- 1Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Clinical Immunology Center, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objective: To define the effectiveness of different anastomosis on clinically relevant postoperative fistula in patients with soft pancreas using the newest version of the fistula definition and criteria.
Background: Different criteria of clinically relevant postoperative pancreatic fistula (POPF) result in the optimal anastomosis technique remaining controversial.
Methods: PubMed, Embase, Web of Science, the Cochrane Central Library, and ClinicalTrials.gov were systematically searched up to 20 April 2020, and were evaluated by Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Randomized controlled trials comparing duct-to-mucosa anastomosis vs. invagination anastomosis in pancreatic surgery were included.
Result: Seven studies involving 1,110 participants were included. Using the postoperative pancreatic fistula definition provided by the International Study Group of Pancreatic Surgery 2016, the incidence rate of grade B/C pancreatic fistula was significantly lower in patients experiencing invagination anastomosis than in those undergoing duct-to-mucosa anastomosis. Four of seven trials comparing invagination with duct-to-mucosa anastomosis in patients with a soft pancreas showed that invagination was significantly better than duct-to-mucosa anastomosis in controlling pancreatic fistula formation, but no significant difference was detected between the two anastomosis techniques in patients with a hard pancreas. No significant difference in the length of hospital stay or postoperative mortality rate was found between the two methods.
Conclusion: This study demonstrated superiority of invagination anastomosis over duct-to-mucosa anastomosis in reducing the risk of Grade B/C postoperative pancreatic fistula using the ISGPS 2016 definition, but it does not significantly reduce the mortality rate or length of hospital stay. The effect of invagination in reducing pancreatic fistula formation is obvious in patients with a soft pancreas, but there is no significant difference between the two anastomosis techniques in patients with a hard pancreas. We found a lower rate of clinically relevant postoperative pancreatic fistula in the invagination group, in patients with a soft pancreas.
Background
Pancreaticoduodenectomy (PD) is one of the most complex and difficult procedures among all surgeries. PD is the most common treatment method for patients with a resectable tumor of the pancreatic head and periampullary region or a suspicious mass or nodule in that area with obvious clinical manifestations. The use of more anastomoses in abdominal surgery increases the risk of developing a postoperative fistula, which may result in various postoperative complications. A postoperative pancreatic fistula (POPF) remains one of the most common complications of PD and can lead to abdominal infection, bleeding, and sepsis, all of which lead to a longer postoperative hospital stay, severe and potentially fatal complications, and higher mortality (1).
Pancreaticojejunostomy and pancreaticogastrostomy are two common methods for digestive reconstruction after PD. Duct-to-mucosa anastomosis and invagination anastomosis are the two major pancreaticojejunostomy techniques, which can also be applied to pancreaticogastrostomy.
Several recent studies have compared the efficacy of invagination and duct-to-mucosa techniques in preventing POPF, but with conflicting results (2–4). Notably, Hua et al. (2). used the definition suggested by the International Study Group of Pancreatic Surgery (ISGPS) in 2005 and found that invagination is not better than duct-to-mucosa anastomosis in terms of reducing POPF but reduce Grade B/C POPF. Sun et al. (3) found no significant difference between invagination and duct-to-mucosa anastomosis; however, they also used the POPF definition proposed by the ISGPS 2005. Ratnayake et al. (5) conducted a network meta-analysis to prove that duct-to-mucosa pancreaticogastrostomy can reduce the rate of POPF, but the ISGPS 2005 definition was still employed. The ISGPS 2005 definition is problematic because clinically non-relevant pancreatic fistulas are not distinguished from total pancreatic fistulas. Therefore, the latest POPF definition released by the ISGPS in 2016 excludes grade A POPF and redefines it as a biochemical fistula, which is more suitable in guiding clinical treatments as well as for meeting clinical needs.
In this study, we used the POPF definition proposed by the ISGPS in 2016 to compare invagination with duct-to-mucosa anastomosis for clinically relevant POPF only (6).
The pancreatic fistula risk score (FRS) is used to predict the risk of a pancreatic fistula after PD. According to the main risk factors for POPF, the FRS summarizes four main indicators: the texture of the pancreas, pathology, diameter of the main pancreatic duct, and intraoperative blood loss. The alternative FRS for PD (a-FRS) is based on three intraoperatively available variables: duct size, pancreatic texture, and body mass index (7). According to both the FRS and a-FRS, patients with a soft pancreas are more likely to develop POPF, but there is a lack of systematic reviews to support this (8). Moreover, few studies have systematically and comprehensively compared the efficacy of the two techniques in preventing clinically relevant POPF or POPF in patients with a soft pancreas.
