- 1Zhejiang Taizhou Hospital, Taizhou, China
- 2Zhejiang Cancer Hospital, University of Chinese Academy of Sciences, Hangzhou, China
- 3Women's Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: The literature reports conflicting results regarding the effect of human papillomavirus (HPV) genotype 16 (HPV-16)/18 (HPV-18) positivity on cervical cancer (CC) prognosis.
Aim: To conduct a meta-analysis to examine the effect of HPV-16/18 positivity on the prognosis of patients with CC.
Methods: PubMed, Embase, and the Cochrane Library were searched for available papers published up to March 2020. The main outcome was the hazard ratio (HR) of overall survival (OS) or disease-free survival (DFS) comparing HPV-16 or HPV-18 positivity and negativity. The random-effects model was used for synthesizing survival outcomes.
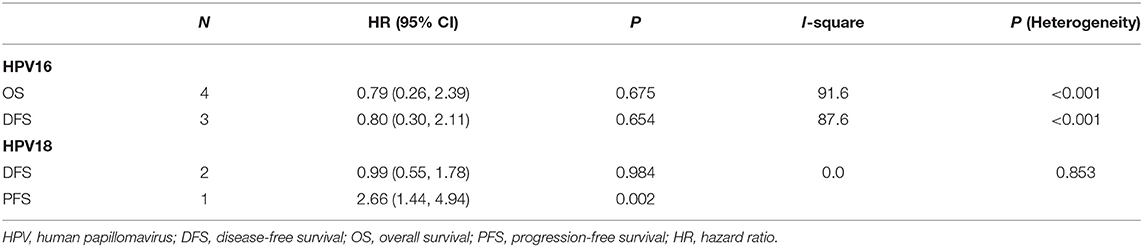
Results: Nine studies and 2,028 patients were included. Four studies reported OS in HPV-16 positivity, and no association was found between HPV-16 positivity and OS to CC (HR = 0.79, 95% CI: 0.26–2.39, P = 0.675). Three studies reported DFS in HPV-16 positivity, and no association was found between HPV-16 positivity and DFS to CC (HR = 0.80, 95% CI: 0.30–2.11, P = 0.654). Two studies reported DFS in HPV-18 positivity, and no association was found between HPV-18 positivity and DFS to CC (HR = 0.99, 95% CI: 0.55–1.78, P = 0.984). One study reported progression-free survival (PFS) in HPV-18 positivity, and an association was observed between HPV-18 positivity and PFS to CC (HR = 2.66, 95% CI: 1.44–4.94, P = 0.002). The sensitivity analyses showed that one study biased the analysis of the association between HPV-16 and OS, and another study biased the association between HPV-16 and DFS.
Conclusion: The presence of HPV-16 and HPV-18 positivity appears to have no significant association with prognosis in CC in either OS or PFS. The presence of HPV-16 or HPV-18 positivity has no significant association with prognosis in CC in either OS or PFS.
Introduction
Cervical cancer (CC) is a malignancy originating in the transformation zone of the cervix, most commonly in squamous cells (1). It is the second most common cancer in women worldwide, with an estimated 569,847 new cases in 2018, and the third most common cause of female cancer mortality, with 311,365 deaths (2, 3). CC has a strong tendency to affect young women, and the peak incidence is in the 40–49 age group (3, 4).
Infection with high-risk human papillomavirus (HPV) is a major risk factor for the development of CC (1, 4–8). It is now well-recognized that the majority of CC is associated with HPV genotypes 16 (HPV-16) and 18 (HPV-18) (6, 9–11). HPV, an epitheliotropic double-stranded DNA oncovirus, typically infects the basal layer of the epithelium through small tears in the mucosa resulting from sexual activity. Active papillomavirus infection occurs when infected basal cells replicate and fill the area. HPV synthesizes six early proteins (E1–E7) and two late capsid proteins (L1 and L2) during replication, and those proteins have immortalizing and transforming properties (1). Persistent HPV infection results in squamous intraepithelial lesions that are graded as cervical intraepithelial neoplasia (CIN) 1, CIN 2, and CIN 3 according to how much epithelium is impacted. The progression from cervical dysplasia to invasive cancer may take years or decades but has been reported to take <1 year in about 10% of patients (12).
Nevertheless, despite the sound pathogenic effect of HPV-16 and HPV-18 for CC, the prognosis of HPV-16 and HPV-18 positivity in patients with CC has not been established. A recent meta-analysis revealed that HPV DNA positivity is associated with good overall survival (OS) and disease-free survival (DFS) in patients with CC (13). Similar meta-analyses reported that HPV positivity was an indicator of favorable prognosis in head and neck cancers (14, 15), but there are studies that failed to support the relationship between HPV-16 or HPV-18 positivity and prognosis of CC (16), and some even indicate that CC associated with HPV-16/18 has a worse survival (17).
Gaining a more comprehensive insight into how the HPV type affects survival is important. We herein hypothesized that HPV-16/18 positivity is associated with poorer prognosis in patients with CC. To test our hypothesis, we conducted this meta-analysis and systematically reviewed the existing literature.
