
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr. , 07 August 2018
Sec. Pediatric Immunology
Volume 6 - 2018 | https://doi.org/10.3389/fped.2018.00215
This article is part of the Research Topic Human Milk Composition and Health Outcomes in Children View all 13 articles
Maternal breast milk (BM) is a complex and unique fluid that evolution adapted to satisfy neonatal needs; in addition to classical nutrients, it contains several bioactive components. BM characteristically shows inter-individual variability, modifying its composition during different phases of lactation. BM composition, determining important consequences on neonatal gut colonization, influences both short and long-term development. Maternal milk can also shape neonatal microbiota, through its glycobiome rich in Lactobacilli spp. and Bifidobacteria spp. Therefore, neonatal nourishment during the first months of life seems the most important determinant of individual's outcomes. Our manuscript aims to provide new evidence in the characterization of BM metabolome and microbiome, and its comparison to formula milk, allowing the evaluation of each nutrient's influence on neonatal metabolism. This result very interesting since potentially offers an innovative approach to investigate the complex relationship between BM components and infant's health, also providing the chance to intervene in a sartorial way on diet composition, according to the nutritional requests. Future research, integrating metabolomics, microbiomics and stem cells knowledge, could make significant steps forward in understanding BM extraordinary properties and functions.
Breast Milk (BM) is a precious fluid which has been considered miraculous since ancient times. Its extraordinary properties have been studied in detail, not resulting fully clarified yet. It can confer protection against a large number of pathologies and exerts a beneficial effect on breastfed newborn's development (1, 2).
BM is the most suitable choice for neonatal nutrition, highly recommended as the exclusive component of the infant's diet for almost 6 months of life (3).
Nutrition in the early neonatal period influences the successive whole life, due to its role in the activation of several metabolic processes, for example, microanatomy development, growth, metabolism, gut microbiological colonization and maturation, immunological system development, brain maturation, and organization (1, 4).
In fact, BM has been associated to many beneficial short-term effects, such as a reduction in necrotizing enterocolitis (NEC) and sepsis (5); a positive influence on long-term outcome (such as neurodevelopment) and a protective effect against infections, overweight, obesity, diabetes and malignant diseases incidence have also been described (6).
BM beneficial effects do not regard exclusively infants' health but could also be exerted on lactating mothers, improving their outcome (6).
BM contains several components, such as lipids, carbohydrates, proteins, vitamins, minerals. Oligosaccharides, the third most abundant constituents of BM, which represent a highly variable fraction of BM, exert several important functions, such as the modulation of neonatal gut microbiota composition, influencing many physiological processes (7).
BM is also defined an “alive” fluid, since it provides to the breastfed newborn maternal soluble bioactive components, growth-factors (GFs), hormones, cytokines, chemokines, immunoglobulins (Ig), and immunological-related cells as leucocytes, cells of both bacterial and maternal origin and finally, as recently demonstrated, also multipotent Stem Cells (SCs) able to integrate in vivo in many neonatal tissues and differentiate into mature cells. Finally, great relevance can also be attributed to the presence of maternal milk microbiota (1, 2, 5, 7–9).
BM composition has the extraordinary property to vary according to gestational age (GA) of the neonate and to the lactation phase (5, 6, 10).
Since the degree of prematurity highly influences BM features, the resulting composition is optimal for preterm newborns needs. Macro- and micronutrients levels vary and determine advantages regarding immunity, neurological development, gastrointestinal maturation (9, 11–17).
The presence of several cytokines and chemokines, showing a higher concentration in colostrum, has been widely evidenced and could represent an additional mechanism of protection, especially against NEC and sepsis (17, 18).
It is not fully known how maternal or pregnancy factors could modify their level, although in peripartum infections, in spontaneous preterm delivery and in VLBW neonates lower concentrations of pro-inflammatory cytokines have been measured, potentially protecting against mucosal damage or pathogens (19).
Even the cellular composition of BM varies among samples deriving from mothers of preterm and full-term newborns, meeting the necessities showed by premature neonates during the first phases of life (9) and confirming the extraordinary ability of this liquid to modify itself according to the newborns features and assuming the best qualities for his optimal development (5).
BM related SCs belong to different lineages such as mammary epithelial cells, neuroepitelial-like SCs and mesenchimal SCs (10–15%) (20).
It seems that, among large amount of SCs ingested by the newborns each day, some can pass from BM through neonatal gut and migrate into brain and other organs; there, they can persist and proliferate as in a microchimerism, restoring the involved organs, potentially even after a damage (1, 5, 21). This interaction between the dyad mother-child result very interesting but all the implications should be deeper clarified.
The great relevance of micronutrients in BM is highlighted by the increasing number of metabolomic studies performed to characterize its metabolic profile and inter- and intra- individual variations (8, 10, 16, 22–30).
BM can be analyzed through nuclear magnetic resonance (NMR) and liquid or gas chromatography coupled with mass spectrometry (LC-MS or GC-MS), to evaluate its unique profile (5).
The first metabolomic study investigating BM composition was conducted by Cesare Marincola et al in 2012 (22). This group demonstrated different metabolic features characterizing subsequent lactation stages. Moreover, they found higher levels of lactose and lower levels of maltose in BM samples, compared to formula milk (FM) (1, 17, 22).
Interesting results were also obtained by the numerous successive studies, performed by several groups. The most relevant findings are reported below, and they can be found in a more detailed way and summarized in 2 tables in the recent papers published by Fanos et al. (5) and Bardanzellu et al. (17).
According to the findings of Spevacek and colleagues, (23) the highest variability can be found in preterm samples. They identified and measured variations in 69 metabolites and also demonstrated that lacto-N-tetraose and lysine decreased during the maturation of full-term milk (23).
Another group (10) demonstrated that preterm BM metabolome mostly varies within 5–7 weeks postpartum; after this period it would probably obtain the composition of term milk after this time and BM dependence on GA seems to be reduced (5, 10, 17, 31).
Moreover, in colostrum samples from preterm delivering mothers, an increased level of fucosylated oligosaccharides, fucose, N-acetylneuramic acid and N-acetylglucosamine, citrate and creatinine have been shown (10).
The group of Villasenor et al. also demonstrated a different composition between full term colostrum and mature milk and moreover, our research group detected a higher sample variability in colostrum instead of mature milk belonging to extremely preterms (27).
Summarizing these findings, the highest variability has been evidenced during the first three months of lactation (25), with a high dependence on GA (28); a different metabolitic pattern comparing human colostrum with transition milk and mature was observed (23).
