
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 September 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.571671
Aim: The purpose of this study was to analyze the incidence, clinical characteristics, prognostic factors and survival of ovarian cancer patients with liver metastases upon initial diagnosis.
Methods: Patients with ovarian cancer liver metastases upon initial diagnosis between 2010 and 2016 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. Univariate and multivariate logistic regression was performed to identify the predictors of the presence of liver metastases in newly diagnosed ovarian cancer patients. Overall survival (OS) was assessed using the Kaplan-Meier method and log-rank test. Univariate and multivariate Cox regression was conducted to determine the independent prognostic factors for OS.
Results: A total of 1,744 ovarian cancer patients with liver metastases was identified from the SEER database, accounting for 6.7% of the entire ovarian cancer patients. As to the unique distant organ provided by SEER, liver was the most common metastatic site of ovarian cancer (4.65%). Age, race, laterality, histology, pathological grade, extrahepatic sites, stage of tumor were the predictors of the presence with liver metastases revealed by multivariable logistic regression model. Median OS for the patients with liver metastases at initial diagnosis of ovarian cancer was 16.0 months. Multivariate Cox regression model confirmed race, histology, extrahepatic metastatic sites, surgery and marital status were independent prognostic factors for OS.
Conclusion: The study provided population-based estimates of the incidence and prognosis of newly diagnosed ovary cancer patients with liver metastases, which could be potentially used for the risk assessment and individualized treatment.
According to the latest cancer statistics in 2019, there were about 22,530 patients newly diagnosed with ovarian cancer and 13,980 patients died of ovarian cancer. Ovarian cancer accounts for 2.5% of all cancers in women and ranks the fifth cause of cancer death among women in the United States (1). Owing to the scarcity of early, specific symptoms and effective screening strategies, patients with ovarian cancer were often diagnosed with synchronous distant metastases at an advanced stage, accounting for about 70% of the whole ovarian cancer population (2). The survival of ovarian cancer patients was stage-dependent. The 5 year survival was 92, 75, 29%, respectively, for the localized cases, regional cases and distant cases (1). Studies of the patterns of distant metastases in stage IV ovarian cancer showed liver was the most common distant metastatic organ of ovarian cancer with the proportion of 37–57%, followed by distant lymph nodes, lung, bone and brain (3–5). Autopsy studies of cases died of primary ovarian cancer showed that the incidence of liver parenchyma was about 48.2–73% (5, 6). The median overall survival (OS) was 30 months for patients with a single liver metastasis (4).
At present, data of prevalence and prognostic factors among ovarian cancer patients with liver metastases is limited. The therapy for ovarian cancer patients with liver metastases is controversial. Here, we explored the Surveillance, Epidemiology, and End Results (SEER) database to analyze the characteristics, incidence, risk factors and prognostic factors of ovarian cancer patients with liver metastases upon initial diagnosis.
All the primary data were acquired from the SEER database, which collected the information of patient demographics, tumor characteristics, treatment and prognosis accounting for ~30% of the whole population in the United States. The datasets of this current study are available from SEER*Stat Version 8.3.6 (http: https://seer.cancer.gov/seerstat/). Patients diagnosed with primary and microscopically confirmed ovarian cancer as the only primary malignancy between 1 January 2010 and 31 December 2016 were included in the study. We excluded those patients younger than 18 years old, diagnosed with carcinoma in situ, benign or borderline tumors, with unknown information of liver metastases, diagnosed by autopsy or death certificate. Finally, there were 26,197 patients eligible for incidence analysis. Among these patients, 1,744 patients had liver metastases upon initially diagnosis of ovarian cancer. Next, we removed patients with unknown follow-up, as well as patients who presented with 0 day of survival record, leaving 25,819 patients and 1,718 patients with liver metastases for the survival analysis.
The incidence of different distant metastatic organs including liver, bone, brain, and lung among the total patients with ovarian cancer was calculated in this study. Incidence was defined the number of ovarian cancer patients with corresponding metastases divided by the whole number of patients with ovarian cancer.
We compared the patient characteristics between patients with liver metastases and those without liver metastases by the Pearson chi-square test or Fisher's exact test appropriately. Study variables in the descriptive statistics were included as follows: age at diagnosis, year of diagnosis, race, laterality, histology, primary tumor stage, regional lymph node stage, pathological grade, surgery, numbers of extrahepatic metastatic sites (bone, brain, and lung), marital status and insurance status. In the SEER database, tumor grades were classified into I (well-differentiated), II (moderately differentiated), III (poorly differentiated), and IV (undifferentiated or anaplastic).
Univariable and multivariable logistic regression was conducted to determine the risk factors for the liver metastases at initial diagnosis of ovarian cancer. Variables including age at diagnosis, race, laterality, histology, pathological grade, the extent of extrahepatic metastatic sites, primary tumor stage, lymph node stage, marital status and insurance status were analyzed in this model. Odds ratios (OR) and 95% confidence intervals (CI) were reported from the logistic regression.
Overall survival (OS) was determined as the date of diagnosis of ovarian cancer to the date of death due to any cause or the date of last follow-up. We utilized the univariable and multivariable Cox proportional hazard models to assess the association of study variables with OS by reporting the hazard ratios (HR) and 95% Cis. In our study, the marital status was enrolled in the analysis of prognostic factors for the following reasons. First, epidemiological studies have reported a reduced risk of ovarian cancer among women who have had children. And infertility has been associated with an increasing risk of ovarian cancer (7, 8). Second, married patients with cancer may have more support from family members, social services and insurance than unmarried patients. Unmarried patients are at significant higher risk of undertreatment and death from cancer (9). Kaplan-Meier method was performed to obtain the survival estimates and the log-rank test was conducted to analyze the difference between subgroups.
