- 1Department of Medical Oncology, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Surgical Oncology, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 3Department of Radiation Oncology, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 4Department of General Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 5Department of Neurosurgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Somatostatin receptor (SSTR) 2, widely expressed in meningioma, is a G-protein-coupled receptor and can be activated by somatostatin or its synthetic analogs. SSTR2 is therefore extensively studied as a marker and target for the diagnosis and treatment of meningioma. Accumulating studies have revealed the crucial clinical significance of SSTR2 in meningioma. Summarizing the progress of these studies is urgently needed as it may not only provide novel and better management for patients with meningioma but also indicate the direction of future research. Pertinent literature is reviewed to summarize the recent collective knowledge and understanding of SSTR2’s clinical significance in meningioma in this review. SSTR2 offers novel ideas and approaches in the diagnosis, treatment, and prognostic prediction for meningioma, but more and further studies are required.
Introduction
Meningiomas, arising from the dura mater of the brain and spinal cord, are currently the most frequent primary intracranial tumors (1). Meningiomas have an estimated annual incidence of 7.86 cases per 100,000 people, accounting for up to 30% of all primary intracranial tumors (2–4). The majority of meningiomas are histologically benign and slow growing and correspond to World Health Organization (WHO) grade I, while up to 20% of the tumors are classified as WHO grade II or grade III meningiomas on account of features of increased malignancy and local invasiveness (5, 6). Progressive enlargement of the tumor and compression of adjacent neural tissue lead to clinical manifestations, such as generalized or focal seizure disorders, focal neurological deficits, and neuropsychological decline (3). The preliminary radiological diagnosis and precise localization of meningioma mainly depend on magnetic resonance imaging (MRI) nowadays (1, 7). Surgical resection remains the standard treatment for meningiomas; however, observation should be considered as a therapeutic option if the clinical situation permits; meanwhile, radiotherapy is becoming increasingly important in the treatment of meningiomas, especially for those surgically inaccessible tumors; in addition, large-scale clinical trials for pharmacotherapy have not presented positive results yet (1, 8–10).
Somatostatin receptors 1–5 (SSTR1–5) pertain to the family of seven-transmembrane G protein-coupled receptors and are widely expressed in both normal tissues and solid tumors (11, 12). These five receptors share some common features underlying structure and signaling mechanisms, but their cellular/subcellular localization and mode of regulation vary from one to another (12, 13). Among these receptors, the overexpression of SSTR2 was the most frequent in meningiomas compared with the other SSTR subtypes (14). In recent years, accumulating studies have reported the correlation between SSTR2 expression and meningiomas. However, to the best of our knowledge, no literature review has been published to summarize it thus far. Hence, we provide a detailed summary of the current understanding of the clinical significance of SSTR2 in meningioma in this review.
Synopsis of SSTR2
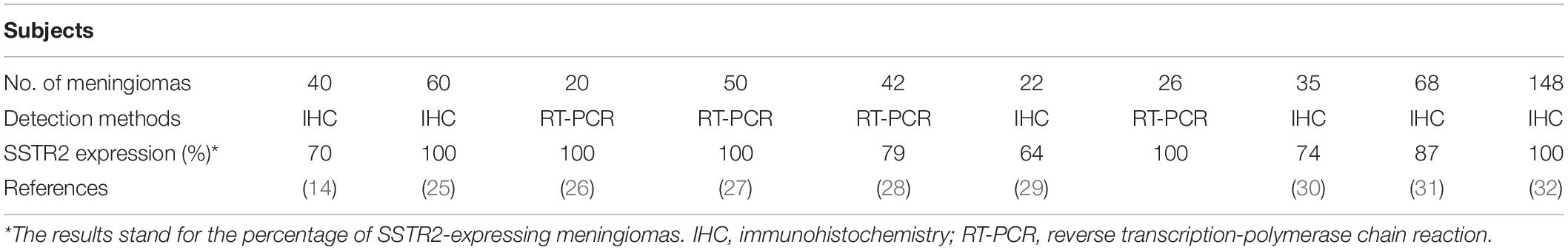
The encoding gene for SSTR2 is localized at chromosome 17q25.1 and comprises two exons. The first exon contains the 5′ untranslated region while exon 2 contains the entire coding region and 3’ untranslated region (13). The SSTR2 gene has a strong tolerance to sequence variations; hardly any disease-related mutations have been discovered in the SSTR2 gene (13, 15). The transcribed SSTR2 mRNA is spliced to produce two isoforms of SSTR2 named SSTR2A (the long form) and SSTR2B (the short form), which differ in the length of the cytoplasmic tail (12, 16). Human tissues include the SSTR2A variant exclusively (13). Typical seven-transmembrane segments and four putative N-glycosylation sites could be displayed in the SSTR2 protein of 369 amino acids (13). The protein can be detected by Western blot as a characteristic band of 70–80 kDa (13, 17, 18). SSTR2 is ubiquitously distributed in normal tissues especially in the central nervous system (CNS) and endocrine system (12, 19–21). SSTR2 is also expressed widely and represents manifold functions in various tumor tissues including neuroendocrine tumors, pituitary adenomas, breast cancer, melanoma, thyroid cancer, and meningioma (20, 22–25). Nevertheless, the expression level of SSTR2 between normal tissues and tumor tissues is different. For instance, SSTR2 was identified as significantly highly expressed in meningioma tissues compared with normal tissues by Anne et al. (26). The expression of SSTR2 can routinely be detected through reverse-transcription polymerase chain reaction and immunohistochemistry (Table 1); the vast majority of meningiomas express SSTR2 (14, 25–32). SSTR2 mediates diverse physiological effects when activated by somatostatin or its synthetic analogs, such as regulating the physiologic secretion of insulin, glucagon, thyroid-stimulating hormone, and growth hormone (GH); protecting retina nerves; and regulating neuronal excitability (13, 23, 33–36).
SSTR2-Related Diagnosis Approaches for Meningioma
A preliminary diagnosis of meningioma typically relies on MRI and computed tomography (CT); further diagnosis includes histological classification, grading, and molecular features (1, 3, 7). However, because the results of CT and MRI are sometimes ambiguous and because biopsy carries potential risks of bleeding, additional approaches (Figure 1) for the diagnosis of meningioma are needed (37). Besides, this “integrated diagnosis” era also calls for other novel and efficient diagnostic methods for accurate diagnoses of meningioma (38, 39).
