- 1Department of Cancer Epidemiology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 2Central Laboratory, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 3Office of Henan Cancer Center, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 4National Office for Maternal and Child Health Surveillance of China, Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 5Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 6Department of Gynecological Oncology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 7Department of Cancer Epidemiology, National Cancer Center, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 8Department of Pathology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 9Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
This study aims to evaluate the clinical performance of the HPV E6/E7 mRNA test in cervical cancer screening in China. A hospital-based study was conducted with mRNA, DNA, and liquid-based cytology (LBC) as primary screening tests. Each woman with a positive result received colposcopy with lesion-targeted-biopsy. Histopathological diagnosis was used as the gold standard. The total agreement of HPV DNA and mRNA was 90.7% (95%CI: 87.9, 92.9) with a kappa value of 0.81. The positive rates of HPV DNA, mRNA, and LBC increased with the severity of histopathology diagnosis, from 25.5, 19.1, and 11.4% in normal to 100.0% in SCC, respectively. The sensitivities for mRNA to detect CIN2+ and CIN3+ were 93.8% (95%CI: 89.7–96.4) and 95.7% (95%CI: 91.3–97.9), respectively, which were not different from HPV DNA testing (95.7% [95%CI: 92.0–97.7], 96.3% [95%CI: 92.1–98.3]), but higher than LBC (80.4% [95%CI: 74.5–85.2] and 88.8% [95%CI: 83.0–92.8]). The specificities for mRNA to detect CIN2+ (79.0% [95%CI: 74.2–83.0]) and CIN3+ (70.5% [95%CI: 65.7–74.9]) were higher than HPV DNA testing (71.0% [95%CI: 65.9–75.7], 62.8% [95%CI: 57.8–67.5]), but lower than LBC (84.5% [95%CI: 80.1–88.0] 79.8% [95%CI: 75.4–83.6]). All tests were more effective in women older than 30 years. HPV mRNA test showed excellent agreement with the DNA test, with similar sensitivity and a higher specificity in detecting high-grade cervical lesions. It is promising that mRNA test could be used for the national cervical cancer screening to reduce false positive without losing sensitivity.
Introduction
It is known that persistent infection with high-risk human papillomavirus (hr-HPV) is a necessary cause for cervical cancer and precancerous lesions (1, 2). With a revealed etiology, cervical cancer is highly preventable (3, 4). Developed countries have begun to use hr-HPV testing in primary cervical cancer screening, either alone or co-testing with cytology (5–8). Evidence shows that HPV-based screening programs provide greater protection against cervical pre-cancer and cancer than other traditional screening methods, such as cytology (9–11). However, since most HPV infections could be cleared spontaneously, HPV-testing identifies numerous infections that will not progress to cervical pre-cancer or cancer, especially in young women (12). Therefore, HPV DNA testing is not recommended to screen women under the age of 30. Moreover, HPV-positive patients referred for subsequent procedures may suffer from unnecessary invasive interventions.
In China, there were 98,900 new cases and 30,500 deaths from cervical cancer in 2015 (13). To curb the increasing trend of this malignancy, the Chinese government has provided a nationwide free cervical cancer screening program for women living in rural areas since 2009, using VIA/VILI, Papanicolaou (Pap) test or HPV test (in pilot sites), based on economic and technological development levels (14). However, due to the nature of these screening methods, the diagnostic accuracy needs to be improved. Moreover, considering China’s large population, novel screening tool with a balance between sensitivity and specificity should be evaluated.
Disease-specific molecular markers of cervical cancer provide a combination of high sensitivity and high specificity to detect cervical pre-cancer. Most of these markers were identified based on the mechanism of HPV-related carcinogenesis. The HPV RNA testing is based on the detection of HR-HPV E6 and E7 mRNA. The oncogenic potential of HPV infection depends on the production of viral E6/E7 oncoproteins. Thus, the detection of E6/E7 mRNA transcripts provides the possibility for a specific test to detect precancerous lesions.
In this study, we evaluated the clinical performance of the HPV E6/E7 mRNA test to detect high-grade cervical intraepithelial neoplasia (CIN) and cancer among Chinese women.
Materials and Methods
Participants and Procedures
This hospital-based study was conducted during April to December 2017 in Henan province, China. Women who visited the department of gynecology of The Second Affiliated Hospital of Zhengzhou University (SAHZU) for colposcopy were invited. The inclusion criteria were as follows: (1) women aged between 25 and 64 years-old; (2) no history of cervical cancer or hysterectomy; (3) no clinical symptoms of pregnancy or 8 weeks after the termination of pregnancy; and (4) understand the study procedures, and voluntarily participated. The study was approved by the Institutional Review Board (IRB) of Henan Cancer Hospital (HCH). Written informed consent was obtained from each participant.
