- 1Center for Tumor Diagnosis and Therapy, Jinshan Hospital, Fudan University, Shanghai, China
- 2Department of Radiation Oncology, Eye and ENT Hospital, Fudan University, Shanghai, China
Background: Both radiotherapy and surgery are now recommended for early stage glottic laryngeal squamous cell carcinoma (LSCC), and both have their own advantages in patients with different characteristics. For each patient, it is hard to determine whether radiotherapy or surgery is more appropriate.
Methods: Patients with T1-2N0M0 glottic LSCC who received radiotherapy or surgery in the 2004–2016 SEER database were reviewed, then randomly divided into training and validation cohorts. Propensity score matching was used to eliminate the baseline variations, and competing risk analyses helped to exclude the effects of other causes of death. Based on univariate and multivariate analyses, we built two nomograms to visually predict the survival of each patient with different characteristics who received radiotherapy or surgery, then validated the accuracy in both training and validation cohorts. Using nomogramEx, we quantified the algorithms of the nomograms and put the nomograms on the websites.
Results: A total of 6538 patients in the SEER database were included. We found that therapy (p = 0.004), T stage (p < 0.001), age (p < 0.001), race (p < 0.044), grade (p = 0.001), and marital status (p < 0.001) were independent prognostic factors. Two nomograms were built to calculate the survival for each patient who received radiotherapy (C-index = 0.668 ± 0.050 in the training cohort and 0.578 ± 0.028 in the validation cohort) or underwent surgery (C-index = 0.772 ± 0.045 in the training cohort and 0.658 ± 0.090 in the validation cohort). Calibration plots showed the accuracy of the nomograms. Using the nomograms, we found that 3872 patients (59.22%) had no difference between the two therapies, 706 patients (10.80%) who received radiotherapy had better survival outcomes, and 1960 patients (29.98%) who underwent surgery had better survival outcome.
Conclusion: Nomograms were used to comprehensively calculate independent factors to determine which treatment (radiotherapy or surgery) is better for each patient. A website was used to offer guidance regarding surgery or radiation for patients and physicians.
Introduction
Laryngeal cancer occurs more frequently in head and neck cancers, and approximately 95% of which are laryngeal squamous cell carcinomas (LSCCs) (1). In China, the incidence of laryngeal cancer is approximately 1.86 per 100,000 annually (2). A total of 23,400 new cases occurred in 2014 (3), most of which were diagnosed in the early stage.
The recommended treatment for early glottic LSCC includes surgery and radiotherapy (4–7). Glottic LSCC is the main site of laryngeal squamous cell carcinomas. In our previous research, we found that the survival of patients with T1a glottic cancer, well-differentiated tumors, who were married, and who received radiotherapy were worse. For patients who had T1b glottic cancer, undifferentiated tumors, and who were unmarried, radiotherapy was not preferable to surgery (8). Individual patients have a complex combination of clinical characteristics, and further exploration of individualized treatment methods for patients with early stage glottic LSCC is warranted to personalize treatment (9–11).
The Surveillance, Epidemiology, and End Results (SEER) program is a source for long-term population-based incidence data. In recent years, nomograms have frequently been used to calculate the proportion of various factors for each patient, and comprehensively consider the impact of multiple factors on survival, which may offer guidance for individual treatment. In this manuscript, we attempted to determine which therapy (radiation or surgery) is a better choice for a patient with T1-2N0M0 glottic LSCC using SEER data and a nomogram.
Materials and Methods
Ethics Statement
The Ethics Committees of Jinshan Hospital and the Eye and ENT Hospital of Fudan University exempted the study because no personal information is included in the SEER database.
Data Selection
We obtained SEER (Incidence – SEER 18 Regs Custom Data with additional treatment fields, November 2018 Sub, 1975 – 2016 varying) data via the SEER∗Stat software (version 8.3.6)1. The selection process to acquire data from the database is shown in Figure 1A. In brief, we selected patients who had early stage glottic LSCC and underwent only radiotherapy or surgery. The old version to the 8th AJCC TMN staging system was converted manually.

Figure 1. (A) Flow diagram of selecting process (B) Mirror histogram of propensity scores for patients with radiotherapy and with surgery. Matched patients are presented in green color. (C) Standardized differences of baseline variables between patients with radiation and with surgery before and after propensity score matching.
Characteristics, including race, age, gender, grade, TMN stage, T stage, marital status, and insurance, were included in the analyses.
Study Design and Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM, Inc., Armonk, NY, United States) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p-value < 0.05 was considered statistically significant.
Patients were divided into two groups (radiotherapy and surgery) and were randomly divided into training and validation cohorts. A propensity score matching system was used to eliminate patient selection bias in the training cohort. In the current study, covariates that may affect the choice of grouped patients were matched as follows: age (≤50, 51–60, 61–70, 71–80, and ≥81 years); race; gender; grade; TMN stage; T stage; and insurance and marital status. Patients who were excluded after propensity score matching were moved to the validation cohort to improve the accuracy of the validation process.
Propensity scores for the training cohort were generated using the “MatchIt” package. The baseline characteristics between the surgery and radiotherapy groups before and after matching were compared using χ2 and Wilcoxon tests (12, 13). All of the patients except the propensity scoring matches were divided into the validation cohort.
The Kaplan-Meier method was used to estimate survival rates. Survival curves were compared using a log-rank test. Competing risk analyses were performed as previously reported (14) because other causes of death were competing outcomes for cancer-specific deaths. A Cox proportional hazards model was used to perform univariate and multivariate analyses.
Two nomograms, as several studies have reported (15, 16), were built on the basis of the results of multivariate analysis. The performance of nomograms was evaluated by the concordance index (C-index). The nomograms were also assessed by comparing the actual probability with the predicted probability and were further validated by comparing the predicted probability in the validation cohort with the observed survival. The “nomogramEx” package was used to extract the polynomial equations to calculate the points of each variable for every patient, and the survival probability corresponding to the total points. Using nomogramEx, the cancer-specific survival rates for each patient who was treated with radiation or underwent surgery at 3 and 5 years were calculated.
