- Thoracic Surgery Laboratory, Department of Thoracic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: The incidence rates of early-stage non-small cell lung cancer (NSCLC) are now increasing, and therapies such as thermal ablation have shown potential therapeutic promise. This study aimed to determine the influence of different surgical methods on overall survival (OS) and cancer-specific survival (CSS) in patients with stage I NSCLC.
Methods: Patients diagnosed with stage I NSCLC who had received thermal ablation or wedge resection between 2004 and 2014 were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. Propensity score matching (PSM) was performed according to the surgical method. Kaplan–Meier curves and a Cox proportional hazard model were used to evaluate OS and CSS.
Results: In all, 4,372 patients with stage I NSCLC were included. Before PSM, the respective 3- and 5-year OS rates were 68.9 and 52.7% in the wedge resection group and 68.5 and 47.8% in the thermal ablation group (p < 0.0001); the corresponding CSS rates were 79.1 and 69.4% and 62.6 and 46.0% (p < 0.0001). After PSM, survival analysis showed that wedge resection had better OS (44.5% vs. 30.1%, p = 0.033) and CSS (63.5% vs. 46%, p = 0.038) than thermal ablation. After PSM, Cox regression showed that treatment was not associated with OS or CSS. For patients aged >75 years, thermal ablation showed similar OS and CSS as wedge resection (OS: 30.6% vs. 41.7%, p = 0.470; CSS: 46.4% vs. 64.1%, p = 0.100). After PSM, thermal ablation still had OS (30.6% vs. 41.0%, p = 0.470) and CSS (46.4% vs. 59.8%, p = 0.100) comparable to wedge resection.
Conclusion: For patients with stage I NSCLC who are unfit for lobectomy, thermal ablation could be a potential therapeutic option, especially for those >75 years old.
Introduction
Surgical resection is the current standard treatment for patients with early-stage non-small cell lung cancer (NSCLC) (1–5). A randomized controlled trial in 1995 showed that lobectomy could be recommended as the standard surgical procedure for stage I (≤3 cm) NSCLC. Patients who cannot tolerate radical surgery are frequently older patients with comorbidities or impaired pulmonary function. Several studies have reported that segmentectomy and wedge resection show similar survival rates for patients who are unfit for lobectomy (6–9). Additionally, although wedge resection is a non-anatomical resection, it may be optimal for patients who cannot undergo lobectomy.
Image-guided percutaneous radiofrequency ablation using lasers and electrocautery have been developed as ablative therapeutic techniques for local tissue destruction (10). Thermal ablation is regarded as a promising treatment for small tumors in patients unfit for surgery (11, 12). However, it remains unclear whether patients unfit for lobectomy can benefit from wedge resection or thermal ablation.
In this study, we used the large Surveillance, Epidemiology, and End Results (SEER) database to compare the outcomes of patients with stage I NSCLC after wedge resection or thermal ablation, based on the 8th edition of the TNM classification.
Materials and Methods
Data Source
We obtained access to the SEER database in November 2019. Our study population included 4,372 patients diagnosed with stages IA and IB NSCLC who underwent thermal ablation or wedge resection between 2004 and 2014. Patients meeting the following criteria were considered eligible for inclusion: (i) histology was identified by ICD-O-3 codes 8140, 8070, 8046, 8250, 8560, 8071, 8012, 8480, 8072, 8481, 8490, 8570, 8255, 8550, or 8260 (13); (ii) no metastasis to the lymph node or other organs; (iii) only one primary tumor; (iv) pathologically confirmed stage IA or IB based on the 8th edition of the TNM classification; and (v) those who underwent thermal ablation or wedge resection. Thermal ablation included laser ablation, cautery, and fulguration in the SEER database. Patients with survival months <1 were excluded from the study. Patients were divided into the thermal ablation and wedge resection groups. Demographic data included age at diagnosis; sex; ethnicity (white, black, Asian, Pacific Islander, or others); and marital status. The cancer characteristics included tumor side (left or right); site (upper lobe, middle lobe, lower lobe, unknown); tumor size (≤1 cm, >1 cm but ≤2 cm, and >2 cm but ≤4 cm); histology (squamous cell carcinoma, adenocarcinoma, or others); and grade (I/II, III/IV, or unknown). Treatment characteristics included lymph node resection and adjuvant therapy (radiotherapy or chemotherapy and none). Survival characteristics included survival months, vital status, and cancer-specific death. Overall survival (OS) and cancer-specific survival (CSS) were derived from the above variables.
Statistical Analysis
Patients were divided into two groups. Categorical variables were presented as frequencies with percentage n (%). Chi-square test or Fisher’s exact test was used to compare these variables between the two groups. Propensity score matching (PSM) was performed using 1:1 nearest neighbor matching with a caliper of 0.001 to obtain a matched pair between the thermal ablation and wedge resection groups. Age at diagnosis (<75 years or ≥75 years), sex, marital status, ethnicity, tumor side and site, tumor size, and histology were used in PSM. Kaplan–Meier (KM) curves for OS and CSS were generated for patients and across strata. Differences between strata were determined by log-rank tests. Cox proportional hazard models were used to assess the relationship of interested variables with OS or CSS. Variables with p < 0.1 in the univariate models were included in the multivariate model. P < 0.05 was considered to indicate statistical significance. Chi-square test, Fisher’s exact test, and Cox analyses were performed with SPSS Statistics 25.0. KM curves were plotted in R (v3.6.2).