The cervical exfoliate cells were obtained from women during the gynecologic examination. The specimen was preserved in 20 ml PreservCyt® transport medium (Hologic Inc., Marlborough, MA, United States) and stored at 4°C. Then, colposcopy examination was performed by a gynecologist. Women with abnormal colposcopy finding underwent lesion-targeted biopsy. If the colposcopy examination was unsatisfactory (the squamocolumnar junction was not completely visible), endocervical curettage (ECC) was performed. Specimens of cervical exfoliated cells were transported to the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS). The specimens were divided into two portions: a 1.5 ml cell mixture into a EP tube for the HR-HPV DNA test and HPV E6/E7 mRNA test. The residual preservcyt with exfoliated cells was used for ThinPrep cytologic test (TCT) (Hologic Inc., Marlborough, MA, United States). The Bethesda reporting system was used for cytology by a senior cytologist (15).
HR-HPV DNA Assay
The 400 μl cell mixture samples from 1.5 ml EP tubes were used for the detection of DNA of 14 hr-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) by Cobas 4800 HPV assay (Roche, Basel, SUI). It reported pooled result of the 14 hrHPV, and the separate result for HPV 16 and 18, simultaneously (16). Cobas 4800 was a PCR-based testing for HPV DNA with nucleic acid hybridization amplification according to the manufacturer’s instructions. Negative and positive quality controls were set in each test. If the ct value was greater than 40, the result was deemed as negative. Otherwise, the result was positive. The positive results included three types: HPV 16, HPV 18, and HPV others (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).
HPV E6/E7 mRNA Testing
One ml samples from 1.5 ml EP tubes were used for the detection of the E6/E7 viral mRNA from the 14 hr-HPV types in aggregate APTIMA HPV test (Hologic Inc., Marlborough, MA, United States), according to the manufacturer’s instructions (17). It was a three steps test, including mRNA extraction, amplification, and amplified product detection. If the copy number was greater than or equal to 1.0, the result was deemed as positive. Otherwise, the result was negative.
Histopathological Diagnosis
Biopsy and ECC tissues were sent to SAHZU for histopathological diagnoses according to the cervical intraepithelial neoplasia (CIN) reporting system. Then histopathologists from HCH reviewed all the slides. Any inconsistent pathological diagnosis was sent to CICAMS for adjudication. The final diagnosis for each woman was based on the worst reading from the panel review. All the detection and diagnosis process were blind.
Statistical Analysis
The final histopathological diagnosis was used as the gold standard. Women with TCT diagnosis of the low-grade squamous intraepithelial lesion (LSIL) or worse (LSIL +) were deemed as liquid-based cytology (LBC) positive. The HPV DNA results were stratified, according to the HPV types: HPV16/18 (i.e., HPV16 and/or HPV18 positive), HPV-others (i.e., any of the 12 hr-HPV types positive excluding HPV16 and HPV18), and HPV-total (i.e., any of the 14 hr-HPV types positive). The absolute estimates and 95% confidential intervals (95%CI) of positive rates, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. The differences in HPV mRNA expressions in different HPV groups were calculated using the chi-square test or Fisher’s exact test. The McNemar test was used to identify the differences in sensitivity and specificity. The α level was set at 0.05, and p < 0.05 (two-sized) was deemed as statistically significant.
Results
In total, 537 women were included in this analysis. The average age was 43.88 ± 10.97 years. The LBC diagnoses were: 183 (34.7%) normal, 135 (25.1%) atypical squamous cells of undetermined significance (ASC-US), 22 (4.1%) atypical squamous cell cannot exclude HSIL (ASC-H), 7 (1.3%) atypical glandular cell (AGC), 82 (15.3%) LSIL, 75 (14.0%) high-grade squamous intraepithelial lesion (HSIL), 31 (5.8%) squamous cell carcinomas (SCC), and 2 (0.4%) adenocarcinoma in situ (AIS). The histopathology diagnoses were 298 (55.5%) normal, 30 (5.6%) cervical intraepithelial neoplasia grade 1 (CIN1), 48 (8.9%) CIN2, 111 (20.7%) CIN3, 43 (8.0%) SCC, and 7 (1.3%) AIS.