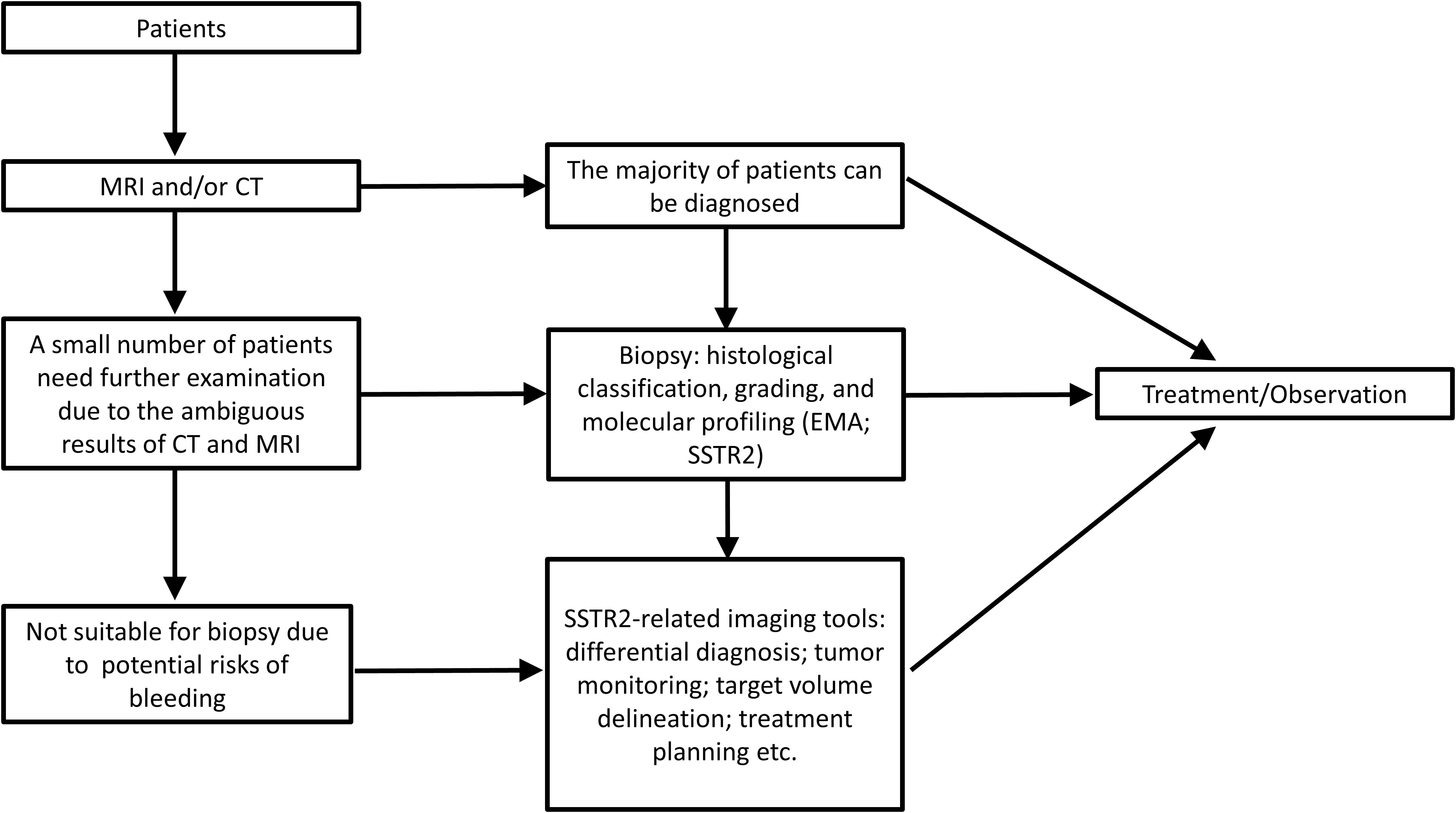
Figure 1. The diagnosis process and application scenarios of meningioma with the utilization of SSTR2.
Somatostatin receptor 2A was found to be a more sensitive diagnostic marker for meningioma than epithelial membrane antigen—a conventional meningioma marker (40). Since then, accumulating evidence has emerged to support the diagnosis value of SSTR2A as it is a highly sensitive and specific marker for meningioma (41, 42). A case report has shown that SSTR2A, combined with epithelial membrane antigen, provides assistance for the diagnosis of an unusual skull tumor with psammomatoid bodies (43).
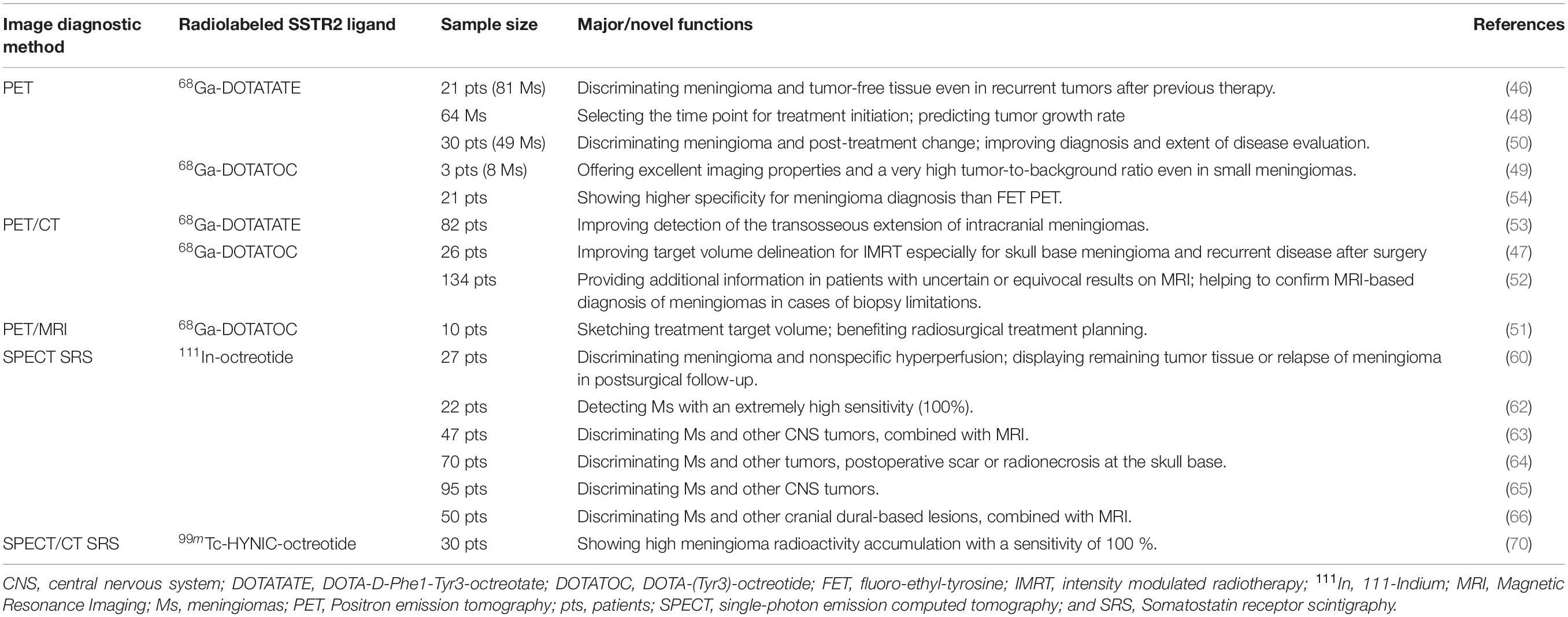
Moreover, given that SSTR2 is expressed in almost 100% meningiomas (14, 26, 30, 44, 45), radiolabeled SSTR2 ligands have been widely utilized in the modern radiological diagnosis of meningioma (Table 2).