Results
Patients Characteristics
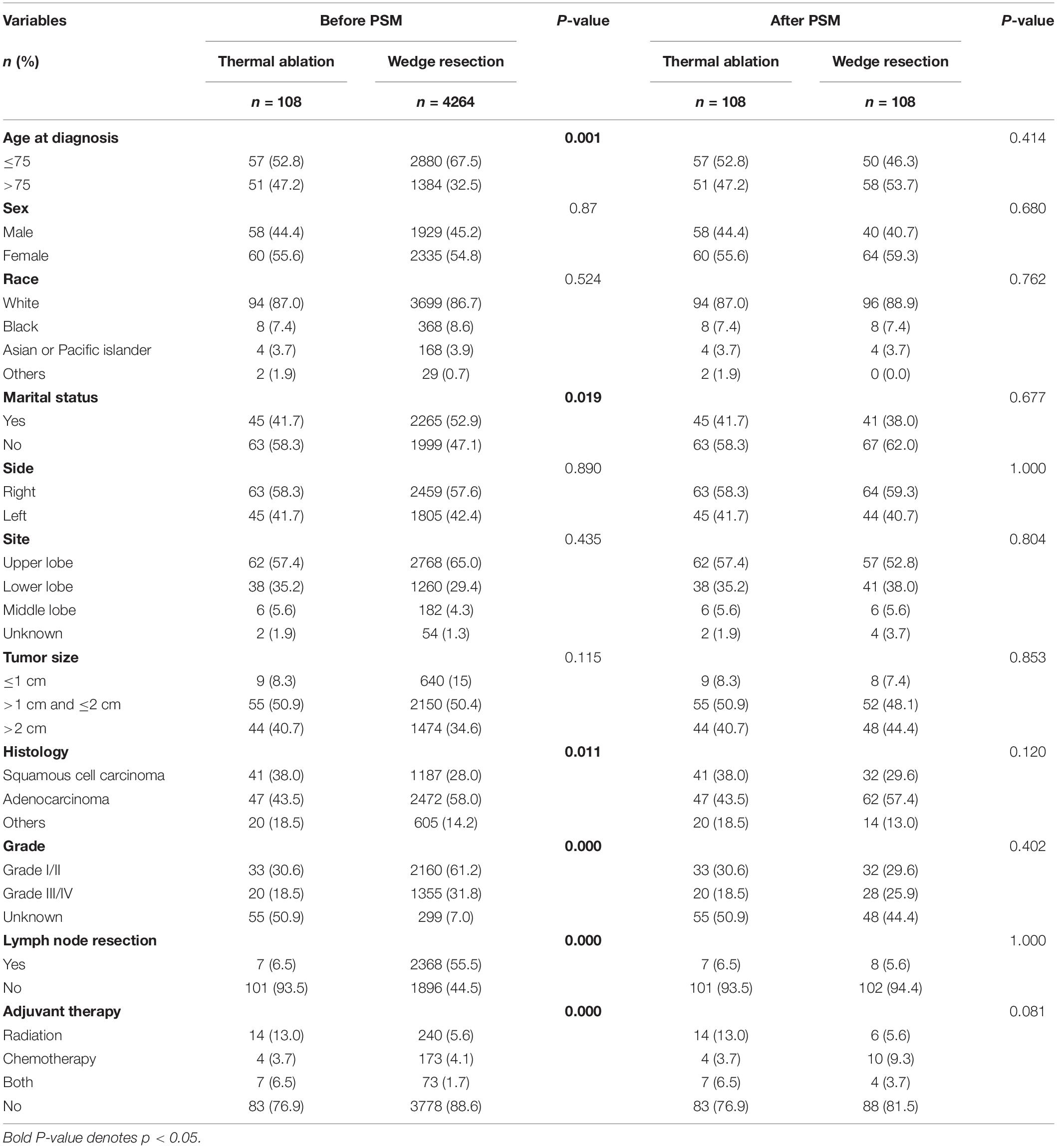
In all, 4,372 patients who received thermal ablation or wedge resection for stage I NSCLC were identified from the SEER database. Patients were divided into two groups based on whether they underwent thermal ablation (n = 108) or wedge resection (n = 4264). In the thermal ablation group, 90 patients underwent laser ablation and 18 underwent cautery or fulguration (data not shown). The clinical characteristics of patients are listed in Table 1. Patients in the thermal ablation group were diagnosed when older (>75 age) (47.2%); were less likely to be married (58.3%); had a histology of squamous cell carcinoma (38.0%); had a lower percentage of grade I/II (30.9%) tumors and lymph node resection (6.5%); and had a higher incidence of adjuvant therapy (23.1%) than those in the wedge resection group. After 1:1 matching of patients based on their respective propensity to undergo thermal ablation, a cohort of 206 patients was selected. There were no significant intergroup differences with respect to any clinical variable.
Survival Analysis in OS and CSS
The OS of patients with wedge resection was better than those with thermal ablation (log rank test, p < 0.0001) (Figure 1A). The estimated 3- and 5-year OS rates were 68.9 and 52.7% in the wedge resection group, respectively, with a median OS of 65 months (95%CI: 62–67). The corresponding rates were 68.5 and 47.8% in the thermal ablation group with a median OS of 37 months (95%CI: 28–46). Meanwhile, the CSS of patients with wedge resection was also better than those with thermal ablation (log rank test, p < 0.0001) (Figure 1B). The estimated 3- and 5-year CSS rates were 79.1 and 69.4%, respectively, in the wedge resection group with no reach for median CSS, while the corresponding rates were 62.6 and 46.0% in the thermal ablation group with a median CSS of 50 months (95%CI: 23–76). The results of the Cox analysis of OS and CSS are shown in Table 2. In the multivariate Cox analysis, treatment was an independent prognostic factor associated with OS and CSS. The thermal ablation group had worse OS (HR: 1.266, 95%CI: 1.003–1.597) and CSS (HR: 1.403, 95%CI: 1.039–1.859) than the wedge resection group. We found that patients who received radiation had worse OS (HR: 1.309, 95%CI: 1.119–1.531) and CSS (HR: 1.309, 95%CI: 1.119–1.531) than those who did not receive adjuvant therapy. Meanwhile, patients who received both radiation and chemotherapy also had worse OS (HR: 1.323, 95%CI: 1.023, 1.711) and CSS (HR 1.730, 95%CI: 1.271, 2.354) than those who did not receive any adjuvant therapies. Other co-variables such as age at diagnosis, sex, tumor size, histology, grade, and lymph node resection were associated with OS and CSS.