Methods
Literature Search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). The search was based on the PICO principle (19), followed by screening using a prespecified protocol and eligibility criteria: (1) population: patients with CC who had a record of HPV genotype; (2) exposure: HPV-16 or HPV-18 positivity; (3) controls: HPV-16 or HPV-18 negativity; (4) outcome: survival; and (5) full text available in English. PubMed, Embase, and the Cochrane Library were searched for available papers published up to March 2020 using the MeSH term “Uterine Cervical Neoplasms,” as well as relevant keywords.
Data Extraction
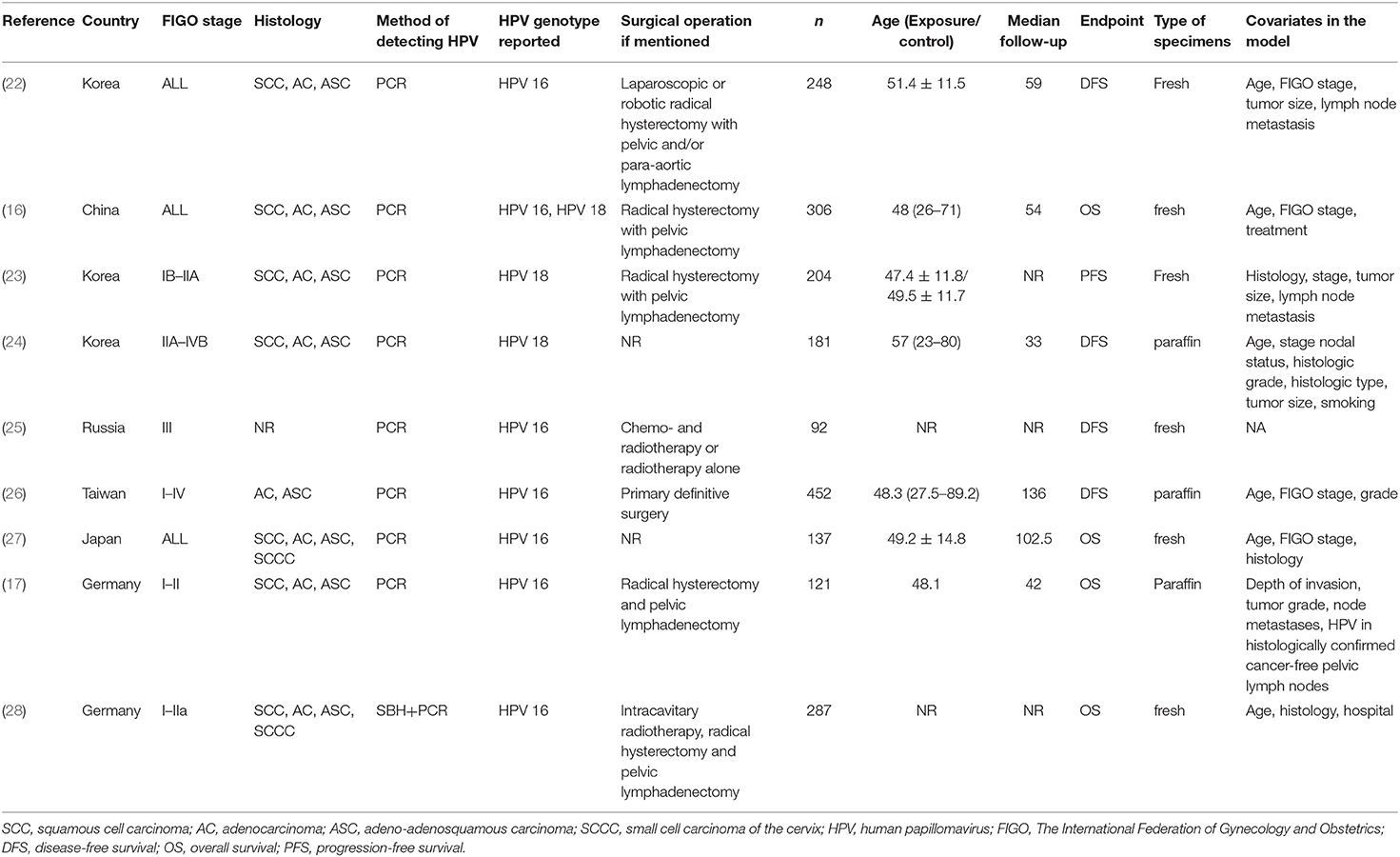
The study characteristics (authors, year of publication, country where the study was performed, median follow-up time, sample size, and mean age in each group), treatment parameters [The International Federation of Gynecology and Obstetrics (FIGO) stage of CC, histology, detection method of HPV genotype, reported HPV genotype, the operation the patients underwent, the endpoint of the study, type of specimens that were used, and covariates if a multivariable model was used], and outcome (OS and DFS) were extracted by two authors independently. Any discrepancy was solved by discussion.
Outcomes
The main outcome was the hazard ratio (HR) of OS or DFS comparing HPV-16 or HPV-18 positivity and negativity on DFS or OS.