Positron emission tomography (PET)-based imaging (including PET, PET/CT, and PET/MRI) applying radiolabeled somatostatin agonists such as 68Ga-DOTATATE (DOTA-D-Phe1-Tyr3-octreotate) and 68Ga-DOTATOC (DOTA-[Tyr3]- octreotide) has been presented to be a precise diagnostic means; this technology is helpful in target volume delineation, radio/surgical treatment planning, diagnosing small meningiomas, and monitoring tumor growth rate, etc. (46–51). Recent researches demonstrated a higher sensitivity of 68Ga-DOTATOC or 68Ga-DOTATATE PET or PET/CT by comparison with contrast-enhanced MRI or fluoroethyl-tyrosine PET in diagnosing meningiomas (52–54). Additionally, the exact delineation seems challenging in some cases with low CT and MRI contrast as a result of osseous infiltration or in skull base meningiomas. PET-based imaging with radiolabeled SSTR2 ligands shows superiority in overcoming this diagnostic difficulty due to the highly specific binding of SSTR2 ligands to SSTR2 in meningiomas and the extremely low absorption in adjacent structures such as bone and brain tissue (7, 53, 55, 56). Furthermore, in the case of atypical meningioma or a rare type of meningioma like optic nerve sheath meningioma, SSTR2-related PET/CT is also deemed to be a useful noninvasive diagnostic method (57–59).
Single photon emission CT (SPECT) somatostatin receptor scintigraphy (SRS) using 111In-octreotide is another valuable tool for the diagnoses of meningiomas based on the general expression of SSTR2 in all meningiomas. SPECT SRS with 111In-octreotide is considered a highly specific imaging approach, and it plays an important role in post-treatment follow-up in meningioma patients (60, 61). Hildebrandt et al. have shown that in vivo detection of SSTRs by 111In-octreotide scintigraphy in meningioma patients had a high sensitivity as a high density of SSTRs was detected in all cases (62). Regarding differential diagnosis in meningioma and other CNS tumors such as craniopharyngiomas, schwannomas, and ependymomas or other cranial dural-based lesions, SPECT SRS with 111In-octreotide has also proven its values (63–66). In the meantime, SPECT SRS could offer aid in the differential diagnosis between meningiomas and radionecrosis or postoperative scar at the skull base, which is meaningful for recurrence screening of meningioma (64). As for cases with an atypical presentation, SPECT SRS can offer support in distinguishing optic nerve sheath meningioma from alternative orbital masses (67, 68).
Other SSTR2-related imaging tools also exhibit diagnostic values. For instance, SPECT/CT SRS using 99mTc-HYNIC-octreotide specifically binding to SSTR2 in meningioma can diagnose primary optic nerve sheath meningioma or allow differentiation of meningiomas from inactive pituitary adenomas, which is seemingly elusive by conventional MRI (69, 70).
These studies suggest that SSTR2-related imaging tools with radiolabeled somatostatin agonists are valuable for precise-positioning tumor detection, evaluation of disease extension, differential diagnosis, and tumor monitoring even in small, asymptomatic, or rare cases.
SSTR2-Related Treatment Approaches for Meningioma
Individualized precision treatment regimens should be employed in treating patients with meningioma since heterogeneity between meningiomas exists and clinical outcomes for different patients vary greatly (1). Correct decision making in the management of meningioma patients is significant in order to achieve optimal clinical consequence and long-time survival (71–73). Surgery is the main treatment for most meningiomas; however, effective treatment modalities for patients with unresectable or recurrent meningioma remain elusive. It is of interest that SSTR2-related/targeted treatments could provide novel therapeutic interventions against meningiomas beyond traditional therapies, especially for those inoperable or recurrent patients.
The exact biological function of SSTR2 in meningioma is hitherto not very sharply defined, but its activation may be correlated to an antiproliferative effect (28, 74–77). Native somatostatin is rapidly metabolized and has a short half-life (1–3 min) in vivo, which limits its clinical use, whereas synthetic somatostatin analogs like octreotide are much more stable (20). Somatostatin analogs have already achieved promising effects in the treatment of high-SSTR2-expression tumors, such as gastroenteropancreatic neuroendocrine tumors and GH-secreting pituitary adenomas (78, 79). The therapeutic efficacy of somatostatin analogs for meningiomas in vitro has been confirmed in various studies (27, 77, 80, 81). For example, Graillon et al. demonstrated that octreotide significantly decreased proliferation in 88% of fresh primary meningioma cells (82). Nonetheless, octreotide has been shown not to induce apoptosis of meningioma cells (82).