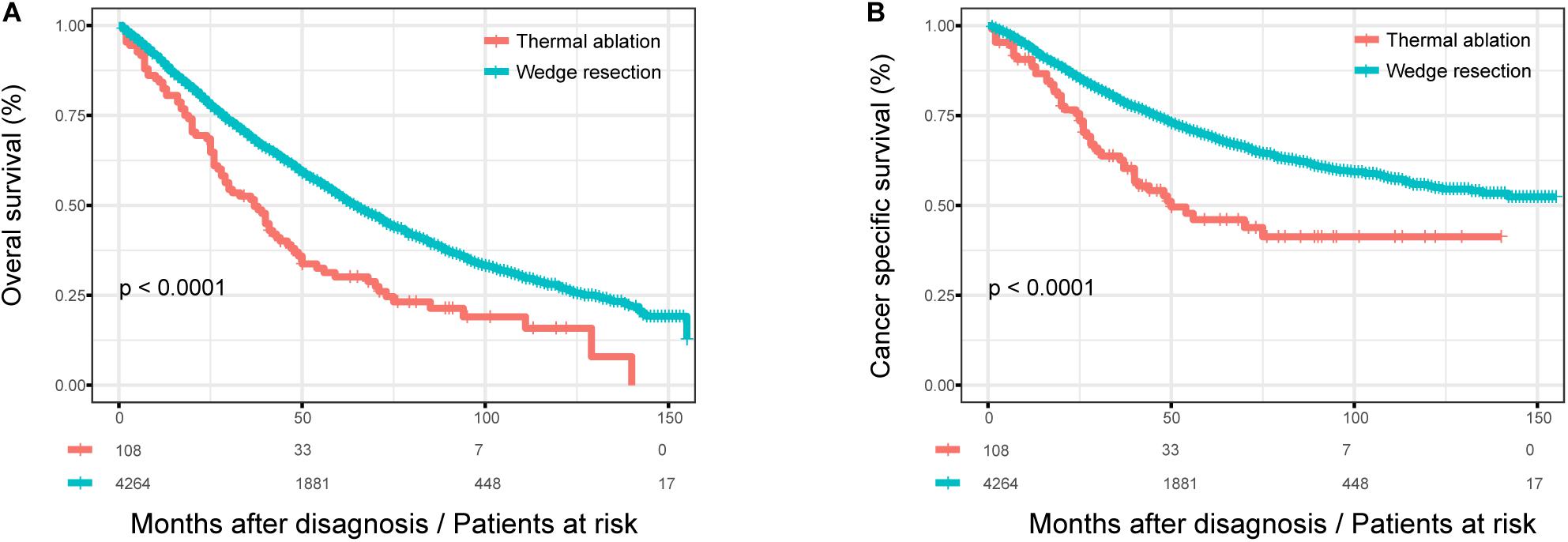
Figure 1. The OS and CSS curves in stage I NSCLC patients who received thermal ablation or wedge resection. (A) The OS curve of stage I NSCLC patients who received thermal ablation or wedge resection (χ2 = 26.247, p < 0.0001). (B) The CSS curve of stage I NSCLC patients who received thermal ablation or wedge resection (χ2 = 22.453, p < 0.0001).
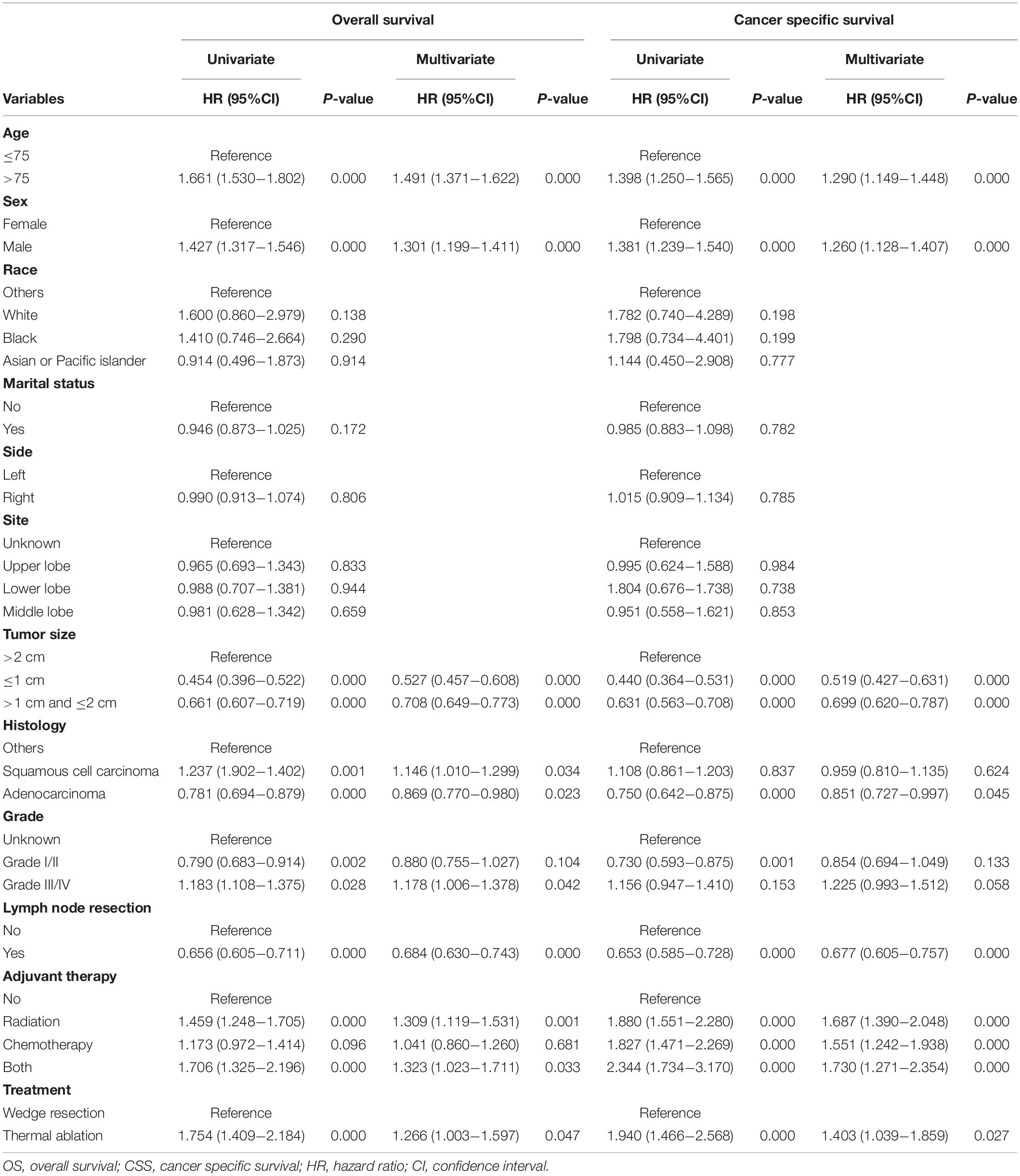
Table 2. Univariate and multivariate Cox regression of OS and CSS in stage I NSCLC patients having thermal ablation and wedge resection.