Results
Patient Characteristics
As shown in Figure 1A, 6538 patients with glottic LSCC (4759 patients treated with radiation and 1779 patients who underwent surgery) were included in our study. A total of 4576 patients were randomly divided into the training cohort. Patients who were excluded after propensity score matching comprised the validation cohort. The baseline characteristics of all participants in the training cohort are summarized in Table 1 and Supplementary Table S1. Compared to the patients who underwent surgery, the patients who underwent radiotherapy were characterized as follows: older (p = 0.071); worse tumor differentiation (p = 0.259); higher T (p < 0.001) and TMN stage (p < 0.001); less likely to be white (p < 0.001); less likely to have insurance (p < 0.001), and less likely to be married (p = 0.134).
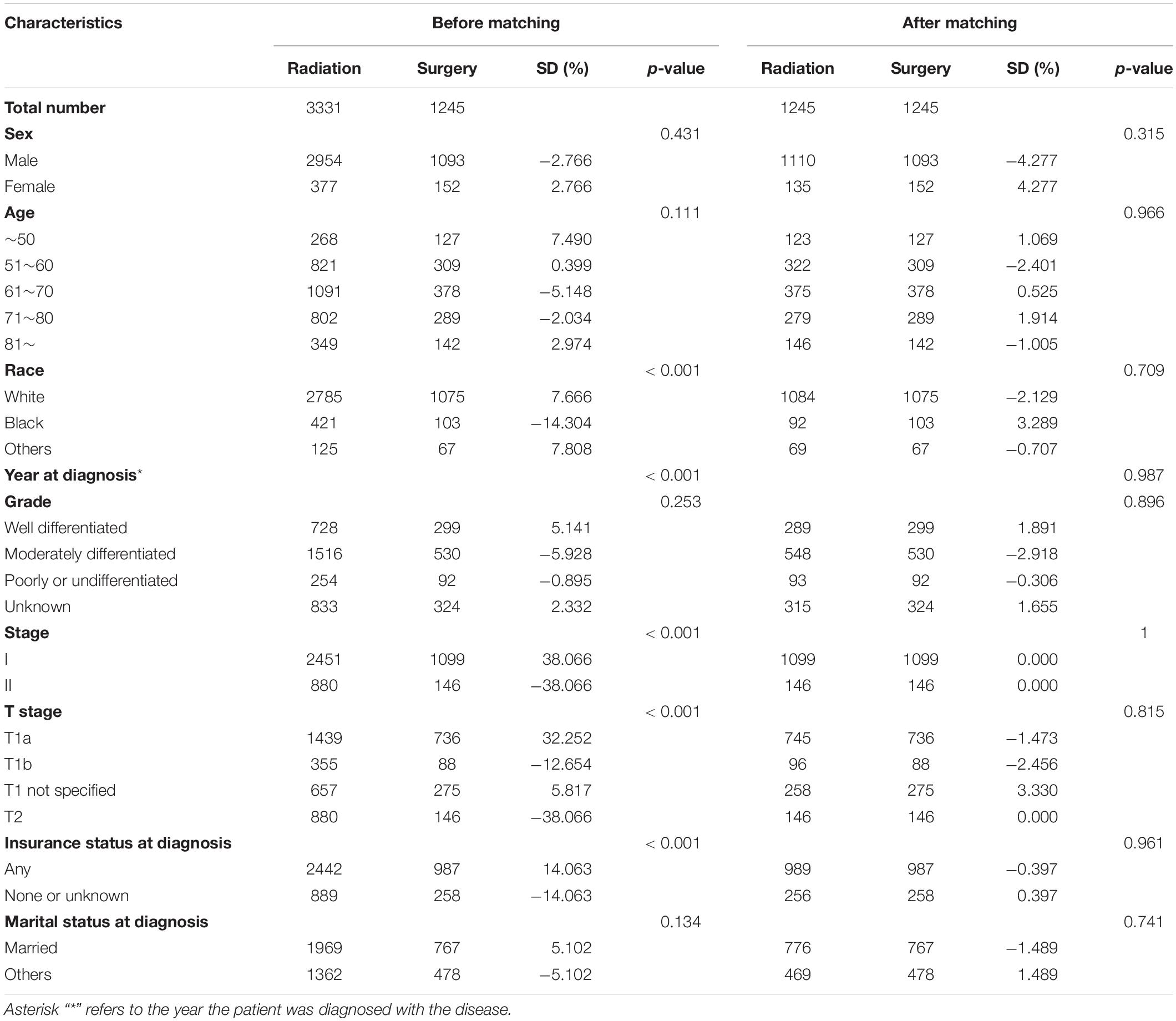
Table 1. Patient characteristics according to the therapy status before and after propensity score matching.
Propensity Score Matching Analyses
After matching, 1245 pairs of patients were selected; one-half received radiotherapy, and the other half underwent surgery. As shown in Table 1 and Supplementary Table S1, there were no significant differences between the radiotherapy and surgery groups after matching. The p-values for variables, including age, race, year of diagnosis, grade, stage, T stage, insurance, and marital status, had been greatly improved. The absolute values of the standardized differences (SD) after matching were all <10%, suggesting that the baseline characteristics were well-balanced. The matched groups had similar propensity score distributions, and the mirror histograms of propensity scores for patients are shown in Figures 1B,C.
Survival Analyses
As shown in Figure 2, patients who received radiation therapy had significantly worse overall survival outcomes compared with patients who underwent surgery (p = 0.0035; Figure 2A). Competing risk analysis also illustrated that the patients who received radiation had a higher risk of cancer-specific mortality (p = 0.003), while there was no apparent difference in the probabilities of other causes of death (p = 0.351; Figure 2B).