The direct and indirect antitumor mechanisms (Figure 2) of the SSTR2 ligands–somatostatin analogs for the treatment of meningioma have been explored in several preclinical researches. Somatostatin or its analogs bind to SSTR2, leading to the activation of specific tyrosine phosphatases (SHP1 and SHP2) and the inhibition of the PI3K/Akt pathways, which mediate its direct antitumor effects through the induction of cyclin-dependent kinase inhibitors and cell cycle arrest (27, 80, 81, 83–87). The indirect antitumor mechanisms of somatostatin analogs incorporate (1) reduction of angiogenesis, particularly by inhibiting vascular endothelial growth factor (VEGF) secretion; (2) suppression of growth factors and hormone secretion that will drive tumor growth; and (3) stimulation of natural antitumor mechanisms (27, 84, 86–88). The synthesis of VEGF, one of the dominant proangiogenic factors, was decreased in meningioma cells by somatostatin analogs, indicating their antiangiogenic effects (27, 84, 86, 87). Somatostatin analogs can inhibit the release of GH from the pituitary gland, which causes the suppression of hepatic production of insulin-like growth factor-1 (IGF-1) (84, 86, 87). Both GH and IGF-1 have been proven to be tumor-promoting factors for meningioma (84, 86–88). Somatostatin and its analogs are also capable of activating the immune system, for SSTR2 are expressed in some immune cells (84, 86, 87, 89).
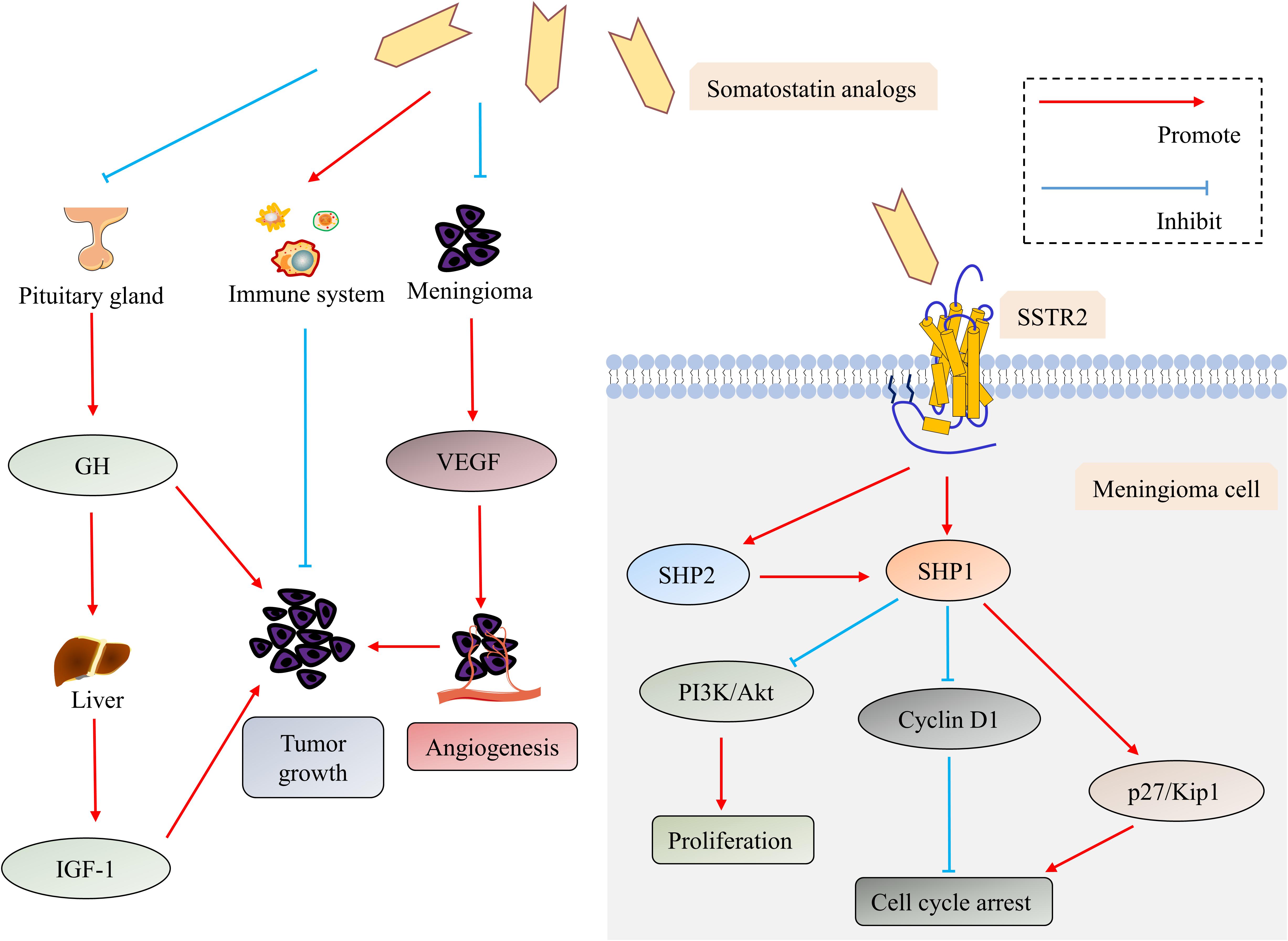
Figure 2. The direct and indirect antitumor mechanisms of the somatostatin analogs in meningioma. Somatostatin analogs exert their direct antitumor effects by binding to SSTR2, which leads to the activation of SHP1 and SHP2. SHP2 can further activate SHP1. SHP1 mediates antiproliferative action through inhibiting the PI3K/Akt pathway and induces cell cycle arrest through down-regulating cyclin D1 while up-regulating p27/Kip 1. Suppressing secretion of VEGF and GH/IGF-1 and activating the immune system are involved in indirect antitumor mechanisms of somatostatin analogs. Abbreviations: Akt, protein kinase B; GH, growth hormone; insulin-like growth factor-1 (IGF-1); PI3K, phosphatidylinositol 3-kinase; SHP1, SH2-containing phosphatase-1; SHP2, SH2-containing phosphatase-2; SSTR, somatostatin receptor; and VEGF, vascular endothelial growth factor.
In some cases, considerable efficacy of somatostatin analogs could even be achieved in the treatment of unresectable or recurrent meningiomas (90–92). Rammo et al. reported a patient with progressive anaplastic meningioma treated with octreotide. Prior to octreotide therapy, repeated surgery and radiation therapy did not help stop the progression of the disease, but surprisingly, this patient remained in remission for over 3 years following octreotide treatment (90).