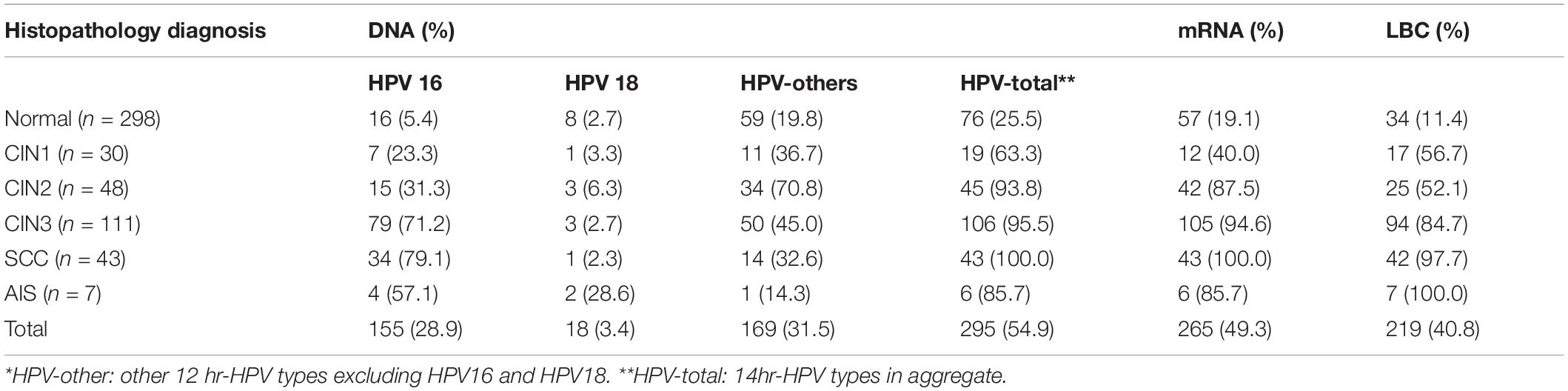
Table 1 showed the correlation between mRNA and DNA detection by HPV types. The total agreement of the two tests was 90.7% (95%CI: 87.9, 92.9) with a kappa value of 0.81. The positive rates of mRNA were 91.7%, 84.6%, and 86.4% in HPV16/18, HPV-others, and HPV-total positive women, respectively. All of the mRNA positive rates were higher in HPV-positive women than HPV-negative women in the same HPV type group (all p < 0.001).
The HPV DNA, mRNA, and LBC positive rates increased with the severity of histopathology diagnosis, ranged from 25.5, 19.1, and 11.4% in normal to 100.0% in SCC, respectively. The positive rates of the three tests in AIS were 85.7, 85.7, and 100.0%, respectively. These three tests had similar positive rates in CIN3, SCC, and AIS. But the positive rates of mRNA were relatively lower in women under CIN2 diagnosis. The rates of HPV16 positive were the highest in CIN3, SCC, and AIS when compared with other HPV DNA groups. HPV18 positive rates were lower than 7% in all squamous cell lesions, but a little higher (28.6%) in AIS (Table 2).
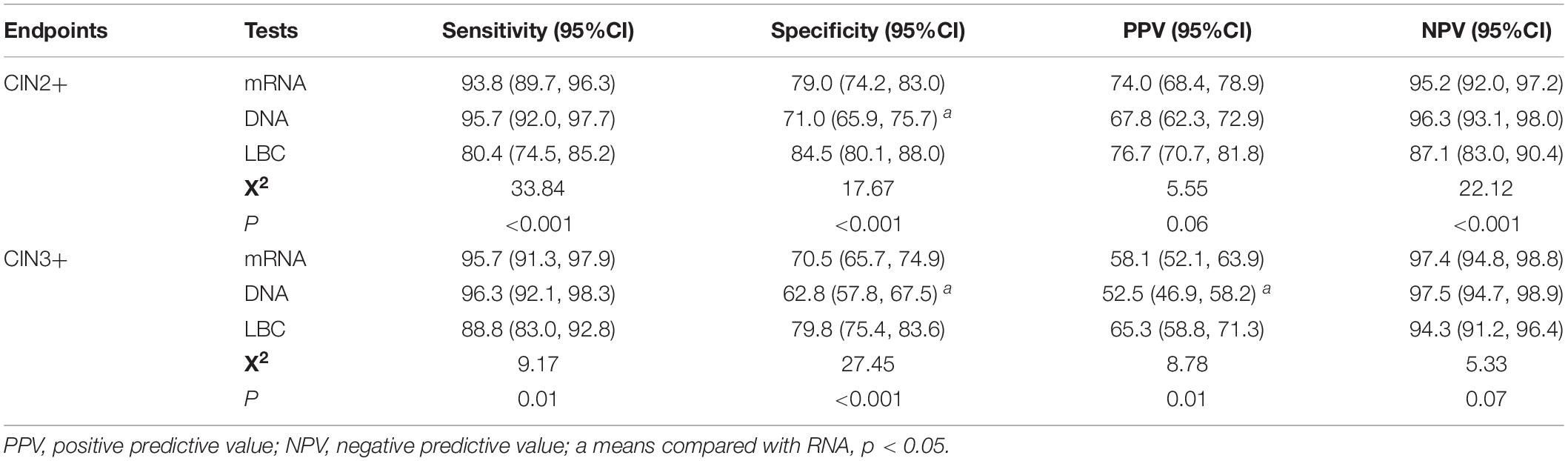