BM from mothers of preterm neonates showed a different composition if compared with full-term newborn mothers' BM. In particular, a higher concentration of proteins and aminoacids, promoting cerebral development and energy production, was observed. This reinforces the concept of BM variability according to the breastfed newborn's peculiarities (17).
Among the genetic factors highly influencing BM composition, four maternal phenotypes, depending on both blood group and expression of two specific genes, were identified.
This influence in particular regards human milk oligosaccharides (HMOs), which constitute the third most abundant solid fraction of BM, following lactose and lipids (1.9–4.5%) (7).
These genes are, firstly, alpha-1-2-fucosyltransferase (secretor gene, FUT2) which is codified by Se gene and allows the classification of secretors (Se+) and non-secretors (Se−) mothers. Secondly, it is considered alpha-1-3-4-fucosyltransferase gene(Lewis gene, FUT3); it indicates positivity or negativity for Lewis Group (Le+ or Le−). According to these considerations, maternal phenotypes can be divided in: Se+/Le+, Se+/Le−, Se−/Le+, and Se-/Le−, showing significant differences in BM metabolites (1, 17, 32, 33).
In fact, Se+/Le+ mother's BM exhibits all the fucosylated oligosaccharides (2′fucosyl-lactose 2′FL, lactodifucotetraose LDFT, Lacto-N-fucopentaose I LNFPI, Lactodifucoesaose I LNDFHI), while the Se−/Le+ mother's phenotype determines the production of samples containing a high concentration of HMOs with (α1-3) and (α1-4)-linked fucose residue, in absence of α1,2-fucosylated structures (32, 34–36).
The great interest concerning BM HMOs composition is also related to their potential influence on microbiota (10, 37, 38).
It has been estimated that FUT2 is expressed in more than 70% of the Caucasian women (34). The absence of α1-2-fucosylated oligosaccharides in BM can lead to several pathophysiological consequences, such as a delayed colonization by Bifidobacteria spp., a higher abundance of Streptococcus spp. and also functional differences of microbiota metabolic activity. According to some authors, Se+/Le+ phenotype results protective against some infections, such as E. coli and Campylobacter spp. and preventive of NEC (35), while infants fed with BM from Se−mothers would show a higher risk for diarrheal diseases (11, 39, 40).
In accordance with these data, Bazanella and colleagues analyzed BM samples from Se+ mothers, demonstrating a higher percentage of fucosylated oligosaccharides instead of Se− mothers, and the presence of B. longum exclusively in the stools of Se+ breastfed neonates (41).
HMOs are known to decrease with milk maturation (10, 27). According to many studies, total HMOs content, sialic acid, lacto-N-tetraose, LNDFH I, 3′- sialyllactose, 6′-siallylactose, fucose, N-acetylglucosamine, N-acetylneuraminic acid resulted higher in preterm milk (10, 23, 42).
In addition to HMO's, also amino acids and lipids showed a great variability across lactation stages and a great dependence on prematurity. Some amino acids increase, while other reduce their concentration during BM maturation (10, 17, 43).
The studies performed in this field allow to conclude that BM, in particular in the first phases and in the samples obtained by premature delivering mothers, is extremely rich in creatinine and amino acids. These factors are involved in two crucial processes, especially for the vulnerable category of preterm babies: brain development and energetic metabolism (17).
In particular, creatinine, betaine, coline, leucine, isoleucine, and valine take part in cerebral maturation (10, 26, 28, 44, 45), while energy production is closely related to the presence of alanine, glutamate, methionine and creatinine (23, 26, 28, 46, 47).
Coline and betaine could play a role in the reduction of cardiovascular diseases (28, 48). Moreover, acetylcarnitine, betaine, lysine, isoleucine, and taurine levels seem to decrease during milk maturation in samples of mother of full term neonates and not in preterm samples (17).
Other detected metabolites may also take part in several immunity processes, hepatic regeneration, lipid and glucidic metabolism (17, 45, 49–51).
It is also been demonstrated that fatty acids' (FA) composition in BM can be influenced by many factors, not fully understood up to now. Among these, maternal age, nationality, parity, body mass index (BMI), diet, newborn's GA, lactation stage, maternal gestational diabetes mellitus, number and duration of breastfed meals and delivery route can be mentioned, although the entity of their influence is not currently attested. The most represented fractions are tryglicerides, palmitic, oleic, linoleic and alpha-linolenic acids (17, 52–60).
FA's content seems to be higher in colostrum from mothers whose neonates' weight was lower than the 20° centile (52, 61, 62) and this mechanism may probably help to compensate the intrauterine growth restriction occurred in these neonates.
According to the analysis of Collado et al. (63), evaluating BM from preterms and full-term delivering mothers, the content of FAs resulted comparable among colostrum and mature milk samples, although different qualitative profiles were found.
Recently, interesting results confirmed the importance of a metabolomic approach to evaluate the differences occurring in newborns fed with BM or formula milk during the early life. Cesare Marincola et al. (64) detected variable urinary profiles in relation to the kind of diet; several and more numerous trials would be needed to fully understand the clinical implications of these findings, improving our knowledge on BM's effects.
Metabolomics also gave promising results analyzing urine and/or blood samples of breastfed neonates or those from their mothers, to understand biological effects of maternal BM. Two recent studies evidenced as different metabolites can be found in urine or blood of breastfed newborns, instead of those described in samples belonging to FM fed newborns (31, 65).
Moreover, the analysis of urine from breastfeeding mothers revealed different profiles too (5, 66).
In conclusion, BM metabolome varies according to GA and lactation phase, depending on neonatal necessities and especially meeting the peculiar requests of the vulnerable category of premature newborns.
Even if BM was considered sterile for long time, it has recently been demonstrated, through culture-dependent and -independent techniques, that the microbial community in BM from healthy mothers can contain more than 200 phylotypes, belonging to about 50 different genera (2, 67, 68).
The technological advances, particularly the cultivation independent methods, such as 16S gene sequencing, allowed a deeper analysis of bacterial diversity, giving more detailed information on the populations present in several human fluids, such as BM.
In this sampling, although they permitted to demonstrate a bacterial load between two and three orders of magnitude, which resulted higher than those estimated by cultures, from a critical point of view these technique are not able to discriminate DNA sequences from non-vital bacteria and extracellular DNA that could interfere the amplification by quantitative PCR (qPCR). However, it is also clear that the early stimulations coming from all the microbial products, alive or residual, could influence the newborn immune system (69).