Survival Analysis in OS and CSS After PSM
After 1:1 matching, the KM curves for patients with different treatment were obtained (Figure 2). The OS of patients with wedge resection were still better than those with thermal ablation after PSM (log rank test, p = 0.033) (Figure 2A). The estimated 3- and 5-year OS rates were 64.3 and 44.5%, respectively, in the wedge resection group, with a median OS of 53 months (95%CI: 39–50); the corresponding rates were 51.6 and 30.1% in the thermal ablation group, with a median OS of 37 months (95%CI: 28–46). The CSS of patients in the wedge resection group was also better than those in the thermal ablation group (log rank test, p = 0.038) (Figure 2B). The estimated 3- and 5-year CSS rates were 75.2 and 63.5%, respectively, in the wedge resection group, with a median CSS of 132 months (95%CI: 59–204), the corresponding rates were 62.6 and 46.0% in the thermal ablation group, with a median OS of 50 months (95%CI: 23–76). The results of the Cox analysis of OS and CSS after PSM are shown in Table 3. Thermal ablation was no longer a risk factor for OS or CSS when compared with wedge resection. Patients who received radiation had worse OS (HR: 1.849, 95%CI: 1.132–3.019) and CSS (HR: 1.879, 95%CI: 1.030–3.425). Co-variables such as race and tumor size were associated with OS. Variable tumor size was associated with CSS.
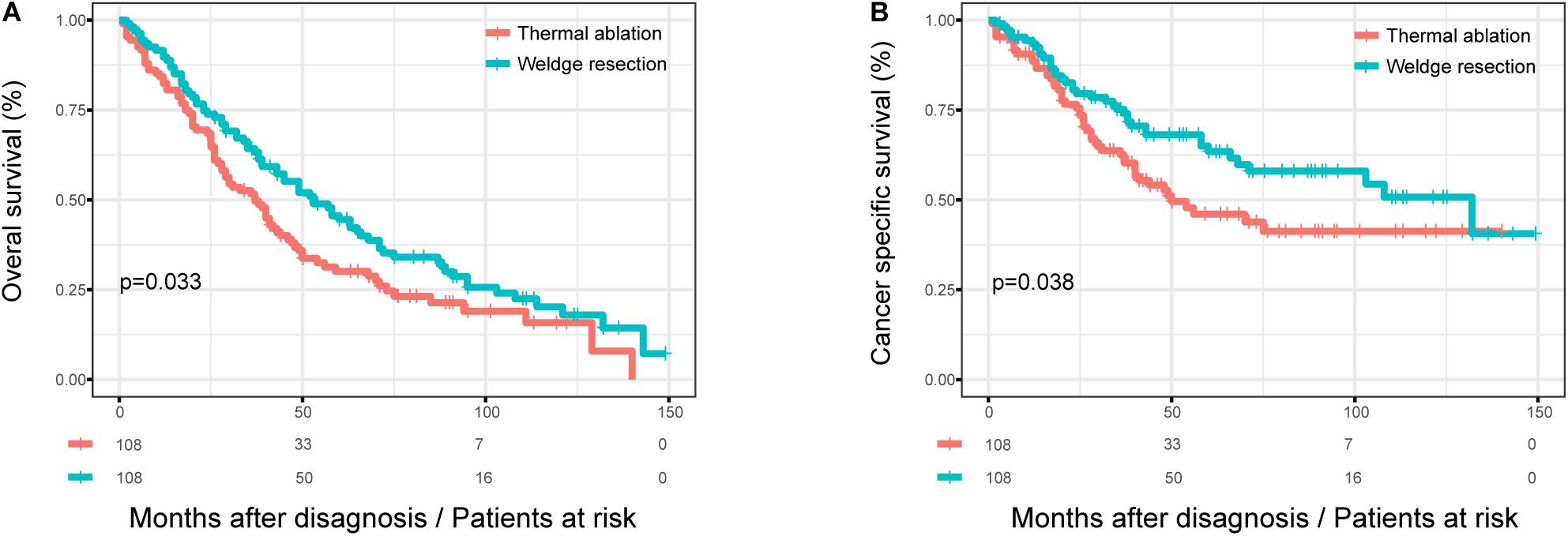
Figure 2. The OS and CSS curves in stage I NSCLC patients who received thermal ablation or wedge resection after PSM. (A) The OS curve of stage I NSCLC patients who received thermal ablation or wedge resection after PSM (χ2 = 4.567, p = 0.033). (B) The CSS curve of stage I NSCLC patients who received thermal ablation or wedge resection after PSM (χ2 = 4.297, p = 0.038).