All the statistical analyses were performed by the SPSS statistical software version 22 (IBM Corporation, USA). All P-values calculated were two-sided, and a p < 0.05 was considered statistically significant.
Total of 26,197 patients were diagnosed with ovarian cancer from 2010, January to 2016, December reported by the SEER database. Among the total 26,197 patients, there were 13,366 patients with serous ovarian cancer (51.0%), 2,321 patients with endometrioid carcinoma (8.9%), 1,765 patients with clear cell ovarian cancer (6.7%), 1,587 patients with mucinous carcinoma (6.1%) and 7,158 patients with other types such as granular cell cancer and Brenner tumor (27.3%). In view of the small size, we categorized the non-serous ovarian cancer into one group. Figure 1 illustrated the incidence of patients with distant metastases at initial diagnosis of ovarian cancer according to the metastatic sites including liver, bone, lung and brain among the entire cohort. 4.65, 0.39, 4.00, 0.10% ovarian cancer patients had liver metastases, bone metastases, lung metastases, brain metastases only, respectively, at initial diagnosis. As to patients with two distant metastatic sites, patients with liver and lung metastases had the highest proportion, accounting for 1.20% among the entire population.
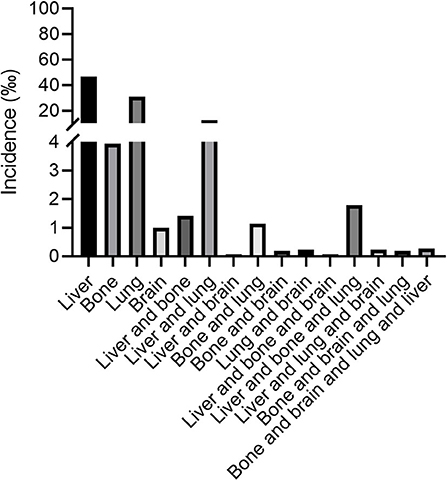
Figure 1. The incidence of ovarian cancer patients with liver, lung, bone, and brain metastases at the time of initial diagnosis.
One thousand seven hundred and forty four patients had liver metastases at initial diagnosis of ovarian cancer, accounting for 6.7% among the total population. Table 1 showed the demographic and clinical characteristics for ovarian cancer patients with and without liver metastases. Compared to patients without liver metastases at initial diagnosis, patients with liver metastases were older (P < 0.001), were more likely to be Black (P < 0.001), had bilateral tumors (P < 0.001), presented with more advanced T stage (P < 0.001), presented with more advanced N stage (P < 0.001), had a higher pathological grade (P < 0.001), had no surgery of primary tumor (P < 0.001), had more extrahepatic metastatic sites to lung, bone and brain (P < 0.001), were more likely to be unmarried (P < 0.001).
