- 1East Midlands Congenital Heart Centre, University Hospitals of Leicester, Leicester, United Kingdom
- 2Cardiovascular Research Center, University of Leicester, Leicester, United Kingdom
Objective: Comparison of early outcomes of normothermic cardiopulmonary bypass (N-CPB, ≥35°C) with hypothermic cardiopulmonary bypass (H-CPB, 28–34°C) for congenital heart defects.
Methods: Data from 99 patients <2 years operated with N-CPB (n = 48) or H-CPB (n = 51) were retrospectively reviewed: aortic X-clamping and CPB duration, vasoactive inotropic score (VIS), arterial lactate, pH and base excess, urine output, extubation, PICU stay, transfusion requirements, chest drain losses, costs of transfusions, and costs of PICU stay.
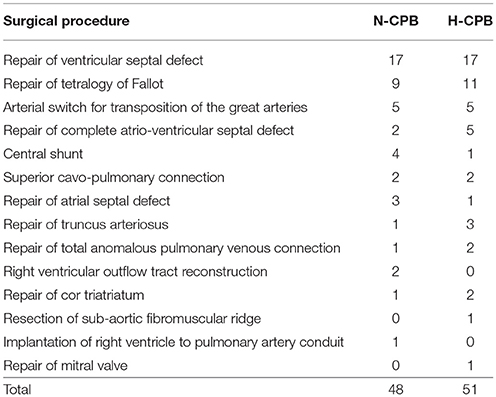
Results: The two groups were homogeneous for diagnosis, risk factors, surgery and demographic variables: N-CPB age 7.7 ± 6.1 months, weight 6.2 ± 2.4 kg, and H-CPB age 6.6 ± 6.5 months, weight 6.1 ± 2.4 kg.
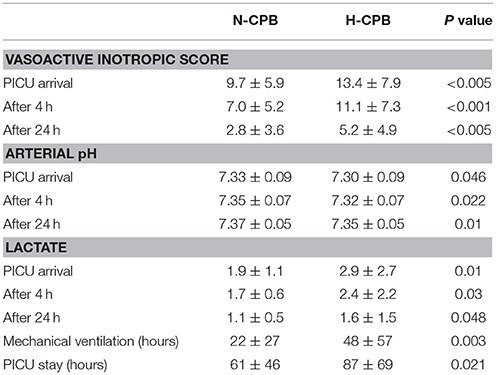
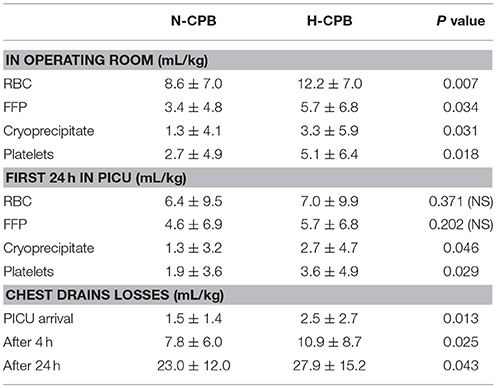
There were no hospital deaths in either group. VIS in N-CPB was lower than H-CPB on PICU arrival (9.7 ± 5.9 vs. 13.4 ± 7.9, P < 0.005), after 4 h (7.0 ± 5.2 vs. 11.1 ± 7.3, P < 0.001) and 24 h (2.8 ± 3.6 vs. 5.6 ± 5.6, P < 0.003); arterial pH was better at PICU arrival (7.33 ± 0.09 vs. 7.30 ± 0.09, P = 0.046) after 4 h (7.35 ± 0.07 vs. 7.32 ± 0.07, P = 0.022) and after 24 h (7.37 ± 0.05 vs. 7.35 ± 0.05, P = 0.01). Extubation was earlier in N-CPB than in H-CPB (22 ± 27 vs. 48 ± 57 h, P = 0.003) as PICU discharge (61 ± 46 h vs. 87 ± 69 h, P = 0.021). Transfusion requirements in operating room were lower in N-CPB vs. H-CPB for RBC, FFP, cryoprecipitate, and platelets, while during the first 24 h in PICU were lower only for cryoprecipitate and platelets.
