- 1Division of Breast Surgery, Department of Surgery, College of Medicine, Seoul St Mary's Hospital, The Catholic University of Seoul, Seoul, South Korea
- 2Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea
- 3Department of Surgery, College of Medicine, Yeungnam University, Daegu, South Korea
Introduction: Best surgical approach of axillary staging remains controversial in locally recurrent breast cancer. We evaluated the reliability of repeat sentinel lymph node biopsy (reSLNB) in patients with ipsilateral breast tumor recurrence (IBTR) after breast conserving surgery (BCS) with sentinel lymph node biopsy (SLNB) in terms of identification rate (IR) and false negative rate (FNR). To address the FNR, we identified patients who underwent sequential axillary lymph node dissection (ALND) after reSLNB.
Methods: A systematic search of PubMed, EMBASE, and Cochrane Library were conducted to identify patient-level data from articles. We searched for data of patients who underwent BCS with SLNB for primary breast cancer and who underwent sequential ALND after reSLNB due to local recurrence. Patients data was also identified by the same criteria at two institutions.
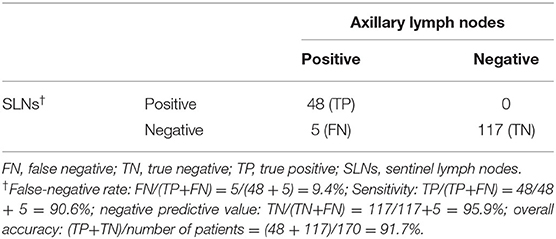
Results: In total, 197 peer-reviewed publications were obtained, of which 20 included patients who met the eligibility criteria. Data from 464 patients were collected. From the two institutions, 31 patients were identified. A total of 495 patients were pooled. The IR of reSLNB was 71.9% (356/495). To address the FNR of reSLNB, 171 patients who underwent ALND after reSLNB were identified. The FNR and accuracy of reSLNB were 9.4% (5/53) and 97.1% (165/170), respectively.
Conclusion: Our pooled data analysis showed that the FNR of reSLNB is lower than 10%, indicating that this operation is a reliable axillary surgery in patients with IBTR after they underwent BCS.
Introduction
Metastasis in axillary lymph nodes is the most important prognostic factor in patients with breast cancer (1). In the past, axillary lymph node dissection (ALND) has been the standard approach for axillary surgery in breast cancer. However, ALND is associated with short-term and long-term morbidities (2–5). Patients treated with sentinel lymph node biopsy (SLNB) have significantly lower post-operative complication such as lymphedema, infection, seroma, and numbness compared to those with ALND (6). Among these complication, lymphedema is one of the most common complication after ALND, and adversely affects the quality of life. Despite of different definition and measurement, the incidence of ALND has been reported up to 56% (7). Nevertheless, the benefits of ALND are limited because most patients with early stage breast cancer are node-negative. SLNB is a less invasive procedure; it can replace ALND in patients with clinically node-negative breast cancer. SLNB has been reported to have a >90% identification rate (IR) and <10% false-negative rate (FNR) (8, 9). Previous studies have reported that SLNB can accurately predict the status of the remaining axillary lymph nodes (10–12).
Because of these advantages, SLNB plays an integral role in the axillary staging for the surgical management of patients with early breast cancer. However, the role of SLNB remains controversial in the surgical management of patients with local recurrence. Ipsilateral breast tumor recurrence (IBTR) after breast conserving surgery (BCS) as the initial surgery has gradually increased; this happens because BCS is currently performed in two of every three cases of surgery for primary breast cancer (13). The 10 year-local recurrence rate after BCS or mastectomy has been reported to be 2–10% (14–16).
For removal of recurred breast lesions in remained breast after BCS, total mastectomy or second lumpectomy can be performed (17). For concurrent axillary surgery, repeat SLNB (reSLNB) might be considered (18, 19). However, most patients with IBTR have a history of undergoing SLNB and radiotherapy that could interrupt their lymphatic channels. Evidence concerning the role of reSLNB for IBTR is still lacking despite the results of previous studies (20–23). The vast majority of earlier studies included few patients and had a retrospective design. In addition, studies that included patients who underwent mastectomy or ALND were heterogeneous (21–23).
In the present study, we focused on the reliability of reSLNB in patients who underwent BCS and SLNB without ALND as the initial surgery. To address the FNR of reSLNB, we further identified patients with IBTR who underwent ALND after reSLNB. To achieve this goal, we conducted a pooled analysis using data from a systematic review and two institutions.
Methods
Search Strategy
A literature search was performed in PubMed, Embase, and Cochrane Library databases of systemic review. All articles including case reports and original articles were searched. These articles were found using the following search terms in the databases: “ipsilateral breast tumor recurrence,” “locally recurrent breast cancer,” “recurrent breast cancer,” “sentinel lymph node biopsy,” “lymphatic mapping,” “repeat,” and “re-operative” (see Search Terms). The articles were independently selected by two researchers, and the literature search was conducted until April 2018.