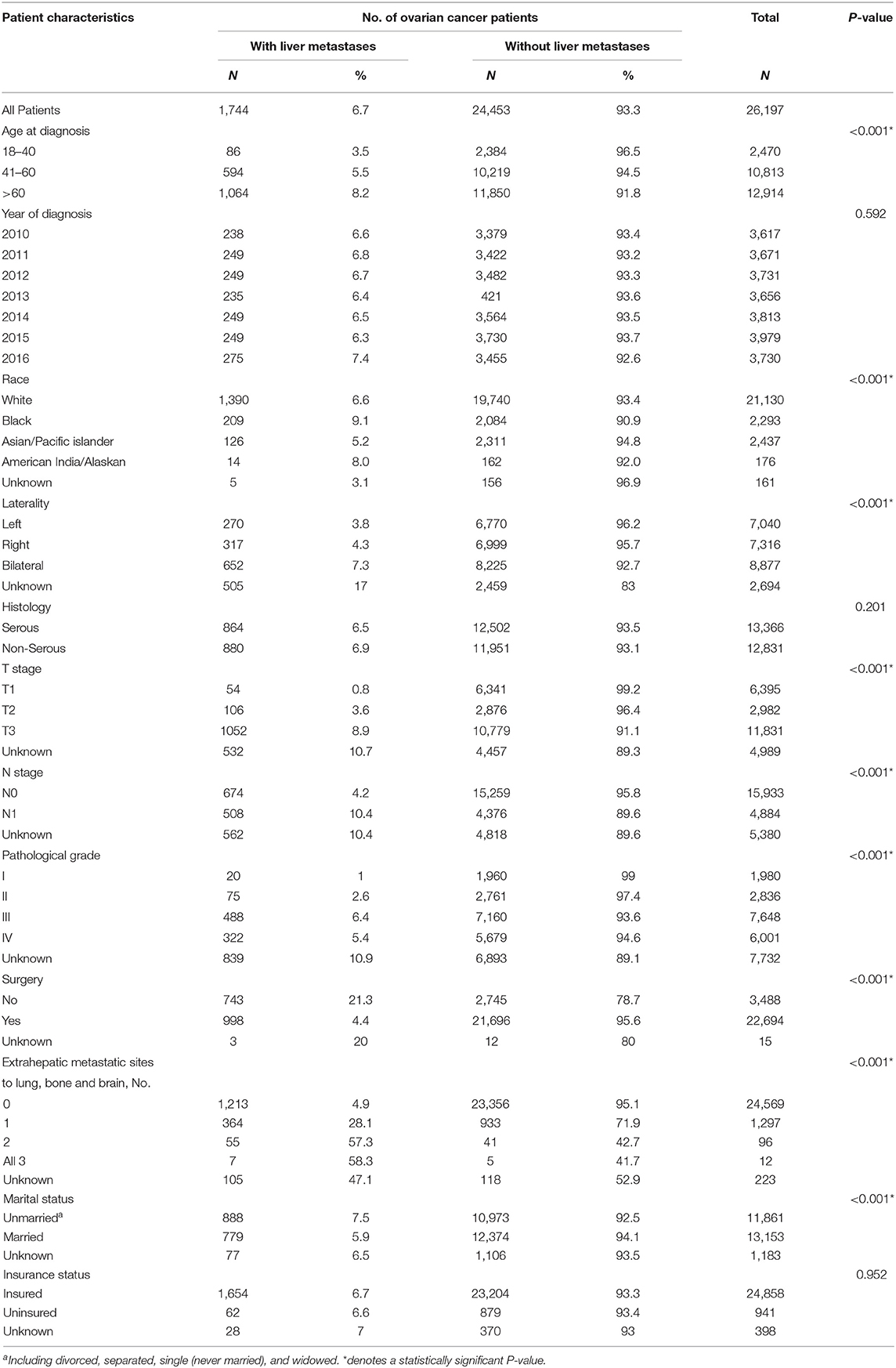
Table 1. Clinicopathological characteristics of ovarian cancer patients with and without liver metastases
The results of univariable and multivariable logistic regression performed among the whole population was displayed in Table 2. On the univariable logistic regression model, age 41–60 years (vs. age 18–40 years, P < 0.001), age greater than 60 years (vs. age 18–40 years, P < 0.001), Black race (vs. White race, P < 0.001), bilateral (vs. left, P < 0.001), pathological grade II (vs. grade I, P < 0.001), grade III (vs. grade I, P < 0.001), grade IV (vs. grade I, P < 0.001), 1 extrahepatic site (vs. 0 extrahepatic site, P < 0.001), 2 extrahepatic sites (vs. 0 extrahepatic site, P < 0.001), 3 extrahepatic sites (vs. 0 extrahepatic site, P < 0.001), T2 stage (vs. T1 stage, P < 0.001), T3 stage (vs. T1 stage, P < 0.001), N1 stage (vs. N0 stage, P < 0.001) were significantly associated with greater odds of liver metastases at initial diagnosis of ovarian cancer. Asian/Pacific islander (vs. White race, P = 0.007), married status (vs. unmarried, P < 0.001) were associated with lower odds of liver metastases at diagnosis. Histology and insurance state were not associated with the risk of having liver metastases at initial diagnosis of ovarian cancer. On the multivariable logistic regression, age >60 years (vs. age 18–40 years, P = 0.032), Black race (vs. White race, P = 0.037); bilateral (vs. left, P < 0.001), non-serous type (vs. serous type, P < 0.001), grade III (vs. grade I, P < 0.001), grade IV (vs. grade I, P < 0.001), 1 extrahepatic site (vs. 0 extrahepatic site, P < 0.001), 2 extrahepatic sites (vs. 0 extrahepatic site, P < 