Chest drain losses (mL/kg) on PICU arrival, after 4 and 24 h were lower with N-CPB vs. H-CPB (respectively 1.5 ± 1.4 vs. 2.5 ± 2.7, P = 0.013, 7.8 ± 6.0 vs. 10.9 ± 8.7, P = 0.025, and 23.0 ± 12.0 vs. 27.9 ± 15.2, P = 0.043). Tranexamic acid infusion was required in 7/48 (14.6%) patients with N-CPB vs. 18/51(= 35.3%) in H-CPB (P = 0.009). The average total costs/patient of blood and blood products (RBC, FFP, cryoprecipitate, platelets) were lower in N-CPB vs. H-CPB for both the first 24 h after surgery (£204 ± 169 vs. £306 ± 254, P = 0.011) as well as during the total duration of PICU stay (£239 ± 193 vs. £427 ± 337, P = 0.001). The average cost/patient/day of stay in PICU was lower in N-CPB than in H-CPB (£4,067 ± 3,067 vs. £5,800 ± 4,600, P = 0.021).
Conclusions: N-CPB may reduce inotropic and respiratory support, shorten PICU stay, and decrease peri-operative transfusion requirements, with subsequent costs reduction, compared to H-CPB. Future studies are needed to validate and support wider use of N-CPB.
Introduction
Surgery for congenital heart defects is generally performed with hypothermic cardiopulmonary bypass (CPB), reduced flow and hemodilution. This technique was historically first used to reduce the metabolic demand during CPB, and to increase the level of safety in the case of unforeseen complications (1).
In addition to the wide spread use of hypothermic CPB (H-CPB) with reduced flow and hemodilution, a large number of operations are still performed with deep hypothermia and circulatory arrest. This technique could be justified in small infants by the reduced duration of CPB, which is used only for cooling and rewarming, by the simplified venous cannulation, particularly in infants with anomalous venous connections, and by the bloodless field offering the surgeons adequate exposure (2).
Unfortunately H-CPB presents side effects, affecting all tissues and organs, with negative influence on clinical outcomes (1, 3–6). To minimize the negative effects of hypothermia, normothermic CPB has been progressively used for repair of congenital heart defects (4–6).
The aim of this retrospective study was to compare early clinical outcomes in patients less than 2 years of age with congenital heart defects who underwent cardiac surgery with normothermic (≥35°C) cardiopulmonary bypass (N-CPB) vs. hypothermic cardiopulmonary bypass (H-CPB).
Methods
Clinical records of patients <2 years of age who underwent cardiac surgery for congenital heart defects with either N-CPB or H-CPB from January 2014 to December 2015 were retrospectively reviewed.
Exclusion criteria were:
a) age >2 years
b) premature patients (gestation age <38 weeks)
c) operations performed without CPB
d) operations performed with temperature lower than 28°C or with a period of circulatory arrest
e) presence of aortic arch hypoplasia or interruption requiring aortic arch reconstruction.
Patients with operations performed at CPB temperatures lower than 28°C were excluded from this study to avoid introducing substantial flow reduction as a potentially important variable in the comparison.
In all patients included in this study the pump flow was maintained at 3.5 L/min/m2 B.S.A. in the group with N-CPB at a temperature always ≥35°C, while in the group with H-CPB the pump flow was reduced to 90% (= 3.15 L/min/m2 B.S.A.) at a temperature = 32°C and to 75% (= 2.63 L/min/m2 B.S.A.) at a temperature = 28°C.
The choice between the use of N-CPB and H-CPB was the decision of the surgeon in charge of the operation.
The following data were analyzed: demographic information, cardiac diagnosis, presence of previous median sternotomy, type of surgery, pre-operative risk evaluation accordingly with the STAT analysis, duration of aortic X-clamping and CPB, vasoactive inotropic score, arterial lactate, pH and base excess (B.E.) on PICU arrival, after 4 and 24 h, urine output (ml/kg/hr) after 4 and 24 h, time to extubation, duration of PICU stay, transfusion requirements of red blood cells (RBC), fresh frozen plasma (FFP), cryoprecipitate and platelets in operating room and during the first 24 h in PICU, chest drain losses on arrival to PICU and after 4 and 24 h, incidence of reoperations due to bleeding, the costs of blood and blood products used for transfusions in the first 24 h after surgery during PICU stay, as well as the cost of stay in PICU.