Furthermore, we also compared invagination with duct-to-mucosa anastomosis in patients with a soft pancreas and hard pancreas, respectively. Finally, we compared the mortality rate and length of hospital stay after PD between patients who underwent duct-to-mucosa and invagination anastomosis.
Materials and Methods
This systematic review was established by the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (9).
Study Selection
Relevant studies were searched and identified by individually searching the following databases: PubMed, Embase, Web of Science, the Cochrane Central Library, and ClinicalTrials.gov up to 20 April 2020. For all databases, the search strategy involved use of the following key terms: “duct-to-mucosa” “pancreatic fistula” “pancreaticoduodenectomy” and “invagination.” The search was limited to publications of randomized controlled trials (RCTs). This meta-analysis adhered to the Critical Appraisal Skills Programme Checklist. An eligibility assessment was performed by two independent reviewers (WL and ZC). Disagreements between reviewers were resolved by group discussions and consensus.
Inclusion and Exclusion Criteria
Eligibility was assessed by two independent reviewers (WL and ZC), with consensus reached by discussing conflicts with a third investigator (TZ). Assessments were performed and repeated twice. First, the titles and abstracts were assessed. The full text of potentially qualified studies was then obtained and assessed. No reviewers were blinded to the authorship of the studies. Dissertations, conference proceedings, and in non-English studies were excluded. There were no restrictions on the history of pancreatic diseases, the follow-up duration, or the reoperation times. To evaluate the effects of reducing pancreatic fistula of invagination and duct-to-mucosa anastomosis, we included those relevant studies.
Outcomes of Interest
The primary outcome measure was the rate of clinically relevant POPF (grades B and C). We use the newest definition of POPF formulated by the ISGPS. The secondary outcome measures were the mortality rate and length of hospital stay. In the subgroup analysis, we compared the rate of clinically relevant POPF between the two anastomosis techniques in patients with a soft pancreas and hard pancreas, respectively.
Data Collection
The data extracted included: first author, year of study, country of origin, number of patients, population characteristics, and POPF rate. The data were extracted and cross-checked independently by two authors (WL and ZC). Disagreements were resolved through discussions with a third reviewer (TZ) until a consensus was reached.
Evaluation of Quality of Evidence
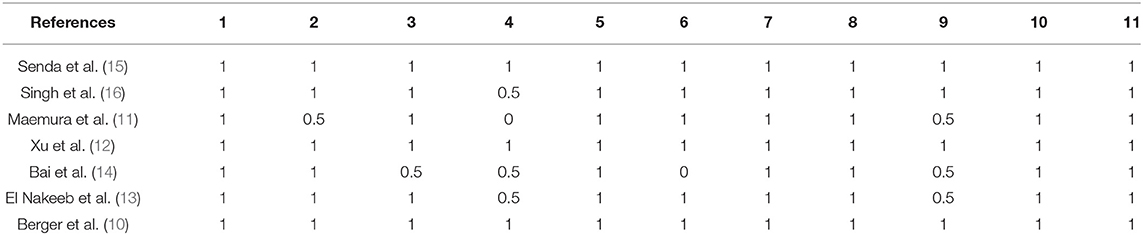
The methodological quality of the selected studies was blindly evaluated by two independent reviewers (WL and ZC). Disagreements were discussed among the group and resolved by a third assessor (TZ). Quality was assessed using the CASP Checklist, which evaluates the risk of bias and comprises 11 items related to methodological quality and statistical reporting. Discrepancies and disagreements were resolved by consensus.
Statistical Analysis
Data were entered into the Cochrane Collaboration's Review Manager program (RevMan version 5.3; Cochrane Collaboration, Oxford, UK). We analyzed the standardized mean differences with 95% confidence intervals (CIs) and performed tests of heterogeneity (I2) for outcomes. Fixed-effects or random-effects models were used accordingly. Subgroup analysis stratified by the effect of pancreatic texture on reducing POPF was performed. Funnel plots were used to detect possible publication bias based on the primary outcome (incidence rate of POPF) and secondary outcomes (mortality and length of hospital stay).
