induce cell-cycle arrest and inhibit tumor growth in Epstein-Barr virus-associated T and natural killer cell lymphoma cells. Clin Cancer Res. (2014) 20:5412–22. doi: 10.1158/1078-0432.CCR-13-3172
15. Onozawa E, Shibayama H, Takada H, Imadome KI, Aoki S, Yoshimori M, et al. STAT3 is constitutively activated in chronic active Epstein-Barr virus infection and can be a therapeutic target. Oncotarget (2018) 9:31077–89. doi: 10.18632/oncotarget.25780
16. Ando S, Kawada JI, Watanabe T, Suzuki M, Sato Y, Torii Y, et al. Tofacitinib induces G1 cell-cycle arrest and inhibits tumor growth in Epstein-Barr virus-associated T and natural killer cell lymphoma cells. Oncotarget (2016) 7:76793–805. doi: 10.18632/oncotarget.12529
17. Zou P, Kawada J, Pesnicak L, Cohen JI. Bortezomib induces apoptosis of Epstein-Barr virus (EBV)-transformed B cells and prolongs survival of mice inoculated with EBV-transformed B cells. J Virol. (2007) 81:10029–36. doi: 10.1128/JVI.02241-06
18. Iwata S, Yano S, Ito Y, Ushijima Y, Gotoh K, Kawada J, et al. Bortezomib induces apoptosis in T lymphoma cells and natural killer lymphoma cells independent of Epstein-Barr virus infection. Int J Cancer (2011) 129:2263–73. doi: 10.1002/ijc.25873
19. Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. (2012) 18:1611–8. doi: 10.1158/1078-0432.CCR-11-1942
20. Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene (2007) 26:1351–6. doi: 10.1038/sj.onc.1210204
21. Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. (2009) 27:5410–7. doi: 10.1200/JCO.2008.21.6150
22. Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood (2011) 117:5827–34. doi: 10.1182/blood-2010-10-312603
23. Kieser A, Sterz KR. The latent membrane protein 1 (LMP1). Curr Top Microbiol Immunol. (2015) 391:119–49. doi: 10.1007/978-3-319-22834-1_4
24. Murata T, Iwata S, Siddiquey MN, Kanazawa T, Goshima F, Kawashima D, et al. Heat shock protein 90 inhibitors repress latent membrane protein 1 (LMP1) expression and proliferation of Epstein-Barr virus-positive natural killer cell lymphoma. PLoS ONE (2013) 8:e63566. doi: 10.1371/journal.pone.0063566
25. Wlodarski P, Kasprzycka M, Liu X, Marzec M, Robertson ES, Slupianek A, et al. Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum. Cancer Res. (2005) 65:7800–8. doi: 10.1158/0008-5472.CAN-04-4180
26. Cen O, Longnecker R. Rapamycin reverses splenomegaly and inhibits tumor development in a transgenic model of Epstein-Barr virus-related Burkitt's lymphoma. Mol Cancer Ther. (2011) 10:679–86. doi: 10.1158/1535-7163.MCT-10-0833
27. Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. (2015) 47:1061–6. doi: 10.1038/ng.3358
28. Dobashi A, Tsuyama N, Asaka R, Togashi Y, Ueda K, Sakata S, et al. Frequent BCOR aberrations in extranodal NK/T-cell lymphoma, nasal type. Genes Chromosomes Cancer (2016) 55:460–71. doi: 10.1002/gcc.22348
Keywords: EBV, EBV-T/NK LPDs, basic-preclinical research, registry database, CAEBV
Citation: Sato Y (2018) Challenges in Managing EBV-Associated T- and NK-Cell Lymphoproliferative Diseases. Front. Pediatr. 6:320. doi: 10.3389/fped.2018.00320
Received: 29 September 2018; Accepted: 08 October 2018;
Published: 06 November 2018.
Edited by:
Shigeyoshi Fujiwara, National Center for Child Health and Development (NCCHD), JapanReviewed by:
Yoji Sasahara, Tohoku University School of Medicine, JapanCopyright © 2018 Sato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshitaka Sato, eXNzYXRvQG1lZC5uYWdveWEtdS5hYy5qcA==
 Yoshitaka Sato
Yoshitaka Sato