0.001), 3 extrahepatic sites (vs. 0 extrahepatic site, P < 0.001), T2 stage (vs. T1 stage, P < 0.001), T3 stage (vs. T1 stage, P < 0.001), N1 stage (vs. N0 stage, P < 0.001) were significantly associated with greater odds of liver metastases at initial diagnosis of ovarian cancer. Married status (vs. unmarried status, P = 0.016) was associated with lower risk of liver metastases at initial diagnosis of ovarian cancer.
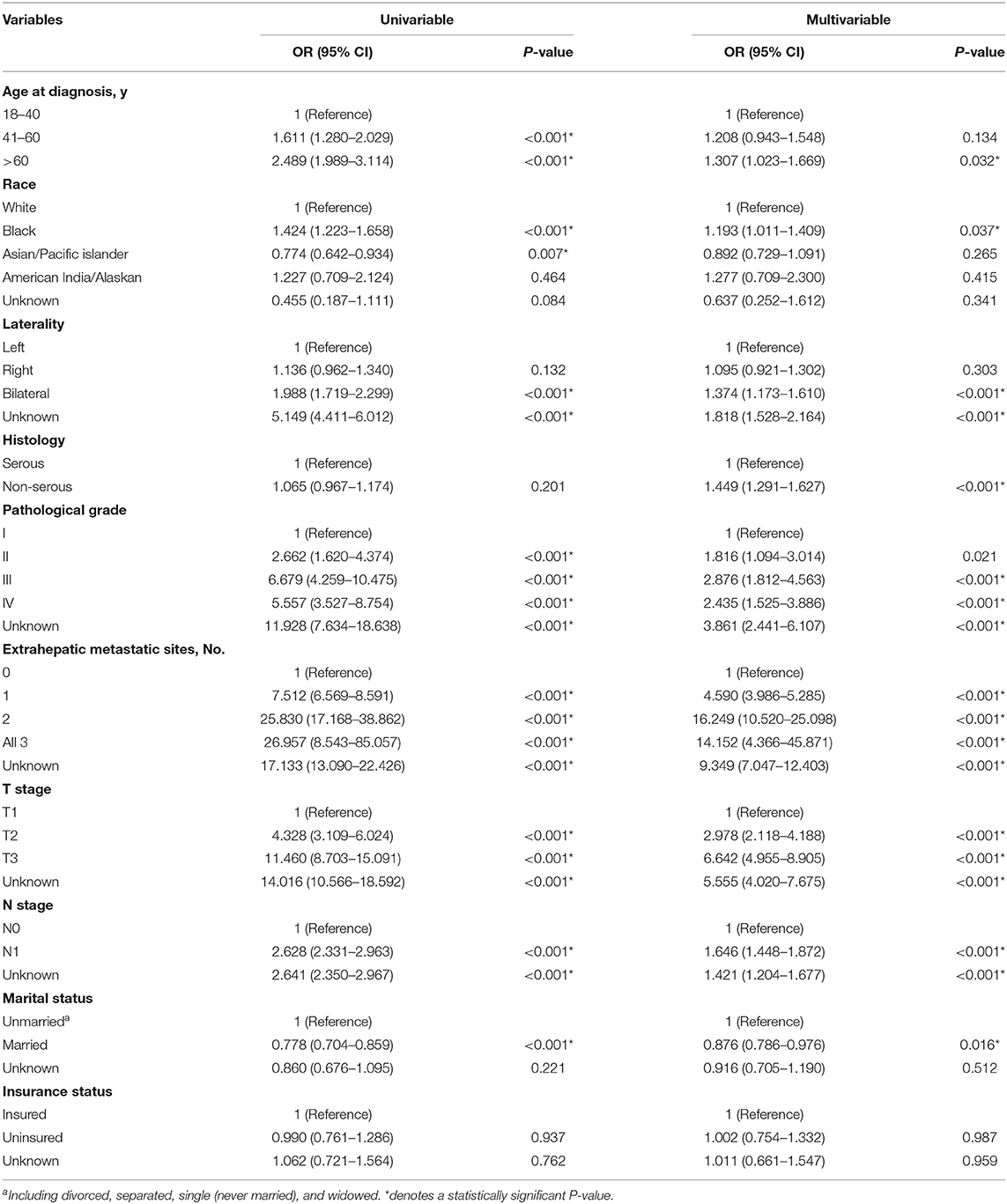
Table 2. Univariable and Multivariable logistic regression for the presence of liver metastases at diagnosis of ovarian cancer.
One thousand seven hundred and eighteen ovarian cancer patients with liver metastases were enrolled in the survival analysis. There were 854 patients with serous ovarian cancer (49.7%), 44 patients with endometrioid carcinoma (2.6%), 48 patients with clear cell ovarian cancer (2.8%), 46 patients with mucinous carcinoma (2.7%) and 726 patients with other types such as granular cell cancer and Brenner tumor (42.3%). The median OS among patients with liver metastases was 16.0 months, and the interquartile range (IQR) was 3.0–50.0 months (Figure 2A). OS curves stratified by ovarian cancer type of pathology was illustrated (Figure 2B). Patients with serous ovarian cancer had better survival than those with non-serous ovarian cancer (median OS: 30.0 months vs. 6.0 months, log-rank test, P < 0.001). The impact of the extent of extrahepatic metastatic disease on the survival of patients with liver metastases was estimated (Figure 2C, log-rank test, P < 0.001). Patients with more numbers of extrahepatic metastatic sites had worse prognosis.
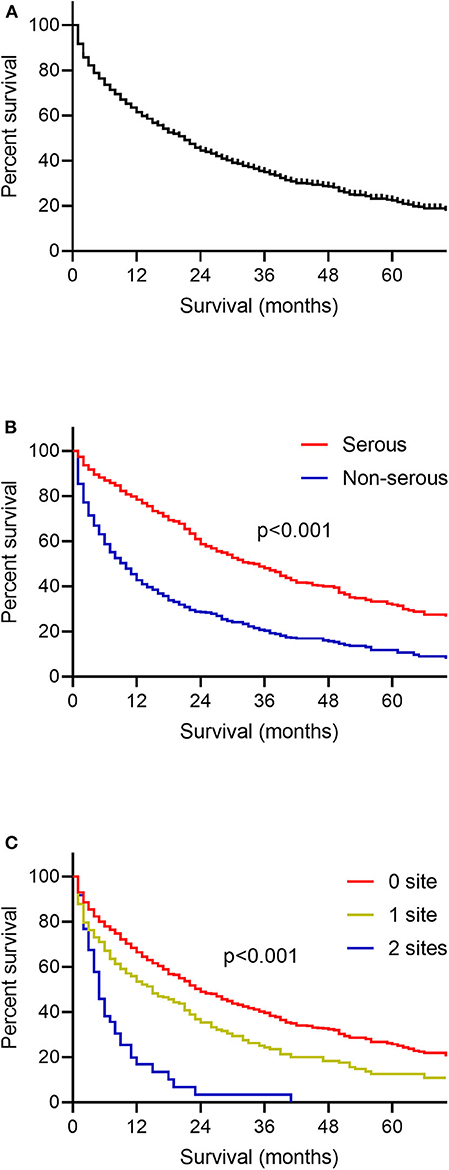
Figure 2. Kaplan-Meier curves for overall survival among patients with ovarian cancer liver metastases. (A) The whole population included in the survival analysis. (B) According to the histology type of ovarian cancer. (C) According to the number of extrahepatic metastatic sites to lung, bone and brain.