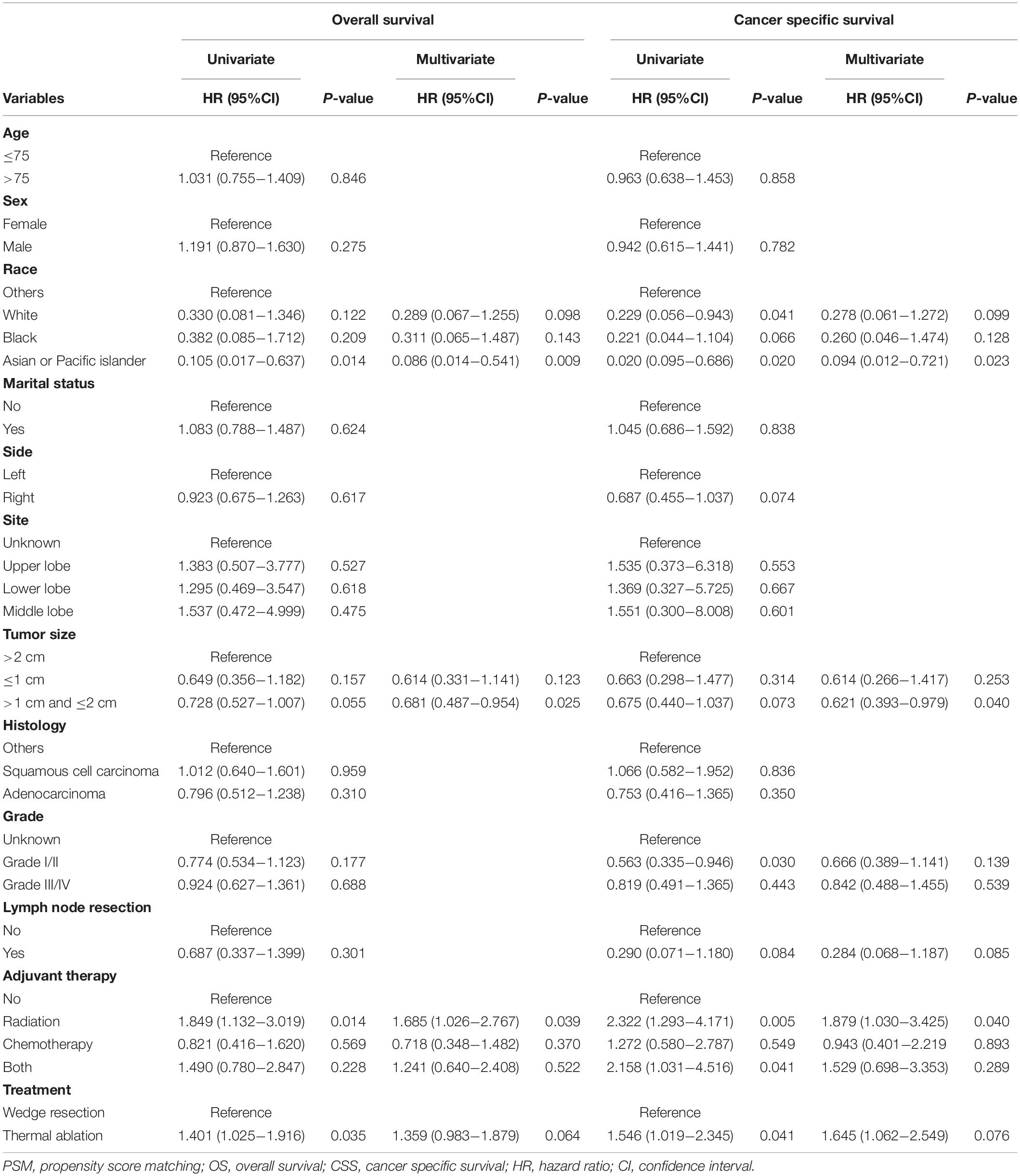
Table 3. Univariate and multivariate Cox regression of OS and CSS in stage I NSCLC patients having thermal ablation and wedge resection after PSM.
Subgroup Analysis
We studied the influence of the surgical method on OS and CSS in patients according to their age at diagnosis before and after PSM. Figure 3 shows the Kaplan–Meier survival curves for patients with different age at diagnosis before PSM. There was no significant difference in OS (log rank test, p = 0.470) and CSS (log rank test, p = 0.100) between the thermal ablation and wedge resection groups when age at diagnosis was >75 years (Figures 3A,B). The 5-year OS and CSS rates were 30.6 and 46.4%, respectively, for those with thermal ablation; the corresponding values were 41.7 and 64.1% for those with wedge resection. However, patients with wedge resection had better OS (log rank test, p < 0.0001) and CSS (log rank test, p < 0.0001) when age at diagnosis was <75 years (Figures 3C,D). The 5-year OS and CSS rates were 30.2 and 46.1%, respectively, for those with thermal ablation; the corresponding rates were 58.3 and 71.9% for those with wedge resection.
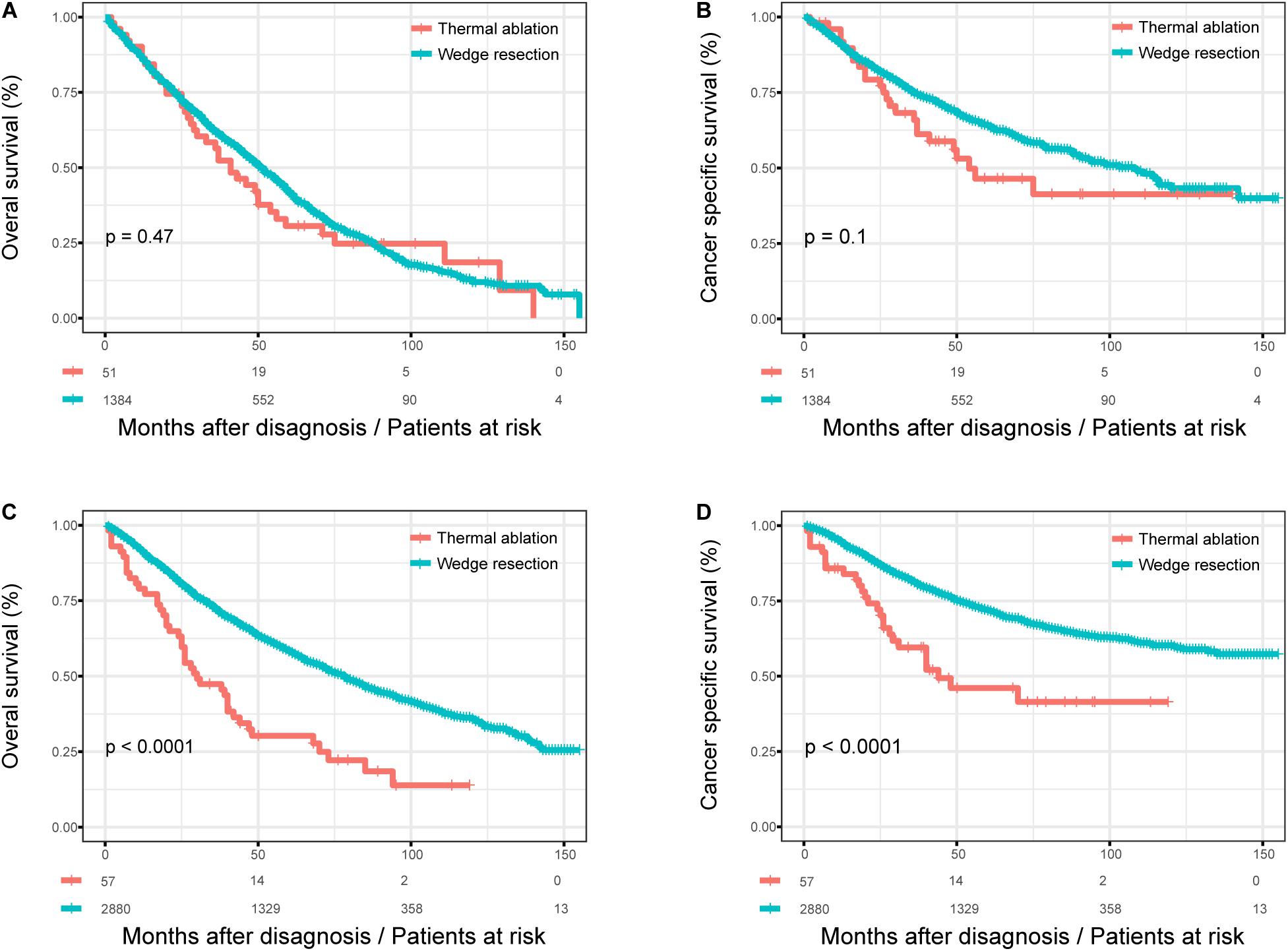
Figure 3. The subgroup analysis of OS and CSS in stage I NSCLC patients who received thermal ablation or wedge resection. (A) The OS curve of stage I NSCLC patients aged >75 years who received thermal ablation or wedge resection (χ2 = 0.515, p = 0.47). (B) The CSS curve of stage I NSCLC patients who received thermal ablation or wedge resection aged over 75 years (χ2 = 2.708, p = 0.1). (C) The OS curve of stage I NSCLC patients <75 years who received thermal ablation or wedge resection (χ2 = 32.190, p < 0.0001). (D) The CSS curve of stage I NSCLC patients <75 years who received thermal ablation or wedge resection (χ2 = 20.966, p < 0.0001).