Results
Literature Search
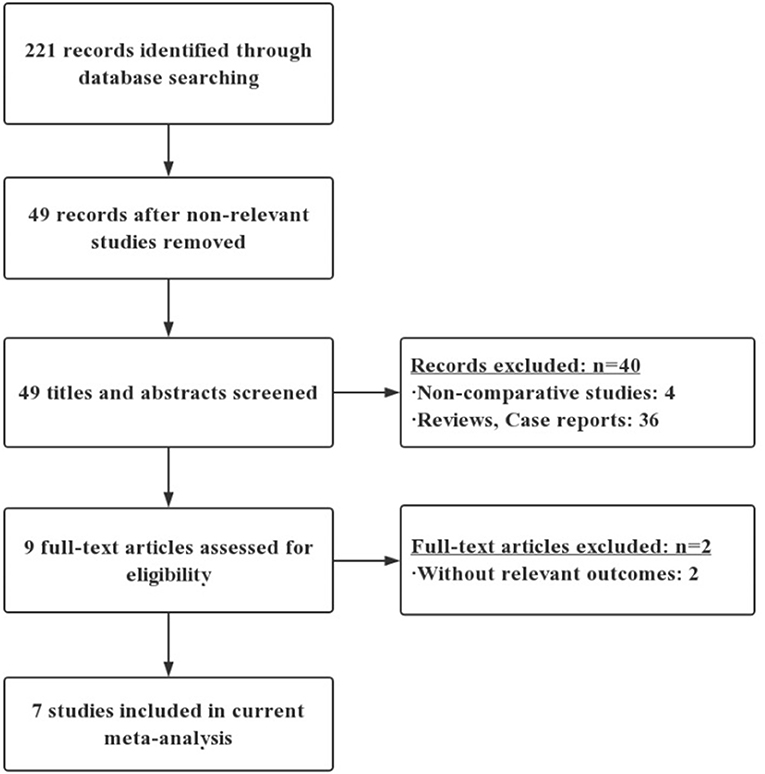
A flow diagram of the literature search is shown in Figure 1. Among 221 unique articles, nine fulfilled the inclusion criteria. Initially, through the electronic database search, we identified 221 citations. Examinations of the reference lists in all relevant papers, recent editorials, and related review articles yielded no further studies for evaluation. Non-RCTs were excluded, leaving 49 citations. Nine articles were then selected after reading the titles and abstracts. After careful reading of these nine full-text articles, two studies were excluded because they did not include POPF outcomes. The remaining seven RCTs were included in the qualitative analysis and the final meta-analysis (10–16).
Study Characteristics
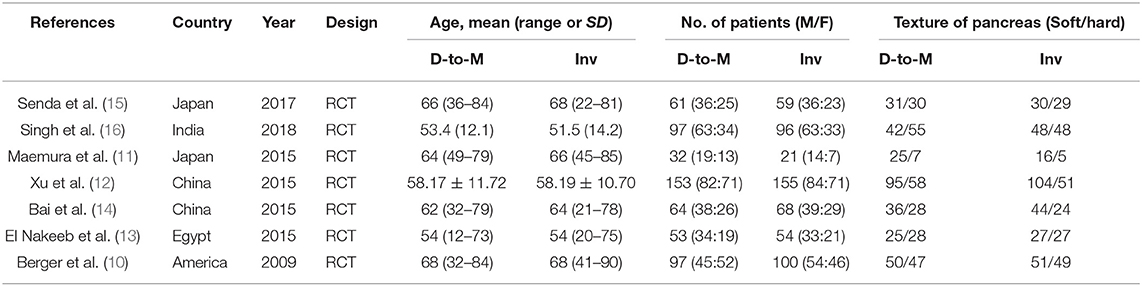
The characteristics of each RCT are presented in Table 1. Our meta-analysis included 1,110 patients (640 men, 470 women) who underwent PD in five different countries. Duct-to-mucosa anastomosis was performed in 557 patients, and invagination anastomosis was performed in 553. The results of the quality assessment of the included RCTs are shown in Table 2.
Primary Outcome
POPF
All of the included studies reported the POPF rate. The POPF rate was 12.4% (69/557) in the duct-to-mucosa group and 7.4% (41/329) in the invagination group. The incidence of Grade B/C POPF was lower in patients experiencing invagination than in those duct-to-mucosa anastomosis (odds ratio [OR] = 1.78, 95% CI = 1.18–2.67, P = 0.006) (Figure 2).