Quality of the Evidence
The quality level of evidence of all articles was assessed independently by two authors according to the Newcastle–Ottawa scale (NOS) for cohort study (20). Discrepancies in the assessment were resolved through discussion until a consensus was reached.
Data Synthesis
The risk estimates of each study were reported as HR or relative risk (RR). We treated RRs as HRs. When possible, multi-adjusted HRs were used in the meta-analysis.
Statistical Analysis
All analyses were performed using STATA SE 14.0 (StataCorp, College Station, Texas, USA). HRs and corresponding 95% confidence intervals (CIs) were used to compare the outcomes. Statistical heterogeneity among studies was calculated using Cochran's Q-test and the I2 index. An I2 > 50% and a Q-test P < 0.10 indicated high heterogeneity, and the random-effects model was used; otherwise, the fixed-effects model was applied. P < 0.05 were considered statistically different. We did not assess potential publication bias by funnel plots and Egger's test because the numbers of studies included in each quantitative analysis were <10, in which case, the funnel plots and Egger's test could yield misleading results (21).
Results
Selection of the Studies
Figure 1 presents the selection flowchart. In the initial search, 184 records were retrieved, and 166 were screened after removing the duplicates. From them, 44 were excluded because of the publication type (notes, conference abstracts, and reviews). Then, 122 full-text papers were assessed and 113 were excluded because of study aim/design (n = 34), outcome (n = 9), population (n = 48), exposures (n = 8), full text not accessible (n = 2), meta-analyses (n = 2), and non-English (n = 10).
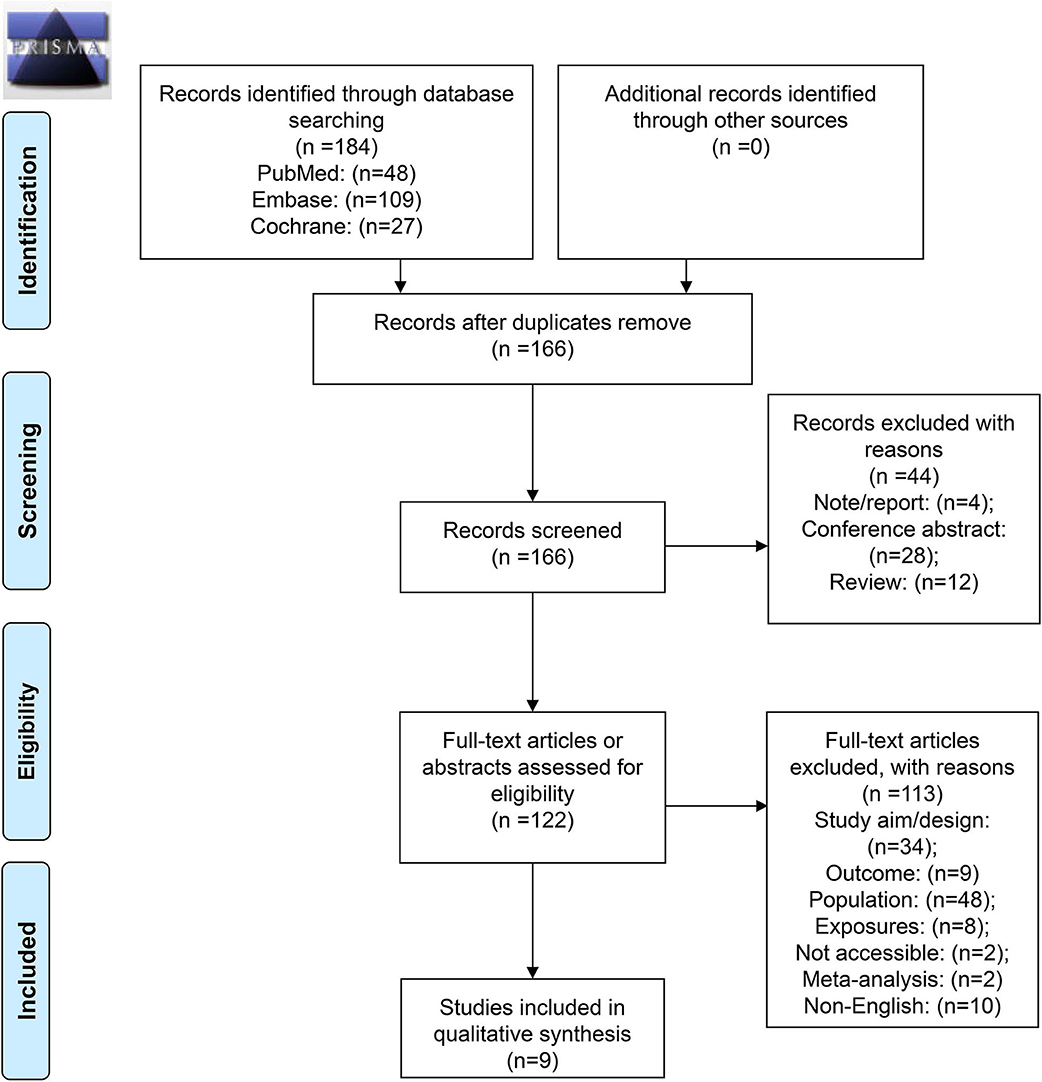
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2009 flow diagram.