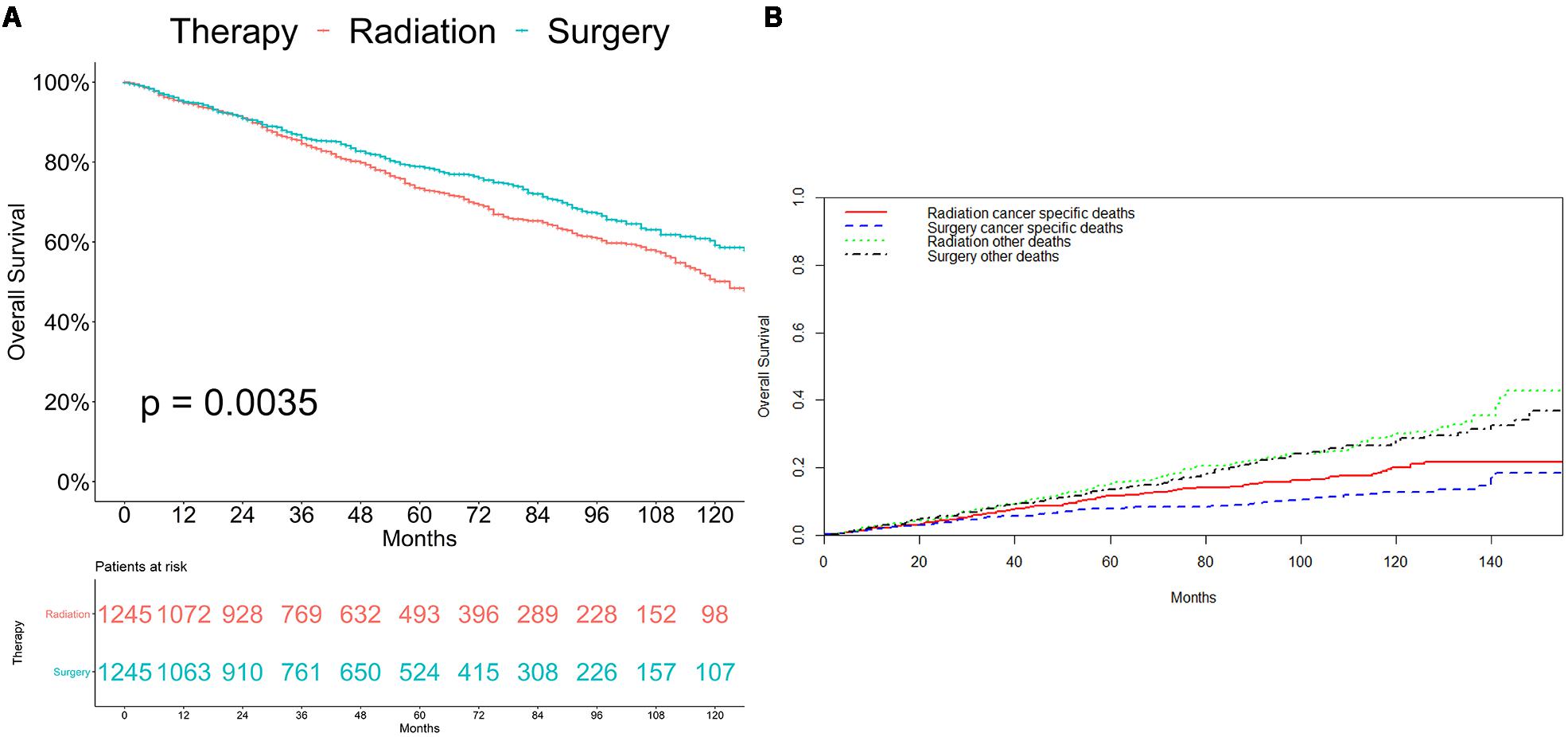
Figure 2. Survival analyses for patients with radiotherapy and with surgery (A) Kaplan-Meier method. (B) Competing risk analysis.
We further analyzed the connection between cancer-specific survival and variations. Multicollinearity was detected to test the independence of the variables included in the regression model, and variance inflation factors (VIF) of all variables were far less than ten indicates there was no multicollinearity problem. As Table 2 and Supplementary Table S2 shows, univariate analyses demonstrated that therapy, age, race, grade, stage, T stage, and marital status were significant predictors of glottic LSCC-specific survival. Gender, year of diagnosis, and insurance status had no significant differences between the two groups. Based on multivariate analysis for patients with glottic LSCC, therapy, race, age, grade, T stage, and marital status were independent prognostic predictors and stage, which were not independent of T stage and not included in the multivariate analysis.
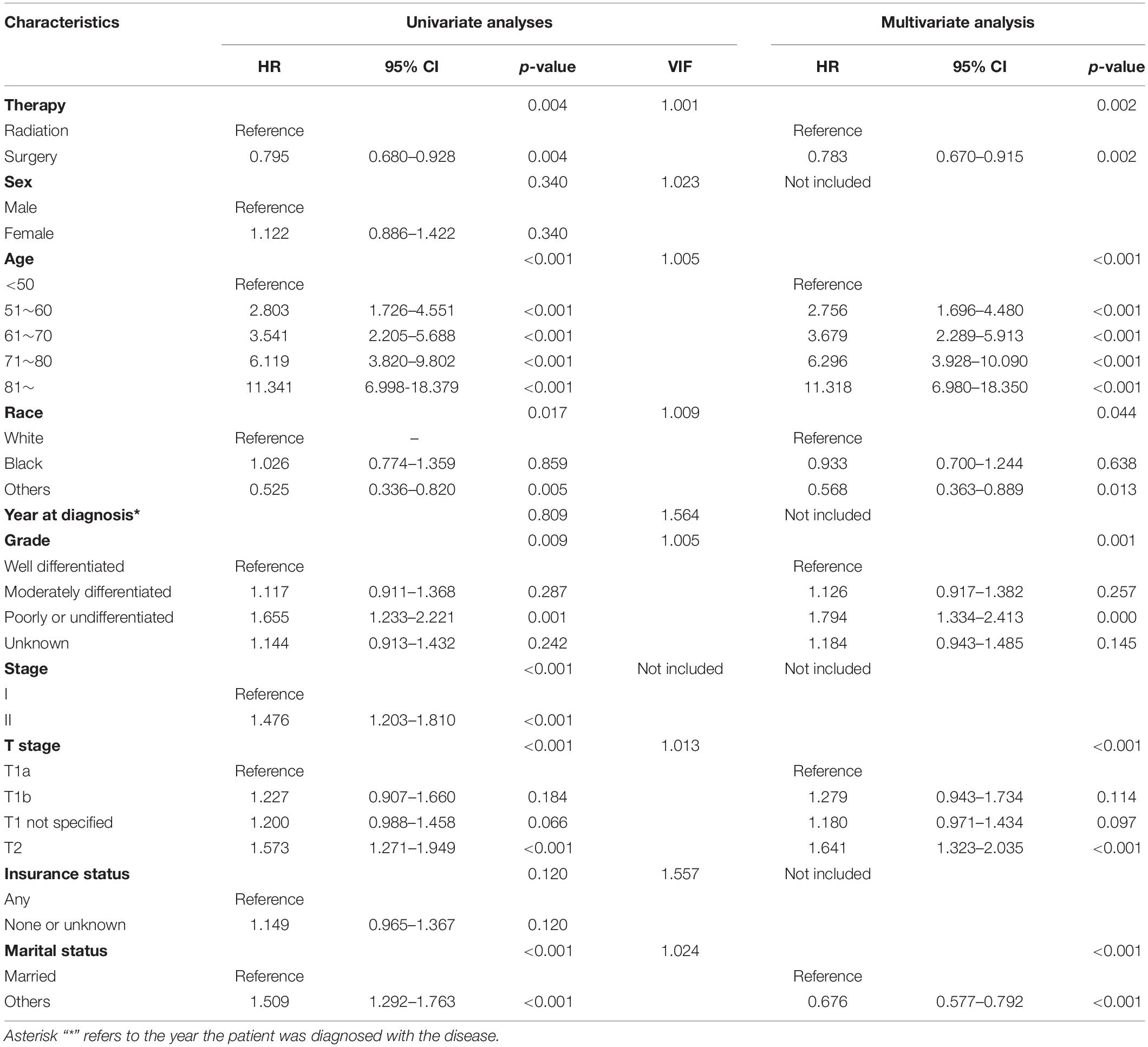
Table 2. Results of univariate and multivariate analyses of cancer-specific survival after matching.