According to the current knowledge, how maternal microbes can reach mammary epithelium and undergo secretion in BM, is still matter of debate. Milk harbored bacteria may derive from the contamination with bacteria from mother's skin (such as S. epidermidis) and from infant's oral cavity. On the other side it has been postulated that microorganisms from maternal intestinal tract can reach the mammary gland through a vascular transport via intestinal immune cells, especially dendritic cells (the so-called entero-mammary pathway hypothesis). This entero-mammary pathway allows to consider as maternal gastrointestinal bacteria during pregnancy and lactation could directly influence the infant's immune system. Moreover, a retrograde flow of newborn's microbes could occur during nursing (17, 67, 68, 70, 71). However, due to its peculiar characterization, this community is even more considered as a site-specific microbiota, as demonstrated by several anaerobic species that are identifiable and that are not present both in the skin or in the oral cavity (2, 67, 68).
This community is represented, for about half, by a constant core microbiota with a limited variability, being BM microbiota dominated by Staphylococcus spp., Pseudomonas spp., Streptococcus spp., Acinetobacter spp., Finegoldia spp., Anaerococcus spp., Actinomyces spp., and Enterobacter spp. (67, 69), with huge differences between colostrum and mature milk (68, 69, 72–77).
On the contrary, the other half seems to be highly dependent on maternal factors, such as ethnicity, diet, drug exposure, environmental factor exposure, mode of delivery (67, 69).
BM is an exceptional source of commensal bacteria for breastfed newborns, representing a dynamic ecosystem for several species, which can modify itself during milk maturation, according to the infant's needs (67, 72). In fact, microbiota shows high variability during the subsequent lactation stages, also highlighted by the possibility to detect different microbial-related metabolites (69, 78). After about one month of lactation, BM microbiota reaches the full maturation and its definitive composition, maintaining therefore a relative stability (67).
Composition and metabolic network of BM microbiota may be considered as an epigenetic determinant of neonatal health (78, 79). Moreover, many microbic-related metabolites represent a linking ring between metabolomics and microbiomics. In particular, BM microbiota is known to shape the newborn intestinal microbiota since the early phases of life. Bacterial genera and species present in colostrum and then in mature milk can positively influence intestinal bacterial network, highly rich in Bifidobacteria spp. and Lactobacilli spp. Bacterial communities produce metabolites, such as short chain fatty acids (SCFA), mostly butyrate, that may be detected in the fluids by metabolomics and are able to influence several health outcomes of the child.
Moreover, an active role is played by sialylated BM HMOs, which can induce transcriptional responses in the intestinal microbiota (i.e., B. Fragilis) potentially influencing even other microbial members, such as E. coli. Therefore, through several routes and deep interactions not fully clarified yet, this leads to infant growth promotion, beneficial metabolic pathways and effects on several organs (brain, liver, respiratory, and urinary tract) (36, 80).
The effect of early diet on pigs' intestinal microbiota has been also evaluated by Piccolo et al. (81), demonstrating that neonatal nutrition could characteristically induce different effects according to the different small bowel's region, mostly influencing duodenum microbial composition, since microbial network seems to be functionally defined by the intestinal segment (81).
This bioregional effect of nutrition on the shape of intestinal microbiota also influences the production of different molecules, highlighting the strict dependence of metabolic profile from microbial communities and the influence played by microbiota itself on the host tissue metabolism, as demonstrated through metabolomic analysis (81).
Hunt et al. (68) observed as BM microbial community represents a unique fingerprint characterizing each mother's sample. BM and therefore neonatal intestinal microbiota can be influenced by several factors, as reported in Table 1, including genetics (such as secretor status, as previously described), delivery route (in relation to newborn's colonization with maternal vaginal microbiota during spontaneous delivery), maternal weight, diet and lifestyle (in relation to ingested foods and even to maternal diseases or metabolic status), antibiotic therapy administered just before delivery (influencing maternal intestinal microbes), environmental factors, lactation stage or GA (recognized as actors influencing intestinal metabolites and HMOs and thus, indirectly, newborn's microbial community), mastitis or maternal dysbiosis (allowing the newborn to become in contact with potentially dangerous microbial communities) (2, 17, 67, 72, 82, 84, 87).
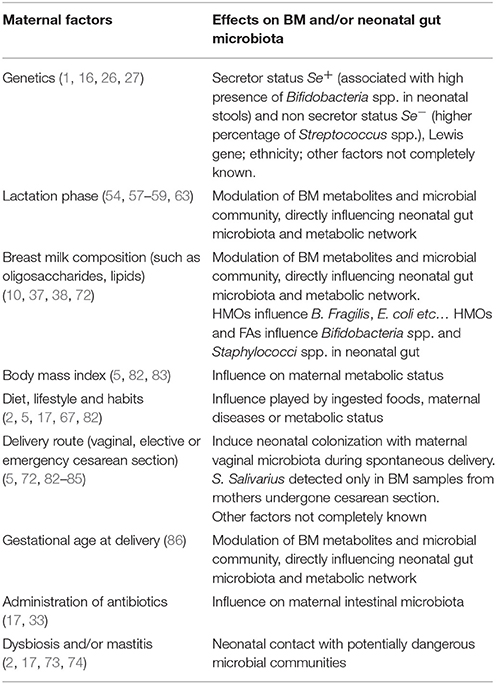
Table 1. Factors influencing the composition of breast milk and/or neonatal intestinal microbiota and some of proposed mechanisms and exerted effects.
Due to such reasons, maternal intestinal microbiota influences breastfed newborn, modulating its intestinal microbial community. In fact, several factors leading to maternal dysbiosis (such as an overgrowth of a microbial species instead of the others) and/or breast infection, which has been associated to a reduced variability in microbiota composition, can show direct effects on breastfed neonate's health (2, 73, 74).
Among the other factors, even BM lipid composition, in addition to maternal BMI, seems potentially influence BM microbiota (72, 74).
Finally, even the modality of delivery can modify qualitative bacterial composition of BM. For example, S. Salivarius, an oral commensal, was detected only in samples collected from mothers who underwent cesarean section (72, 85).
It is clear that BM microbiota plays a central role even in the early colonization of neonatal gastro-enteric tract (67, 88), considering that the breastfed newborn swallows about 1 x 105−8 bacteria/day (67–69, 71, 72, 88, 89). Since BM contains both probiotics (such as Bifidobacteria spp. and Lactobacilli spp.) and prebiotics (mostly HMOs) it can be considered a natural symbiotic mixture (72, 75).
Thus, neonatal commensal bacteria could be involved in gut tolerance modulation, immune system stimulation and may even inhibit reactions vs. some DNA fragments (78, 90, 91). This would result in a greater protection against several diseases, reducing the rate of enteric and respiratory infections (67, 71, 92).
Moreover, in BM, there are many anaerobic and lactic acid bacteria (69), which could confer further anti-microbial protection and improve nutrients' absorption (67, 68, 71, 72, 88–91, 93).