Definition, Inclusion Criteria, and Data Extraction for reSLNB
IBTR was defined as recurred breast tumors or new ipsilateral primary breast tumors because it was impossible to distinguish the two diagnoses. A positive reSLNB outcome was defined as the presence of micro-metastasis (>0.2 mm and/or >200 cells, but not larger than 2 mm) and macro-metastasis, according to the American Joint Committee on Cancer, 7th edition (24). Isolated tumor cells (clusters of cells <0.2 mm and/or <200 cells) were defined as node-negative. The most selected articles did not specify exact radiation field and dose. Thus, we excluded analysis of radiotherapy because we could not distinguish whether a patient received radiation on the breast and/or axilla.
Patients included in this analysis had to meet the following criteria: (i) history of BCS for former breast cancer or ductal carcinoma in situ with histologically clear margins and of SLNB without ALND, (ii) IBTR or new ipsilateral primary breast tumor, and (iii) reSLNB and sequential ALND to assess the FNR of reSLNB. The following cases were excluded from the analysis: (i) presence of distant metastasis, and (ii) presence of inflammatory breast cancer. Even if the first and second operations were performed at different hospitals, patients were included if medical records from both hospitals were confirmed (see Information data extraction). With the corrected data, we attempted to answer the question (see Review questions).
This study was guided by the Preferred Reporting Items for Systemic Reviews and Meta-analyses (PRISMA) statement (25). The selection process with PRISMA standards in our study is depicted in Figure 1. All articles were searched independently by Chang Ik Yoon and Sung Gwe Ahn. In the literature search, patient-level data were collected. Articles not published in English, articles in which full-text articles were unavailable, review articles, duplicated articles, commentaries, editorials, poster, conference papers, and letters to the editor were excluded. Discrepancies were resolved through discussion (Chang Ik Yoon and Sung Gwe Ahn). Data obtained from the literature search and two institutions, Gangnam Severance Hospital and Yeungnam University Hospital, were analyzed together. Inclusion and exclusion criteria for patient data from the two institutions were the same as those mentioned above. The injection methods, doses, and sites for lymphatic mapping varied among studies (Table 1 and Supplementary Table 1). The injection and SLNB protocol at Gangnam Severance Hospital and Yeungnam University was as follows. A radioisotope was injected into the subdermal layer of the periareolar site 15 min before surgery. Sentinel lymph nodes (SLNs) were identified and removed using a Gamma-ray detecting probe. Sequential ALND was performed in all patients after reSLNB. Breast surgery was performed with second lumpectomy or mastectomy. Harvested SLNs were fixed using 10% formalin solution. Each SLN was sectioned into 2–3-mm-thick slices, and all slices were frozen and examined microscopically.
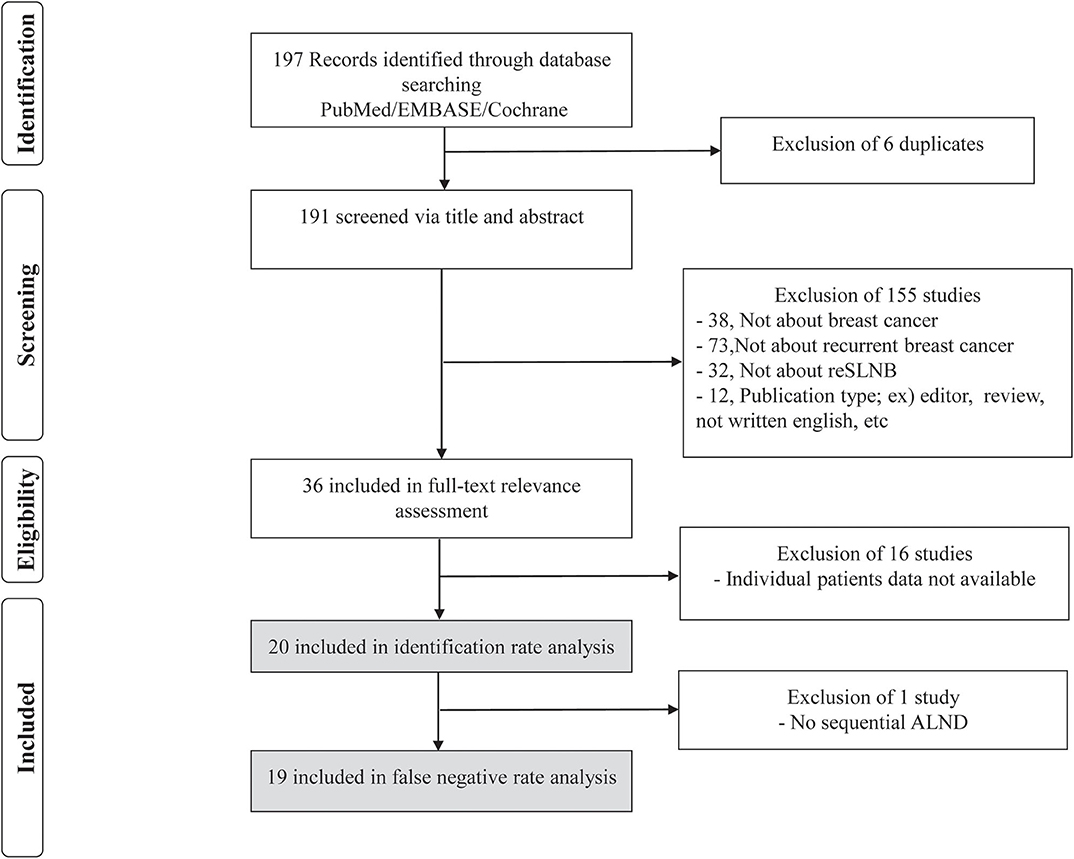
Figure 1. Flowchart for the search strategy on repeat sentinel lymph node biopsy in patients with local recurrence. Our search strategy found 197 abstracts. Of these 197 abstracts, 6 were excluded due to duplication, and 155 were excluded based on predefined exclusion criteria. The remaining 36 articles were fully reviewed, of which 16 were excluded because they failed to meeting inclusion criteria.