Among the whole population, there were 1,385 patients with extrahepatic metastatic disease. The presence of liver metastases or not on the median survival of these patients stratified by the extent of extrahepatic metastatic sites was concluded in Table 3. Broadly speaking, patients with no baseline of liver metastases had better survival than patients with liver metastases. Specifically, significant difference was shown in patients with bone and liver metastases (median OS, 6.0 months; IQR, 1.0–10.0 months) vs. those with bone metastases only (median OS, 10.0 months; IQR, 3.0–44.0 months) (log-rank test, P = 0.003), patients with lung and liver metastases (median OS, 13.0 months; IQR, 2.0–33.0 months) vs. those with lung metastases only (median OS, 21.0 months; IQR, 6.0–41.0 months) (log-rank test, P = 0.001), patients with brain and liver metastases (median OS, 1.0 months; IQR, 1.0–1.0 months) vs. those with brain metastases only (median OS, 7.0 months; IQR, 2.0-NR) (log-rank test, P = 0.016).
Univariate and multivariate Cox proportional hazards models were performed to evaluate the prognostic factors of ovarian cancer patients with liver metastases (Table 4). In the univariate Cox models, Black race (vs. White race, HR,1.500; 95% CI, 1.261–1.784; P < 0.001); American India/Alaskan (vs. White race, HR, 1.776; 95% CI, 1.027–3.071; P = 0.04), non-serous type(vs. serous type, HR, 2.493; 95% CI, 2.206–2.819; P < 0.001), 1 extrahepatic site (vs. 0 extrahepatic site, HR, 1.458; 95% CI, 1.262–1.683; P < 0.001), 2 extrahepatic sites (vs. 0 extrahepatic site, HR, 2.687; 95% CI, 1.988–3.632; P < 0.001) were significantly associated with increased all-cause mortality. Surgery (vs. non-surgery, HR, 0.255; 95% CI, 0.225–0.289; P < 0.001); married state (vs. unmarried, HR, 0.661; 95% CI, 0.585–0.747; P < 0.001) reduced the risk of death. In the multivariate Cox models, Black race (vs. White race, HR,1.252; 95% CI, 1.049–1.494; P = 0.013); American India/Alaskan (vs. White race, HR, 2.325; 95% CI, 1.337–4.044; P = 0.003), non-serous type(vs. serous type, HR, 1.651; 95% CI, 1.440–1.892; P < 0.001), 1 extrahepatic site (vs. 0 extrahepatic site, HR, 1.168; 95% CI, 1.008–1.352; P = 0.038), 2 extrahepatic sites (vs. 0 extrahepatic site, HR, 1.682; 95% CI, 1.238–2.286; P = 0.001), 3 extrahepatic sites (vs. 0 extrahepatic site, HR, 2.758; 95% CI, 1.230–6.187; P = 0.014) were significantly correlated with a higher risk of all- cause mortality. Surgery (vs. non-surgery, HR, 0.324; 95% CI, 0.278–0.378; P < 0.001); married state (vs. unmarried, HR, 0.821; 95% CI, 0.723–0.931; P = 0.002) reduced the risk of death. Age at diagnosis, pathological grade and insurance state were not correlated with all-cause mortality.
The dissemination types of ovarian cancer were divided into the transcoelomic metastasis, hematogenous and lymphatic spread metastasis, which individually had distinct molecular metastases mechanisms (10). Some research focused on the mechanisms of distant metastases including liver metastases in ovarian cancer. Kim et al. found the reduction of chemokine receptor the lymphotactin receptor (XRC1) suppressed the colon, spleen and liver metastases of SKOV3-xenograft mouse model (11). A study from Li et al. revealed that high level of insulin-like growth factor-1 (IGF1) was associated with advanced clinical stage and liver metastases of ovarian cancer patients by analyzing the expression of IGF1 in epithelial ovarian cancer clinical specimens. Further basic research manifested the IGF1 promoted the proliferation and migration of ovarian cancer cells and inhibition of IGF1 receptor and the downstream molecules effectively suppressed the malignant phenotype of tumor cells. Therefore, targeting the IGF1 pathway may be promising for the treatment for ovarian cancer patients with liver metastases (12). Joelle et al. found that the cell surface glycoprotein CD44 contributed to the spheroid formation, mesothelial adhesion and mesenteric metastasis in epithelial ovarian carcinoma. However, decrease of CD44 expression promoted the peritoneal metastases like liver and the thoracic cavity (13). Wang et al. found the overexpression of miR-203 attenuated the TGFβ pathway and inhibited the epithelial to mesenchymal transition. And necropsy of the orthotopic ovarian cancer mouse model showed the miR-203 suppressed primary tumor growth and peritoneal metastases including liver and spleen (14). A study from Yang et al. focused on the role of SMAD4 in ovarian cancer development and invasion. Research results showed that knocking out of SMAD4 impaired the vessel endothelial cell tubule formation. Although nude mice experiment indicated the loss of SMAD4 did not influence the tumor growth, it inhibited the barrier integrity in endothelial cell and promoted the ovarian cancer liver metastases (15). Ponnusamy et al. found MUC4 mucin promoted the process of epithelial to mesenchymal transition, and overexpression of MUC4 induced significantly larger tumors and was associated with a higher incidence of metastasis to distant sites including colon, liver and diaphragm (16). Yu et al. found the lysophosphatidic acid (LPA) receptors including LPA1, LPA2, LPA3 were involved the process of tumor proliferation and invasion by regulating VEGF and the cytokines including IL-6 and IL-8. And the overexpression of LPA receptors was associated with distant metastases including liver, kidney and pancreas by necropsy of the SKOV3 xenografts tumors (17).