Table 3 showed the clinical performance of the three tests in detecting CIN2+ and CIN3+ lesions. The sensitivities for mRNA to detect CIN2+ and CIN3+ were 93.8% (95%CI: 89.7–96.4) and 95.7% (95%CI: 91.3–97.9), respectively, which were not different from those detected by DNA (95.7% [95%CI: 92.0–97.7] for CIN2+, 96.3% [95%CI: 92.1–98.3] for CIN3+), but higher than those detected by LBC (80.4% [95%CI: 74.5–85.2] for CIN2+ and 88.8% [95%CI: 83.0–92.8] for CIN3+). The specificities for mRNA to detect CIN2+ (79.0% [95%CI: 74.2–83.0]) and CIN3+ (70.5% [95%CI: 65.7–74.9]) were significantly higher than those detected by DNA (71.0% [95%CI: 65.9–75.7] for CIN2+, 62.8% [95%CI: 57.8–67.5] for CIN3+) (p < 0.05), but lower than those detected by LBC (84.5% [95%CI: 80.1–88.0] for CIN2+, 79.8% [95%CI: 75.4–83.6] for CIN3+). The PPVs for mRNA to detect CIN2+ and CIN3+ were 74.0% (95%CI: 68.4–78.9) and 58.1% (95%CI: 52.1–63.9), and the NPVs were 95.2% (95%CI: 92.0–97.2) and 97.4% (95%CI: 94.8–98.8), respectively.
Tables 4, 5 showed the positive rates and clinical performance of the three tests in different age groups. In summary, all the tests showed better performance for women older than 30 years than younger women. HPV DNA test had the highest sensitivity, and LBC had the highest specificity for detecting CIN2+ and CIN3+ in women younger than 30 years.
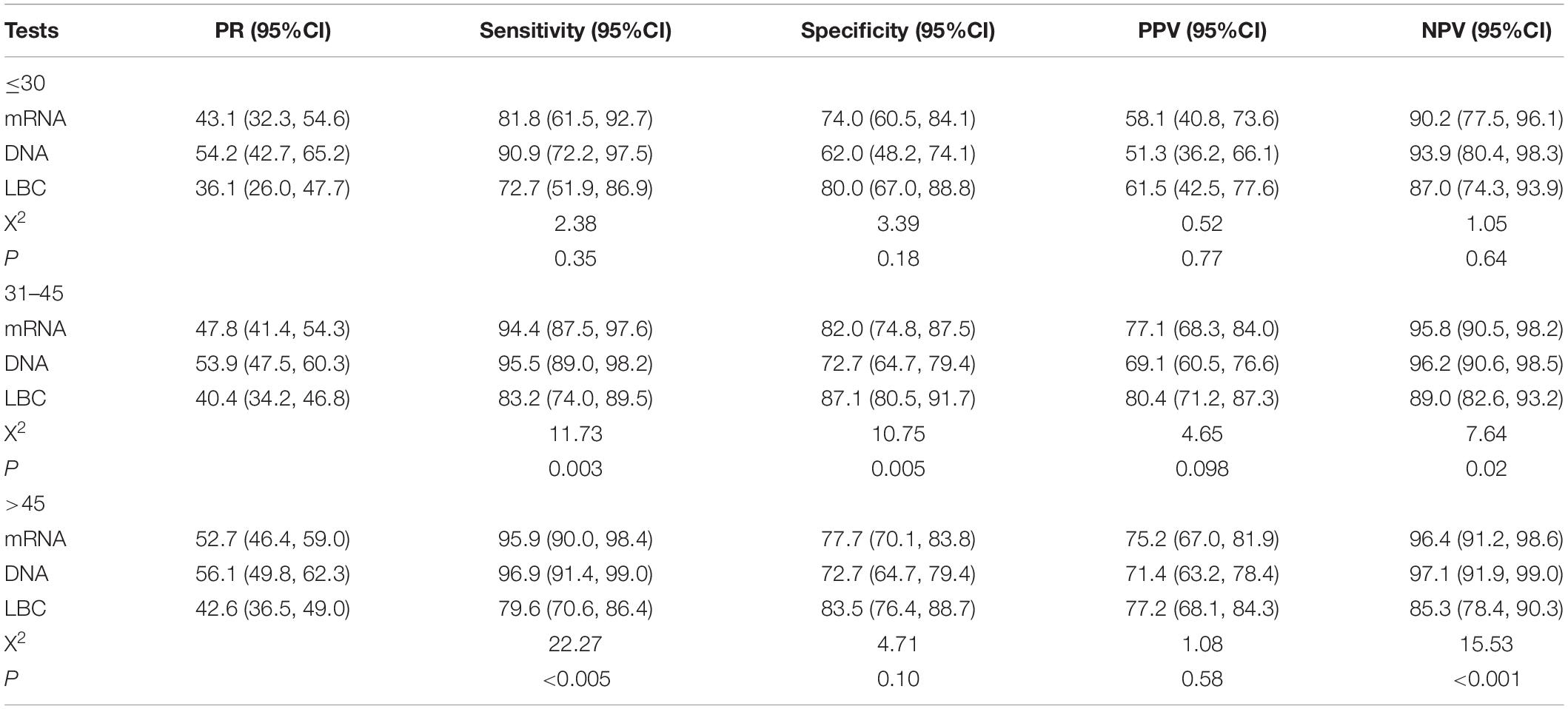
Table 4. The positive rates and clinical performance of tests to detect CIN2+ in different age groups.