Subgroup Analysis After PSM
Figure 4 shows the KM survival curves for patients with different age at diagnosis after PSM. There was still no significant difference in OS (log rank test, p = 0.470) and CSS (log rank test, p = 0.100) between the thermal ablation and wedge resection groups after PSM when age at diagnosis was >75 years (Figures 4A,B). The 5-year OS and CSS rates were 30.6 and 46.4%, respectively, for those with thermal ablation; the corresponding values were 41.0 and 59.8% for those with wedge resection. Patients in the wedge resection group had better OS than those in the thermal ablation group (log rank test, p < 0.0001), but no significant difference was noted between the two groups with respect to CSS (log rank test, p = 0.057), when age at diagnosis was <75 years (Figures 4C,D). The 5-year OS and CSS rates were 30.2 and 46.1%, respectively, for those with thermal ablation; the corresponding rates were 48.9 and 68.2% for those with wedge resection.
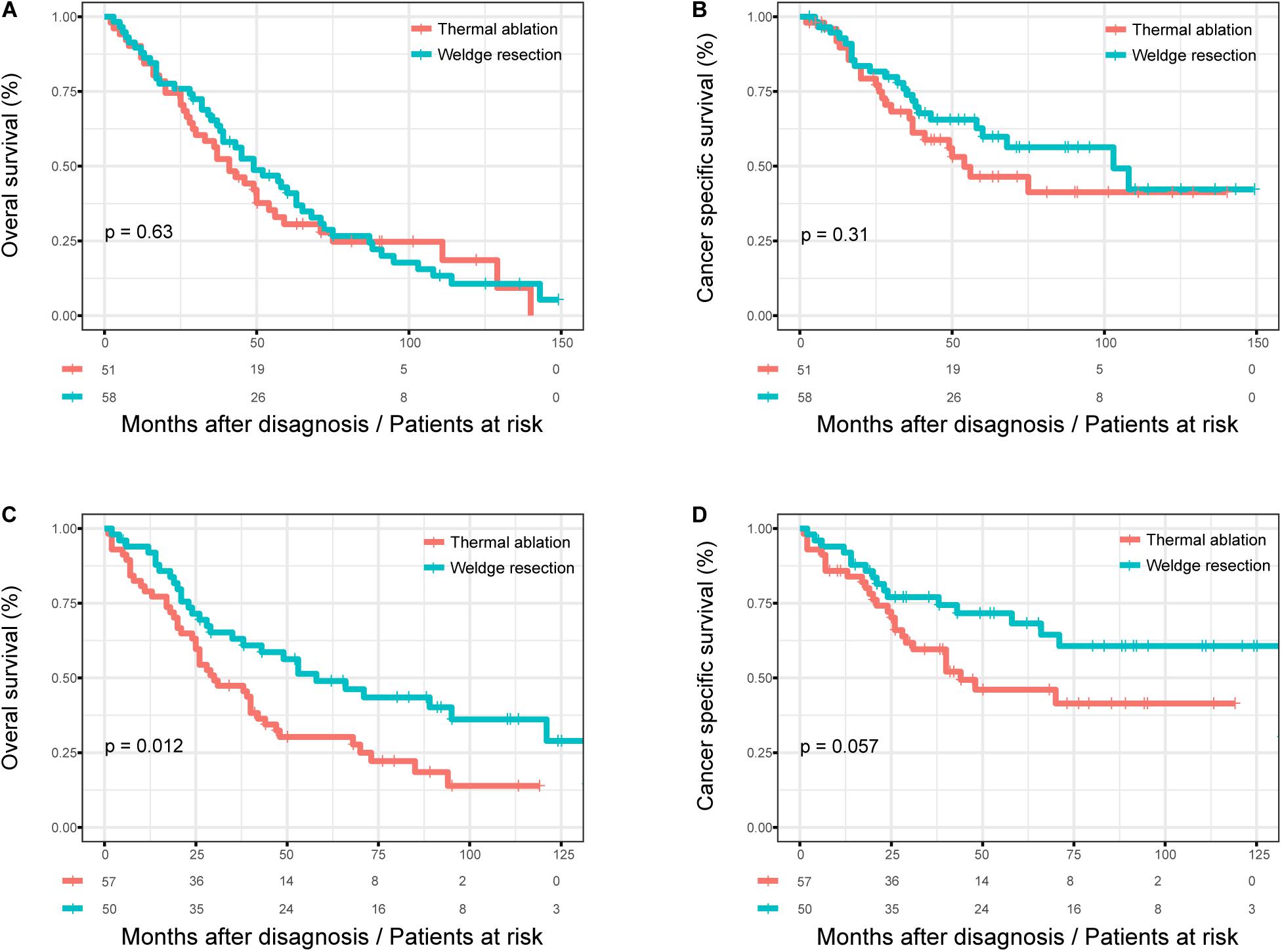
Figure 4. The subgroup analysis of OS and CSS in stage I NSCLC patients who received thermal ablation or wedge resection. (A) The OS curve of stage I NSCLC patients >75 years who received thermal ablation or wedge resection after PSM (χ2 = 0.233, p = 0.63). (B) The CSS curve of stage I NSCLC patients >75 years who received thermal ablation or wedge resection after PSM (χ2 = 1.04, p = 0.31). (C) The OS curve of stage I NSCLC patients <75 years who received thermal ablation or wedge resection after PSM (χ2 = 6.384, p = 0.012). (D) The CSS curve of stage I NSCLC patients <75 years who received thermal ablation or wedge resection after PSM (χ2 = 3.633, p = 0.057).