All data were expressed as mean ± Standard Deviation. As the small sample size did not allow for meaningful multivariable comparisons, a univariable comparison between the two groups was performed with Student's T-test, accepting as statistical significance P < 0.05.
The study received approval by the Institutional Review Board and Ethical Committee.
Results
A total of 99 patients were identified, 48 with mean age 7.7 ± 6.1 months, mean weight 6.2 ± 2.4 kg, operated on with N-CPB, and 51 with mean age 6.6 ± 6.5 months, mean weight 6.1 ± 2.4 kg operated on with H-CPB.
The two groups, N-CPB and H-CPB, were homogeneous (no statistical difference) regarding the demographic variables of age (P = 0.20) and body weight (P = 0.36).
In the N-CPB group 2/48 (= 4.2%) of patients had a previous median sternotomy, one before bidirectional Glenn and the other before implantation of right ventricle to pulmonary artery conduit, and in the H-CPB group 5/51 (9.8%), three before repair of ventricular septal defect and two before bidirectional Glenn, but the difference did not reach statistical significance (P = 0.37).
Table 1 shows the distribution of cardiac diagnosis and type of surgical procedure between the two groups.
Pre-operative risk evaluation accordingly with the STAT analysis showed no difference between the two groups, with mean value 2.0 ± 1.2 in N-CPB and mean value 2.0 ± 1.1 in H-CPB (P = 0.46).
There was a statistically significant difference between N-CPB and H-CPB regarding the duration of aortic X-clamping, shorter in N-CPB vs. H-CPB (mean 58 ± 37′, vs. mean 76 ± 37′, P = 0.01) as well a shorter duration of CPB in N-CPB vs. H-CPB (mean 94 ± 41′, vs. mean 116 ± 41′, P = 0.005).
There were no hospital deaths in either group.
Table 2 shows the results of the early clinical outcomes for vasoactive inotropic score, arterial pH, lactate, duration of mechanical ventilation (time to tracheal extubation) and stay in PICU.
Although there was a trend in favor of N-CPB, no statistical difference was reached for arterial B.E. and urine output.
Table 3 shows the transfusion requirements for RBC, FFP, platelets, and cryoprecipitate, as well as the chest drains losses, in the operating room and during the first 24 h in PICU.
There was no obvious correlation between chest drains losses and presence of previous median sternotomy: 1/2 patients with previous median sternotomy in N-CPB group and 2/5 in H-CPB group did not require any intra-operative or post-operative transfusion.
No statistical difference was reached in the incidence of reoperation due to chest bleeding, which occurred in 0/48 (= 0%) patients in N-CPB vs. 2//51 (= 3.9%) in H-CPB (P = 0.08).
In the group with N-CPB 3/48 (= 6.3%) of the patients required no transfusion vs. 2/51 (= 3.9%) in the group with H-CPB, but the difference failed to reach statistical significance (P = 0.30).
Tranexamic acid infusion was required in 7/48 (14.6%) of the patients with N-CPB vs. 18/51 (= 35.3%) in the group with H-CPB (P = 0.009).
The total costs per patient of blood and blood products (RBC, FFP, cryoprecipitate, platelets) were lower in N-CPB vs. H-CPB for both the first 24 h after surgery (mean £204 ± 169 vs. mean £306 ± 254, P = 0.011) as well as during the total duration of PICU stay (mean £239 ± 193 vs. mean £427 ± 337, P = 0.001).
The average cost/patient/day of stay in PICU was lower in N-CPB than in H-CPB (mean £4,067 ± 3,067 vs. £5,800 ± 4,600, P = 0.021).
Discussion
Hypothermia, Flow Reduction and Hemodilution
Despite positive outcomes reported with H-CPB in surgical repair of complex congenital heart defects (7), several experimental and clinical studies over the years have demonstrated serious negative effects of hypothermia with flow reduction and hemodilution at both the cellular and tissue level (1, 3–6, 8–32).
Hypothermia, flow reduction and hemodilution have been demonstrated to decrease ATP levels, glycogen levels, intracellular pH, efficiency of membrane-based ion pumps, mitochondrial function, and intracellular enzyme function, as well as increased anaerobic metabolism, lactate production, cell swelling, and increased Calcium influx (1, 3–6, 8–32).