To better compare the difference of treatment on cancer-specific survival in glottic LSCC patients, we stratified the patients after matching by variables in univariate analysis. As is shown in Supplementary Figures S1–S8, glottic LSCC patients with a worse cancer-specific survival had the following characteristics: not black; male; insurance; T1a stage; well-differentiated tumor; married, 51–60 or 71–80 years of age. For glottic LSCC patients with other characteristics, radiotherapy and surgery had equivalent efficacy.
Construction and Validation of the Nomogram
The final multivariate model uncovered six independent variables, including therapy, age, race, grade, T stage, and marital status. As shown in Figures 3A, 4A, the nomograms were developed based on the variables.
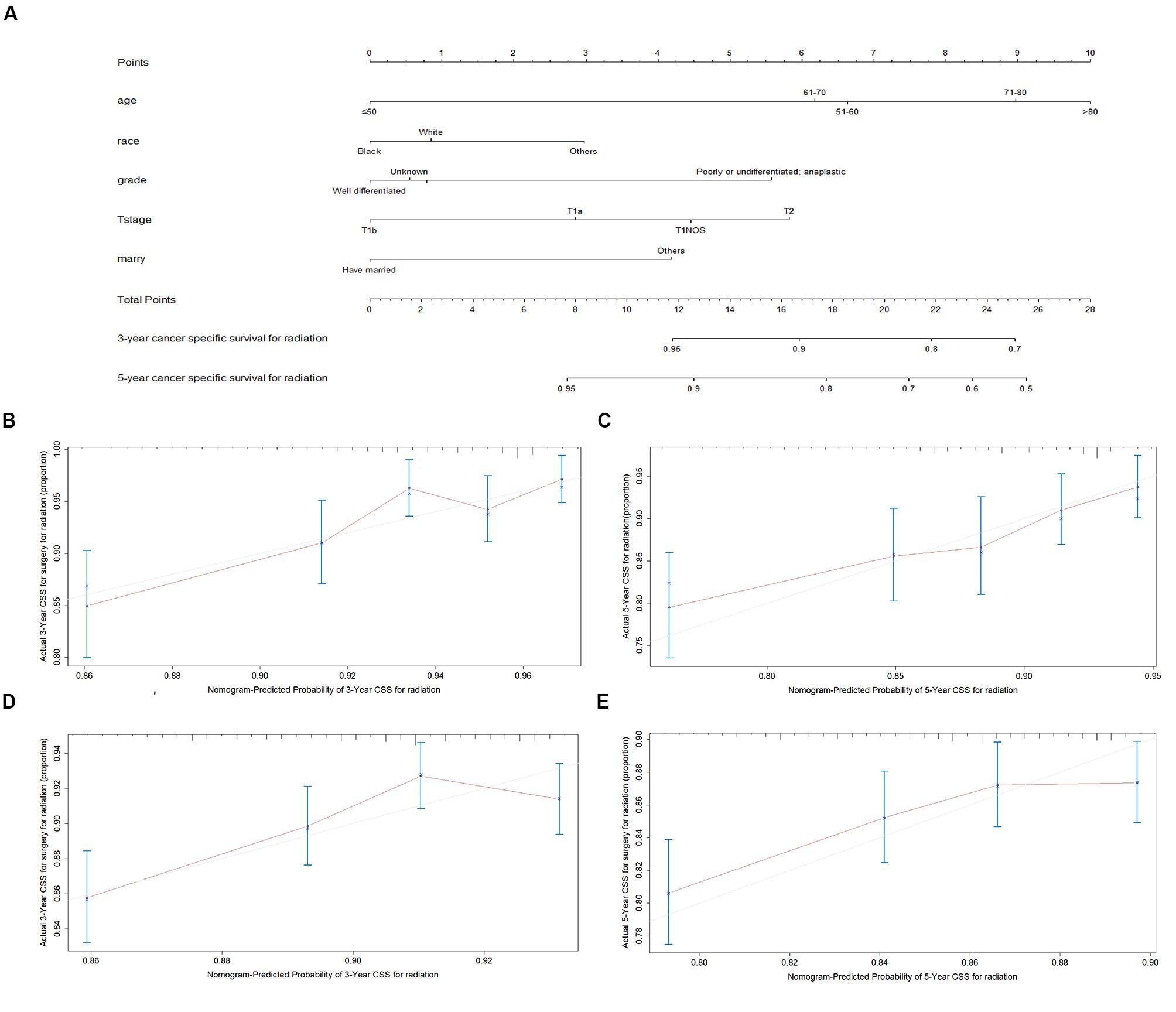
Figure 3. Nomogram analyses for patients with radiotherapy (A) A nomogram for prediction of 3- and 5-year CSS rates of patients (B) Calibration curve of the nomogram predicting 3-year CSS rates in training cohort. (C) Calibration curve of the nomogram predicting 5-year CSS rates in training cohort. (D) Calibration curve of the nomogram predicting 3-year CSS rates in validation cohort (E) Calibration curve of the nomogram predicting 3-year CSS rates in validation cohort.