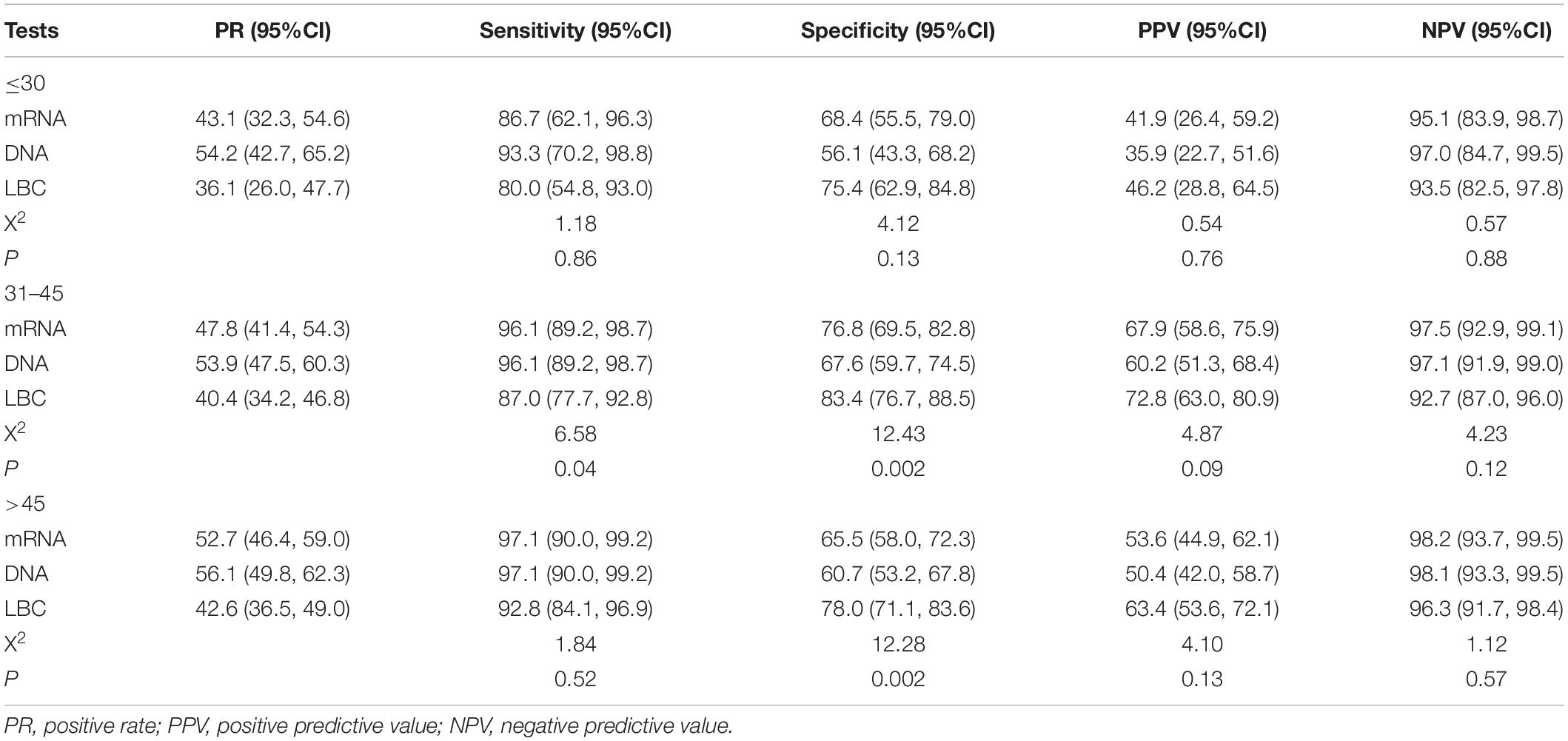
Table 5. The positive rates and clinical performance of tests to detect CIN3+ in different age groups.