These changes in parenchymal cells, endothelial cells, and inflammatory cells are responsible for the inflammatory response and the ischemia/reperfusion injury, with the observed clinical consequences of low cardiac output syndrome requiring inotropic support, pulmonary dysfunction requiring respiratory support, metabolic derangement with acidosis and renal failure, coagulation derangement with excessive chest bleeding, and neurologic complications and neurodevelopmental impairment (1, 3–6, 11, 14–19, 21).
Introduction of Normothermia
The reported negative consequences of H-CPB have motivated the search for an alternative modality for perfusion in the pediatric population.
The first substantial change introduced in clinical practice was to reduce the degree of hemodilution, with a higher hematocrit on CPB (28, 31).
However the most important modification to conventional CPB with hypothermia and hemodilution was the introduction of normothermic high flow CPB.
This technique of perfusion was first used in Paris, France, by Lecompte and Durandy (33), who later reported the use of their technique in a very large number of pediatric cardiac patients (34, 35). Visits to their hospital, with direct exposure to their technique, persuaded other surgeons to introduce this method in their own clinical practice, resulting in an increase in the number of units using normothermic high flow CPB with reduced hemodilution (1, 4–6, 36–42).
The basic principles of N-CPB are maintaining CPB flow at 3.5 L/min/m2 B.S.A., nasopharynx and rectal temperature between 35.0 and 36.5°C, and hematocrit ≥30%. These conditions are closer to normal physiology, where the systemic flow is 3.0–5.5 L/m2 B.S.A./min, the temperature 37°C, and the hematocrit 45% (6).
The CPB flow used in conventional H-CBP is generally reported at 2.0–2.4 L/m2 BSA/min or 100–120 mL/min/kg of body weight, and frequently is further reduced during the central part of the operation when requested by the surgeon to facilitate the surgical exposure, or even to circulatory arrest with deep hypothermia.
In this study only patients with operations performed at a temperature ≥28°C were included in the H-CPB group, to avoid introducing a substantial flow reduction as this is potentially a confounding variable in the comparison.
The total duration of CPB is shorter with N-CPB than with H-CPB because the period of cooling at the beginning of CPB and the rewarming at the end of CPB, required for H-CPB, is avoided in N-CPB. This alone can contribute to reducing the negative effects of CPB.
We have also observed a reduction in the duration of aortic X-clamping in N-CPB. This is probably due to the fact that aortic X-clamping was limited to the closure of intra-cardiac defects and the right heart reconstruction is performed with beating heart, while in the H-CPB group the entire procedure was performed with the aortic X-clamp on, knowing that the heart reperfusion could have been accomplished during the required rewarming period.
Potential Concerns
The three potential concerns of using N-CPB are the reduced margin of safety against potential incidents during CPB, inadequate surgical exposure and adequate neurological protection.
As demonstrated in this study, as well as reported by other hospitals, the clinical advantages provided in the immediate post-operative period by N-CPB in comparison with H-CPB outweigh all potential risks of incidents (1, 5, 6, 33–38).
Adequate surgical exposure can be achieved with appropriate venous cannulation and left heart venting, even in small cyanotic neonates with large collateral circulation and in the presence of anomalous venous connections (1, 5, 6, 33–38).
With regard to the neurological complications reported by the H-CPB, the N-CPB was associated with contradictory neurologic outcomes, varying from the safety in relationship to the neurodevelopmental status (42) to the observation with magnetic resonance imaging suggesting that normothermic perfusion is associated with few new lesions in comparison with the pre-operative investigations (43).
Limits of This Study
The limits of this study are the following:
a) retrospective
b) non-randomized
c) single center
d) relatively small sample size
e) small sample size did not allow for meaningful multivariable comparisons.
One of our aims is to share our experience and stimulate prospective multi-center randomized controlled trials comparing the outcomes of N-CPB with H-CPB.
Our retrospective study was motivated by the literature report of two studies comparing N-CPB with H-CPB without evidence of substantial differences (44, 45), while a very large systematic review showed that N-CPB is at least as safe as the conventional H-CPB (46). To the best of our knowledge we are not aware of any study comparing the early clinical outcomes and the blood requirements in N-CPB with H-CPB in patients younger than 2 years operated on for congenital heart defects.
Conclusion
This experience shows that N-CPB is safe and reproducible, reduces inotropic and respiratory support, shortens the duration of PICU stay, and decreases the peri-operative transfusion requirements, with subsequent costs reduction, compared with H-CPB.