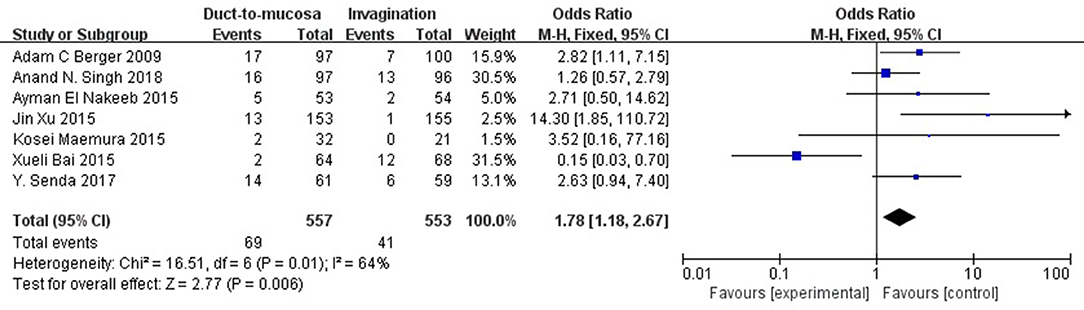
Figure 2. The comparison of incidence of POPF (grade B/C) between patients undergoing invagination and duct-to-mucosa. Incidence of clinically relevant POPF (grade B/C) was lower in patients undergoing invagination than in those undergoing duct-to-mucosa anastomosis (odds ratio [OR] = 1.78, 95% CI = 1.18–2.67, P = 0.006).
Secondary Outcomes
Length of Hospital Stay
Three RCTs reported the length of hospital. No significant difference was found between the two techniques. No significant heterogeneity was present (Q statistic = 2.42, P = 0.30, I2 = 17%). Data were analyzed using the fixed-effects model, which showed no significant heterogeneity (relative risk = −0.33, 95% CI = −1.80–1.14, P = 0.66) (Figure 3).
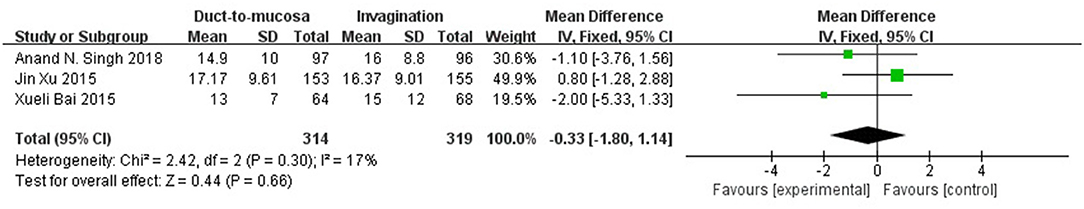
Figure 3. Three RCTs reported the length of hospital stay with useful data for analysis. No significant difference was found between the duct-to-mucosa and invagination techniques. No significant heterogeneity was present (Q statistic = 2.42, P = 0.30, I2 = 17%). The fixed-effects model was indicated; again, no significant difference was observed between the two groups (relative risk = −0.33, 95% CI = −1.80–1.14, P = 0.66).
Overall Mortality
The mortality rates were reported in five RCTs. The overall mortality rate was 2.45% (2.16% in the duct-to-mucosa group and 2.75% in the invagination group). The fixed-effects model was indicated but no significant difference was observed between the two groups (Q statistic = 3.09, P = 0.54, I2 = 0%). No significant difference was observed between the two groups (OR = 0.79, 95% CI = 0.36–1.75, P = 0.56) (Figure 4).
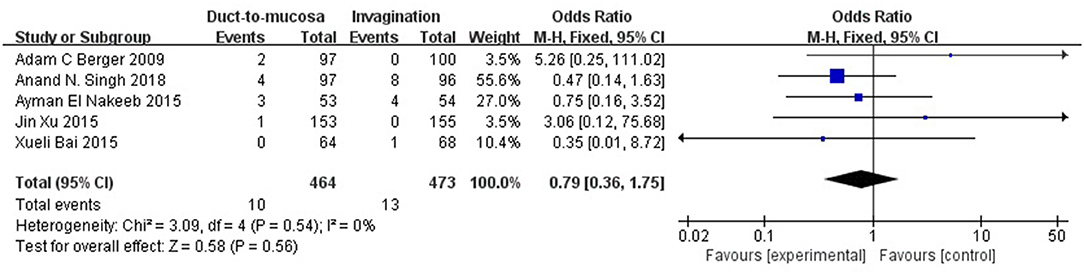
Figure 4. The postoperative mortality rates were reported in five RCTs. The overall mortality rate was 2.45% (2.16% in the duct-to-mucosa group and 2.75% in the invagination group). Data were analyzed using the fixed-effects model, which showed no significant heterogeneity (Q statistic = 3.09, P = 0.54, I2 = 0%). No significant difference was observed between the two groups (OR = 0.79, 95% CI = 0.36–1.75, P = 0.56).