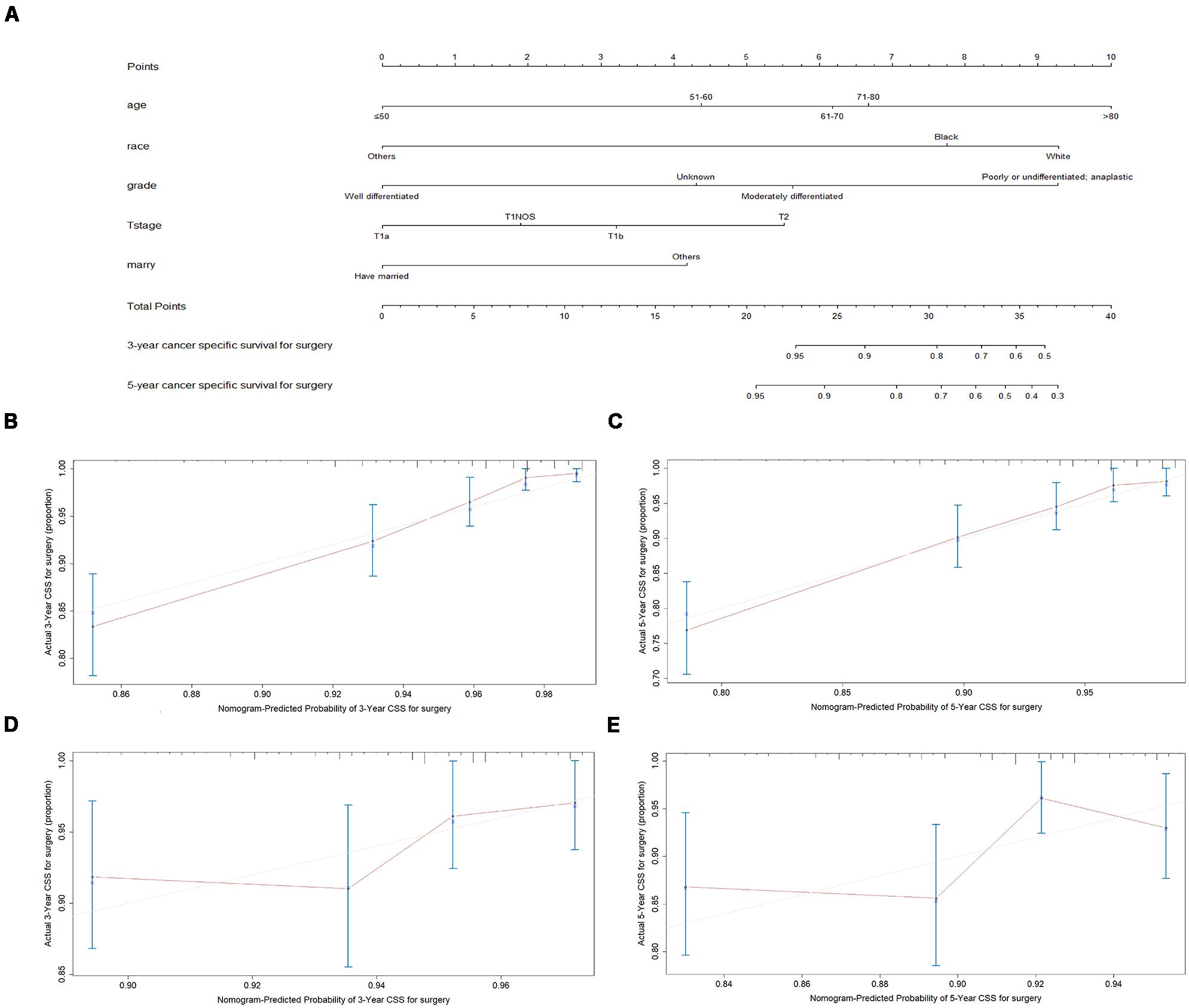
Figure 4. Nomogram analyses for patients with surgery (A) A nomogram for prediction of 3- and 5-year CSS rates of patients (B) Calibration curve of the nomogram predicting 3-year CSS rates in training cohort. (C) Calibration curve of the nomogram predicting 5-year CSS rates in training cohort. (D) Calibration curve of the nomogram predicting 3-year CSS rates in validation cohort (E) Calibration curve of the nomogram predicting 3-year CSS rates in validation cohort.
A total of 4576 patients were randomly divided into the training cohort and patients excluded after propensity score matching comprised the validation cohort. As shown in Figures 3B,C, 4B,C, the calibration plots are based on internal validation of the training cohort. The C-index for the prediction of cancer-specific survival in glottic LSCC patients who underwent surgery and received radiotherapy was 0.772 ± 0.045 and 0.668 ± 0.050, respectively. In addition, external validation of the nomograms was performed in the validation cohort. The C-index for surgery (0.658 ± 0.090) and radiotherapy (0.578 ± 0.028) was calculated based on the calibration plots shown in Figures 3D,E, 4D,E. The C-index for internal and external validation indicated that the nomograms have a good fit with the actual observations. Therefore, the 3- and 5-year cancer-specific survival rates predicted by the nomograms were reliable.
Nomogram Analyses and Website Application
We used the nomogramEx function in R software to calculate the specific algorithms for the above two nomograms and uploaded the algorithms to our website2. Patients and physicians can enter age, grade, marital status, and other personal details on our website, which automatically calculates the predicted 3- and 5-year survival rates of patients who received radiotherapy or underwent surgery. The patients and physicians can compare outcomes of radiation and surgery to determine which is the better treatment option. For example, an 82-year-old Chinese patient with a stage T1a moderately differentiated glottic LSCC who is divorced can use our website to find out that the 3- and 5-year survival rates for surgery are 98.16 and 94.78%, respectively, while the corresponding rates for radiotherapy are 78.40 and 68.45%, respectively. At this time, surgery may be a better choice for this patient.