Wider use of N-CPB in pediatric cardiac surgery should be taken into consideration, and prospective multi-center randomized controlled trials comparing the outcomes with H-CPB in a risk adjusted population of patients with complex congenital heart defects are required to support these results.
Author Contributions
AC: design of the study, analysis of the results and preparation of the manuscript; CB, SC, and JW: data collection and revision; M-TT: study design, revision of the manuscript; BM and MC: revision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Corno AF, Von Segesser LK. Is hypothermia necessary in pediatric cardiac surgery? Eur J Cardiothorac Surg. (1999) 15:110–1. doi: 10.1016/S1010-7940(98)00291-7
2. Ungerleider RM, Shen I. Optimizing response of the neonate and infant to car-diopulmonary bypass. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2003) 6:140–6. doi: 10.1053/pcsu.2003.50008
3. Bellinger DC, Wernovsky G, Rappaport LA, Mayer JE Jr, Castaneda AR, Farrell DM, et al. Cognitive development of children following early repair of transposition of the great arteries using deep hypothermic circulatory arrest. Pediatrics (1991) 87:701–7.
4. Caputo M, Bays S, Rogers CA, Pawade A, Parry AJ, Suleiman S, et al. Randomized comparison between normothermic and hypothermic cardiopulmonary bypass in pediatric open-heart surgery. Ann Thorac Surg. (2005) 80:982–8. doi: 10.1016/j.athoracsur.2005.03.062
5. Durandy Y. Warm pediatric cardiac surgery: European experience. Asian Car-diovasc Thorac Ann. (2010) 18:386–95. doi: 10.1177/0218492310376675
6. Shamsuddin AM, Nikman AM, Ali S, Zain MRM, Wong AR, Corno AF. Normothermia for pediatric and congenital heart surgery: an expanded horizon. Front Pediatr. (2015) 3:23. doi: 10.3389/fped.2015.00023
7. Barratt-Boyes BG, Simpson M, Neutze JM. Intracardiac surgery in neonates and infants using deep hypothermia with surface cooling and limited cardiopulmonary bypass. Circulation (1971) 43(5 Suppl.):I25–30. doi: 10.1161/01.CIR.43.5S1.I-25
8. Bellinger DC, Wernovsky G, Rappaport LA, Mayer JE Jr, Castaneda AR, Farrell DM, et al. Cognitive development of children following early repair of transpo-sition of the great arteries using deep hypothermic circulatory arrest. Pediatrics (1991) 87:701–7.
9. Terblanche J, Isaacson LC, Eales L, Barnard CN. Renal function during and immediately after profound hypothermia. Surgery (1961) 50:869–76.
10. Monroe RG, Strang RH, LaFarge CG, Levy J. Ventricular performance pressure volume relationship ona O2 consumption during hypothermia. Am J Physiol. (1964) 206:67–73.
11. Baum D, Dillard DH, Porte D. Inhibition of insulin release in infants undergo-ing deep hypothermic cardiovascular surgery. N Engl J Med. (1968) 279:1309–14. doi: 10.1056/NEJM196812122792404
12. McConnell DH, White FN, Peters F, Nelson RL, Goldstein SM, Maloney JV, et al. Importance of alkalosis in maintenance of “ideal” blood pH during hypothermia. Surg Forum (1975) 26:263–5.
13. DuPlessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL. Periop-erative effects of alpha-stat versus pH-stat strategies for deep hypothermic circulatory bypass in infants. J Thorac Cardiovasc Surg. (1977) 114:991–1000. doi: 10.1016/S0022-5223(97)70013-8
14. Landymore RW, Murphy DA, Longley WJ. Effects of cardiopulmonary bypass and hypothermia on pancreatic function and peripheral utilization of glucose. Can J Surg. (1979) 22:248–50.
15. Swain JA, White FN, Peters RM. The effect of pH on the hypothermic ventricular fibrillation threshold. J Thorac Cardiovasc Surg. (1984) 87:445–51.
16. Kurihara S, Sakai T. Effect of rapid cooling on mechanical and electrical responses in ventricular muscle of the guinea pig. J Physiol. (1985) 361:361–78. doi: 10.1113/jphysiol.1985.sp015650
17. Quiroga MM, Miyagishima R, Haendschen LC, Glovsky M, Martin BA, Hogg JC. The effect of body temperature on leukocyte kinetics during cardiopulmonary bypass. J Thorac Cardiovasc Surg. (1985) 90:91–6.