The recent study of Damaceno et al. (72), investigating BM microbiota in healthy mothers, revealed a bacterial concentration ranging from 1.5 to 4.0 log10 CFU/mL, with the highest concentration in colostrum. In their sample, S. epidermidis resulted the predominant species.
Our group (67) evaluated microbiota network in italian mothers, detecting a variable microbial composition duringprogressive lactation stages and even some differences occurring among different populations. In particular, colostrum of Italian mothers mostly contained Abiotrophia spp., Actynomicetospora spp., Aerococcus spp., Alloicoccus spp., Amaricoccus spp., Bergeyella spp., Citrobacter spp., Desulfovibrio spp., Dolosigranulum spp., Faecalibacterium spp., Parasutterella spp., Rhodanobacter spp., Rubellimicrobium spp. (67, 88, 94). Other authors also demonstrated a high prevalence of Weisella spp., Leuconostoc spp., Staphylococci spp., Streptococci spp., and Lactobacilli spp. (82).
In mature BM from italian mothers, Abiotrophia spp. and Aerococcus spp. were also present, in addition to Acetanaerobacterium spp., Aciditerrimonas spp., Acidocella spp., Aminobacter spp., Bacillus spp., Caryophanon spp., Delftia spp., Microvirga spp., Parabacteroides spp., Phascolarctobacterium spp., and Alistipes spp. (67). Other authors also reported the presence of Veillonella spp., Leptotrichia spp., Prevotella spp. (82)., Enterococcus spp., Lactococcus spp., Actinomyces spp., Corynebacterium spp., Kecuria spp., Escherichia coli spp., Klebsiella spp., and Raistonia spp. species (88).
In the same study evaluations were conducted in colostrum and mature milk from mothers living in Burundi, where so many genetic but also environmental factors can be taken into account to explain huge differences in microbial composition. Several differences in the microbiota network have been observed also in different lactation stages of the same population and it is clear that the differences between these two populations may influence the findings (67).
Analyzing the gut microbiota of breastfed neonates, and comparing it to FM fed infants, different levels of Proteobacteria spp., Bacteroides spp., Actinobacteria spp., and Firmicutes spp. were detected (95); moreover, Bifidobacteria spp. resulted one of the most represented species, especially Bifidobacterium longum subsp. longum and infantis, and B. breve (96), which also showed a high concentration in breastfed neonate's stools (2, 86).
Bifidobacteria spp., Lactobacilli spp., and Bacteroides spp. proliferation is useful to face intestinal aggressive pathogens' invasion (such as Salmonella spp., Lysteria spp., and Campilobacter spp. (97, 98). Moreover, BM has a buffering capacity, that allows acidifying the intestinal content in order to make it more fermentable by the bacteria of the proximal colon. BM shows an inhibitory effect on the growth of Clostridi spp., Bacteriodes spp. and other anaerobic bacteria.
The great influence exerted by BM on neonatal intestinal microbial composition allow to indicate this community with the expression milk-oriented microbiota (MOM) (1).
This effect mainly occurs through the action of the glycans, constituted by free HMOs, glycolipids and glycoproteins and highly contained in BM. In fact, as previously reported, they act as prebiotics, since they do not undergo absorption in proximal gut (38, 99) and represent growth substrates for specific hugs (67, 68, 71, 72, 88, 96, 100–103).
Therefore HMOs, and even FAs, influence the growth of some beneficial species in neonatal gut, such as Bifidobacteria spp. and Staphylococci spp., related to several positive effects (38, 96, 104–106). For example, a better response to vaccines, an improved function of the intestinal barrier and a protection against intestinal infections (38, 107–109).
Moreover, bacterial species like Bacteroides spp., Bifidobacteria spp. and Lactobacilli spp. are very important for HMOs' metabolism (72), promoting their degradation into sugars available for energy production. In addition, Bifidobacteria spp., Lactobacilli spp., and Bacteroides spp. can induce short chain fatty acids (SCFAs) production, playing a role in gut mucosa homeostasis and in lipid metabolism (97, 98).
A recent study of Karav et al. (96) demonstrated that a endo-β-N-acetylglucosaminidase (EndoBI-1) found in several Bifidobacteria spp. promotes the cleavage of N-linked glycans fragments. These can influence bacterial selective growth, especially allowing Bifidobacterium longum subsp. Infantis (B. infantis) proliferation and even interfering with other subspecies' metabolism. On the contrary, in the same study, B. infantis did not result able to grow exclusively in presence of the de-glycosylated protein fraction, confirming the role played by glycans in bacterial growth.
In this perspective, Bifidobacteria spp. and other species able to perform the initial de-glycosylation seem advantaged, since this represents a key passage. In fact, many studies demonstrated that the shape of gut microbiota, through the influence of microbial growth, is better promoted by HMOs and deconjugated glycans instead of the whole glycoprotein or glycolipid (96, 110–120).
This topic represents an interesting field of research, being not full clarified up to now. It would be very promising to find a link among the exact and inter-individual BM composition, the exerted influence on intestinal microbiota of the newborn and its resulting clinical phenotype. In fact it could be a suitable substrate for therapeutic beneficial applications modifying the final outcomes.
For example, BM content in HMOs and even neonatal gut microbiota showed some differences in under nutrition models, leading to an impaired infantile development. Therefore, it could be very useful to perform some dietetic strategies, adapted to these needs, which could treat or prevents several disorders, including under nutrition (38, 121).
BM exceptional features make it a very precious fluid, whose extraordinary properties and functions have not been fully clarified yet. It would be very interesting to understand all maternal factors influencing BM composition, also regarding SCs, in terms of quality and quantity (21, 122).
In the last years the importance of metabolomics has been highlighted, especially due to its role in characterizing metabolites related to microbial network. This integrated approach to the triad nutrients-microbes-metabolites can allow the identification of the effective bacterial taxa in BM and therefore transferred to the newborn (78, 123, 124), since we know that BM is the best modulator of neonatal microbiota (125). These findings would help to clarify, and even predict, BM influence on neonatal short- and long-term outcomes. Moreover, such observations may result useful to perform a sartorial approach through targeted strategies which potentially could, improve neonatal or even maternal health through the modulation of BM microbiota (2).
Finally, these evidences suggest the possible importance of bacterial supplementation of FM. The detailed knowledge of BM composition could allow to produce the best artificial products to provide to the nourished newborn a FM resembling, in the most accurate way, BM characteristics (78, 126).
DP is affiliated with the International Inflammation Network (in-FLAME) of the World Universities Network.
FB made the selection of the papers from the literature. FB wrote the paper. VF and DP conceived the paper and FS and PA revisioned the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LA and handling Editor declared their shared affiliation.