Discussion
In recent years, the detection rate of pulmonary nodules has increased owing to high-resolution computed tomography (CT) enabling the detection of early-stage lung cancer (14). Although surgery is the standard of treatment for early stage patients, there were other therapies to choose from. Lobectomy is a standard surgical procedure for stage I NSCLC (1). For those who cannot tolerate lobectomy, segmentectomy and wedge resection can be considered. Although wedge resection is a non-anatomical resection, several studies have shown wedge resection to be beneficial in stage I NSCLC when the tumor size is small (15, 16). However, patients who are older or have multiple morbidities may have reduced cardiopulmonary function after surgical resection. Local-treatment options such as stereotactic body radiation therapy (SBRT) and thermal ablation have been regarded as viable alternatives for patients that are unfit for surgery (17–19). Multiple trials have shown that SBRT in treating early-stage NSCLC in elderly people is safe and effective. For elderly people with a greater number of comorbidities, SBRT has proven to be a curative modality, with comparable local tumor control and 3-year OS rates to lobectomy (20–23). On the other hand, Chaitan et al. (24) suggested that thermal ablation could be used to effectively treat or control stage IA NSCLC in inoperable patients (3-year OS, 50%). However, few studies have investigated whether thermal ablation is adequate for stage I NSCLC compared with wedge resection when lobectomy or segmentectomy is unfit for patients or for those unwilling to undergo invasive surgery. In this study, we compared the outcome of patients undergoing wedge resection or thermal ablation. We also explored whether thermal ablation is an option for those who cannot undergo lobectomy but can tolerate wedge resection.
In this study, before PSM, we found that the thermal ablation group had worse OS and CSS than the wedge resection group. Apart from the surgery method, seven factors including age, sex, tumor size, histology, grade, lymph node resection, and adjuvant therapy were found to be associated with OS and CSS outcomes in the multivariate Cox regression analysis. We performed subgroup survival analysis, and found that thermal ablation had comparable OS and CSS to wedge resection, when patients were >75 years old. After PSM, the thermal ablation group was no longer a risk factor of OS or CSS. Patients who received radiation had worse OS and CSS than those who did not. The other two factors including ethnicity and tumor size were found to be associated with OS and CSS outcomes in the multivariate Cox regression. However, upon subgroup analysis after PSM, thermal ablation was still equivalent to wedge resection with respect to OS and CSS in patients >75 years old. The number of comorbidities increases with age (25), and elderly NSCLC patients would likely benefit from thermal ablation rather than surgery. Several studies have also revealed that thermal ablation showed safety and efficacy in both primary and secondary lung malignancies, and after propensity score matching, the 2-year OS survival rates could be comparable with surgery and SBRT (26, 27). Moreover, our large database study suggested that compared with wedge resection, thermal ablation may be the optimal procedure for patients >75 years with stage I NSCLC, based on the 8th edition of TNM classification. However, for patients <75 years who are unfit for lobectomy, we still recommend wedge resection, provided they can tolerate it.
To our best knowledge, this is the first database study assessing the equivalency of thermal ablation vs. wedge resection in stage I NSCLC based on the 8th edition of TNM classification. This study used a multicenter population-based database and included 4,372 patients with precise long-term survival data. We used several statistical methods including PSM, Cox regression analysis, and KM curves to confirm the results. This study also revealed the current treatment for stage I NSCLC patients who are unfit for radical surgery in the real word.
Our study has some limitations. Firstly, thermal ablation is offered to patients with stage I NSCLC who are unfit for surgical resection because of cardiorespiratory comorbidity or insufficient vital lung function (28). However, information on multiple comorbidities and cardio-pulmonary function is not available in the SEER database. The reason for these patients choosing thermal ablation is not known, which may cause a degree of bias. Secondly, information regarding tumor grade was unknown in some of the patients, which may further bias our results. Thirdly, this study is a retrospective study with inevitable intrinsic shortcomings. Despite these limitations, we believe that our findings may assist future research designs and proposals.
Conclusion
In conclusion, this study demonstrated that for stage I NSCLC, thermal ablation could be a potential option for patients unfit for lobectomy, especially those aged >75 years. We still recommended wedge resection for patients who are <75 years. More studies are needed to investigate the equivalency of thermal ablation vs. wedge resection in stage I NSCLC.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
Author Contributions
XF and YT designed and directed the project. CZ and JL planned and carried out the majority of the project and took the lead in writing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. (1995) 60:615–22; discussion 622–13.
2. Sugarbaker DJ. Lung cancer. 6: the case for limited surgical resection in non-small cell lung cancer. Thorax. (2003) 58:639–41. doi: 10.1136/thorax.58.7.639
3. Miller DL, Rowland CM, Deschamps C, Allen MS, Trastek VF, Pairolero PC. Surgical treatment of non-small cell lung cancer 1 cm or less in diameter. Ann Thorac Surg. (2002) 73:1545–50; discussion 1550–41.