Therefore, nine studies were included (16, 17, 22–28) (Table 1). Those studies included a total of 2,028 patients. The mean age range was 47–57 years. The median follow-up ranged from 33 to 136 months. Four studies scored 7 on the NOS (24, 26–28), four studies scored 8 (16, 22, 23, 25), and one study scored 9 (17) (Supplementary Table 1).
Survival According to Human Papillomavirus Subtype 16
Four studies reported OS in HPV-16 positivity (16, 17, 27, 28), and no association was found between HPV-16 positivity and OS to CC (HR = 0.79, 95% CI: 0.26–2.39, P = 0.675; I2 = 91.6%, Pheterogeneity < 0.001) (Figure 2, Table 2). Three studies reported DFS in HPV-16 positivity (22, 25, 26), and no association was found between HPV-16 positivity and DFS to CC (HR = 0.80, 95% CI: 0.30–2.11, P = 0.654; I2 = 87.6%, Pheterogeneity < 0.001; Figure 3, Table 2).
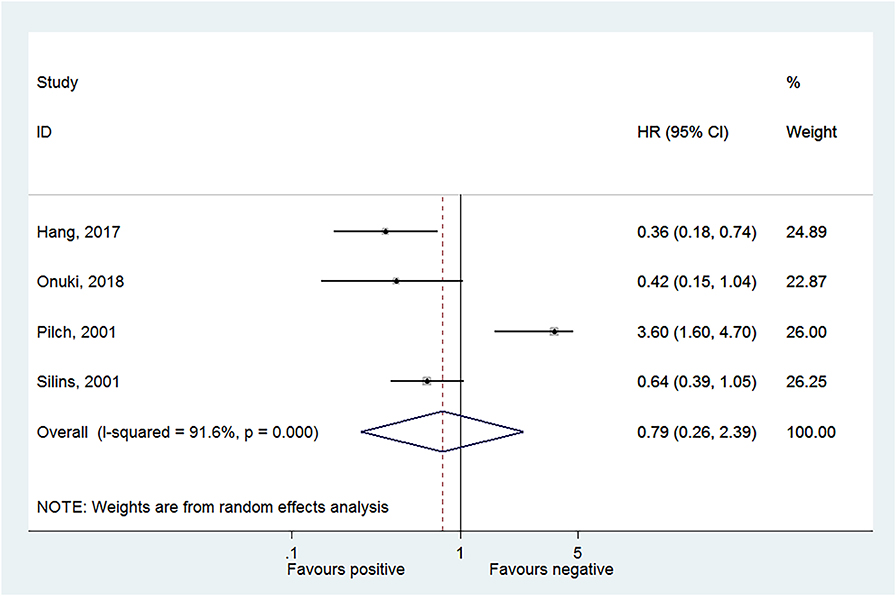
Figure 2. Forest plot of overall survival comparing the human papillomavirus subtype 16 (HPV-16) positive vs. negative groups.
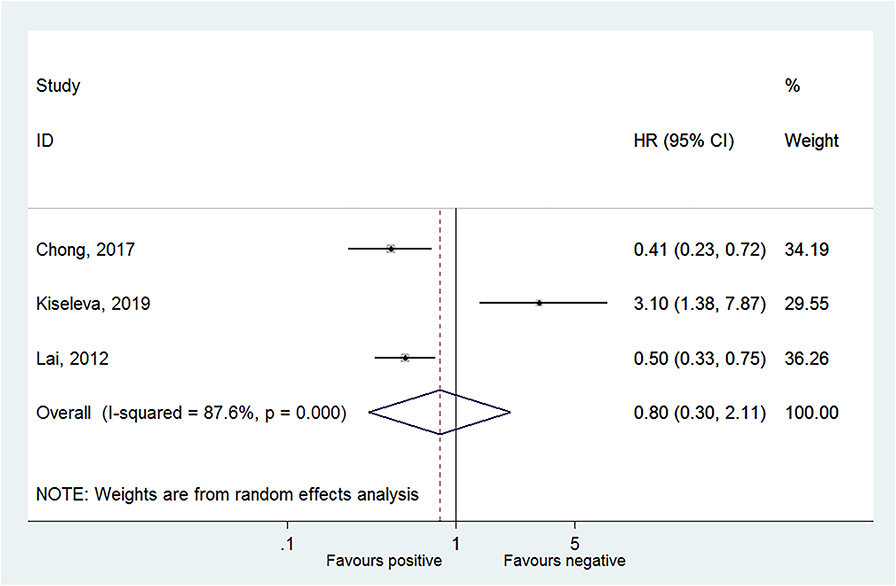
Figure 3. Forest plot of disease-free survival comparing the human papillomavirus subtype 16 (HPV-16) positive vs. negative groups.