18. Murkin JM, Farrar JK, Tweed WA, McKenzie FN, Guiraudon G. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of PaCO2. Anesth Analg (1987) 66:825–32. doi: 10.1213/00000539-198709000-00003
19. Greely WJ, Kern FH, Ungerleider RM, Boyd JL, Quill T, Smith LR. The effect of hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral metabolism in neonates, infants and children. J Thorac Cardiovasc Surg. (1991) 101:783–94.
20. McMahon WS, Gillette PC, Hinton RB, Stratton JR, Crawford FA, Spinale FG. Developmental differences in myocyte contractile response after cardioplegic arrest. J Thorac Cardiovasc Surg. (1996) 111:1257–66.
21. Rady MY, Ryan T, Starr NJ. Early onset of acute pulmonary dysfunction after cardiovascular surgery: risk factors and clinical outcome. Crit Care Med. (1997) 25:1831–9. doi: 10.1097/00003246-199711000-00021
22. Corno AF. Evaluation of resting coronary blood flow and coronary flow reserve in infants after cardiac operations. J Thorac Cardiovasc Surg. (1998) 116:182–3. doi: 10.1016/S0022-5223(98)70267-3
23. Birdi L, Caputo M, Underwood M, Bryan AJ, Angelini GD. The effects of cardiopulmonary bypass temperature on inflammatory response following cardiopulmonary bypass. Eur J Cardiothorac Surg. (1999) 16:540–5. doi: 10.1016/S1010-7940(99)00301-2
24. Heltne JK, Koller ME, Lund T, Bert J, Rynning SE, Stangeland L. Dynamic evaluation of fluid shifts during normothermic and hypothermic cardiopulmonary bypass in piglets. Acta Anaesthesiol Scand. (2000) 44:1220–5. doi: 10.1034/j.1399-6576.2000.441006.x
25. Honore PM, Jacquiet LM, Beale RJ, Renauld JC, Valadi D, Noirhomme P. Effects of normothermia versus hypothermia on extravascular lung water and serum cytokines during cardiopulmonary bypass: a randomized, controlled trial. Crit Care Med. (2001) 29:1903–9. doi: 10.1097/00003246-200110000-00009
26. Clancy RR, McGaurn SA, Wernovsky G, Gaynor JW, Spray TL, Norwood WI. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics (2003) 111:592–601. doi: 10.1542/peds.111.3.592
27. Wypij D, Newburger JW, Rappaport LA, DuPlessis AJ, Jonas RA, Wernovsky G. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston circulatory arrest trial. J Thorac Cardiovasc Surg. (2003) 126:1397–403. doi: 10.1016/S0022-5223(03)00940-1
28. Jonas RA, Wypij D, Roth SJ, Bellinger DC, Visconti KJ, DuPlessis AJ. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. (2003) 126:1765–74. doi: 10.1016/j.jtcvs.2003.04.003
29. Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ. Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med. (2003) 31:28–33. doi: 10.1097/00003246-200301000-00004
30. Karl TR, Hall S, Ford G, Kelly EA, Brizard CP, Mee RB. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevel-opmental outcome. J Thorac Cardiovasc Surg. (2004) 127:213–22. doi: 10.1016/j.jtcvs.2003.06.001
31. Newburger JW, Jonas RA, Soul J, Kussman BD, Bellinger DC, Laussen PC. Randomized trial of hematocrit 25% versus 35% during hypothermic car-diopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. (2008) 135:347–54. doi: 10.1016/j.jtcvs.2007.01.051
32. Bellinger DC, Wypij D, DuPlessis AJ, Rappaport LA, Riviello J, Jonas RA. Developmental and neurologic effects of alpha-stat versus pH stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. (2001) 121:374–83. doi: 10.1067/mtc.2001.111206
33. Durandy Y, Hulin S, Lecompte Y. Normothermic cardiopulmonary bvpass in pediatric surgery. J Thorac Cardiovasc Surg. (2002) 123:194. doi: 10.1067/mtc.2001.120005
34. Durandy YD, Hulin S. Normothermic bypass in pediatric surgery: technical aspects and clinical experience with 1400 cases. ASAIO J. (2006) 52:539–42. doi: 10.1097/01.mat.0000242597.92625.e9
35. Durandy YD, Younes M, Mahut B. Pediatric warm open heart surgery and prolonged cross-clamp time. Ann Thorac Surg. (2008) 86:194–7. doi: 10.1016/j.athoracsur.2008.08.004
36. Milella L, Da Cruz E, Gajraj R, Corno AF. Computerized anaesthesia and nor-mothermic perfusion in paediatric cardiac surgery. In: 2nd World Congress of Pediatric Cardiology and Cardiac Surgery. Honolulu, HI (1997). p. 376.