4. Izbicki JR, Passlick B, Pantel K, Pichlmeier U, Hosch SB, Karg O, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg. (1998) 227:138–44. doi: 10.1097/00000658-199801000-00020
5. Landreneau RJ, Sugarbaker DJ, Mack MJ, Hazelrigg SR, Luketich JD, Fetterman L, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. (1997) 113:691–8; discussion 698-700.
6. Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. (2001) 71:956–60; discussion 961. doi: 10.1016/S0003-4975(00)02223-2
7. Allen MS, Darling GE, Pechet TT, Mitchell JD, Herndon JE II, Landreneau RJ, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. (2006) 81:1013–9; discussion 1019–20.
8. Bando T, Yamagihara K, Ohtake Y, Miyahara R, Tanaka F, Hasegawa S, et al. A new method of segmental resection for primary lung cancer: intermediate results. Eur J Cardiothorac Surg. (2002) 21:894–9; discussion 900.
9. Tsutani Y, Kagimoto A, Handa Y, Mimae T, Miyata Y, Okada M. Wedge resection versus segmentectomy in patients with stage I non-small-cell lung cancer unfit for lobectomy. Jpn J Clin Oncol. (2019) 49:1134–42.
10. Uhlig A, Uhlig J, Trojan L, Kim HS. Stereotactic body radiotherapy for stage I renal cell carcinoma: national treatment trends and outcomes compared to partial nephrectomy and thermal ablation. J Vasc Interv Radiol. (2020) 31:564–71. doi: 10.1016/j.jvir.2019.11.009
11. Nishida T, Inoue K, Kawata Y, Kinoshita H, Matsuoka T, Toyoshima M, et al. Percutaneous radiofrequency ablation of lung neoplasms: a minimally invasive strategy for inoperable patients. J Am Coll Surg. (2002) 195:426–30. doi: 10.1016/s1072-7515(02)01281-4
12. Herrera LJ, Fernando HC, Perry Y, Gooding WE, Buenaventura PO, Christie NA, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. (2003) 125:929–37. doi: 10.1067/mtc.2003.18
13. Hansen RN, Zhang Y, Seal B, Ryan K, Yong C, Darilay A, et al. Long-term survival trends in patients with unresectable stage III non-small cell lung cancer receiving chemotherapy and radiation therapy: a SEER cancer registry analysis. BMC Cancer. (2020) 20:276.
14. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. (2011) 365:395–409. doi: 10.1056/nejmoa1102873
15. Yendamuri S, Sharma R, Demmy M, Groman A, Hennon M, Dexter E, et al. Temporal trends in outcomes following sublobar and lobar resections for small (</= 2 cm) non-small cell lung cancers–a Surveillance Epidemiology End Results database analysis. J Surg Res. (2013) 183:27–32. doi: 10.1016/j.jss.2012.11.052
16. Altorki NK, Kamel MK, Narula N, Ghaly G, Nasar A, Rahouma M, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small ct1n0 non-small cell lung cancer. J Thorac Oncol. (2016) 11:1984–92. doi: 10.1016/j.jtho.2016.06.031
17. Ashwin S, Li R, Kim J, Salgia R, Hurria A, Amin A. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer in the elderly. Semin Oncol. (2018) 45:210–9. doi: 10.1053/j.seminoncol.2018.06.002
18. Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. (2008) 247:871–9. doi: 10.1148/radiol.2473070996
19. Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol. (2013) 57:466–74. doi: 10.1111/1754-9485.12068
20. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. (2015) 16:630–7. doi: 10.1016/s1470-2045(15)70168-3
21. Takeda A, Sanuki N, Eriguchi T, Kaneko T, Morita S, Handa H, et al. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. (2013) 86:257–63. doi: 10.1016/j.ijrobp.2013.01.006
22. Van Der Voort Van Zyp NC, van der Holt B, van Klaveren RJ, Pattynama P, Maat A, Nuyttens JJ, et al. Stereotactic body radiotherapy using real-time tumor tracking in octogenarians with non-small cell lung cancer. Lung Cancer. (2010) 69:296–301. doi: 10.1016/j.lungcan.2009.12.008
23. Sandhu AP, Lau SKM, Rahn D, Fuster MM, Bazhenova L, Mundt AJ, et al. Stereotactic body radiation therapy in octogenarians with stage i lung cancer. Clin Lung Cancer. (2014) 15:131–5.
24. Narsule CK, Sridhar P, Nair D, Gupta A, Oommen RJ, Ebright MI, et al. Percutaneous thermal ablation for stage IA non-small cell lung cancer: long-term follow-up. J Thorac Dis. (2017) 9:4039–45. doi: 10.21037/jtd.2017.08.142
25. Girones R, Torregrosa D, Gomez-Codina J, Maestu I, Tenias JM, Rosell R. Prognostic impact of comorbidity in elderly lung cancer patients: use and comparison of two scores. Lung Cancer. (2011) 72:108–13. doi: 10.1016/j.lungcan.2010.07.001
26. Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. (2015) 121:3491–8. doi: 10.1002/cncr.29507
27. Petre EN, Solomon SB, Sofocleous CT. The role of percutaneous image-guided ablation for lung tumors. Radiol Med. (2014) 119:541–8. doi: 10.1007/s11547-014-0427-7
Keywords: thermal ablation, wedge resection, NSCLC, stage I, survival, SEER
Citation: Zeng C, Lu J, Tian Y and Fu X (2020) Thermal Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Based on the Eighth Edition of the TNM Classification: A Population Study of the US SEER Database. Front. Oncol. 10:571684. doi: 10.3389/fonc.2020.571684
Received: 11 June 2020; Accepted: 18 August 2020;
Published: 14 October 2020.
Edited by:
Meng Xu Welliver, The Ohio State University, United StatesReviewed by:
Meghan Mooradian, Massachusetts General Hospital and Harvard Medical School, United StatesCalvin Sze Hang Ng, The Chinese University of Hong Kong, China
Copyright © 2020 Zeng, Lu, Tian and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yitao Tian, eWl0YW9fdGlhbkAxNjMuY29t; Xiangning Fu, ZnV4aWFuZ25pbmcwNkAxNjMuY29t
†These authors have contributed equally to this work
 Chenxi Zeng†
Chenxi Zeng† Jiawei Lu
Jiawei Lu Yitao Tian
Yitao Tian Xiangning Fu
Xiangning Fu