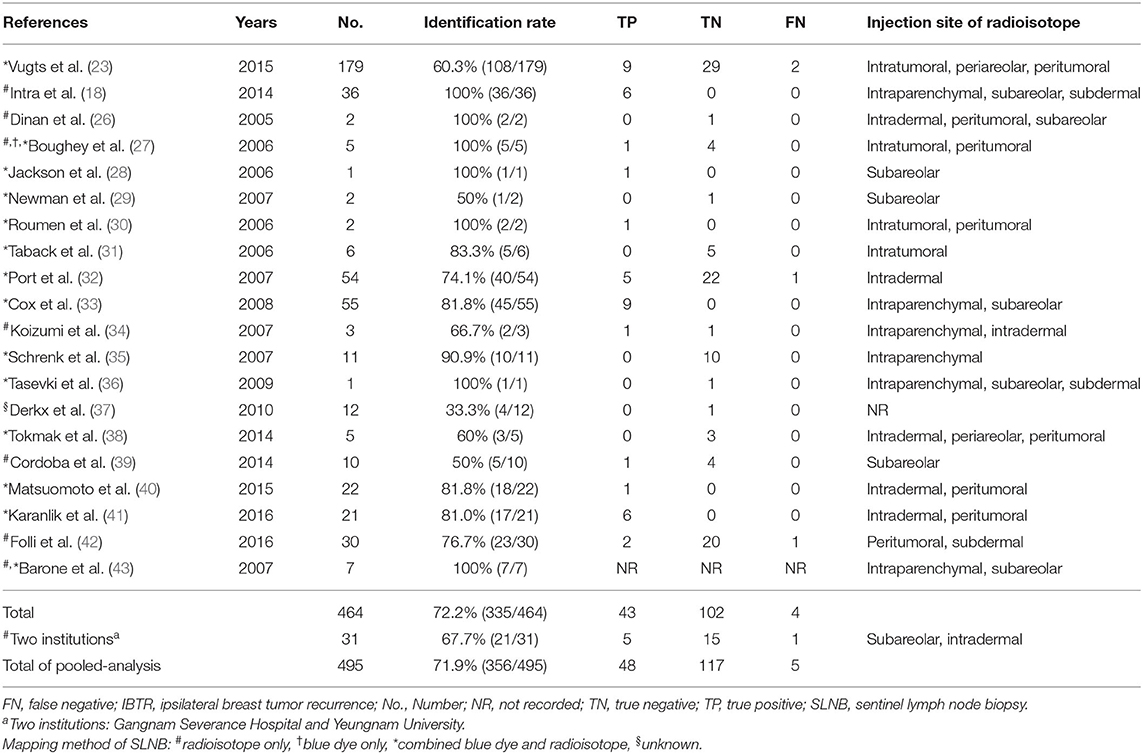
Table 1. Information of publication year, number of patients, identification rate and pathologic status for 20 studies on repeat sentinel lymph node biopsy (reSLNB).
The study was conducted in accordance with the good clinical practice guidelines and the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Gangnam Severance Hospital (Local IRB number: 3-2018-0344).
Statistical Analysis
IR of reSLNB was defined as the number of successful cases divided by the total number of patients who underwent reSLNB. The FNR, accuracy, true-positive rate, and negative predictive value of reSLNB were calculated, respectively. The FNR of reSLNB is considered too high if the FNR is <10%. Differences in IR according to the mapping methods (dual mapping/radioisotope only/blue dye only) were compared using the chi-square test. SPSS version 23 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. Statistical significance was defined as p-value of <0.05.