This work was funded in part by Progetto Ricerca Ateneo 2017 – 2018, University of Pisa, Project number PRA_2017_38, DR n.83/2017.
1. Fanos V. Metabolomics, milk-oriented microbiota (MOM) and multipotent stem cells: the future of research on breast milk. J Pediatr Neonat Individual Med. (2015) 4:e040115. doi: 10.7363/040115
2. Bode L, McGuire M, Rodriguez JM, Geddes DT, Hassiotou F, Hartmann PE, et al. It's alive: microbes and cells in human milk and their potential benefits to mother and infant. Am Soc Nutr Adv Nutr. (2014) 5:571–3. doi: 10.3945/an.114.006643
3. American Academy of Pediatrics. Section on breastfeeding, breastfeeding and the use of human milk. Pediatrics (2012) 129, 827–41. doi: 10.1542/peds.2011-3552
4. German BJ, Smilowitz JT, Lebrilla CB, Mills DA, Freeman SL. Metabolomics and milk: the development of the microbiota in breastfed infants. In: Kochhar S, Martin F-P editors. Metabonomics and Gut Microbiota in Nutrition and Disease (Molecular and integrative toxicology). London: Humana Press (Springer) (2015). p. 147–67.
5. Fanos V, Pintus R, Reali A, Dessì A. Miracles and mysteries of breast milk: from Egyptians to the 3 M's (Metabolomics, Microbiomics, Multipotent stem cells). J Pediatr Neonat Individual Med. (2017) 6:e060204. doi: 10.7363/060204
6. Anatolitou F. Human milk benefits and breastfeeding. J Pediat Neonat Individ Med. (2012) 1:11–8. doi: 10.7363/010113
7. Fanos V, Reali A, Marcialis MA, Bardanzellu F. What you have to know about human milk oligosaccharides. J Pediatr Neonat Individual Med. (2018) 7:e070137. doi: 10.7363/070137
8. Cesare Marincola F, Dessì A, Corbu S, Reali A, Fanos V. Clinical impact of human breast milk metabolomics. Clin Chim Acta (2015) 451:103–6. doi: 10.1016/j.cca.2015.02.021
9. Kaingade P, Somasundaram I, Nikam A, Behera P, Kulkarni S, Patel J. Breast milk cell components and its beneficial effects on neonates: need for breast milk cell banking. J Ped Neonat Individual Med. (2017) 6:060115. doi: 10.7363/060115
10. Sundekilde UK, Downey E, O'Mahony JA, O'Shea CA, Ryan CA, Kelly AL, et al. The effect of gestational and lactational age on the human milk metabolome. Nutrients (2016) 8:304. doi: 10.3390/nu8050304
11. Hurst NM. The 3 M's of breast-feeding the preterm infant. J Perinat Neonatal Nurs. (2007) 21:234–9. doi: 10.1097/01.JPN.0000285813.59269.6e
12. Kobata R, Tsukahara H, Ohshima Y, Ohta N, Tokuriki S, Tamura S, et al. High levels of growth factors in human breast milk. Early Hum Dev. (2008) 84:67–9. doi: 10.1016/j.earlhumdev.2007.07.005
13. Underwood MA. Human milk for the premature infant. Pediatr Clin North Am. (2013) 60:189–207. doi: 10.1016/j.pcl.2012.09.008
14. Bhatia J. Human milk and the premature infant. Ann Nutr Metab. (2013) 62:8–14. doi: 10.1159/000351537
15. Paul VK, Singh M, Srivastava LM, Arora NK, Deorari AK. Macronutrient and energy content of breast milk of mothers delivering prematurely. Indian J. Pediatr. (1997) 64:379–82. doi: 10.1007/BF02845209
16. Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr. (2011) 30:215–20. doi: 10.1016/j.clnu.2010.08.003
17. Bardanzellu F, Fanos V, Reali A. “Omics” in human colostrum and mature milk: looking to old data with new eyes. Nutrients (2017) 9:843. doi: 10.3390/nu9080843
18. Yang M, Cao X, Wu R, Liu B, Ye W, Yue X, et al. Comparative proteomic exploration of whey proteins in human and bovine colostrum and mature milk using iTRAQ-coupled LC-MS/MS. Int J Food Sci Nutr. (2017) 68:671–81. doi: 10.1080/09637486.2017.1279129
19. Zambruni M, Villalobos A, Somasunderam A, Westergaard S, Nigalye M, Turin CG, et al. Maternal and pregnancy-related factors affecting human milk cytokines among Peruvian mothers bearing low-birth-weight neonates. J Reprod Immunol. (2017) 120:20–6. doi: 10.1016/j.jri.2017.04.001
20. Reali A, Puddu M, Pintus MC, Marcialis MA, Pichiri G, Coni P, Manus D, et al. Multipotent stem cells of mother's milk. J Pediatr Neonatal Individ Med. (2016) 5:1 e50103. doi: 10.7363/050103
21. Briere CE, McGrath JM, Jensen T, Matson A, Finck C. Breast milk stem cells. Clin Issues Neonat Care (2016) 16:410–9. doi: 10.1097/ANC.0000000000000338
22. Cesare Marincola F, Noto A, Caboni P, Reali A, Barberini L, Lussu M, et al. A metabolomic study of preterm human and formula milk by high resolution NMR and GC/MS analysis: preliminary results. J Matern Fet Neonat Med. (2012) 25:62–7. doi: 10.3109/14767058.2012.715436
23. Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB, Slupsky CM. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. J Nutr. (2015) 145:1698–708. doi: 10.3945/jn.115.210252
24. Longini M, Tataranno ML, Proietti F, Tortoriello M, Belvisi E, Vivi A. A metabolomic study of preterm and term human and formula milk by proton MRS analysis: preliminary results. J Matern Fet Neonat Med. (2014) 27:27–33. doi: 10.3109/14767058.2014.955958
25. Wu J, Domellöf M, Zivkovic AM, Larsson G, Öhman A, Nording ML. NMR-based metabolite profiling of human milk: a pilot study of methods for investigating compositional changes during lactation. Biochem Biophys Res Commun. (2015) 469:626–32. doi: 10.1016/j.bbrc.2015.11.114
26. Andreas NJ, Hyde MJ, Gomez-Romero M, Lopez-Gonzalvez MA, Villase-or A, Wijeyesekera A, et al. Multiplatform characterization of dynamic changes in breast milk during lactation. Electrophoresis (2015) 36:2269–85. doi: 10.1002/elps.201500011
27. Villase-or A, Garcia-Perez I, Garcia A, Posma JM, Fernández-López M, Nicholas AJ, et al. Breast milk metabolome characterization in a single-phase extraction, multiplatform analytical approach. Anal Chem. (2014) 86:8245–52. doi: 10.1021/ac501853d
28. Ghisu A, Fanos V. Metabolomic Longitudinal Analysis Longitudinale of Breast Milk from Mothers Delivering Preterm Newborns of Different Gestational Ages. Bachelor's Thesis, University of Cagliari, Cagliari (2016).