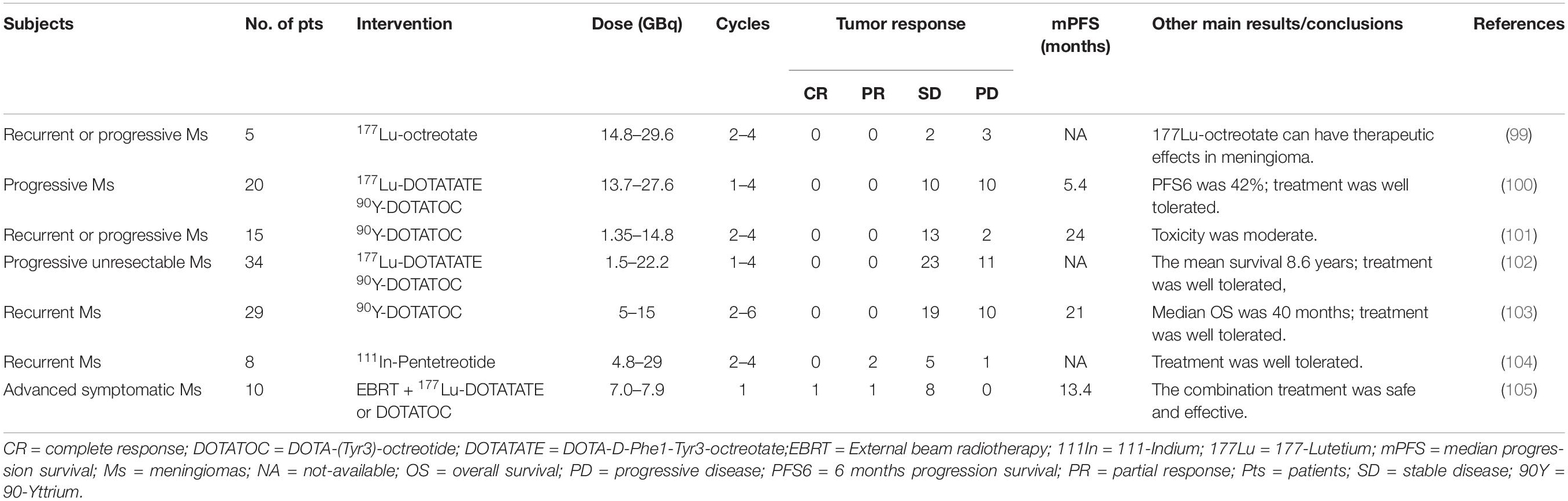
A few clinical studies have been carried out to evaluate the efficacy and safety of somatostatin analogs in the treatment for patients with meningioma (Table 3). A prospective pilot trial was carried out by Chamberlain et al. with a sustained-release somatostatin analog (Sandostatin LAR) treating 16 recurrent meningioma patients. The median overall survival (OS) was 7.5 months; 31% of patients achieved partial radiographic response, and 44% achieved 6 months progression-free survival (PFS); the toxicity of Sandostatin LAR was small (74). These results revealed that Sandostatin LAR might be a useful and tolerable alternative therapy option for recurrent meningiomas. Johnson et al. conducted a phase II study of subcutaneous octreotide treatment for recurrent meningioma patients. The results of this study were less satisfactory: even though octreotide was well tolerated and 2 of 11 patients experienced prolonged stability, it had not been able to produce objective tumor response (93). Complete resection of skull base meningiomas is always challenging; to this end, Schulz et al. treated patients harboring a progressive residual meningioma after surgery with a somatostatin analog. Disregarding the fact that no case of tumor disappearance was observed, the disease appeared to have stabilized in all cases (94). This study offered a perspective on additional therapy for post-surgery skull base meningiomas with somatostatin analogs. Regretfully, it was not a randomized controlled prospective clinical trial. For the treatment of recurrent high-grade meningioma, the efficacy of somatostatin analog might be limited, according to a phase II study showing that none of nine patients achieved radiographic partial response (82). In another trial, a somatostatin analog called pasireotide LAR (SOM230C) was prescribed monthly to patients with recurrent or progressive meningioma; unfortunately, it also failed to increase the proportion of patients with 6 months PFS significantly (95). Studies have manifested that the low levels of Raf kinase inhibitory protein or the mutations of aryl hydrocarbon receptor interacting protein were related to the unsatisfactory response to somatostatin analogs for the treatment of GH-secreting pituitary adenomas (96, 97), notwithstanding the fact that there is a paucity of similar studies in meningioma. Taken collectively, somatostatin analogs represent a safe but undefined therapeutic option in meningioma management. Notably, these clinical trials suffer from limited sample size and short duration, so more and larger trials are urgently warranted.
SSTR2-directed peptide receptor radionuclide therapy (PRRT) has also exhibited their potential therapeutic use for patients with meningiomas. Beta-emitters 90-yttrium (90Y) and 177-lutetium (177Lu) are the most widely used radiometals in PRRT at present (98). Certain amounts of clinical studies (Table 4) have investigated the therapeutic effect of SSTR2-targeted PRRT in treating meningioma patients. In a clinical study, five meningioma patients, among which three had tumors that were very large with standard medical therapies that all failed, were treated with 177Lu-octreotate. Consequently, two of them had stable disease (SD) while three of them had progressive disease after PRRT treatment (99). A retrospective study also presented the activity of SSTR2-targeted PRRT using 177Lu-DOTATATE or 90Y-DOTATOC in patients with meningioma, with the results that 10 of 20 patients achieved SD for a median time of 17 months (100). Gerster-Gilliéron et al. have recommended 90Y-DOTATOC as a second- or third-line option for recurrent or progressive meningiomas, since median PFS (Figure 3) of patients receiving systemic 90Y-DOTATOC treatment was 57 months and the treatment was safe (101). Moreover, the results of a phase II prospective clinical trial, in which 67.6% of patients achieved SD and the mean survival of all enrolled patients was 8.6 years, lent further support to the use of SSTR2-directed PRRT in patients with progressive unresectable meningioma (102). Many more clinical studies have confirmed the efficacy and safety of SSTR2-targeted PRRT in the treatment of meningiomas (103–105). Indeed, the recent European Association of Neuro-Oncology guidelines on meningioma have declared PRRT a promising approach to treat refractory meningiomas across all WHO grades in the future (1). The selective accumulation of radiolabeled somatostatin analogs in meningioma cells enhances the efficacy while reducing the toxicity of PRRT. Nevertheless, because these traces are mainly excreted by the kidney, renal toxicity seems inevitable, which may limit the application of PRRT (98, 106, 107). Generally, in patients with recurrent or complex unresectable meningiomas, especially in those where standard treatments have failed, the use of SSTR2-targeted PRRT should be considered; for those who accept PRRT, we should pay close attention to their renal function, and renal protection should be provided.