Discussion
The presented results showed that the mRNA test and DNA teat reached a high agreement as 90.7%. The HPV DNA, mRNA, and LBC positive rates increased with the severity of histopathology diagnosis, from 25.5, 19.1, and 11.4% in normal to 100.0% in SCC, respectively. The sensitivities for mRNA to detect CIN2 and CIN3+ were 93.8% and 95.7%, which were similar to those detected by DNA (95.7% for CIN2+ and 96.3% for CIN3+). But the specificities for mRNA (79.0% for CIN2+ and 70.5% for CIN3+) were significantly higher than those detected by DNA (71.0% for CIN2+ and 62.8% for CIN3+).
In this study, the HPV E6/E7 mRNA test and HPV DNA test showed high agreement. Of those HPV 16/18 DNA positive women, 91.7% were also positive on mRNA, which is similar to other studies, that reported an overall agreement of over 90% between APTIMA HPV test and HPV DNA tests (17, 18), and consistently higher positive rates for HPV DNA test in different populations (19, 20). We noticed higher positive rates in older women than younger women, which was slightly different from other studies (21–23). This variation may be attributed to the study design (i.e., hospital-based study), which limits the extrapolation of the findings into the general population. Interestingly, the discordant rate was higher in women aged 30 years or younger (25.6% vs. 8.6%). Studies have demonstrated a higher rate of spontaneous clearance for HPV infection in younger women, which indicated a lower possibility of HPV integration (24).
As we know, DNA-based HPV tests detect the presence or absence of HPV DNA. However, most HPV infections are transient, cleared spontaneously within 1 year, which would not progress to cervical pre-cancer or cancer (25). E6/E7 mRNA expression only occurs in actively infected cells and increase during CIN development and progression (26). Therefore, the HPV mRNA test is supposed to be more specific in detecting high-grade cervical lesions. Our data confirmed this hypothesis. We found that the mRNA test was as sensitive as the DNA test, but more specific in detecting cervical pre-cancer and cancer. Other researchers drew similar conclusions in either primary screening (20) or triage of women with minor abnormal cytology (27). In our study, 50 women had discordant results between HPV DNA and mRNA test. Among them, 34 DNA + /mRNA- versus 8 mRNA + /DNA- were diagnosed as normal or CIN1; 6 versus 2 were diagnosed as CIN2 or CIN3; no test difference was observed in cancers. Therefore, if we replace the HPV DNA test with the mRNA test, 34 women would not be referred for unnecessary colposcopy, but 4 CIN2 and 2 CIN3 would be missed. In spite of 6 cases missing, there was no sensitivity lost, but specificity increased. Literature showed 40–60% of CIN2 cases would regressed within 2 years (28, 29), and not all CIN3 cases were true pre-cancerous lesions (30). Cook et al. also found a low CIN2+ rate among mRNA-/DNA + women (17). Therefore, the risk of invasive cancer for the 6 women was low in a short time interval.
We also evaluated the test performance in older and younger women. The age-specific analysis found that the three tests functioned better in women older than 30 years. Based on the fact that younger women with CIN lesions had a higher possibility of regression, we suggest a conservative management of younger women. Studies have found that HPV infection was common in young women, however, a large portion of them were transient infections, and may be cleared spontaneously (31, 32). The short duration of most HPV infections in these women suggests that the associated cervical dysplasia should be managed conservatively (12). Therefore, the HPV DNA test may not be a suitable screening method for women under 30 years of age, due to the high false-positive rate. The screening performance of cytology and E6/E7 mRNA comprehensively in young women should be evaluated. In our study, data showed that cytology had the highest specificity in women under 30 years, but the sensitivity is lower than the HPV DNA test. However, for E6/E7 mRNA, it had a moderate performance in women younger than 30 years, which had higher sensitivity than cytology accompanied by higher specificity than HPV DNA, suggesting that E6/E7 mRNA testing may be a promising option for the screening of women under 30.