29. Praticò G, Capuani G, Tomassini A, Baldassarre ME, Delfini M, Miccheli A. Exploring human breast milk composition by NMR-based metabolomics. Nat Prod Res. (2014) 28:95–101. doi: 10.1080/14786419.2013.843180
30. Smilowitz JT, O'Sullivan A, Barile D, German JB, Lonnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. (2013) 143:1709–18. doi: 10.3945/jn.113.178772
31. Dessì A, Murgia A, Agostino R, Pattumelli MG, Schirru A, Scano P, et al. Exploring the role of different neonatal nutrition regimens during the first week of life by urinary GC-MS metabolomics. Int J Mol Sci. (2016) 17:265. doi: 10.3390/ijms17020265
32. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology (2012) 22:1147–62. doi: 10.1093/glycob/cws074
33. Urbaniak C, McMillan A, Angelini M, Gloor GB, Sumarah M, Burton JP, et al. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome (2014) 2:24. doi: 10.1186/2049-2618-2-24
34. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. (2015) 91:619–22. doi: 10.1016/j.earlhumdev.2015.09.001
35. Pruneddu G, Cesare Marincola F, Briana DD, Gavrili S, Georgantzi S, Dessì A, et al. Metabolomics of human breast milk: preliminary results on the importance of secretor phenotype. In: Selected Abstracts of the 13th International Workshop on Neonatology; Cagliari (Italy); October 25-28, 2017 (2017).
36. Donovan SM, Comstock SS. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab. (2016) 69:42–51. doi: 10.1159/000452818
37. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut (2012) 61:1417–25. doi: 10.1136/gutjnl-2011-301404
38. Charbonneau MR, O'Donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell (2016) 164:859–71. doi: 10.1016/j.cell.2016.01.024
39. Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome (2015) 3:13. doi: 10.1186/s40168-015-0071-z
40. Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. (2005) 25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553
41. Bazanella M, Maier TV, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gomez MX, et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr. (2017) 106:1274–86. doi: 10.3945/ajcn.117.157529
42. De Leoz MLA, Gaerlan SC, Strum JS, Dimapasoc LM, Mirmiran M, Tancredi DJ, et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res. (2012) 11:4662–72. doi: 10.1021/pr3004979
43. Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr. (1996) 64, 572–6. doi: 10.1093/ajcn/64.4.572
44. Kinney HC, Harthigasan J, Borenshteyn NI, Flax JD, Kirschner DA. Myelination in the developing human brain: biochemical correlates. Neurochem Res. (1994) 19:983–96. doi: 10.1007/BF00968708
45. Jakobsen LH. Effect of a high protein meat diet on muscle and cognitive functions: a randomized controlled dietary intervention trial in healthy men. Clin Nutr. (2011) 30:303–11. doi: 10.1016/j.clnu.2010.12.010
46. Fritz IB, Yue KT. Long-chain carnitine acyltransferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J Lipid Res. (1963) 4:279–88.
47. Stephens FB, Wall BT, Marimuthu K, Shannon CE, Constantin-Teodosiu D, Macdonald IA, et al. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J Physiol. (2013) 591:4655–66. doi: 10.1113/jphysiol.2013.255364
48. Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathways Metabolic. Nutrients (2013) 5:3481–95. doi: 10.3390/nu5093481
49. Negro M. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fit. (2008) 48:347–51.
50. Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol. (2013) 43:7620–9. doi: 10.3748/wjg.v19.i43.7620
51. Nagata C, Nakamura K, Wada K, Tsuji M, Tamai Y, Kawachi T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. Am J Epidemiol. (2013) 178:1226–32. doi: 10.1093/aje/kwt112
52. Sinanoglou VJ, Cavouras D, Boutsikou T. Factors affecting human colostrum fatty acid profile: a case study. PLoS ONE (2017) 14:12. doi: 10.1371/journal.pone.0175817
53. Salamon S, Csapo J. Composition of the mothers' milk. protein contents, amino acid composition, biological value. a review. Acta Univ Sapientae Alimentaria (2009) 2:174–195.
54. Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T. Physiological aspects of human milk lipids. Early Hum Dev. (2001) 65:3–18. doi: 10.1016/S0378-3782(01)00204-3
55. Marin MC, Sanjurjo A, Rodrigo MA, De Alaniz MJT. Long-chain polyunsaturated fatty acids in breast milk in La Plata, Argentina: relationship with maternal nutritional status. Prostaglandins Leukotrienes Essent Fatty Acids (2005) 73:355–60. doi: 10.1016/j.plefa.2005.07.005
56. Da Cunha J, Macedo Da Costa TH, Ito MK. Influences of maternal dietary intake and suckling on breast milk lipid and fatty acid composition in low-income women from Brasilia, Brazil. Early Hum Dev. (2005) 81:303–11. doi: 10.1016/j.earlhumdev.2004.08.004
57. Innis SM. Fatty acids and early human development. Early Hum Dev J Immunol. (2007) 83:761–6. doi: 10.1016/j.earlhumdev.2007.09.004
58. Smit EN, Martini IA, Mulder H, Boersma ER, Muskiet FAJ. Estimated biological variation of the mature human milk fatty acid composition. Prostaglandins Leukotrienes Essent Fatty Acids (2002) 66:549–55. doi: 10.1054/plef.2002.0398
59. Azulay Chertok IR, Haile ZT, Eventov-Friedman S, Silani Kove N, Argov-Argaman N. Influence of gestational diabetes mellitus on fatty acid concentrations in human colostrum. Nutrition (2017) 36:17–21. doi: 10.1016/j.nut.2016.12.001
60. Zhao JP, Levy E, Fraser WD, Julien P, Delvin E, Montoudis A, et al. Circulating docosahexaenoic acid levels are associated with fetal insulin sensitivity. PLoS ONE (2014) 9:85054. doi: 10.1371/journal.pone.0085054
61. Armoni DK, Mandel D, Hausman KM, Lubetzky R. Breast milk fat content of mothers to small-for-gestational-age infants. J Perinatol. (2015) 35:444–6. doi: 10.1038/jp.2014.200
62. Lubetzky R, Argov-Argaman N, Mimouni FB. Fatty acids composition of human milk fed to small for gestational age infants. J Matern Fetal Neonatal Med. (2016) 29, 3041–4. doi: 10.3109/14767058.2015.1114082