Survival According to Human Papillomavirus Subtype 18
Two studies reported DFS in HPV-18 positivity (22, 24), and no association was found between HPV-18 positivity and DFS to CC (HR = 0.99, 95% CI: 0.55–1.78, P = 0.984; I2 = 0.0%, Pheterogeneity = 0.853; Figure 4, Table 2). One study reported progression-free survival (PFS) in HPV-18 positivity (23), and an association was observed between HPV-18 positivity and PFS to CC (HR = 2.66, 95% CI: 1.44–4.94, P = 0.002; Figure 4, Table 2).
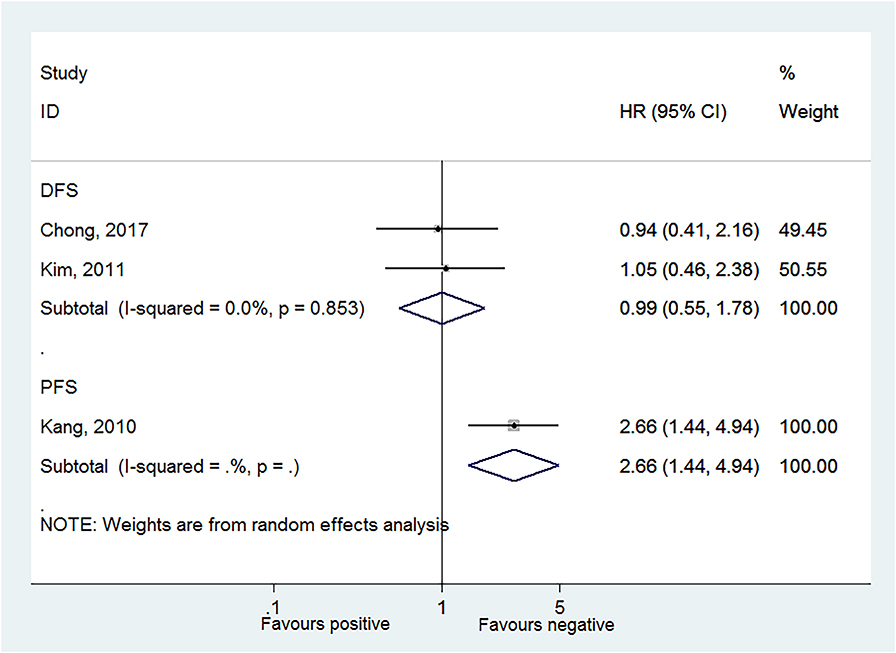
Figure 4. Forest plot of disease-free survival and progression-free survival comparing the human papillomavirus subtype 18 (HPV-18) positive vs. negative groups.
Sensitivity Analyses
Regarding the association between HPV-16 and OS, the sensitivity analysis showed that omitting Pilch et al. (17) affected the conclusion (Figure 5). Regarding the association between HPV-16 and DFS, the sensitivity analysis showed that omitting Kiseleva et al. (25) affected the conclusion (Figure 6).
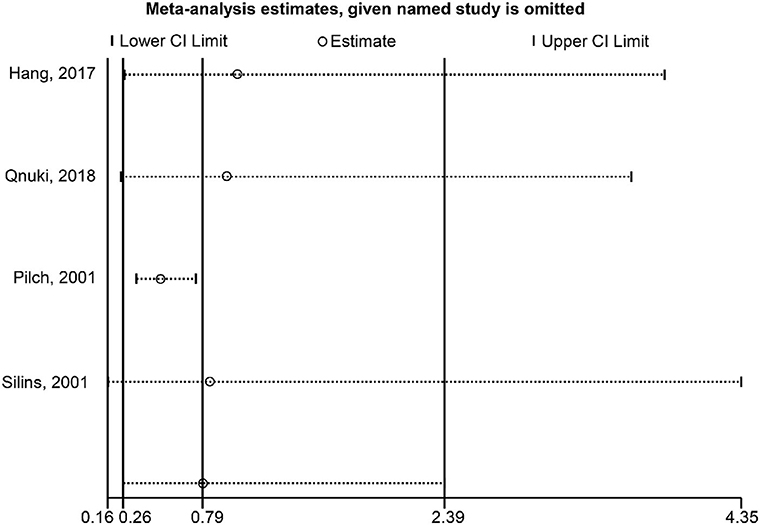
Figure 5. Sensitivity analysis of overall survival comparing the human papillomavirus subtype 16 (HPV-16) positive vs. negative groups.
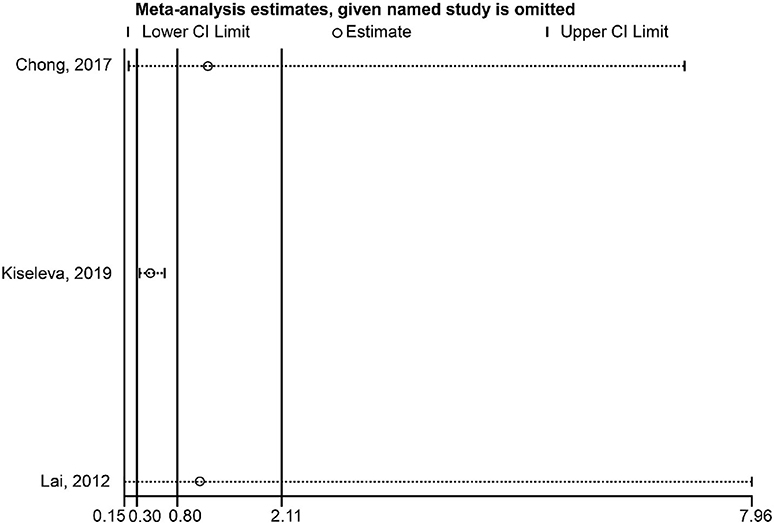
Figure 6. Sensitivity analysis of disease-free survival comparing the human papillomavirus subtype 16 (HPV-16) positive vs. negative groups.