Although the China Food and Drug Administration approved three prophylactic HPV vaccines (i.e., Cervarix, Gardasil, and Cecolin), cervical cancer prevention still relies on screening. In 2009, the Chinese government launched a nationwide free cervical cancer screening for rural women. In 2015, the HPV DNA test was used as a primary screening tool in pilot sites for the first time. However, due to its large population, HPV DNA-based screening would result in unavoidable huge false positives, leading to a waste of health resources and unnecessary anxiety. It is promising that mRNA could be used in the national cervical cancer screening program to reduce the false positive without losing any sensitivity.
Several limitations should be addressed in this study. First, this is a cross-sectional study, and we could not evaluate the risk of lesion progression associated with HPV E6/E7 mRNA. However, we stratified our data by histological grade, which provides information on the correlation. Second, it is important to note that the results cannot be fully generalized to the general population because the study participants were recruited from the outpatient in hospital.
In conclusion, the APTIMA mRNA test had good agreement with Cobas 4800 HPV DNA test with similar sensitivity and higher specificity to detect high-grade cervical lesions. It is promising that mRNA could be used in China’s national cervical cancer screening program to reduce the false positive without losing any sensitivity. Further studies are needed to evaluate the clinical performance of mRNA test in younger women in China.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the role of co-expression of HPV E6/E7 mRNA and p16/Ki-67 protein in predicting the risk of cervical cancer. By Life Science Ethics Review Committee of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
S-KZ contributed to the design and wrote the manuscript. ZG contributed to the manuscript’s writing and revision. PW, M-MJ, and P-PG contributed to the investigation and HPV DNA test. L-NK and Z-NW contributed the HPV mRNA test. D-MZ contributed to cytology and histology examination. QC and X-QC performed the statistical analysis. X-BS helped to conceive the study and assisted with the statistical analyses. Y-LQ and J-GZ helped to conceive the study and assisted with the manuscript’s writing. J-GZ assisted with the implementation of the study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81502475) and Science and Technology Project of Henan Province (Grant No. 172102310067).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All the women involved in this study are acknowledged for their participancy. We also appreciated the contribution from all relevant doctors for this study and the help of the National Cancer Center, Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College. We also appreciated 32nd International Papillomavirus Conference (IPVC 2018) for providing a platform to present the abstract of the study (title: Clinical Performance of HPV E6/E7 mRNA test to Detect Cervical High-grade Intraepithelial Neoplasia and Cancer: A Hospital-based Study in China, IPVC8-0744 Poster Session).
Abbreviations
hr-HPV, high-risk human papillomavirus; VIA/VILI, visual inspection with acetic acid/visual inspection with Lugol’s Iodine solution; Pap, papanicolaou; SAHZU, Second Affiliated Hospital of Zhengzhou University; IRB, Institutional Review Board; HCH, Henan Cancer Hospital; CICAMS, Cancer Institute and Hospital Chinese Academy of Medical Sciences; 95%CI, 95% confidential intervals; ECC, endocervical curettage; TCT, ThinPrep cytologic test; CIN, cervical intraepithelial neoplasia; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; LBC, liquid-based cytology; PPV, positive predictive value; NPV, negative predictive value; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cell cannot exclude HSIL; AGC, atypical glandular cell; SCC, squamous cell carcinomas; AIS, adenocarcinoma in situ.
References
1. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9. doi: 10.1002/(sici)1096-9896(199909)189:1<12::aid-path431>3.0.co;2-f
2. Bosch FX, de Sanjosé S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers. (2007) 23:213–27.
3. Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Curr Opin Obstet Gynecol. (2016) 28:4–10.
4. Denny L, Prendiville W. Cancer of the cervix: early detection and cost-effective solutions. Int J Gynaecol Obstet. (2015) 131(Suppl. 1):S28–32.
5. Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. (2007) 357:1579–88. doi: 10.1056/nejmoa071430
6. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. (2015) 136:189–97. doi: 10.1016/j.ygyno.2014.11.076
7. Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. (2007) 370:1764–72. doi: 10.1016/s0140-6736(07)61450-0
8. Zorzi M, Del Mistro A, Farruggio A, de’Bartolomeis L, Frayle-Salamanca H, Baboci L, et al. Use of a high-risk human papillomavirus DNA test as the primary test in a cervical cancer screening programme: a population-based cohort study. BJOG. (2013) 120:1260–7. doi: 10.1111/1471-0528.12272
9. Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. (2006) 119:1095–101. doi: 10.1002/ijc.21955
10. Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. NEngl J Med. (2009) 360:1385–94.
11. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. (2014) 383:524–32. doi: 10.1016/s0140-6736(13)62218-7
12. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. (1998) 338:423–8.
13. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32.