63. Collado MC, Santaella M, Mira-Pascual L, Martinez Arias E, Khodayar-Pardo P, et al. Longitudinal study of cytokine expression, lipid profile and neuronal growth factors in human breast milk from term and preterm deliveries. Nutrients (2015) 7:577–91. doi: 10.3390/nu7105415
64. Cesare Marincola F, Corbu S, Lussu M, Noto A, Dessì A, Longo S, et al. Impact of early post natal nutrition on the NMR urinary metabolic profile of infant. J. Prot. Res. (2016) 15:3712–23. doi: 10.1021/acs.jproteome.6b00537
65. Acharjee A, Prentice P, Acerini C, Smith J, Hughes IA, Ong K, et al. The translation of lipid profiles to nutritional biomarkers in the study of infant metabolism. Metabolomics (2017) 13:25. doi: 10.1007/s11306-017-1166-2
66. Sachse D, Bærug A, Sletner L, Birkeland KI, Nakstad B, Jenum AK, et al. Urine NMR metabolomics analysis of breastfeeding biomarkers during and after pregnancy in a large prospective cohort study. Scand J Clin Lab Invest. (2014) 74:264–72. doi: 10.3109/00365513.2014.884240
67. Drago L, Toscano M, De Grandi R, Grossi E, Padovani EM, Peroni DG. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy vs. Burundi. ISME J. (2016) 11:1–10. doi: 10.1038/ismej.2016.183
68. Hunt KM, Foster JA, Forney LJ, Schutte UME, Back DI, Abdo Z, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE (2011) 6:e21313. doi: 10.1371/journal.pone.0021313
69. Boix-Amoros A, Collado MC, Mira A. Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front Microbiol. (2016) 7:492. doi: 10.3389/fmicb.2016.00492
70. Rodriguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. (2014) 5:779–84. doi: 10.3945/an.114.007229
71. Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. (2013) 69:1–10. doi: 10.1016/j.phrs.2012.09.001
72. Damaceno QS, Souza JP, Nicoli JR, Paula RL, Assis GB, Figueiredo HC, et al. Evaluation of potential probiotics isolated from human milk and colostrum. Prob Antimicro Prot. (2017) 9:371–9. doi: 10.1007/s12602-017-9270-1
73. Obermajer T, Lipoglavsek L, Tompa G, Treven P, Lorbeg PM, Matijasic BB, et al. Colostrum of healthy Slovenian mothers: microbiota composition and bacteriocin gene prevalence. PLoS ONE (2015) 10:e0123324. doi: 10.1371/journal.pone.0123324
74. Jiménez E, Andrés J, Manrique M, Pareja-Tobes P, Tobes R, Martinez-Blanch JF, et al. Metagenomic analysis of milk of healthy and mastitis-suffering women. J Hum Lact. (2015) 31:406. doi: 10.1177/0890334415585078
75. Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. The first prebiotico in humans. human milk oligosaccharides. J Clin Gastroenterol. (2004) 38:S80–3.
76. McGuire MK, McGuire MA. Human milk: mother nature's prototypical probiotic food? Adv Nutr. (2015) 6:112–23. doi: 10.3945/an.114.007435
77. Jiménez E, Delgado S, Fernandez L, Garcia N, Albujar M, Gomez A, et al. Assessment of the bacterial diversity of human colostrum and screening of staphylococcal and enterococcal populations for potential virulence factors. Res Microbiol. (2008) 159:595–601. doi: 10.1016/j.resmic.2008.09.001
78. Obermajer T, Pogacic T. Commentary: relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front Microbiol. (2016) 7:1281. doi: 10.3389/fmicb.2016.01281
79. Moya A, Ferrer M. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol. (2016) 24:402–13. doi: 10.1016/j.tim.2016.02.002
80. Leviton A, Dammann O, Engelke S, Allred E, Kuban KC, O'Shea TM, et al. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatr. (2010) 99:1795–800. doi: 10.1111/j.1651-2227.2010.01973.x
81. Piccolo BD, Mercer KE, Bhattacharyya S, Bowlin AK, Saraf MK, Pack L, et al. Early postnatal diets affect the bioregional small intestine Microbiome and Ileal Metabolome in neonatal pigs. J Nutr. (2017) 147:1499–509. doi: 10.3945/jn.117.252767
82. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. (2012) 96:544–51. doi: 10.3945/ajcn.112.037382
83. Fanos V. Diet and Microbiota. Foods, Bacteria, Probiotics and Health. Cagliari: Hygeia Press (2017).
84. Toscano M, De Grandi R, Peroni DG, Grossi E, Facchin V, Comberiati P, et al. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. (2017) 17:205. doi: 10.1186/s12866-017-1109-0
85. Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR. Evaluation of safetyand human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol. (2011) 49:2356–64. doi: 10.1016/j.fct.2011.06.038
86. Khodayar-Pardo P, Mira-Pascual I, Collado MC, Martinez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol. (2014) 34:599–605. doi: 10.1038/jp.2014.47
87. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. (2010) 107:14691–6. doi: 10.1073/pnas.1005963107
88. Martín R, Langa S, Reviriego C, Jimínez E, Marin ML, Xaus J, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. (2003) 143:754–8. doi: 10.1016/j.jpeds.2003.09.028
89. Heikkila MP, Saris PE. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol. (2003) 95:471–8. doi: 10.1046/j.1365-2672.2003.02002.x
90. Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Brück WM, Berger B, et al. Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes (2015) 6:321–5. doi: 10.1080/19490976.2015.1078051
91. Ward TL, Hosid S, Loshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC Microbiol. (2013) 13:116. doi: 10.1186/1471-2180-13-116
92. Bergmann H, Rodríguez JM, Salminen S, Szajewska H. Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report. BR J Nutr. (2014) 112:1119–28. doi: 10.1017/S0007114514001949
93. Gilliland SE. Health and nutritional benefits from lactic acid bacteria. FEMS Microb Rev. (1990) 7:175–88. doi: 10.1111/j.1574-6968.1990.tb04887.x
94. Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, et al. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics (2010) 11:488. doi: 10.1186/1471-2164-11-488
95. Donovan SM, Wang M, Li M, Friedberg I, Schwartz SL, Chapkin RS. Host-microbe interaction in the neonatal intestine: role of human milk oligosaccharides. Adv Nutr. (2012) 3:450S–5S. doi: 10.3945/an.112.001859
96. Karav S, Le Park A, Leite Nobrega de Moura Bell JM, Freese SA, Kirmiz N, Block DE, Barile D, et al. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Am Soc Microbiol. (2016) 82:3622–30. doi: 10.1128/AEM.00547-16