Discussion
The literature reports conflicting results regarding the effect of HPV-16/18 positivity on CC prognosis. Therefore, this meta-analysis aimed to examine the effect of HPV-16/18 positivity on the prognosis of patients with CC. The results suggest that the presence of HPV-16 and HPV-18 positivity appears to have no significant association with prognosis in CC in either OS or PFS. The presence of HPV-16 or HPV-18 positivity has no significant association with the prognosis of CC (either OS or PFS). This is in contradiction to the aggressive feature of HPV-16/18-positive lesions during the development of CIN to CC.
In HPV-associated CC, tumorigenesis is driven by the E6 and E7 oncogenes from the viral DNA integrated into the host cells (29), but in HPV-negative CC, tumorigenesis is driven by the intrinsic oncogenes (30), and the two types of CC could be distinct diseases (13). A recent meta-analysis showed that pretreatment HPV DNA positivity in patients with CC was associated with a better prognosis in Mongoloids and Caucasians (13). Similar results were observed for head and neck cancers (14, 15), but these previous meta-analyses did not examine the HPV types.
It is now well-known that the different HPV types differ widely in terms of epidemiology and potential for CIN and CC (31–36). Among them, HPV-16 and HPV-18 are generally considered as being those at the highest risk of CC (31–36). Available studies suggest a positive effect of HPV16/18 positivity on CC outcomes (16, 22, 26), a negative effect (17, 25), or no effect (27, 28). A nationwide study that was not eligible for the present meta-analysis showed that patients with CC positive for a high-risk HPV type had a better prognosis than patients negative for such types (37), but other non-eligible studies also report conflicting results (30, 38–43). Indeed, Cuschieri et al. (39) showed that patients with CC and HPV-16/18 had better survival than those without HPV-16/18. Wang et al. (42) showed that CC caused by both HPV α-7 (which includes HPV-18) and HPV α-8 (which includes HPV-16) had a better prognosis than CC caused by HPV α-7 alone. Dahlgren et al. (43) reported a better prognosis for CC with HPV-16, but Lai et al. (40) reported a worse prognosis for HPV-18. When considering the eligible studies, the present meta-analysis suggests that there is no association between HPV-16/18 positivity and CC outcomes.
The results of the present meta-analysis must be considered in light of its limitations. We failed to conclude the prognostic effect of HPV-16 and HPV-18 in CC because the eligible studies had conflicting results. Of note, a number of studies could not be included because they did not report results specifically for HPV-16 or HPV-18. To our knowledge, there are only a few studies that investigated the prognostic effect of HPV-16 and HPV-18 in patients with CC, and the survival outcomes in each study were reported differently. Nevertheless, the non-eligible studies also had conflicting conclusions. Despite that nine studies were included in the meta-analysis, never more than four studies were analyzed together for a given outcome. One study reported the risk estimates as RR instead of HR. We treated the RR as HR for the analysis purpose, but it could introduce a bias. The nine studies were all observational studies, decreasing the strength of the conclusion, but a randomized control trial is not possible in this context. In addition, false-negatives could not be taken into account because of nonuniform reporting or non-reporting among the included studies. Finally, the risk estimates of survival outcomes were not reported at the same duration after the patients were discharged from the hospital.
In conclusion, the presence of HPV-16 and HPV-18 positivity appears to have no significant association with prognosis in CC in either OS or PFS, but the sensitivity analysis indicated that the study by Pilch et al. (17) has a strong impact on the outcome. Eliminating this study from the analysis would lead to a conclusion of a better prognosis of HPV-16 positivity in CC. Despite the limitations, the present meta-analysis observed different results on the prognostic effect of HPV-16 and HPV-18 among the existing studies. Future studies with larger numbers of patients in different countries and various ethnicities should be encouraged.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
XC substantially contributed to conception or design, contributed to acquisition, analysis, or interpretation of data, drafted the manuscript for important content, critically revised the manuscript for important intellectual content, and gave final approval. PZ substantially contributed to conception or design, contributed to acquisition, analysis, or interpretation of data, drafted the manuscript for important content, and critically revised the manuscript for important intellectual content. SC contributed to acquisition, analysis, or interpretation of data. HZ drafted the manuscript for important content. XDC gave final approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Zhejiang Provincial Natural Fund Public Welfare Technology Applied Research Funding Project (LGF20H160022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01733/full#supplementary-material
References
1. Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. (2017) 123:2219–29. doi: 10.1002/cncr.30588
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cervical Cancer. Version 1.2020. Fort Washington: National Comprehensive Cancer Network (2020).