14. Wen C. China’s plans to curb cervical cancer. Lancet Oncol. (2005) 6:139–41. doi: 10.1016/S1470-2045(05)01761-4
15. Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 bethesda system: terminology for reporting results of cervical cytology. JAMA. (2002) 287:2114–9.
16. Cui M, Chan N, Liu M, Thai K, Malaczynska J, Singh I, et al. Clinical performance of Roche Cobas 4800 HPV Test. J Clin Microbiol. (2014) 52:2210–1.
17. Cook DA, Smith LW, Law J, Mei W, van Niekerk DJ, Ceballos K, et al. Aptima HPV assay versus hybrid capture® 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. J Clin Virol. (2017) 87:23–9.
18. Castle PE, Eaton B, Reid J, Getman D, Dockter J. Comparison of human papillomavirus detection by Aptima HPV and cobas HPV tests in a population of women referred for colposcopy following detection of atypical squamous cells of undetermined significance by Pap cytology. J Clin Microbiol. (2015) 53:1277–81. doi: 10.1128/JCM.03558-14
19. Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjänen K, Halfon P, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Int J Cancer. (2011) 129:691–701. doi: 10.1002/ijc.25726
20. Ge Y, Christensen P, Luna E, Armylagos D, Xu J, Schwartz MR, et al. Aptima human papillomavirus E6/E7 mRNA test results strongly associated with risk for high-grade cervical lesions in follow-up biopsies. J Low Genit Tract Dis. (2018) 22:195–200. doi: 10.1097/LGT.0000000000000393
21. de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. (2007) 7:453–9.
22. Franceschi S, Herrero R, Clifford GM, Snijders PJ, Arslan A, Anh PT, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. (2006) 119:2677–84.
23. Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. (2008) 43(4 Suppl.):S5–25.
24. Martin CM, O’Leary JJ. Histology of cervical intraepithelial neoplasia and the role of biomarkers. Best Pract Res Clin Obstet Gynaecol. (2011) 25:605–15. doi: 10.1016/j.bpobgyn.2011.04.005
25. Rodríguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. (2008) 100:513–7.
26. Haedicke J, Iftner T. A review of the clinical performance of the Aptima HPV assay. J Clin Virol. (2016) 76 Suppl 1:S40–8. doi: 10.1016/j.jcv.2015.10.027
27. Arbyn M, Roelens J, Cuschieri K, Cuzick J, Szarewski A, Ratnam S, et al. The APTIMA HPV assay versus the hybrid capture 2 test in triage of women with ASC-US or LSIL cervical cytology: a meta-analysis of the diagnostic accuracy. Int J Cancer. (2013) 132:101–8. doi: 10.1002/ijc.27636
28. Munro A, Powell RG, Cohen P A, Bowen S, Spilsbury K, O’Leary P, et al. Spontaneous regression of CIN2 in women aged 18-24 years: a retrospective study of a state-wide population in Western Australia. Acta Obstet Gynecol Scand. (2016) 95:291–8.
29. Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. (2009) 113:18–25. doi: 10.1097/AOG.0b013e31818f5008
30. McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. (2008) 9:425–34.
31. Cuschieri K, Ronco G, Lorincz A, Smith L, Ogilvie G, Mirabello L, et al. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. (2018) 143:735–45. doi: 10.1002/ijc.31261
Keywords: HPV – human papillomavirus, mRNA, cervical cancer, sensitiviity, specificity
Citation: Zhang S-K, Guo Z, Wang P, Kang L-N, Jia M-M, Wu Z-N, Chen Q, Cao X-Q, Zhao D-M, Guo P-P, Sun X-B, Zhang J-G and Qiao Y-L (2020) The Potential Benefits of HPV E6/E7 mRNA Test in Cervical Cancer Screening in China. Front. Oncol. 10:533253. doi: 10.3389/fonc.2020.533253
Received: 07 February 2020; Accepted: 11 September 2020;
Published: 02 October 2020.
Edited by:
Tianhui Chen, University of Chinese Academy of Sciences, ChinaReviewed by:
Sunyoung Park, Yonsei University, South KoreaYimin Zhu, Zhejiang University, China
Sveinung Wergeland Sorbye, University Hospital of North Norway, Norway
Copyright © 2020 Zhang, Guo, Wang, Kang, Jia, Wu, Chen, Cao, Zhao, Guo, Sun, Zhang and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Gong Zhang, emhhbmdqZ0B6enUuZWR1LmNu; You-Lin Qiao, cWlhb3lAY2ljYW1zLmFjLmNu
 Shao-Kai Zhang1
Shao-Kai Zhang1 Peng Wang
Peng Wang Qiong Chen
Qiong Chen Xi-Bin Sun
Xi-Bin Sun You-Lin Qiao
You-Lin Qiao