Subgroup Analysis
Considering that soft pancreatic texture may be a significant risk factor for POPF, a subgroup analysis of soft pancreas patients was performed. Four studies involving 441 patients with a soft pancreas were pooled and analyzed. These four trials, comparing invagination with duct-to-mucosa anastomosis in patients with a soft pancreas, showed that invagination is much better than duct-to-mucosa anastomosis in controlling pancreatic fistula (OR = 2.47, 95% CI = 1.57–3.90, P < 0.0001). Four trials comparing invagination with duct-to-mucosa anastomosis in patients with a hard pancreas showed no significant difference between the two techniques in controlling pancreatic fistula (OR = 1.06, 95% CI = 0.50–2.27, P = 0.87) (Figure 5).
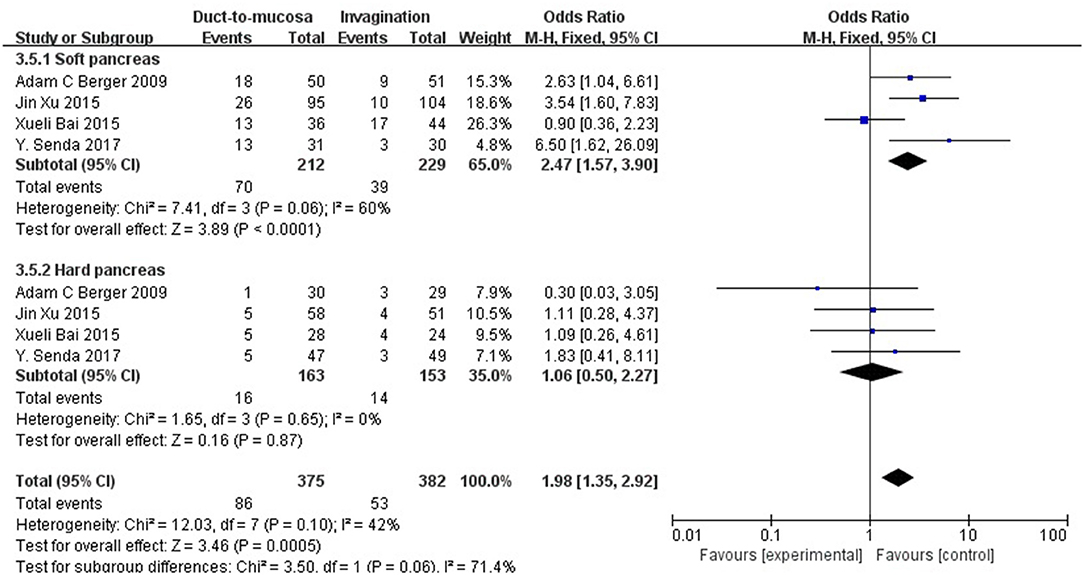
Figure 5. A subgroup analysis for patients with a soft pancreas. These four trials comparing invagination with duct-to-mucosa anastomosis in patients with a soft pancreas showed that invagination is much better than duct-to-mucosa anastomosis in controlling pancreatic fistula (OR = 2.47, 95% CI = 1.57–3.90, P < 0.0001). Four trials comparing invagination with duct-to-mucosa anastomosis in patients with a hard pancreas showed no significant difference between the two techniques in controlling pancreatic fistula (OR = 1.06, 95% CI = 0.50–2.27, P = 0.87).
Publication Bias
A funnel plot based on the incidence of POPF, length of hospital stay, and mortality rate is presented in Figure 6. The funnel plot does not show obvious asymmetry, and only one study lay outside the limits of the 95% CI for the incidence rate of POPF. No study lay outside the limits for mortality and length of hospital stay, indicating no obvious publication bias.
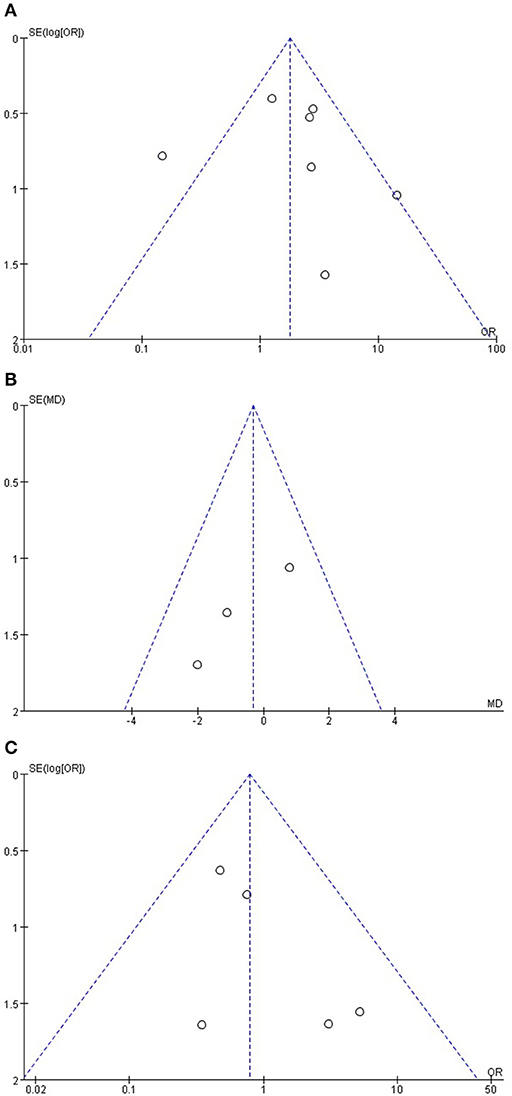
Figure 6. (A) Funnel plot based on the incidence of POPF, (B) length of hospital stay, and (C) mortality rate.