Results
Search Results
In PubMed, EMBASE, and Cochrane database, we found 194 articles using the above mentioned searching terms (Figure 1). All articles retrieved from Embase and Cochrane Library databases were included to those extracted from PubMed. Adding the three articles from references in the previous meta-analysis of reSLNB (21, 22), a total of 197 articles were initially identified. Of these, there were six duplicated articles. A total of 191 abstracts were reviewed, and 155 abstracts were excluded for the following reasons: (i) not about breast cancer (n = 38), (ii) not about recurrent breast cancer (n = 73), (iii) not about reSLNB (n = 32), (iv) inappropriate publication types such as editorial, review, or articles not written in English (n = 12). A full-text review of 36 articles was conducted. A total of 20 articles finally met the inclusion criteria. From these, 19 articles analyzed the FNR of reSLNB. These articles were published from 2005 to 2016 (Table 1).
In addition, from 1995 to 2017, a total of 31 patients with IBTR after BCS met the inclusion criteria in the Gangnam Severance Hospital and Yeungnam University Hospital databases. These patients underwent reSLNB for their axillary staging.
Identification Rate of reSLNB
A total of 464 cases of reSLNB were found in the literature search (Figure 2). Of these, 335 were successful. The IR of reSLNB in articles was 72.2% (335/464). Among the 31 cases of reSLNB performed at the two institutions, 10 cases of sentinel failure occurred. The IR was 67.7% (21/31). The total IR of the pooled analysis was 71.9% (356/495) (Table 1).
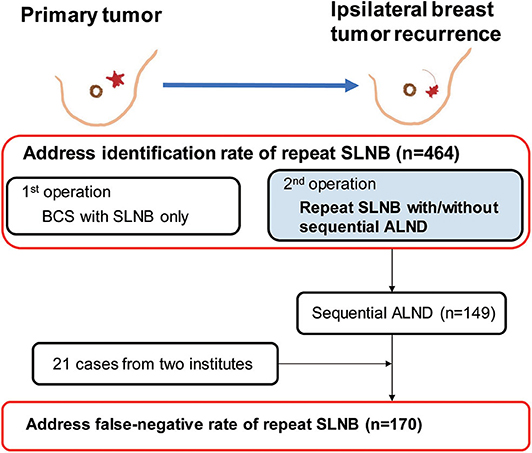
Figure 2. Flowchart for data curation in order to address the identification rate and false-negative rate of repeat sentinel lymph node biopsy. A total of 1,212 cases of 20 articles were found, 464 subjects met inclusion criteria about first breast cancer surgery. Among these, repeat sentinel lymph node was performed in 335 patients, resulting in 72.2% of identification rate for reSLNB. Except 1 article, sequential ALND was performed in 149 patients in 19 articles. Then we added 21 patients who underwent sequential ALND after reSLNB from two institutions. Finally, a total of 170 patients were analyzed for false-negative rate of reSLNB. BCS, breast conserving surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
The IR according to mapping tracers was described in Supplementary Table 2. The IR of dual mapping was 69.9% (251/359) and that of single mapping with a radioisotope was 79.5% (89/112). In three other reports where tracers were not clearly distinguished, the IR was 66.7%. There was no significant difference in IR according to mapping tracers (Supplementary Table 2, p = 0.122).
FNR/Diagnostic Performance of reSLNB
In 19 articles, the results of ALND following reSLNB were obtained from patient-level data of 149 patients (Figure 2). True positive-, false negative-, and true negative cases of reSLNB were 43, 4, and 102, respectively (Table 1). In addition, data from 21 patients with sequential ALND of the two institutions were added.
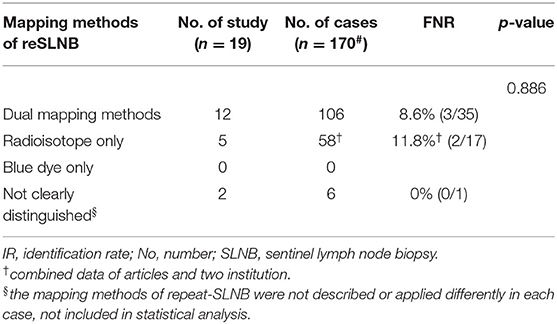
In a total of 170 patients who underwent ALND after reSLNB, the overall FNR was 9.4% (5/53) (Table 2). The overall accuracy, true positive rate (same as sensitivity), and negative predictive value of reSLNB were 97.1, 90.6, and 95.9%, respectively (Table 2). The FNRs of reSLNB using single or dual tracers were 11.8% (2/17) and 8.6% (3/35), respectively (Table 3). There were no statistically significant differences in FNR of reSLNB according to mapping method (Table 3, p = 0.886).