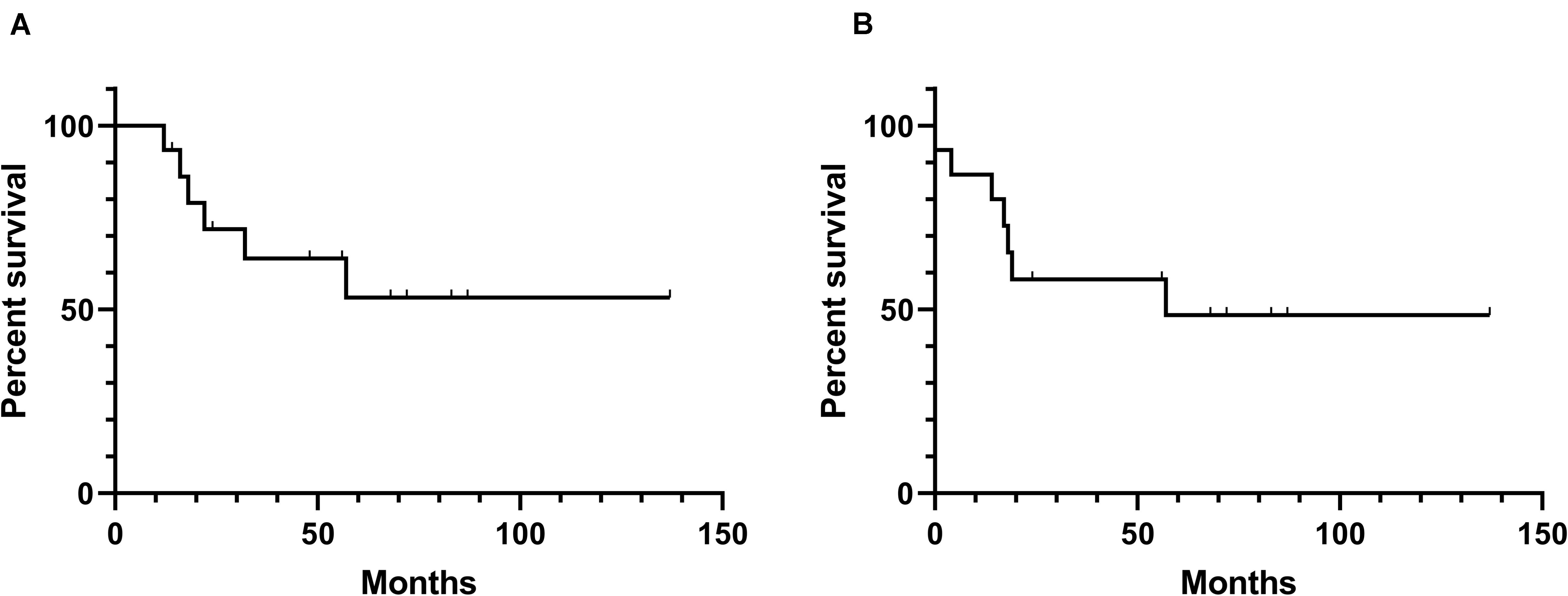
Figure 3. Kaplan–Meier curves of overall survival and progression-free survival of the reported effects (101) of SSTR-related radiation therapy. (A) Overall survival. (B) Progression-free survival.
Taken together, SSTR2-related/targeted treatments are promising approaches for the treatment of unresectable or refractory meningiomas. Somatostatin analogs can only inhibit the proliferation but fail to induce the apoptosis of meningioma cells; meanwhile, somatostatin analog treatment for meningioma exhibits efficacy in vitro and some special cases, but clinical studies have not achieved satisfactory results. Consequently, the effectiveness of somatostatin analog treatment for meningioma is actually controversial currently; further studies are required to identify and select the patients in whom treatment with somatostatin analogs is potentially effective. Importantly, SSTR2-targeted PRRT has shown an effect on the treatment of meningiomas in some clinical studies.
SSTR2 in Prognostic Prediction of Meningioma
It is of clinical importance to predict the prognosis of meningioma patients, since it can provide a valuable reference for the proper management of patients, such as making treatment and follow-up strategies. Previous studies have manifested several potential prognostic indicators for meningioma, including the WHO tumor grade, the extent of resection, expression of progesterone and estrogen receptors, mitotic index, and bone involvement (72, 108–111). However, additional prognostic factors are still sorely needed to better predict the outcomes of meningioma patients.
Barresi et al. have attempted to draw the association between SSTR2 and tumor grade by analyzing SSTR2 immunohistochemical expression in 35 different-grade meningiomas; their results have shown that SSTR2 was frequently expressed in high-grade meningiomas and related to higher microvessel density (30). Explicitly, 57% grade I, 75% grade II, and 66% grade III meningiomas were characterized by a high expression of SSTR2 (30). Somatostatin or its analogs might be effective in the therapy of meningiomas by reducing their blood supply based on this study (112). Nevertheless, Durand et al. have found that SSTR2 levels were not grade related but histotype related, with significantly higher expression levels in the meningothelial subtype than in the fibroblastic subtype (29). This finding may support the use of somatostatin or its analogs to treat this subtype. Silva et al. have argued that SSTR2 levels might correlate to the risk of recurrence because the high expression of SSTR2 was observed in partially resected meningiomas with tumor regrowth (25). Additionally, Seystahl et al. have also observed that the expression level of SSTR2 was not correlated with the WHO grade of meningiomas; yet the expression level of SSTR could be a predictive biomarker for the outcome of meningioma patients treated with PRRT; a higher expression of SSTR2 was revealed to be associated with better PFS after PRRT treatment (100). These researches indicate that whether SSTR2 levels are grade related in meningiomas remains controversial; meanwhile, SSTR2 could still offer some implications for prognosis prediction in spite of this controversy.
Conclusion and Prospects
Meningiomas are the most frequent intracranial tumors. SSTR2 expressed in almost all meningiomas, which provides novel ideas and approaches in the diagnosis, treatment, and prognostic prediction for meningiomas. Certain progress regarding the clinical significance of SSTR2 in meningioma has been made in the past few decades. SSTR2-related imaging tools with radiolabeled somatostatin agonists, including PET, PET/CT, PET MRI, SPECT SRS, and SPECT/CT SRS, have significant value in (preclinical) diagnosis, differential diagnosis, and disease evaluation. Despite accumulating evidence that SSTR2-related/targeted treatments (e.g., somatostatin analogs and SSTR2-targeted PRRT) are promising and safe therapeutic options for unresectable or refractory meningiomas, several controversial areas remain. More and larger multicenter long-term follow-up and randomized prospective trials are urgently needed, especially in uncovering the precise underlying signaling pathways of SSTR2 ligands–somatostatin analogs’ antitumor effects as well as identifying and selecting candidate patients who may benefit from these treatments.