4. Wipperman J, Neil T, Williams T. Cervical cancer: evaluation and management. Am Fam Phys. (2018) 97:449–54.
5. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. (2007) 370:890–907. doi: 10.1016/S0140-6736(07)61416-0
6. Bernard E, Pons-Salort M, Favre M, Heard I, Delarocque-Astagneau E, Guillemot D, et al. Comparing human papillomavirus prevalences in women with normal cytology or invasive cervical cancer to rank genotypes according to their oncogenic potential: a meta-analysis of observational studies. BMC Infect Dis. (2013) 13:373. doi: 10.1186/1471-2334-13-373
7. Bohmer G, van den Brule AJ, Brummer O, Meijer CL, Petry KU. No confirmed case of human papillomavirus DNA-negative cervical intraepithelial neoplasia grade 3 or invasive primary cancer of the uterine cervix among 511 patients. Am J Obstet Gynecol. (2003) 189:118–20. doi: 10.1067/mob.2003.439
8. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv72–iv83. doi: 10.1093/annonc/mdx220
9. Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. (2011) 128:927–35. doi: 10.1002/ijc.25396
10. Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. (2003) 348:518–27. doi: 10.1056/NEJMoa021641
11. Tornesello ML, Losito S, Benincasa G, Fulciniti F, Botti G, Greggi S, et al. Human papillomavirus (HPV) genotypes and HPV16 variants and risk of adenocarcinoma and squamous cell carcinoma of the cervix. Gynecol Oncol. (2011) 121:32–42. doi: 10.1016/j.ygyno.2010.12.005
12. Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, et al. Cervical cancer: a global health crisis. Cancer. (2017) 123:2404–12. doi: 10.1002/cncr.30667
13. Li P, Tan Y, Zhu LX, Zhou LN, Zeng P, Liu Q, et al. Prognostic value of HPV DNA status in cervical cancer before treatment: a systematic review and meta-analysis. Oncotarget. (2017) 8:66352–9. doi: 10.18632/oncotarget.18558
14. Liu H, Li J, Zhou Y, Hu Q, Zeng Y, Mohammadreza MM. Human papillomavirus as a favorable prognostic factor in a subset of head and neck squamous cell carcinomas: a meta-analysis. J Med Virol. (2017) 89:710–25. doi: 10.1002/jmv.24670
15. Rainsbury JW, Ahmed W, Williams HK, Roberts S, Paleri V, Mehanna H. Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head Neck. (2013) 35:1048–55. doi: 10.1002/hed.22950
16. Hang D, Jia M, Ma H, Zhou J, Feng X, Lyu Z, et al. Independent prognostic role of human papillomavirus genotype in cervical cancer. BMC Infect Dis. (2017) 17:391. doi: 10.1186/s12879-017-2465-y
17. Pilch H, Gunzel S, Schaffer U, Tanner B, Brockerhoff P, Maeurer M, et al. Human papillomavirus (HPV) DNA in primary cervical cancer and in cancer free pelvic lymph nodes–correlation with clinico-pathological parameters and prognostic significance. Zentralbl Gynakol. (2001) 123:91–101. doi: 10.1055/s-2001-12411
18. Selcuk AA. A guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. (2019) 57:57–8. doi: 10.5152/tao.2019.4058
19. Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. (2010) 31:47–50. doi: 10.4103/0253-7184.69003
20. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
21. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. London: Cochrane Collaboration. (2019) (Updated July 2019).
22. Chong GO, Lee YH, Han HS, Lee HJ, Park JY, Hong DG, et al. Prognostic value of pre-treatment human papilloma virus DNA status in cervical cancer. Gynecol Oncol. (2018) 148:97–102. doi: 10.1016/j.ygyno.2017.11.003
23. Kang WD, Kim CH, Cho MK, Kim JW, Cho HY, Kim YH, et al. HPV-18 is a poor prognostic factor, unlike the HPV viral load, in patients with stage IB-IIA cervical cancer undergoing radical hysterectomy. Gynecol Oncol. (2011) 121:546–50. doi: 10.1016/j.ygyno.2011.01.015
24. Kim JY, Nam BH, Lee JA. Is human papillomavirus genotype an influencing factor on radiotherapy outcome? Ambiguity caused by an association of HPV 18 genotype and adenocarcinoma histology. J Gynecol Oncol. (2011) 22:32–8. doi: 10.3802/jgo.2011.22.1.32
25. Kiseleva VI, Mkrtchyan LS, Ivanov SA, Lyubina LV, Bezyaeva GP, Panarina LV, et al. The presence of human papillomavirus DNA integration is associated with poor clinical results in patients with third-stage cervical cancer. Bull Exp Biol Med. (2019) 168:87–91. doi: 10.1007/s10517-019-04654-2
26. Lai CH, Chou HH, Chang CJ, Wang CC, Hsueh S, Huang YT, et al. Clinical implications of human papillomavirus genotype in cervical adeno-adenosquamous carcinoma. Eur J Cancer. (2012) 49:633–41. doi: 10.1016/j.ejca.2012.09.008
27. Onuki M, Matsumoto K, Tenjimbayashi Y, Tasaka N, Akiyama A, Sakurai M, et al. Human papillomavirus genotype and prognosis of cervical cancer: favorable survival of patients with HPV16-positive tumors. Papillomavirus Res. (2018) 6:41–5. doi: 10.1016/j.pvr.2018.10.005
28. Silins I, Avall-Lundqvist E, Tadesse A, Jansen KU, Stendahl U, Lenner P, et al. Evaluation of antibodies to human papillomavirus as prognostic markers in cervical cancer patients. Gynecol Oncol. (2002) 85:333–8. doi: 10.1006/gyno.2002.6628
29. zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. (1989) 49:4677–81.