Discussion
In primary breast cancer with early stage, SLNB has been preferred procedure for axillary staging because it offers oncologic safety with fewer complications such as lymphedema, pain, range of motion, and sensory defect compared to ALND (5, 44, 45). However, in locally recurrent breast cancer, it lacks evidence that reSLNB could be performed for axillary staging method in terms of FNR. Since a large multi-institutional randomized study showed that the FNR of SLNB was 9.8% in clinically node-negative breast cancer (4), recent trials aimed to demonstrate a safety of SLNB if the FNR wound not be >10% in clinically node-positive breast cancer treated with preoperative chemotherapy (46–48). On the basis of these studies, we consider that reSLNB would be acceptable if the FNR of reSLNB is not >10%.
With this background, our pooled analysis used abundant data concerning reSLNB performed in patients with IBTR and demonstrated that the procedure was reliable. The FNR was 9.4%, and followed by an accuracy of 97.1% and a negative predictive value of 95.9%, although the IR was low as 71.9%
Our study has several strengths compared with previous studies evaluating reSLNB for locally recurred breasts cancer. The FNR was accurately addressed as back-up ALND was conducted after successful reSLNB in about half of patients. To address the FNR of SLNB, ALND is an inevitable procedure to rule out the chance of metastases in lymph nodes not retrieved by SLNB. However, the vast majority of patients included in earlier studies underwent axillary staging through reSLNB alone. An accurate assessment of FNR of reSLNB by sequential ALND for patients with IBTR is the novelty of our study.
In addition, we only included patients with true IBTR. Our study population underwent BCS and SLNB alone for their primary breast cancer. The homogeneity of the first surgery is distinguished from that in other studies including patients who underwent mastectomy or ALND. Moreover, we enrolled a relatively large number of patients from previously published articles and local institutional database that has strength in delicate information of patients.
Traditionally, in patients with IBTR, complete axillary clearance has been considered essential, regardless of axillary nodal involvement. However, recent advances in non-invasive diagnostic imaging have raised questions against whether sequential ALND is mandatory because more than half of the patients with IBTR had no axillary metastases (21, 22). Thus, many investigators have interests in de-escalating axillary surgery, and may accept the concept of limited axillary management (19), as long as credible sentinel lymph node detection is guaranteed. Because, in cases of IBTR, preceding axillary surgery may lead to disruption of lymphatic flow that undermines reliability of reSLNB. Also, another study reported that aberrant lymphatic drainage was visualized in two-fifths of the patients with locally recurred cancer (43.2%) (21).
However, SLNB is a minimally invasive method and has a lower chance of fully destroying common lymphatic routes from the breast to axillary SLNs than ALND. It is at least indirectly supported by the results of a previous study in which the rate of aberrant lymphatic drainage was significantly lower in patients with a history of SLNB than in those with a history of ALND (17.4 vs. 69.2%) (21).
Moreover, some studies provided evidence that a few common afferent lymphatic channels exist and drain breast tumors to axillary SLNs through several major lymphatic trunks (49, 50), implying a possibility of an alternative path from the breast to axillary lymph nodes after previous axillary surgery. Our data showed that reSLNB was successfully performed in 71.9% of the patients with IBTR, suggesting that lymphatic tracts between the breast and SLNs are intact in more than two-third of patients undergoing previous SLNB. As a consequence, reSLNB could be more reliably performed in patients with a history of SLNB alone.
A fundamental limitation of this study was the heterogeneity among the included studies. There are several differences such as surgical techniques, mapping methods of SLNB, and radiation therapy. Regarding prior radiotherapy affecting lymphatic drainage, information was missed in most patients from the articles, although a majority of patients might be treated with radiotherapy after breast conservative surgery. In addition, most articles had very few patients and a retrospective design. Also, we did not perform a statistical analysis to confirm heterogeneity among studies due to the study design of pooled data analysis which collected data of identifiable patients in each study. Despite these limitations, our study was a large-scale pooled analysis that showed that reSLNB is reliable for axillary staging in patients with IBTR and who were formerly treated with BCS and SLNB.
In conclusion, our study found that the reSLNB FNR is lower than 10% indicating that this procedure is reliable for axillary staging in patients with IBTR, even though they already underwent SLNB. It could be a feasible axillary surgery in these patients like those with primary cancer. Further validation through prospectively designed studies is warranted for these findings.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The study was conducted in accordance with the good clinical practice guidelines and the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Gangnam Severance Hospital (Local IRB number: 3-2018-0344). The need for informed consent was waived under the approval of the IRB due to the retrospective design.
Author Contributions
CY, SA, and JJ contributed conception and design of the study. CY, SA, JC, and SB organized the database. DK, CC, and SP assisted to first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Search Terms in the Databases
In PubMed
((((((ipsilateral breast tumor recurrence) OR locally recurrent breast cancer) OR recurrent breast cancer)) AND (((“Sentinel Lymph Node Biopsy”) OR sentinel lymph node biopsy) OR lymphatic mapping))) OR (((((“Sentinel Lymph Node Biopsy”) OR sentinel lymph node biopsy) OR lymphatic mapping)) AND ((repeat) OR re-operative)).