Author Contributions
YZ, WW, and AS conceptualized the research project. WW, YZ, YW, and LL drafted the manuscript. YW and JL drew the figures. PZ and AS reviewed and modified the manuscript. AS, YD, and PZ supervised the research and led the discussion. All authors approved the final version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (81701144).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
111 In, 111-indium; 177Lu, 177-lutetium; 90Y, 90-yttrium; CNS, central nervous system; CT, computed tomography; DOTATATE, DOTA-D-Phe1-Tyr3-octreotate; DOTATOC, DOTA-(Tyr3)-octreotide; IGF-1, insulin-like growth factor-1; MRI, magnetic resonance imaging; PET, positron emission tomography; PFS, progression-free survival; PRRT, peptide receptor radionuclide therapy; SD, stable disease; SHP1, SH2-containing phosphatase-1; SHP2, SH2-containing phosphatase-2; SPECT, single-photon emission computed tomography; SRS, somatostatin receptor scintigraphy; SSTR, somatostatin receptor; VEGF, vascular endothelial growth factor; WHO, World Health Organization.
References
1. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. (2016) 17:e383–91. doi: 10.1016/s1470-2045(16)30321-7
2. Preusser M, Brastianos PK, Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. (2018) 14:106–15. doi: 10.1038/nrneurol.2017.168
3. Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. (2004) 363:1535–43. doi: 10.1016/s0140-6736(04)16153-9
4. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. (2019) 21(Suppl 5):v1–100. doi: 10.1093/neuonc/noz150
5. Harter PN, Braun Y, Plate KH. Classification of meningiomas-advances and controversies. Chin Clin Oncol. (2017) 6(Suppl 1):S2. doi: 10.21037/cco.2017.05.02
6. Villa C, Miquel C, Mosses D, Bernier M, Di Stefano AL. The 2016 World Health Organization classification of tumours of the central nervous system. Presse Med. (2018) 47(Pt 2):e187–200. doi: 10.1016/j.lpm.2018.04.015
7. Huang RY, Bi WL, Griffith B, Kaufmann TJ, la Fougère C, Schmidt NO, et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol. (2019) 21(Suppl. 1):i44–61. doi: 10.1093/neuonc/noy143
8. Debus J, Wuendrich M, Pirzkall A, Hoess A, Schlegel W, Zuna I, et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. (2001) 19:3547–53. doi: 10.1200/jco.2001.19.15.3547
9. Wang C, Kaprealian TB, Suh JH, Kubicky CD, Ciporen JN, Chen Y, et al. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro Oncol. (2017) 19:1263–70. doi: 10.1093/neuonc/nox007
10. Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother. (2018) 18:241–9. doi: 10.1080/14737175.2018.1429920
11. Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol. (2013) 34:228–52. doi: 10.1016/j.yfrne.2013.07.005
12. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. (1999) 20:157–98. doi: 10.1006/frne.1999.0183
13. Günther T, Tulipano G, Dournaud P, Bousquet C, Csaba Z, Kreienkamp HJ, et al. International union of basic and clinical pharmacology. CV. Somatostatin receptors: structure, function, ligands, and new nomenclature. Pharmacol Rev. (2018) 70:763–835. doi: 10.1124/pr.117.015388
14. Schulz S, Pauli SU, Schulz S, Händel M, Dietzmann K, Firsching R, et al. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin Cancer Res. (2000) 6:1865–74.
15. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. (2016) 536:285–91. doi: 10.1038/nature19057
16. Patel YC, Greenwood M, Kent G, Panetta R, Srikant CB. Multiple gene transcripts of the somatostatin receptor SSTR2: tissue selective distribution and cAMP regulation. Biochem Biophys Res Commun. (1993) 192:288–94. doi: 10.1006/bbrc.1993.1412
17. Hoffman GA, Garrison TR, Dohlman HG. Endoproteolytic processing of Sst2, a multidomain regulator of G protein signaling in yeast. J Biol Chem. (2000) 275:37533–41. doi: 10.1074/jbc.M005751200
18. Helboe L, Møller M, Nørregaard L, Schiødt M, Stidsen CE. Development of selective antibodies against the human somatostatin receptor subtypes sst1-sst5. Brain Res Mol Brain Res. (1997) 49:82–8. doi: 10.1016/s0169-328x(97)00127-7
19. Lamberts SW, van der Lely AJ, Hofland LJ. New somatostatin analogs: will they fulfil old promises? Eur J Endocrinol. (2002) 146:701–5. doi: 10.1530/eje.0.1460701
20. Rai U, Thrimawithana TR, Valery C, Young SA. Therapeutic uses of somatostatin and its analogues: current view and potential applications. Pharmacol Ther. (2015) 152:98–110. doi: 10.1016/j.pharmthera.2015.05.007
21. Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. (2003) 20:255–64. doi: 10.1385/endo:20:3:255
22. Grozinsky-Glasberg S, Shimon I, Korbonits M, Grossman AB. Somatostatin analogues in the control of neuroendocrine tumours: efficacy and mechanisms. Endocr Relat Cancer. (2008) 15:701–20. doi: 10.1677/erc-07-0288
23. Smitha MC, Maggi M, Orlando C. Somatostatin receptors in non-endocrine tumours. Dig Liver Dis. (2004) 36(Suppl 1):S78–85. doi: 10.1016/j.dld.2003.11.019
24. Mizutani G, Nakanishi Y, Watanabe N, Honma T, Obana Y, Seki T, et al. Expression of somatostatin receptor (SSTR) subtypes (SSTR-1, 2A, 3, 4 and 5) in neuroendocrine tumors using real-time RT-PCR method and immunohistochemistry. Acta Histochem Cytochem. (2012) 45:167–76. doi: 10.1267/ahc.12006
25. Silva CB, Ongaratti BR, Trott G, Haag T, Ferreira NP, Leães CG, et al. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int J Clin Exp Pathol. (2015) 8:131 85–92.