In our study, the incidence of patients with liver metastases upon initial diagnosis of ovarian cancer was 4.65%. A study from Dauplat et al. showed the incidence of parenchymal liver metastases including the initial diagnosis and later recurrence was 9.4% and the median survival was 5.0 months among the total 255 patients (18). The discordancy may be caused by the difference of study population. Our study showed that old age, Black race, bilateral tumors, non-serous type, high grade, extrahepatic metastases, advanced stage and lymph nodes involvement were risk factors associated with the presence of liver metastases upon initial diagnosis of ovarian cancer. Previous study found that advanced stage, high grade and lymph node involvement were significant risk factors associated with distant metastases (19). The single-institution study of 244 serous ovarian cancer patients manifested that the increasing age, high grade tumor and advanced stage were risk factors associated with the presence of liver metastases (20). Loizzi et al. analyzed the clinical characteristics and survival of 29 ovarian cancer patients with hepatic metastases. Results indicated that 76% patients presented with papillary serous histology and 62% patients presented with poorly differentiated tumors (21). However, No significant difference was seen when compared the distant metastatic patterns for different histologic variants of ovarian cancer in the study from Rose et al. (22).
In our study, the median OS of patients with ovarian cancer was 16 months (IQR, 3–50 months). The subgroup analysis indicated that the patients with non-serous ovarian cancer and more numbers of extrahepatic sites had worse outcome. The multivariate Cox model showed the Black race, non-serous type and extrahepatic metastatic sites were correlated with increased risks of all- cause mortality, which was basically in accordance with previous studies (4, 21, 23). A study from Loizzi et al. revealed the OS among ovarian cancer patients with liver metastases upon initial diagnosis, with liver metastases as first recurrence, with liver metastases as second relapse was 19 months (IOR: 6–23 months), 24 months (IQR: 3–44 months), 10.0 months (IQR: 1–33 months), respectively, and no significant difference was seen among the three subgroups. The patients with liver metastases only had better survival than those with other metastatic sites (median OS: 25 months vs. 8 months, IQR: 9–44 months vs. 1–20 months, P = 0.033). And patients with serous ovarian cancer had better survival than those with other type of ovarian cancer (median OS: 23 vs. 8 months, IQR: 1–44 vs. 1–15 months, P = 0.005, HR, 2.875, 95% CI, 2.51–3.23) (21).
The treatment for ovarian cancer patients with liver metastases was still uncertain. Our study showed the surgery of primary site reduced the risk of all-cause death (HR, 0.255; 95% CI, 0.225–0.289; P < 0.001). A study enrolled in 105 patients with stage IV ovarian cancer from Curtin et al. showed surgery was an important determinant prognosis (24). Gallotta et al. retrospectively analyzed the clinical outcome of laparoscopic secondary cytoreduction for 29 patients with localized recurrent ovarian cancer. The rate of complete debulking was 96.2% and the median DFS was 14.0 months (25). A study of analyzing the safety of laparoscopic secondary cytoreductive surgery in 58 patients with platinum-sensitive recurrent ovarian cancer showed that the median PFS was 28.0 months and the 2 year OS was 90.7% (26). Wang et al. found that the OS of ovarian cancer patients with liver metastases who received R0 liver resection and cytoreductive surgery were 50.1 months, however, the OS of patients who received R0 cytoreductive surgery and non-R0 liver resection was 20.0 months (27). Some selected patients with cytoreductive surgery and liver resection had better outcome. A complete cytoreduction to no residual disease, good performance status, negative resection margins, less numbers of liver lesions and long progression-free interval were significant factors correlated with favorable outcome of ovarian cancer patients with liver metastases. However, it is worth noting that liver resection may cause some relevant complications like bilioma, abnormality of liver function, diaphragmatic injury, chest complications, and bile leakage (28). Transarterial chemoembolization (TACE) had some role in the treatment of ovarian cancer patients with liver metastases. The survival rates after receiving TACE was 58% after 1 year, 19% after 2 years, and 13% after 3 years (29). A study of 109 patients with stage IV ovarian cancer from Giovanni et al. found patients with multiple unresectable liver metastases had worse survival than those with resectable liver involvement (median OS, 14 months vs. NR, P = 0.003) (30). In a retrospective study of 37 patients with stage IV epithelial ovarian cancer with liver metastases, Naik et al. found that optimal cytoreduction is an independent prognostic factors associated with more favorable outcome (31). Zhuo et al. analyzed the survival difference of 29 ovarian cancer patients with liver metastases receiving microwave ablation (MWA) or surgical resection (SR). And no significant difference was seen between the two groups (5 year OS rate: SR vs. MWA, 64.3% vs. 51.3%, P = 0.198) (32).