In Embase
(ipsilateral AND breast AND tumor AND recurrence OR (locally AND recurrent AND breast AND cancer) OR (recurrent AND breast AND cancer)) AND (sentinel AND lymph AND node AND biopsy OR (lymphatic AND mapping)) AND (repeat OR “re operative”).
In Cochrane Library Database
((ipsilateral breast tumor recurrence) OR (locally recurrent breast cancer)) OR ((recurrent breast cancer) OR (sentinel lymph node biopsy) OR (lymphatic mapping)) AND ((repeat) OR (re-operative)).
Information Data Extraction: The Following Information Was Collected
1. Number of patients with ipsilateral breast tumor recurrence (locally recurrent breast cancer)
2. Primary breast treatment: mastectomy with breast conserving surgery/lumpectomy
3. Primary axillary treatment: sentinel lymph node biopsy (SLNB), axillary lymph node dissection (ALND), or none
4. Adjuvant radiotherapy after primary event
5. Secondary axillary treatment: repeat sentinel lymph node biopsy (reSLNB), sequential ALND, or none
6. Mapping methods of reSLNB
7. Identification rate and false negative rate of reSLNB
8. Pathologic status of reSLNB
9. Sequential ALND and pathologic outcome.
Review Questions: With the Extracted Data, an Attempt Was Made to Answer the Following Questions
1. What is the identification rate for a reSLNB?
2. What is the mapping methods for the reSLNB procedure?
3. What is pathologic status of the repeat sentinel lymph node (reSLN)?
4. What is the false-negative rate and the identification of reSLNB?
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.518568/full#supplementary-material
Abbreviations
reSLNB, repeat sentinel lymph node biopsy; IBTR, ipsilateral breast tumor recurrence; BCS, breast conserving surgery; SLNB, sentinel lymph node biopsy; IR, identification rate; FNR, false negative rate; ALND, axillary lymph node dissection; PRISMA, Preferred Reporting Items for Systemic Reviews and Meta-analysis; SLN, sentinel lymph node.
References
1. Fisher ER, Costantino J, Fisher B, Redmond C. Pathologic findings from the national surgical adjuvant breast project (Protocol 4). Discriminants for 15-year survival. National Surgical Adjuvant Breast and Bowel Project Investigators. Cancer. (1993) 71(6 Suppl.):2141–50. doi: 10.1002/1097-0142(19930315)71:6+<2141::aid-cncr2820711603>3.0.co;2-f
2. Roses DF, Brooks AD, Harris MN, Shapiro RL, Mitnick J. Complications of level I and II axillary dissection in the treatment of carcinoma of the breast. Ann Surgery. (1999) 230:194–201. doi: 10.1097/00000658-199908000-00009
3. Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. (2006) 95:279–93. doi: 10.1007/s10549-005-9025-7
4. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. (2007) 8:881–8. doi: 10.1016/S1470-2045(07)70278-4
5. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J Clin Oncol. (2007) 25:3657–63. doi: 10.1200/JCO.2006.07.4062
6. Kell MR, Burke JP, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta-analysis. Breast Cancer Res Treat. (2010) 120:441–7. doi: 10.1007/s10549-009-0705-6
7. Sakorafas GH, Peros G, Cataliotti L, Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. (2006) 15:153–65. doi: 10.1016/j.suronc.2006.11.003
8. Clarke D, Newcombe RG, Mansel RE. The learning curve in sentinel node biopsy: the ALMANAC experience. Ann Surg Oncol. (2004) 11 (3 Suppl):211s−5s. doi: 10.1007/BF02523631
9. McMasters KM, Wong SL, Chao C, Woo C, Tuttle TM, Noyes RD, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg. (2001) 234:292–9. doi: 10.1097/00000658-200109000-00003
10. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. (1994) 220:391–8. doi: 10.1097/00000658-199409000-00015
11. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer–a multicenter validation study. N Engl J Med. (1998) 339:941–6. doi: 10.1056/NEJM199810013391401
12. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. (1997) 349:1864–7. doi: 10.1016/S0140-6736(97)01004-0
13. Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. (2010) 28:3437–41. doi: 10.1200/JCO.2009.27.6774
14. Arriagada R, Le MG, Guinebretiere JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol. (2003) 14:1617–22. doi: 10.1093/annonc/mdg452
15. Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. (2014) 21:717–30. doi: 10.1245/s10434-014-3480-5
16. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. (2002) 347:1227–32. doi: 10.1056/NEJMoa020989
17. Burger AE, Pain SJ, Peley G. Treatment of recurrent breast cancer following breast conserving surgery. Breast J. (2013) 19:310–8. doi: 10.1111/tbj.12105
18. Intra M, Viale G, Vila J, Grana CM, Toesca A, Gentilini O, et al. Second axillary sentinel lymph node biopsy for breast tumor recurrence: experience of the european institute of oncology. Ann Surg Oncol. (2015) 22:2372–7. doi: 10.1245/s10434-014-4282-5
19. Ugras S, Matsen C, Eaton A, Stempel M, Morrow M, Cody HS 3rd. Reoperative sentinel lymph node biopsy is feasible for locally recurrent breast cancer, but is it worthwhile? Ann Surg Oncol. (2016) 23:744–8. doi: 10.1245/s10434-015-5003-4
20. Kothari MS, Rusby JE, Agusti AA, MacNeill FA. Sentinel lymph node biopsy after previous axillary surgery: a review. Eur J Surg Oncol. (2012) 38:8–15. doi: 10.1016/j.ejso.2011.10.003
21. Maaskant-Braat AJ, Voogd AC, Roumen RM, Nieuwenhuijzen GA. Repeat sentinel node biopsy in patients with locally recurrent breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Res Treat. (2013) 138:13–20. doi: 10.1007/s10549-013-2409-1
22. Poodt IGM, Vugts G, Schipper RJ, Nieuwenhuijzen GAP. Repeat sentinel lymph node biopsy for ipsilateral breast tumor recurrence: a systematic review of the results and impact on prognosis. Ann Surg Oncol. (2018) 25:1329–39. doi: 10.1245/s10434-018-6358-0
23. Vugts G, Maaskant-Braat AJ, Voogd AC, van Riet YE, Roumen RM, Luiten EJ, et al. Improving the success rate of repeat sentinel node biopsy in recurrent breast cancer. Ann Surg Oncol. (2015) 22(Suppl. 3):S529–35. doi: 10.1245/s10434-015-4787-6
24. Stephen B. Edge DRB, Compton CC, Fritz AG, Greene FL, Trotti III A. AJCC cancer Staging Handbook, From the AJCC Cancer Staging Manual. 7th ed. Chicago, IL: Springer (2009). p. 417–60.
25. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
26. Dinan D, Nagle CE, Pettinga J. Lymphatic mapping and sentinel node biopsy in women with an ipsilateral second breast carcinoma and a history of breast and axillary surgery. Am J Surg. (2005) 190:614–7. doi: 10.1016/j.amjsurg.2005.06.025
27. Boughey JC, Ross MI, Babiera GV, Bedrosian I, Feig BW, Hwang RF, et al. Sentinel lymph node surgery in locally recurrent breast cancer. Clin Breast Cancer. (2006) 7:248–53. doi: 10.3816/CBC.2006.n.037
28. Jackson BM, Kim S, Davidson R, Schuchter L, Acs G, Czerniecki BJ. Repeat operative sentinel lymph node biopsy. Clin Breast Cancer. (2006) 6:530–2. doi: 10.3816/CBC.2006.n.007
29. Newman LA. Lymphatic mapping and sentinel lymph node biopsy for locally recurrent breast cancer: new clues to understanding the biology of chest wall relapse. Ann Surg Oncol. (2007) 14:2182–4. doi: 10.1245/s10434-007-9374-z
30. Roumen RM, Kuijt GP, Liem IH. Lymphatic mapping and sentinel node harvesting in patients with recurrent breast cancer. Eur J Surg Oncol. (2006) 32:1076–81. doi: 10.1016/j.ejso.2006.08.007
31. Taback B, Nguyen P, Hansen N, Edwards GK, Conway K, Giuliano AE. Sentinel lymph node biopsy for local recurrence of breast cancer after breast-conserving therapy. Ann Surg Oncol. (2006) 13:1099–104. doi: 10.1245/ASO.2006.08.026
32. Port ER, Garcia-Etienne CA, Park J, Fey J, Borgen PI, Cody HS 3rd. Reoperative sentinel lymph node biopsy: a new frontier in the management of ipsilateral breast tumor recurrence. Ann Surg Oncol. (2007) 14:2209–14. doi: 10.1245/s10434-006-9237-z
33. Cox CE, Furman BT, Kiluk JV, Jara J, Koeppel W, Meade T, et al. Use of reoperative sentinel lymph node biopsy in breast cancer patients. J Am Coll Surg. (2008) 207:57–61. doi: 10.1016/j.jamcollsurg.2008.01.017
34. Koizumi M, Koyama M, Tada K, Nishimura S, Miyagi Y, Makita M, et al. The feasibility of sentinel node biopsy in the previously treated breast. Eur J Surg Oncol. (2008) 34:365–8. doi: 10.1016/j.ejso.2007.04.007
35. Schrenk P, Tausch C, Wayand W. Lymphatic mapping in patients with primary or recurrent breast cancer following previous axillary surgery. Eur J Surg Oncol. (2008) 34:851–6. doi: 10.1016/j.ejso.2007.11.006