We predicted the prognosis of patients with early glottic LSCC who underwent surgery and radiotherapy. Greater than 6000 patients in the SEER databases were included in this study, and we listed the results as a histogram in Figure 5.
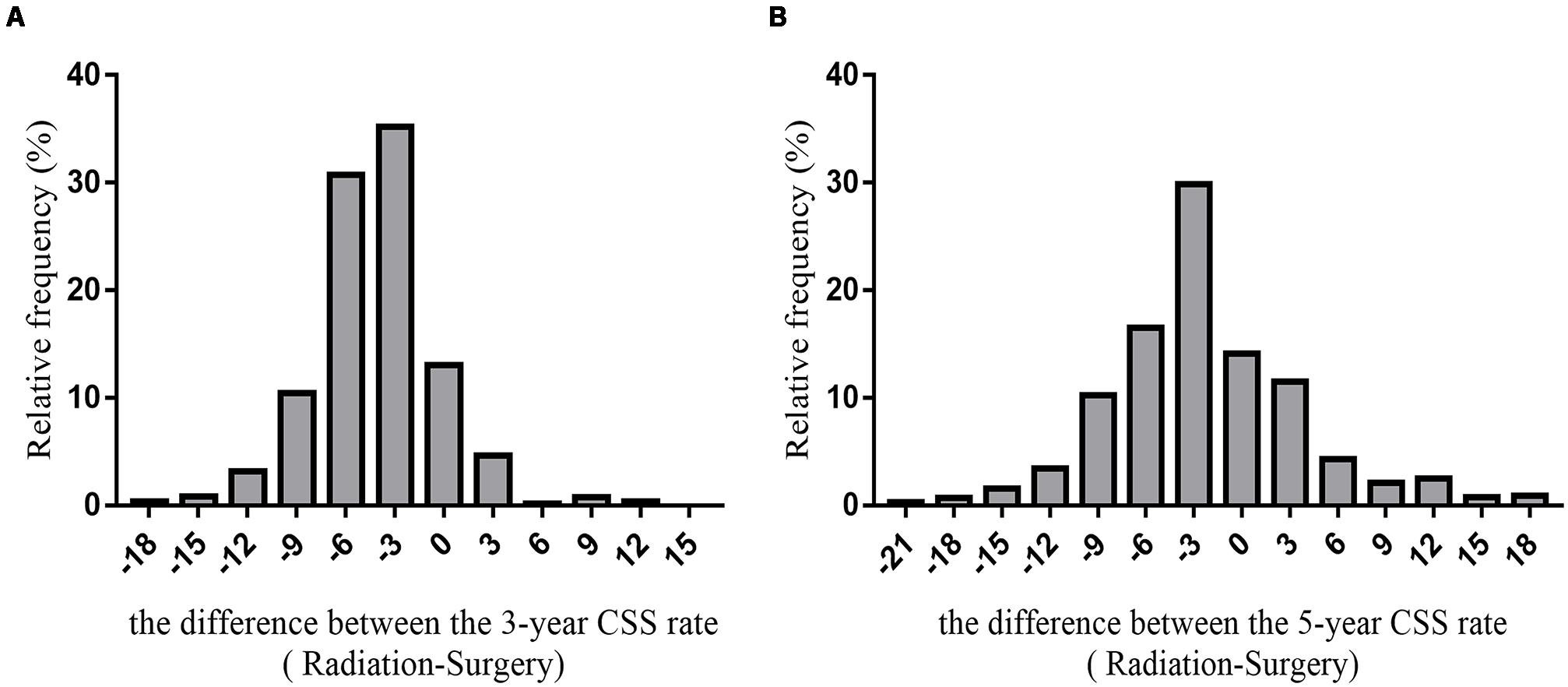
Figure 5. The histogram of the difference between CSS rates (radiotherapy-surgery) (A) the difference between 3-year CSS rates (B) the difference between 5-year CSS rates.
The average difference between the 3-year cancer-specific survival rates assuming all patients received radiotherapy or underwent surgery was −4.29 ± 3.94%. The difference between the 5-year cancer-specific survival rates assuming all patients received radiotherapy or underwent surgery was −2.04 ± 6.74%.
Based on the SEER database, the 5-year survival rate for surgery for 1960 patients (29.98%) was >5% higher than the 5-year survival rate for radiotherapy. The 5-year survival rate for 706 patients undergoing surgery was <5% than the 5-year survival rate for patients who received radiotherapy. The difference in the 5-year survival rate between 3872 patients (59.22%) who underwent surgery or received radiotherapy was between −5 and 5%; thus, this group of patients may choose surgery or radiotherapy.
Discussion
In this study, we compared the cancer-specific survival of 6538 patients with early stage glottic LSCC, who received radiotherapy or underwent surgery. We built and validated two web-based nomograms to predict the cancer-specific survival for each patient who received radiotherapy or underwent surgery. Patients can input relevant information, such as age, grade, T stage, and marital status, on our website and estimate which treatment is superior. Our findings will be of great benefit to help patients and physicians to make treatment decisions.
We showed that radiotherapy and surgery each have their own advantages in the glottic LSCC population (17). Of the 6538 patients from the SEER database, surgery was superior in 29.98%, radiotherapy was superior in 10.80%, and operation and radiotherapy had similar efficacy in 59.22%. Patients and physicians can use our web-based nomograms to predict which therapy is more appropriate for them.
A nomogram is a simple graphical representation of a statistical prediction model that is frequently applied to patients and has gained popularity among oncologists and patients participating in clinical trials (18–23). In the current study, we used two nomograms to visually and individually predict the survival of glottic LSCC patients with different clinicopathologic characteristics to help them choose a superior treatment modality. We believe that our nomogram-based method can be used to compare the outcomes of several therapies and can play an increasingly significant role in future clinical analyses.
Inverso et al. (24) reported that marital status has a positive effect on metastatic laryngeal cancer. Common symptoms of laryngeal cancer included hoarseness, otalgia, dysphagia, and voice changes (25). Such symptoms can attract the early attention of partners or spouses, who may urge patients to receive timely diagnosis and treatment. Investigators have reported that patients who have fee-for-service insurance are more likely to undergo cancer screening tests that may affect stage at the time of diagnosis (26). Our analysis showed that insurance was not an independent factor in patients with early stage laryngeal cancer. The relationship between insurance and survival outcome needs further exploration.