Discussion
Although several studies have compared duct-to-mucosa and invagination anastomosis, increasing conflicting outcomes have been obtained. This is the first study to prove the statistically significant superiority of invagination over duct-to-mucosa anastomosis in terms of the rate of clinically relevant POPF after PD. The use of different definitions of POPF and the lack of a standardized surgical techniques make it difficult to compare results from different studies. In this article, a pancreatic fistula was defined using the newest version of the ISGPF criteria (2016), in which grades B and C POPF are termed clinically significant, while grade A is a biochemical fistula (7).
We found that in contrast to duct-to-mucosa anastomosis, invagination is easier to perform, and more of the pancreatic juice can be drained into the jejunum. Mechanically, when the duct-to-mucosa technique is applied, the main pancreatic duct is anastomosed, and other branch pancreatic ducts are closed by adhesion between the pancreatic stump and the jejunal or gastric wall. The technical requirements of duct-to-mucosa anastomosis are a dilated duct and a firm pancreas, and there are concerns about incomplete drainage of the pancreatic stump. The strengths of duct-to-mucosa technique include sufficient drainage of the main duct into the connected intestine as well as long-term preservation of duct patency. However, this technique does not allow drainage of the other branch ducts on the cut surface of the stump and requires a longer operative time. In patients with small pancreatic ducts, the operation is technically difficult and prolonged, and the risk of pancreatic injury and complications such as POPF are increased. Berger et al. (8) released a significantly higher rate of POPF after the duct-to-mucosa technique than the invagination technique and surprisingly found that the duct-to-mucosa technique was an independent risk factor of POPF. Therefore, the main advantage of duct-to-mucosa anastomosis is that it is suitable for patients with a small intestinal lumen, thicker pancreatic stump, or a hard pancreas. However, the disadvantage is that the method only facilitates drainage of the main pancreatic duct; it ignores the branch ducts. If the stump is not sutured well, a severe POPF may develop because of the presence of the branch pancreatic duct. A narrow main pancreatic duct makes this technique more difficult. In addition, the duct-to-mucosa technique is slightly complicated, involving many sutures and anastomoses with a high risk of needle-induced leakage.
The present study showed that invagination anastomosis is significantly superior in terms of the rate of clinically relevant POPF after PD. The mechanism underlying this superiority may be as follows; first, the operation time is much longer for duct-to-mucosa anastomosis than invagination because the former is a more complicated operation with a high number of anastomoses, making POPF more likely to develop; second, invagination involves the creation of a larger anastomosis to wrap the pancreatic duct for prevention of POPF, unlike the duct-to-mucosa technique which does not.
Patients with an exceedingly narrow main pancreatic duct, which cannot be seen on the pancreatic cut surface, are more likely to be treated using the invagination technique. The invagination technique is capable of complete drainage of both the pancreatic duct and the stump. But the need to mobilize a long length of the pancreatic stump and the need to place circumferential sutures, culminate in impairment, and disruption of the stump's blood supply (17). Therefore, the main advantages of invagination anastomosis are that both the pancreatic duct and pancreatic stump are buried in the jejunum and the operation is relatively simple. It is suitable for patients with a narrow pancreatic duct and a soft pancreas. However, because the pancreatic stump is exposed to the intestinal lumen with long-term exposure to digestive juice, the pancreatic stump is prone to erode and bleed, and the pancreatic duct is easily obstructed. This is a common shortcoming of all invagination anastomosis procedures. Patients with an excessive pancreatic stump are not suitable for invagination, especially through laparoscopy. If the anastomosis is forcibly inserted, not only will ischemia of the pancreatic stump occur but the pancreas will tear because of excessive tension on the suture.