36. Tasevski R, Gogos AJ, Mann GB. Reoperative sentinel lymph node biopsy in ipsilateral breast cancer relapse. Breast. (2009) 18:322–6. doi: 10.1016/j.breast.2009.09.009
37. Derkx F, Maaskant-Braat AJ, van der Sangen MJ, Nieuwenhuijzen GA, van de Poll-Franse LV, Roumen RM, et al. Staging and management of axillary lymph nodes in patients with local recurrence in the breast or chest wall after a previous negative sentinel node procedure. Eur J Surg Oncol. (2010) 36:646–51. doi: 10.1016/j.ejso.2010.05.009
38. Tokmak H, Kaban K, Muslumanoglu M, Demirel M, Aktan S. Management of sentinel node re-mapping in patients who have second or recurrent breast cancer and had previous axillary procedures. World J Surg Oncol. (2014) 12:205. doi: 10.1186/1477-7819-12-205
39. Cordoba O, Perez-Ceresuela F, Espinosa-Bravo M, Cortadellas T, Esgueva A, Rodriguez-Revuelto R, et al. Detection of sentinel lymph node in breast cancer recurrence may change adjuvant treatment decision in patients with breast cancer recurrence and previous axillary surgery. Breast. (2014) 23:460–5. doi: 10.1016/j.breast.2014.03.007
40. Matsumoto A, Jinno H, Nakamura T, Saito J, Takahashi M, Hayashida T, et al. Technical feasibility of sentinel lymph node biopsy in patients with ipsilateral breast tumor recurrence and previous axillary surgery. Int J Surg. (2015) 22:28–31. doi: 10.1016/j.ijsu.2015.07.709
41. Karanlik H, Ozgur I, Kilic B, Fathalizadeh A, Sanli Y, Onder S, et al. Sentinel lymph node biopsy and aberrant lymphatic drainage in recurrent breast cancer: findings likely to change treatment decisions. J Surg Oncol. (2016) 114:796–802. doi: 10.1002/jso.24423
42. Folli S, Falco G, Mingozzi M, Buggi F, Curcio A, Ferrari G, et al. Repeat sentinel lymph node biopsy in patients with ipsilateral recurrent breast cancer after breast-conserving therapy and negative sentinel lymph node biopsy: a prospective study. Minerva Chirurgica. (2016) 71:73–9. Available online at: https://www.researchgate.net/publication/280388560
43. Barone JL, Feldman SM, Estabrook A, Tartter PI, Rosenbaum Smith SM, Boolbol SK. Reoperative sentinel lymph node biopsy in patients with locally recurrent breast cancer. Am J Surg. (2007) 194:491–3. doi: 10.1016/j.amjsurg.2007.07.011
44. Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection vs. axillary dissection. J Surg Oncol. (2010) 102:111–8. doi: 10.1002/jso.21535
45. Gill G. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy vs. axillary clearance (SNAC): a randomized controlled surgical trial. Ann Surg Oncol. (2009) 16:266–75. doi: 10.1245/s10434-008-0229-z
46. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. (2013) 310:1455–61. doi: 10.1001/jama.2013.278932
47. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. (2013) 14:609–18. doi: 10.1016/S1470-2045(13)70166-9
48. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. (2015) 33:258–64. doi: 10.1200/JCO.2014.55.7827
49. Goyal A, Mansel RE. Multifocality and sentinel node biopsy in breast cancer. Eur J Surg Oncol. (2004) 30:3–4. doi: 10.1016/j.ejso.2003.10.022
Keywords: repeat sentinel lymph node biopsy, false negative rate (FNR), recurrent breast cancer, identification rate (IR), SLNB
Citation: Yoon CI, Ahn SG, Kim D, Choi JE, Bae SJ, Cha CH, Park S and Jeong J (2020) Repeat Sentinel Lymph Node Biopsy for Ipsilateral Breast Tumor Recurrence After Breast Conserving Surgery With Sentinel Lymph Node Biopsy: Pooled Analysis Using Data From a Systematic Review and Two Institutions. Front. Oncol. 10:518568. doi: 10.3389/fonc.2020.518568
Received: 08 January 2020; Accepted: 18 August 2020;
Published: 23 September 2020.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Ziv Radisavljevic, Brigham and Women's Hospital and Harvard Medical School, United StatesBenedetto Ielpo, Hospital del Mar, Spain
Copyright © 2020 Yoon, Ahn, Kim, Choi, Bae, Cha, Park and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joon Jeong, gsjjoon@yuhs.ac
†These authors have contributed equally to this work
 Chang Ik Yoon
Chang Ik Yoon Sung Gwe Ahn
Sung Gwe Ahn Dooreh Kim2
Dooreh Kim2 Soong June Bae
Soong June Bae Joon Jeong
Joon Jeong