Our study had several limitations. First, the information in the SEER database is not detailed, such as radiation technology, radiation dose, and surgery regimen. We were not able to calculate the influence of these factors. Second, owing to the absence of life quality data in the SEER database, we only focused on survival outcome rather than assess functional outcomes, while a trial revealed that patients with radiotherapy had less hoarseness-related inconvenience (27, 28). Du et al. (2) reported that surgery had preferable fundamental frequency values over radiotherapy in T1aN0M0 glottic carcinoma (29). With the development of surgery in recent years, the effect of surgery on pronunciation and other functions has greatly improved (7, 30–34). Third, the results might not be applicable to other populations because patients included in this research were from the United States. The last, we only analyzed the clinical characteristics but didn’t include several molecular factors such as HPV, or TP53 mutations since such information was not included in the SEER database. The last, but most important limitation was that it should be noted that our results may not be a reference before prospective trials are conducted because the study was retrospective.
Conclusion
In our study, we analyzed the independent prognostics factors for early stage glottic LSCC patients and built nomograms to comprehensively calculate independent factors and help determine which treatment, radiotherapy, or surgery, is better for each patient. A website is also available to offer guidance about surgery or radiation for patients and physicians.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The Ethics Committees of Jinshan Hospital and the Eye and ENT Hospital of Fudan University exempted the study because no personal information is included in the SEER database.
Author Contributions
YD, SS, and ML: conception and design. YD, LY, and YZ: administrative support. YD, TQ, and SS: provision of study materials or patients. YD and ML: collection and assembly of data. LY, YZ, and TQ: data analysis and interpretation. All authors: manuscript writing and approval of final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81703023) (http://www.nsfc.gov.cn/), and Foundation of Shanghai Municipal Commission of Health and Family Planning (20184Y0204), Medical Guidance Fund of Shanghai Science and Technology Commission (19401931700), and Key Subject Construction Program of Shanghai Health Administrative Authority (ZK2019B30).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01669/full#supplementary-material
FIGURE S1 | Survival analyses for patients with radiotherapy and with surgery stratified by age after matching (A) ≤50 years of age. (B) 51–60 years of age. (C) 61–70 years of age. (D) 71–80 years of age. (E) >80 years of age.
FIGURE S2 | Survival analyses for patients with radiotherapy and with surgery stratified by race. (A) White. (B) Black. (C) Other race.
FIGURE S3 | Survival analyses for patients with radiotherapy and with surgery stratified by sex. (A) Male. (B) Female.
FIGURE S4 | Survival analyses for patients with radiotherapy and with surgery stratified by differentiation after matching (A) Well differentiated. (B) Moderately differentiated. (C) Poorly or undifferentiated, anaplastic. (D) Differentiation unknown.
FIGURE S5 | Survival analyses for patients with radiotherapy and with surgery stratified by stage. (A) Stage I. (B) Stage II.
FIGURE S6 | Survival analyses for patients with radiotherapy and with surgery stratified by T stage after matching (A) T1a. (B) T1b. (C) T1NOS. (D) T2.
FIGURE S7 | Survival analyses for patients with radiotherapy and with surgery stratified by insurance. (A) Patient who had insurance. (B) Patients at other insurance status.
FIGURE S8 | Survival analyses for patients with radiotherapy and with surgery stratified by marital status. (A) Married patient. (B) Patients at other marital status.
TABLE S1 | Characteristics of year at diagnosis of patients according to the therapy status before and after propensity score matching.
TABLE S2 | Results of year at diagnosis in univariate and multivariate analyses of cancer-specific survival after matching.
Footnotes
References
1. Almadori G, Bussu F, Cadoni G, Galli J, Paludetti G, Maurizi M. Molecular markers in laryngeal squamous cell carcinoma: towards an integrated clinicobiological approach. Eur J Cancer. (2005) 41:683–93. doi: 10.1016/j.ejca.2004.10.031
2. Liu Y, Zhao Q, Ding G, Zhu Y, Li W, Chen W. Incidence and mortality of laryngeal cancer in China, 2008-2012. Chin J Cancer Res. (2018) 30:299–306. doi: 10.21147/j.issn.1000-9604.2018.03.02
3. Wei KR, Zheng RS, Liang ZH, Sun KX, Zhang SW, Li ZM, et al. [Incidence and mortality of laryngeal cancer in China, 2014]. Zhonghua Zhong Liu Za Zhi. (2018) 40:736–43.
4. Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA Cancer J Clin. (2017) 67:31–50. doi: 10.3322/caac.21386
5. Baird BJ, Sung CK, Beadle BM, Divi V. Treatment of early-stage laryngeal cancer: a comparison of treatment options. Oral Oncol. (2018) 87:8–16. doi: 10.1016/j.oraloncology.2018.09.012
6. Koroulakis A, Agarwal M. Cancer, Laryngeal. StatPearls. Treasure Island, FL: StatPearls Publishing LLC (2019).
7. Hendriksma M, van Loon Y, Klop WMC, Hakkesteegt MM, Heijnen BJ, El Hasnaoui I, et al. Quality of life and voice outcome of patients treated with transoral CO2 laser microsurgery for early glottic carcinoma (T1-T2): a 2-year follow-up study. Eur Arch Otorhinolaryngol. (2019) 276:805–14. doi: 10.1007/s00405-019-05348-1
8. Zhan C, Yang X, Song X, Yan L. Radiotherapy vs surgery for T1-2N0M0 laryngeal squamous cell carcinoma: a population-based and propensity score matching study. Cancer Med (2018) 7:2837–47. doi: 10.1002/cam4.1525
9. Obid R, Redlich M, Tomeh C. The treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am. (2019) 31:1–11.
10. Chung SY, Kim KH, Keum KC, Koh YW, Kim SH, Choi EC, et al. Radiotherapy versus cordectomy in the management of early glottic cancer. Cancer Res Treat. (2018) 50:156–63. doi: 10.4143/crt.2016.503
11. Mehel DM, Ozgur A, Sahin N, Vural AA, Yemis T, Celebi M, et al. Voice quality after radiotherapy and cordectomy in early-stage glottic carcinomas. Ear Nose Throat J. (2019) 23:145561319876905.