97. Gura T. Nature's first functional food. Science (2014) 345:747–9. doi: 10.1126/science.345.6198.747
98. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
99. Engfer MB, Stahl B, Finke B, Sawatzki G, Danial H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. (2000) 71:1589–96. doi: 10.1093/ajcn/71.6.1589
100. Solis G, Reyes-Gavilan CG, Fernandez N, Margolles A, Gueimonde M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast milk and the infant gut. Anaerobe (2010) 16:307–10. doi: 10.1016/j.anaerobe.2010.02.004
101. Patras KA, Wescombe PA, Rosler B, Hale JD, Tagg JR, Doran KS. Streptococcus salivarius K12 limits group B streptococcus vaginal colonization. Infect Immun. (2015) 83:3438–44. doi: 10.1128/IAI.00409-15
102. Thomas DW, Greer FR. Committee on nutrition: section on gastroenterology, hepatology and nutrition probiotics and prebiotics in pediatrics. Pediatrics (2010) 126:1217–31. doi: 10.1542/peds.2010-2548
103. Li Y, Shimuzu T, Hosaka A, Kanedo N, Ohtsuka Y, Yamashiro Y. Effects of Bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediat Int. (2004) 46:509–15. doi: 10.1111/j.1442-200x.2004.01953.x
104. Garrido D, Dallas DC, Mills DA. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: mechanisms and implications. Microbiology (2013) 159:649–64. doi: 10.1099/mic.0.064113-0
105. Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr. (2014) 34:143. doi: 10.1146/annurev-nutr-071813-105721
106. Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. (2000) 20:699–722. doi: 10.1146/annurev.nutr.20.1.699
107. Huda MS, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, et al. Stool microbiota and vaccine responses of infants. Pediatrics (2014) 134:362–72. doi: 10.1542/peds.2013-3937
108. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature (2011) 469:543–7. doi: 10.1038/nature09646
109. Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastroenterol. (2008) 295:1025–34. doi: 10.1152/ajpgi.90227.2008
110. Collin M, Olsén A. EndoS: a novel secreted protein from streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. (2001) 20:3046–55. doi: 10.1093/emboj/20.12.3046
111. Collin M, Fischetti VA. A novel secreted endoglycosidase from enterococcus faecalis with activity on human immunoglobulin G and ribonuclease B. J Biol Chem. (2004) 279:22558–70. doi: 10.1074/jbc.M402156200
112. Muramatsu H, Tachikui H, Ushida H, Song XJ, Qiu Y, Yamamoto S, et al. Molecular cloning and expression of endo-β-N-acetylglucosaminidase D, which acts on the core structure of complex type asparagine-linked oligosaccharides. J Biochem. (2001) 129:923–8. doi: 10.1093/oxfordjournals.jbchem.a002938
113. Garrido D, Nwosu C, Ruiz-Moyano S, Aldredge D, German JB, Lebrilla CB, et al. Endo-beta-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol Cell Proteom. (2012) 11:775–85. doi: 10.1074/mcp.M112.018119
114. Byers HL, Tarelli E, Homer KA, Beighhton D. Sequential deglycosylation and utilization of the N-linked, complex-type glycans of human a1-acid glycoprotein mediates growth of Streptococcus oralis. Glycobiology (1999) 9:469–79. doi: 10.1093/glycob/9.5.469
115. Ward RE, Ni-onuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res. (2007) 51:1398–405. doi: 10.1002/mnfr.200700150
116. Lo Cascio RG, Ni-onuevo MR, Kronewitter SR, Freeman SL, German JB, Lebrilla CB, et al. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb Biotechnol. (2009) 2:333–42. doi: 10.1111/j.1751-7915.2008.00072.x
117. Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem. (2011) 286:34583–92. doi: 10.1074/jbc.M111.248138
118. Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, Lebrilla CB, Mills DA. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl Envir Microbiol. (2013) 79:6040–9. doi: 10.1128/AEM.01843-13
119. Ruas-Madiedo P, Gueimonde M, Fernandez-Garcia M, Clara G, Margolles A. Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl Envir Microbiol. (2008) 74:1936–40. doi: 10.1128/AEM.02509-07
120. Kiyohara M, Nakatomi T, Kurihara S, Fushinobu S, Suzuki H, Tanaka T, et al. α-N-Acetylgalactosaminidase from infant associated bifidobacteria belonging to novel glycoside hydrolase family 129 is implicated in alternative mucin degradation pathway. J Biol Chem. (2012) 287:693–700. doi: 10.1074/jbc.M111.277384
121. Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbioma immaturity in malnourished Bangladeshi children. Nature (2014) 510:417–21. doi: 10.1038/nature13421
122. Twigger AJ, Hodgetts S, Filgueira L, Hartmann PE, Hassiotou F. From breast milk to brains: the potential of stem cells in human milk. J Hum Lact. (2013) 29:136–9. doi: 10.1177/0890334413475528
123. Chow J, Panasevich MR, Alexander D, Vester Boler BM, Rossoni Serao MC, Faber TA, et al. Fecal metabolomics of healthy breast-fed vs. formula-fed infants before and during in vitro batch culture fermentation. J Prot Res. (2014) 13:2534–42. doi: 10.1021/pr500011w
124. Scano P, Murgia A, Demuru M, Consonni R, Caboni P. Metabolite profiles of formula milk compared to breast milk. Food Res Int. (2016) 87:76–82. doi: 10.1016/j.foodres.2016.06.024
125. Bashiardes S, Thaiss CA, Elinav E. It's in the milk: feeding the microbiome to promote infant growth. Cell Metab. (2016) 23:393–4. doi: 10.1016/j.cmet.2016.02.015
Keywords: human milk, metabolomics, microbiota, microbiomics, human milk oligosaccharides, preterm, newborn
Citation: Bardanzellu F, Fanos V, Strigini FAL, Artini PG and Peroni DG (2018) Human Breast Milk: Exploring the Linking Ring Among Emerging Components. Front. Pediatr. 6:215. doi: 10.3389/fped.2018.00215
Received: 20 April 2018; Accepted: 13 July 2018;
Published: 07 August 2018.
Edited by:
Valerie Verhasselt, University of Western Australia, AustraliaReviewed by:
Niels Van Best, Maastricht University Medical Centre, NetherlandsCopyright © 2018 Bardanzellu, Fanos, Strigini, Artini and Peroni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flaminia Bardanzellu, YmFyZGFuemVsbHUuZmxhbWluaWFAdmlyZ2lsaW8uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.