The occurrence of POPF depends on several factors, including the diameter of the pancreatic duct, pancreatic texture, and anastomotic technique (18). Among the various factors that can contribute to POPF, the texture of the pancreas was clearly a major contributing factor in the present study. We found that patients with a soft pancreas were more likely to develop POPF after duct-to-mucosa than after invagination anastomosis. This is because it is difficult to anastomose a soft pancreas using the duct-to-mucosa technique. The diameter of the pancreatic duct is another important factor that can lead to POPF. We aimed to conduct a subgroup analysis of different diameters of the pancreatic duct, but only four articles included relevant data, and only two of these four articles had comparable data.
In the duct-to-mucosa technique, the size of the anastomosis is small with relatively less leakage than in the invagination technique, and tissue corrosion caused by the pancreatic fistula is limited. In contrast, invagination anastomosis involves a double-layer suture technique, which provides enough space for the accumulation of pancreatic juice. In addition, the anastomosis is relatively large, usually about 3 cm. Once POPF occurs, the amount of leakage is relatively large with a higher risk of infection. Each anastomosis has its own strengths and weaknesses.
The present meta-analysis showed no significant difference in the mortality rate or length of hospital stay between the two anastomosis techniques. The reason may be that invagination reduces the POPF rate but cannot reduce the overall complication rate. We searched relevant studies from all databases and found that all relevant RCTs revealed no significant difference between invagination and duct-to-mucosa anastomosis (10, 13, 14, 19, 20), which can support the hypothesis that invagination reduces the POPF rate but cannot reduce the overall complication rate. Additional studies are needed to define the optimal technique of pancreatic reconstruction after PD. Such studies could provide a stimulus for future prospective randomized trials focusing on variations in technique and interventions in pancreatic surgery.
Our study has several strengths. We included high-quality studies, and all of them were RCTs. In addition, we used the newest standard definition of POPF (ISGPS 2016). Moreover, we identified a new method with which to reduce the rate of clinically relevant POPF without increasing the mortality rate or length of hospital stay.
Our study also has several limitations. First, some studies reported in languages other than English and, possibly, some unpublished studies were excluded; this may have contributed to selection bias. Second, several known risk factors for POPF, such as the diameter of the main pancreatic duct, stent use, and octreotide use, could not be explored in the subgroup analysis because of the lack of comparable information in the included trials. Third, indication for PD may be a potential bias due to heterogenicity. Fourth, although we believe that the conclusion of this meta-analysis is the most dependable conclusion obtained in this field to date, it might not be the final conclusion. Future studies may change the conclusion, although the probability seems low.
Conclusion
This study demonstrated superiority of invagination anastomosis over duct-to-mucosa anastomosis in reducing the risk of a Grade B/C postoperative pancreatic fistula using the ISGPS 2016 definition. The effect of invagination in reducing pancreatic fistula formation is obvious in patients with a soft pancreas. We found a lower rate of clinically relevant postoperative pancreatic fistula in the invagination group, in patients with a soft pancreas.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
WL and ZC: study design and data interpretation. WL: literature search, data retrieval, data analysis, and writing of the manuscript. WL, ZC, and TZ: critical revision and final approval. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81772639, No. 81802475, No. 81972258, No. 81974376); the Natural Science Foundation of Beijing (No. 7192157); the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1-001); the China Postdoctoral Science Foundation (No. 198831); the National Fundamental Research Program of China (2018YFE0118600); the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge all the participants for searching, analyzing, and performing the studies that contributed to this piece of work and all the collaborators who make such studies possible. We acknowledge the clinic staff and managers of Peking Union Medical College Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College for their valuable contributions to this research.
Abbreviations
PD, Pancreaticoduodenectomy; POPF, Postoperative pancreatic fistula; FRS, fistula risk score; ISGPS, International Study Group of Pancreatic Surgery; CIs, Confidence intervals.