37. Corno AF. What are the best temperature, flow, and hematocrit levels for pediatric cardiopulmonary bypass? J Thorac Cardiovasc Surg. (2002) 124:856–7. doi: 10.1067/mtc.2002.126388
38. Pouard P, Mauriat P, Ek F, Haydar A, Gioanni S, Laquay N, et al. Normoth-ermic cardiopulmonary bypass and myocardial cardioplegic protection for neonatal arterial switch operation. Eur J Cardiothorac Surg. (2006) 30:695–9. doi: 10.1016/j.ejcts.2006.07.032
39. Corno AF. Normal temperature and flow:are the “physiological” values so scary? Eur J Cardiothorac Surg. (2007) 31:756. doi: 10.1016/j.ejcts.2007.01.009
40. Cassano V, Milella L. Warm surgery: our experience. Eur J Cardiothorac Surg. (2007) 31:754–5. doi: 10.1016/j.ejcts.2007.01.015
41. Caputo MD, Patel N, Angelini GD, de Siena P, Stoica S, Parry AJ, et al. Effect of normothermic cardiopulmonary bypass on renal injury in pediatric cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. (2011) 142:1114–21. doi: 10.1016/j.jtcvs.2011.08.008
42. Poncelet AJ, van Steenberghe M, Moniotte S, Detaille T, Beauloye C, Bertrand L, et al. Cardiac and neurological assessment of normothermia/warm blood cardioplegia vs. hypothermia/cold crystallolid cardioplegia in pediatric cardiac surgery. Eur J Cardiothorac Surg. (2011) 40:1384–90. doi: 10.1016/j.ejcts.2011.03.047
43. Durandy Y, Rubatti M, Couturier R, Rohnean A. Pre-and post-operative mag-netic resonance imaging in neonatal arterial switch operation using warm per-fusion. Artif Organs (2011) 35:1111–25. doi: 10.1111/j.1525-1594.2011.01325.x
44. Lema G, Aeschlimann N, Becker P. Normothermia versus hypothermia during pediatric cardiac surgery: no answer as yet. J Thorac Cardiovasc Surg. (2012) 143:758. doi: 10.1016/j.jtcvs.2011.10.091
45. Raos S, Sheehan K, Culliford L, Pike K, Elllis L, Parry AJ, et al. Normothermic versus hypothermic cardiopulmonary bypass in children undergoing open heart surgery (thermic-2): study protocol for a randomized controlled trial. JMIR Res Protoc. (2015) 4:e59. doi: 10.2196/resprot.4338
Keywords: cardiac surgery, normothermia, hypothermia, cardiopulmonary bypass, pediatric intensive care unit
Citation: Corno AF, Bostock C, Chiles SD, Wright J, Tala M-TJ, Mimic B and Cvetkovic M (2018) Comparison of Early Outcomes for Normothermic and Hypothermic Cardiopulmonary Bypass in Children Undergoing Congenital Heart Surgery. Front. Pediatr. 6:219. doi: 10.3389/fped.2018.00219
Received: 14 May 2018; Accepted: 17 July 2018;
Published: 17 August 2018.
Edited by:
Utpal S. Bhalala, Baylor College of Medicine, United StatesReviewed by:
Meena Nathan, Boston Children's Hospital, Harvard University, United StatesYasuhiro Fujii, Okayama University, Japan
Copyright © 2018 Corno, Bostock, Chiles, Wright, Tala, Mimic and Cvetkovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio F. Corno, afc10@leicester.ac.uk
 Antonio F. Corno
Antonio F. Corno Claire Bostock1
Claire Bostock1 Branko Mimic
Branko Mimic Mirjana Cvetkovic
Mirjana Cvetkovic