30. Riou G, Favre M, Jeannel D, Bourhis J, Le Doussal V, Orth G. Association between poor prognosis in early-stage invasive cervical carcinomas and non-detection of HPV DNA. Lancet. (1990) 335:1171–4. doi: 10.1016/0140-6736(90)92693-C
31. Bosch FX, de Sanjose S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers. (2007) 23:213–27. doi: 10.1155/2007/914823
32. Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. (2008) 110(3 Suppl 2):S4–7. doi: 10.1016/j.ygyno.2008.07.045
33. Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, et al. Cervical cancer: screening, diagnosis and staging. J BUON. (2016) 21:320–5.
34. Siegler E, Reichman Y, Kugelman N, Mackuli L, Lavie O, Ostrovsky L, et al. Low-risk human papillomavirus types in cervical intraepithelial neoplasia 2-3 and in invasive cervical cancer patients. J Lower Genital Tract Dis. (2019) 23:248–52. doi: 10.1097/LGT.0000000000000486
35. Sand FL, Munk C, Frederiksen K, Junge J, Iftner T, Dehlendorff C, et al. Risk of CIN3 or worse with persistence of 13 individual oncogenic HPV types. Int J Cancer. (2019) 144:1975–82. doi: 10.1002/ijc.31883
36. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. (2003) 16:1–17. doi: 10.1128/CMR.16.1.1-17.2003
37. Lei J, Ploner A, Lagheden C, Eklund C, Nordqvist Kleppe S, Andrae B, et al. High-risk human papillomavirus status and prognosis in invasive cervical cancer: a nationwide cohort study. PLoS Med. (2018) 15:e1002666. doi: 10.1371/journal.pmed.1002666
38. Barreto CL, Martins DB, de Lima Filho JL, Magalhaes V. Detection of human Papillomavirus in biopsies of patients with cervical cancer, and its association with prognosis. Arch Gynecol Obstet. (2013) 288:643–8. doi: 10.1007/s00404-013-2803-2
39. Cuschieri K, Brewster DH, Graham C, Nicoll S, Williams AR, Murray GI, et al. Influence of HPV type on prognosis in patients diagnosed with invasive cervical cancer. Int J Cancer. (2014) 135:2721–6. doi: 10.1002/ijc.28902
40. Lai CH, Chang CJ, Huang HJ, Hsueh S, Chao A, Yang JE, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. (2007) 25:3628–34. doi: 10.1200/JCO.2007.11.2995
41. Lo KW, Cheung TH, Chung TK, Wang VW, Poon JS, Li JC, et al. Clinical and prognostic significance of human papillomavirus in a Chinese population of cervical cancers. Gynecol Obstet Invest. (2001) 51:202–7. doi: 10.1159/000052925
42. Wang CC, Lai CH, Huang HJ, Chao A, Chang CJ, Chang TC, et al. Clinical effect of human papillomavirus genotypes in patients with cervical cancer undergoing primary radiotherapy. Int J Radiat Oncol Biol Phys. (2010) 78:1111–20. doi: 10.1016/j.ijrobp.2009.09.021
Keywords: human papillomavirus 16, human papillomavirus 18, uterine cervical neoplasms, prognosis, meta-analysis
Citation: Chen X, Zhang P, Chen S, Zhu H, Wang K, Ye L, Wang J, Yu J, Mei S, Wang Z and Cheng X (2020) Better or Worse? The Independent Prognostic Role of HPV-16 or HPV-18 Positivity in Patients With Cervical Cancer: A Meta-Analysis and Systematic Review. Front. Oncol. 10:1733. doi: 10.3389/fonc.2020.01733
Received: 30 April 2020; Accepted: 03 August 2020;
Published: 07 October 2020.
Edited by:
Angeles Alvarez Secord, Duke University, United StatesReviewed by:
Lorenzo Gerratana, University of Udine, ItalyLuigi Pedone Anchora, Catholic University of the Sacred Heart, Italy
Copyright © 2020 Chen, Zhang, Chen, Zhu, Wang, Ye, Wang, Yu, Mei, Wang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Cheng, Y2hlbnhpbmcmI3gwMDA0MDtlbnplbWVkLmNvbQ==
 Xing Chen1
Xing Chen1 Xiaodong Cheng
Xiaodong Cheng