References
1. Kennedy EP, Yeo CJ. Dunking pancreaticojejunostomy versus duct-to-mucosa anastomosis. J Hepatobiliary Pancreat Sci. (2011) 18:769–74. doi: 10.1007/s00534-011-0429-y
2. Hua J, He Z, Qian D, Meng H, Zhou B, Song Z. Duct-to-mucosa versus invagination pancreaticojejunostomy following pancreaticoduodenectomy: a systematic review and meta-analysis. J Gastrointest Surg. (2015) 19:1900–9. doi: 10.1007/s11605-015-2913-1
3. Sun X, Zhang Q, Zhang J, Lou Y, Fu Q, Zhang X, et al. Meta-analysis of invagination and duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: an update. Int J Surg. (2016) 36:240–7. doi: 10.1016/j.ijsu.2016.11.008
4. Lyu Y, Li T, Wang B, Cheng Y, Zhao S. Selection of pancreaticojejunostomy technique after pancreaticoduodenectomy: duct-to-mucosa anastomosis is not better than invagination anastomosis: a meta-analysis. Medicine. (2018) 97:e12621. doi: 10.1097/MD.0000000000012621
5. Ratnayake CBB, Wells CI, Kamarajah SK, Loveday B, Sen G, French JJ, et al. Critical appraisal of the techniques of pancreatic anastomosis following pancreaticoduodenectomy: a network meta-analysis. Int J Surg. (2020) 73:72–7. doi: 10.1016/j.ijsu.2019.12.003
6. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
7. Mungroop TH, van Rijssen LB, van Klaveren D, Jasmijn Smits F, van Woerden V, Linnemann RJ, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. (2019) 269:937–43. doi: 10.1097/SLA.0000000000002822
8. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. (2013) 216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002
9. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
10. Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. (2009) 208:738–47. doi: 10.1016/j.jamcollsurg.2008.12.031
11. Maemura K, Mataki Y, Kurahara H, Mori S, Higo N, Sakoda M, et al. Pancreaticogastrostomy after pancreaticoduodenectomy using twin square wrapping with duct-to-mucosa anastomosis. Eur Surg Res. (2015) 55:109–18. doi: 10.1159/000433425
12. Xu J, Zhang B, Shi S, Qin Y, Ji S, Xu W, et al. Papillary-like main pancreatic duct invaginated pancreaticojejunostomy versus duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Surgery. (2015) 158:1211–8. doi: 10.1016/j.surg.2015.04.020
13. El Nakeeb A, El Hemaly M, Askr W, Abd Ellatif M, Hamed H, Elghawalby A, et al. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study. Int J Surg. (2015) 16:1–6. doi: 10.1016/j.ijsu.2015.02.002
14. Bai X, Zhang Q, Gao S, Lou J, Li G, Zhang Y, et al. Duct-to-mucosa vs invagination for pancreaticojejunostomy after pancreaticoduodenectomy: a prospective, randomized controlled trial from a single surgeon. J Am Coll Surg. (2016) 222:10–8. doi: 10.1016/j.jamcollsurg.2015.10.003
15. Senda Y, Shimizu Y, Natsume S, Ito S, Komori K, Abe T, et al. Randomized clinical trial of duct-to-mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br J Surg. (2018) 105:48–57. doi: 10.1002/bjs.10727
16. Singh AN, Pal S, Mangla V, Kilambi R, George J, Dash NR, et al. Pancreaticojejunostomy: does the technique matter? A randomized trial. J Surg Oncol. (2018) 117:389–96. doi: 10.1002/jso.24873
17. Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, Ehlers JP, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. (2002) 194:746–58. doi: 10.1016/S1072-7515(02)01202-4
18. Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. (2016) 22:7797–805. doi: 10.3748/wjg.v22.i34.7797
19. Chou FF, Sheen-Chen SM, Chen YS, Chen MC, Chen CL. Postoperative morbidity and mortality of pancreaticoduodenectomy for periampullary cancer. Eur J Surg. (1996)162:477–81.
Keywords: duct-to-mucosa, surgery, invagination, pancreatic fistula, soft pancreas, pancreatic texture, new criteria
Citation: Cao Z, Luo W, Qiu J, Liu Y, Zheng L and Zhang T (2020) Is Invagination Anastomosis More Effective in Reducing Clinically Relevant Pancreatic Fistula for Soft Pancreas After Pancreaticoduodenectomy Under Novel Fistula Criteria: A Systematic Review and Meta-Analysis. Front. Oncol. 10:1637. doi: 10.3389/fonc.2020.01637
Received: 27 May 2020; Accepted: 27 July 2020;
Published: 21 August 2020.
Edited by:
Jun Li, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Riccardo Casadei, University of Bologna, ItalyAlessandra Bertacco, Padua University Hospital, Italy
Copyright © 2020 Cao, Luo, Qiu, Liu, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taiping Zhang, dHBpbmd6aGFuZ0B5YWhvby5jb20=
†These authors have contributed equally to this work. Wenhao Luo is considered to be the joint first author.
 Zhe Cao
Zhe Cao Wenhao Luo
Wenhao Luo Jiangdong Qiu1
Jiangdong Qiu1 Yueze Liu
Yueze Liu Taiping Zhang
Taiping Zhang