Ailbhe et al. compared the clinical characteristics and survival between ovarian cancer patients with liver parenchymal invasion (LPI) from peritoneal metastases and those with hematogenous liver metastases (HLM). Results showed increasing age and suboptimal cytoreduction were factors associated with LPI while increasing age, high grade tumor and advanced stage were risk factors correlated with HLM. Survival analysis showed that ovarian cancer patients with LPI had similar survival to those without LPI (median OS: 80 vs. 123 months, IQR: 50-NR vs. 49–279 months, P = 0.6) while ovarian cancer patients with HLM had worse survival than those without HLM (median OS: 63 vs. 145 months, IQR: 43–139 months vs. 50-NR, P = 0.006). Therefore, it may be important to elucidate the clear criteria and identify the metastases type among ovarian cancer patients with liver metastases for individual clinical management (20). A study from Charlie et al. compared the frequency of visceral metastases between BRCA1/2 deficient ovarian cancer patients and BRCA1/2 proficient ovarian cancer patients, BRCA1/2 deficient ovarian cancer had increased incidence of visceral metastases (BRCA1/2 deficient vs. BRCA1/2 proficient, 58% vs. 5%, P < 0.001) and liver metastases (BRCA1/2 deficient vs. BRCA1/2 proficient, 42% vs. 0%, P < 0.001). BRCA1/2 sequencing should be considered among the ovarian cancer patients for better clinical management (33). Gallotta et al. analyzed the survival of 34 recurrent ovarian cancer patients with liver metastases who underwent liver resection within secondary cytoreductive surgery. Results indicated that patients with BRCA mutation had better survival than those with BRCA wild type (3 year post-liver resection progression free survival: 81.0% vs. 15.2%, P = 0.001). The assessment of BRCA mutational status may be important for risk stratification among the ovarian cancer patients with liver metastases (34). Sood et al. evaluated the status of p53 mutation in 130 ovarian cancer patients. Results revealed patients with a null mutation had a higher incidence of distant metastases than those with missense mutations or wild type p53 (66% vs. 8% vs. 8%, P < 0.001). Twenty five percentage patients with null p53 mutation presented with distant metastases including liver, spleen, brain, and thorax at initial diagnosis of ovarian cancer. It may be important to evaluate the p53 status for clinicians when dealing with the ovarian cancer patients (35).
To our knowledge, this current study was the largest study about ovarian cancer patients with liver metastases. However, several limitations should be acknowledged. First, the information provided by SEER database is insufficient, including the other metastatic sites such as peritoneal metastases of ovarian cancer, the detailed data about size and number of liver metastases and the information of individual treatment. Second, the study population are mainly in the ovarian cancer patients with liver metastases upon initial diagnosis excluding those developed with liver metastases during the recurrence. Third, the SEER database is based on the register in the United States. The study results may cause deviations in the other parts of the whole world.
In conclusion, this present study provided valuable information including incidence, risk factors and prognostic factors for newly diagnosed ovarian cancer patients with liver metastases. These findings assist clinicians make clinical management decisions of prognostic assessment and risk stratification. In the future, basic research and large sample prospective clinical trials are warranted to further evaluate the molecular characteristics and treatment for ovarian cancer patients with liver metastases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethical Committee Review Board of Fudan University Shanghai Cancer Centre (Shanghai, China). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HZ, JL, and XW contributed to the conception and design of this study. HZ, FX, and MN analyzed the data. HZ and XW contributed with a critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Science Foundation of China (No. 81672569 and 81972431) and the Science and Technology Commission of Shanghai Municipality (16411950200, KW1711, 17411963000).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank SEER for providing open access to the database.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. (2011) 61:183–203. doi: 10.3322/caac.20113
3. Gardner AB, Charo LM, Mann AK, Kapp DS, Eskander RN, Chan JK. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: most common locations and outcomes. Clin Exp Metastasis. (2020) 37:107–13. doi: 10.1007/s10585-019-10007-0
4. Deng K, Yang C, Tan Q, Song W, Lu M, Zhao W, et al. Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol. (2018) 150:460–5. doi: 10.1016/j.ygyno.2018.06.022
5. Kataoka A, Nisihda T, Sugiyama T, Ohta S, Yakushiji M, Kojiro M, et al. Metastases observed autopsy in 70 women with ovarian-cancer - relationship to treatment. Oncol Rep. (1995) 2:557–62. doi: 10.3892/or.2.4.557
6. Güth U, Huang DJ, Bauer G, Stieger M, Wight E, Singer G. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer. (2007) 110:1272–80. doi: 10.1002/cncr.22919
7. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. (2017) 14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084
8. Gaitskell K, Green J, Pirie K, Barnes I, Hermon C, Reeves GK, et al. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective million women study. Int J Cancer. (2018) 142:281–9. doi: 10.1002/ijc.31063
9. Aizer AA, Chen M-H, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. (2013) 31:3869–76. doi: 10.1200/JCO.2013.49.6489
10. Barbolina MV. Molecular mechanisms regulating organ-specific metastases in epithelial ovarian carcinoma. Cancers. (2018) 10:444. doi: 10.3390/cancers10110444
11. Kim M, Rooper L, Xie J, Rayahin J, Burdette JE, Kajdacsy-Balla AA, et al. The lymphotactin receptor is expressed in epithelial ovarian carcinoma and contributes to cell migration and proliferation. Mol Cancer Res. (2012) 10:1419–29. doi: 10.1158/1541-7786.MCR-12-0361
12. Liu L, Wang X, Li X, Wu X, Tang M, Wang X. Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol Rep. (2018) 39:818–26. doi: 10.3892/or.2017.6148
13. Sacks Suarez J, Gurler Main H, Muralidhar GG, Elfituri O, Xu H-L, Kajdacsy-Balla AA, et al. CD44 regulates formation of spheroids and controls organ-specific metastatic colonization in epithelial ovarian carcinoma. Mol Cancer Res. (2019) 17:1801–14. doi: 10.1158/1541-7786.MCR-18-1205
14. Wang B, Li X, Zhao G, Yan H, Dong P, Watari H, et al. miR-203 inhibits ovarian tumor metastasis by targeting BIRC5 and attenuating the TGFβ pathway. J Exp Clin Cancer Res. (2018) 37:235. doi: 10.1186/s13046-018-0906-0
15. Yang J, Wang Y, Zeng Z, Qiao L, Zhuang L, Gao Q, et al. Smad4 deletion in blood vessel endothelial cells promotes ovarian cancer metastasis. Int J Oncol. (2017) 50:1693–700. doi: 10.3892/ijo.2017.3957
16. Ponnusamy MP, Lakshmanan I, Jain M, Das S, Chakraborty S, Dey P, et al. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. (2010) 29:5741–54. doi: 10.1038/onc.2010.309
17. Yu S, Murph MM, Lu Y, Liu S, Hall H S, Liu J, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. (2008) 100:1630–42. doi: 10.1093/jnci/djn378
18. Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. (1987) 60:1561–6.
19. Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. (2003) 13:125–9. doi: 10.1046/j.1525-1438.2003.13054.x
20. O'Neill AC, Somarouthu B, Tirumani SH, Braschi-Amirfarzan M, van den Abbeele AD, Ramaiya NH, et al. Patterns and prognostic importance of hepatic involvement in patients with serous ovarian cancer: a single-institution experience with 244 patients. Radiology. (2017) 282:160–70. doi: 10.1148/radiol.2016152595
21. Loizzi V, Rossi C, Cormio G, Cazzolla A, Altomare D, Selvaggi L. Clinical features of hepatic metastasis in patients with ovarian cancer. Int J Gynecol Cancer. (2005) 15:26–31. doi: 10.1136/ijgc-00009577-200501000-00005
22. Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. (1989) 64:1508–13.
23. Winter WE, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a gynecologic oncology group study. J Clin Oncol. (2008) 26:83–9. doi: 10.1200/JCO.2007.13.1953
24. Curtin JP, Malik R, Venkatraman ES, Barakat RR, Hoskins WJ. Stage IV ovarian cancer: impact of surgical debulking. Gynecol Oncol. (1997) 64:9–12. doi: 10.1006/gyno.1996.4550
25. Gallotta V, Fagotti A, Fanfani F, Ferrandina G, Nero C, Costantini B, et al. Laparoscopic surgical management of localized recurrent ovarian cancer: a single-institution experience. Surg Endosc. (2014) 28:1808–15. doi: 10.1007/s00464-013-3390-9
26. Gallotta V, Conte C, Giudice MT, Nero C, Vizzielli G, Gueli Alletti S, et al. Secondary laparoscopic cytoreduction in recurrent ovarian cancer: a large, single-institution experience. J Minim Invasive Gynecol. (2018) 25:644–50. doi: 10.1016/j.jmig.2017.10.024
27. Wang M, Zhou J, Zhang L, Zhao Y, Zhang N, Wang L, et al. Surgical treatment of ovarian cancer liver metastasis. Hepatobiliary Surg Nutr. (2019) 8:129–37. doi: 10.21037/hbsn.2018.12.06
28. Gasparri ML, Grandi G, Bolla D, Gloor B, Imboden S, Panici PB, et al. Hepatic resection during cytoreductive surgery for primary or recurrent epithelial ovarian cancer. J Cancer Res Clin Oncol. (2016) 142:1509–20. doi: 10.1007/s00432-015-2090-3
29. Vogl TJ, Naguib NNN, Lehnert T, Nour-Eldin N-EA, Eichler K, Zangos S, et al. Initial experience with repetitive transarterial chemoembolization (TACE) as a third line treatment of ovarian cancer metastasis to the liver: indications, outcomes and role in patient's management. Gynecol Oncol. (2012) 124:225–9. doi: 10.1016/j.ygyno.2011.11.001
30. Aletti GD, Podratz KC, Cliby WA, Gostout BS. Stage IV ovarian cancer: disease site-specific rationale for postoperative treatment. Gynecol Oncol. (2009) 112:22–7. doi: 10.1016/j.ygyno.2008.09.010
31. Naik R, Nordin A, Cross PA, Hemming D, de Barros Lopes A, Monaghan JM. Optimal cytoreductive surgery is an independent prognostic indicator in stage IV epithelial ovarian cancer with hepatic metastases. Gynecol Oncol. (2000) 78:171–5. doi: 10.1006/gyno.2000.5841
32. Zhuo S, Zhou J, Ruan G, Zeng S, Ma H, Xie C, et al. Percutaneous microwave ablation vs. surgical resection for ovarian cancer liver metastasis. Int J Hyperthermia. (2020) 37:28–36. doi: 10.1080/02656736.2019.1706767
33. Gourley C, Michie CO, Roxburgh P, Yap TA, Harden S, Paul J, et al. Increased incidence of visceral metastases in Scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol. (2010) 28:2505–11. doi: 10.1200/JCO.2009.25.1082
34. Gallotta V, Conte C, D'Indinosante M, Capoluongo E, Minucci A, de Rose AM, et al. Prognostic factors value of germline and somatic BRCA in patients undergoing surgery for recurrent ovarian cancer with liver metastases. Eur J Surg Oncol. (2019) 45:2096–102. doi: 10.1016/j.ejso.2019.06.023
Keywords: liver metastases, ovarian cancer, incidence, prognosis, SEER
Citation: Zhao H, Xu F, Li J, Ni M and Wu X (2020) A Population-Based Study on Liver Metastases in Women With Newly Diagnosed Ovarian Cancer. Front. Oncol. 10:571671. doi: 10.3389/fonc.2020.571671
Received: 11 June 2020; Accepted: 28 August 2020;
Published: 25 September 2020.
Edited by:
Connie Irene Diakos, Royal North Shore Hospital, AustraliaReviewed by:
Valerio Gallotta, Catholic University of the Sacred Heart, ItalyCopyright © 2020 Zhao, Xu, Li, Ni and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Wu, d3UueGhAZnVkYW4uZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.