12. Yang X, Sun F, Chen L, Shi M, Shi Y, Lin Z, et al. Prognostic value of visceral pleural invasion in non-small cell lung cancer: a propensity score matching study based on the SEER registry. J Surg Oncol. (2017) 116:398–406. doi: 10.1002/jso.24677
13. Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. (2015) 16:286–96. doi: 10.3348/kjr.2015.16.2.286
14. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. (2007) 40:381–7. doi: 10.1038/sj.bmt.1705727
15. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. (2008) 26:1364–70. doi: 10.1200/jco.2007.12.9791
16. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/s1470-2045(14)71116-7
17. Guimaraes AV, Dedivitis RA, Matos LL, Aires FT, Cernea CR. Comparison between transoral laser surgery and radiotherapy in the treatment of early glottic cancer: a systematic review and meta-analysis. Sci Rep. (2018) 8:11900.
18. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. (2015) 33:861–9. doi: 10.1200/jco.2014.56.6661
19. Ohori Tatsuo G, Riu Hamada M, Gondo T, Hamada R. [Nomogram as predictive model in clinical practice]. Gan To Kagaku Ryoho. (2009) 36:901–6.
20. Tang F, Zhang H, Lu Z, Wang J, He C, He Z. Prognostic factors and nomograms to predict overall and cancer-specific survival for children with wilms. Tumor. Dis Markers. (2019) 2019:1092769.
21. Touijer K, Scardino PT. Nomograms for staging, prognosis, and predicting treatment outcomes. Cancer. (2009) 115(Suppl. 13):3107–11. doi: 10.1002/cncr.24352
22. Tan W, Xie X, Huang Z, Chen L, Tang W, Zhu R, et al. Construction of an immune-related genes nomogram for the preoperative prediction of axillary lymph node metastasis in triple-negative breast cancer. Artif Cells Nanomed Biotechnol. (2020) 48:288–97. doi: 10.1080/21691401.2019.1703731
23. Cai BB, Hou XQ, Zhou X, Ye TT, Fang G, Huang HZ, et al. Use of a novel index, the A-index, and its associated nomogram to predict overall survival rates after resection of primary hepatocellular carcinoma. Clin Chim Acta. (2020) 500:34–41. doi: 10.1016/j.cca.2019.10.001
24. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. (2015) 121:1273–8. doi: 10.1002/cncr.29171
25. Chen AY, Schrag NM, Halpern M, Stewart A, Ward EM. Health insurance and stage at diagnosis of laryngeal cancer: does insurance type predict stage at diagnosis? Arch Otolaryngol Head Neck Surgery. (2007) 133:784–90. doi: 10.1001/archotol.133.8.784
26. Potosky AL, Breen N, Graubard BI, Parsons PE. The association between health care coverage and the use of cancer screening tests. Results from the 1992 National Health Interview Survey. Med Care. (1998) 36:257–70. doi: 10.1097/00005650-199803000-00004
27. Aaltonen LM, Rautiainen N, Sellman J, Saarilahti K, Makitie A, Rihkanen H, et al. Voice quality after treatment of early vocal cord cancer: a randomized trial comparing laser surgery with radiation therapy. Int J Radiat Oncol Biol Phys. (2014) 90:255–60. doi: 10.1016/j.ijrobp.2014.06.032
28. Arias F, Arraras JI, Asin G, Uzcanga MI, Maravi E, Chicata V, et al. Quality of life and voice assessment in patients with early-stage glottic cancer. Head Neck. (2015) 37:340–6. doi: 10.1002/hed.23603
29. Du G, Liu C, Yu W, Li J, Li W, Wang C, et al. Voice outcomes after laser surgery vs. radiotherapy of early glottic carcinoma: a meta-analysis. Int J Clin Exp Med. (2015) 8:17206–13.
30. Fink DS, Sibley H, Kunduk M, Schexnaildre M, Kakade A, Sutton C, et al. Subjective and objective voice outcomes after transoral laser microsurgery for early glottic cancer. Laryngoscope. (2016) 126:405–7. doi: 10.1002/lary.25442
31. Gandhi S, Gupta S, Rajopadhye G. A comparison of phonatory outcome between trans-oral CO2 Laser cordectomy and radiotherapy in T1 glottic cancer. Eur Arch Otorhinolaryngol. (2018) 275:2783–6. doi: 10.1007/s00405-018-5152-8
32. Kono T, Saito K, Yabe H, Uno K, Ogawa K. Comparative multidimensional assessment of laryngeal function and quality of life after radiotherapy and laser surgery for early glottic cancer. Head Neck. (2016) 38:1085–90. doi: 10.1002/hed.24412
33. Lee HS, Kim JS, Kim SW, Noh WJ, Kim YJ, Oh D, et al. Voice outcome according to surgical extent of transoral laser microsurgery for T1 glottic carcinoma. Laryngoscope. (2016) 126:2051–6. doi: 10.1002/lary.25789
Keywords: glottic laryngeal squamous cell carcinoma, radiotherapy, surgery, SEER, nomogram
Citation: Du Y, Shao S, Lv M, Zhu Y, Yan L and Qiao T (2020) Radiotherapy Versus Surgery–Which Is Better for Patients With T1-2N0M0 Glottic Laryngeal Squamous Cell Carcinoma? Individualized Survival Prediction Based on Web-Based Nomograms. Front. Oncol. 10:1669. doi: 10.3389/fonc.2020.01669
Received: 26 February 2020; Accepted: 28 July 2020;
Published: 26 August 2020.
Edited by:
Christian Simon, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Yong Yang, Nanjing Medical University, ChinaHaige Zhang, Luoyang Central Hospital Affiliated to Zhengzhou University, China
Xiaoyun Shen, Sir Run Run Shaw Hospital, China
Copyright © 2020 Du, Shao, Lv, Zhu, Yan and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiankui Qiao, cWlhb3RrQDE2My5jb20=; Li Yan, eWFubDEzQGZ1ZGFuLmVkdS5jbg==
 Yajing Du
Yajing Du Shali Shao1
Shali Shao1 Li Yan
Li Yan