26. Dutour A, Kumar U, Panetta R, Ouafik L, Fina F, Sasi R, et al. Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. (1998) 76:620–7. doi: 10.1002/(sici)1097-0215(19980529)76:53.0.co;2-s
27. Graillon T, Romano D, Defilles C, Saveanu A, Mohamed A, Figarella-Branger D, et al. Octreotide therapy in meningiomas: in vitro study, clinical correlation, and literature review. J Neurosurg. (2017) 127:660–9. doi: 10.3171/2016.8.Jns16995
28. Arena S, Barbieri F, Thellung S, Pirani P, Corsaro A, Villa V, et al. Expression of somatostatin receptor mRNA in human meningiomas and their implication in in vitro antiproliferative activity. J Neurooncol. (2004) 66:155–66. doi: 10.1023/b:neon.0000013498.19981.55
29. Durand A, Champier J, Jouvet A, Labrousse F, Honnorat J, Guyotat J, et al. Expression of c-Myc, neurofibromatosis Type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: relation to grades or histotypes. Clin Neuropathol. (2008) 27:334–45. doi: 10.5414/npp27334
30. Barresi V, Alafaci C, Salpietro F, Tuccari G. Sstr2A immunohistochemical expression in human meningiomas: is there a correlation with the histological grade, proliferation or microvessel density? Oncol Rep. (2008) 20:485–92. doi: 10.3892/or_00000032
31. Agaimy A, Buslei R, Coras R, Rubin BP, Mentzel T. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. (2014) 65:60–70. doi: 10.1111/his.12366
32. Dijkstra BM, Motekallemi A, den Dunnen WFA, Jeltema JR, van Dam GM, Kruyt FAE, et al. SSTR-2 as a potential tumour-specific marker for fluorescence-guided meningioma surgery. Acta Neurochir. (2018) 160:1539–46. doi: 10.1007/s00701-018-3575-z
33. Ben-Shlomo A, Melmed S. Pituitary somatostatin receptor signaling. Trends Endocrinol Metab. (2010) 21:123–33. doi: 10.1016/j.tem.2009.12.003
34. Kailey B, van de Bunt M, Cheley S, Johnson PR, MacDonald PE, Gloyn AL, et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic β- and α-cells. Am J Physiol Endocrinol Metab. (2012) 303:E1107–16. doi: 10.1152/ajpendo.00207.2012
35. Bassant MH, Simon A, Poindessous-Jazat F, Csaba Z, Epelbaum J, Dournaud P. Medial septal GABAergic neurons express the somatostatin sst2A receptor: functional consequences on unit firing and hippocampal theta. J Neurosci. (2005) 25:2032–41. doi: 10.1523/jneurosci.4619-04.2005
36. Vasilaki A, Thermos K. Somatostatin analogues as therapeutics in retinal disease. Pharmacol Ther. (2009) 122:324–33. doi: 10.1016/j.pharmthera.2009.03.010
37. Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, et al. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J Nucl Med. (2005) 46:763–9.
38. Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, et al. International society of neuropathology–haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. (2014) 24:429–35. doi: 10.1111/bpa.12171
39. Nowosielski M, Galldiks N, Iglseder S, Kickingereder P, von Deimling A, Bendszus M, et al. Diagnostic challenges in meningioma. Neuro Oncol. (2017) 19:1588–98. doi: 10.1093/neuonc/nox101
40. Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. (2015) 130:441–3. doi: 10.1007/s00401-015-1459-3
41. Anis SE, Lotfalla M, Zain M, Kamel NN, Soliman AA. Value of SSTR2A and claudin – 1 in differentiating meningioma from schwannoma and hemangiopericytoma. Open Access Maced J Med Sci. (2018) 6:248–53. doi: 10.3889/oamjms.2018.062
42. Boulagnon-Rombi C, Fleury C, Fichel C, Lefour S, Marchal Bressenot A, Gauchotte G. Immunohistochemical approach to the differential diagnosis of meningiomas and their mimics. J Neuropathol Exp Neurol. (2017) 76:289–98. doi: 10.1093/jnen/nlx008
43. Richardson TE, Georgescu MM, Kapur P, Hwang H, Barnett SL, Raisanen JM, et al. Unusual skull tumors with psammomatoid bodies: a diagnostic challenge. Clin Neuropathol. (2017) 36:114–20. doi: 10.5414/np300997
44. Meewes C, Bohuslavizki KH, Krisch B, Held-Feindt J, Henze E, Clausen M. Molecular biologic and scintigraphic analyses of somatostatin receptor-negative meningiomas. J Nucl Med. (2001) 42:1338–45.
45. Matsuyama A, Jotatsu M, Uchihashi K, Tsuda Y, Shiba E, Haratake J, et al. MUC4 expression in meningiomas: under-recognized immunophenotype particularly in meningothelial and angiomatous subtypes. Histopathology. (2019) 74:276–83. doi: 10.1111/his.13730
46. Rachinger W, Stoecklein VM, Terpolilli NA, Haug AR, Ertl L, Pöschl J, et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. (2015) 56:347–53. doi: 10.2967/jnumed.114.149120
47. Gehler B, Paulsen F, Oksüz MO, Hauser TK, Eschmann SM, Bares R, et al. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. (2009) 4:56. doi: 10.1186/1748-717x-4-56
48. Sommerauer M, Burkhardt JK, Frontzek K, Rushing E, Buck A, Krayenbuehl N, et al. 68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate? Neuro Oncol. (2016) 18:1021–7. doi: 10.1093/neuonc/now001
49. Henze M, Schuhmacher J, Hipp P, Kowalski J, Becker DW, Doll J, et al. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. (2001) 42:1053–6.
50. Ivanidze J, Roytman M, Lin E, Magge RS, Pisapia DJ, Liechty B, et al. Gallium-68 DOTATATE PET in the evaluation of intracranial meningiomas. J Neuroimaging. (2019) 29:650–6. doi: 10.1111/jon.12632
51. Acker G, Kluge A, Lukas M, Conti A, Pasemann D, Meinert F, et al. Impact of 68Ga-DOTATOC PET/MRI on robotic radiosurgery treatment planning in meningioma patients: first experiences in a single institution. Neurosurg Focus. (2019) 46:E9. doi: 10.3171/2019.3.Focus1925
52. Afshar-Oromieh A, Giesel FL, Linhart HG, Haberkorn U, Haufe S, Combs SE, et al. Detection of cranial meningiomas: comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. (2012) 39:1409–15. doi: 10.1007/s00259-012-2155-3
53. Kunz WG, Jungblut LM, Kazmierczak PM, Vettermann FJ, Bollenbacher A, Tonn JC, et al. Improved detection of transosseous meningiomas using (68)Ga-DOTATATE PET/CT compared with contrast-enhanced MRI. J Nucl Med. (2017) 58:1580–7. doi: 10.2967/jnumed.117.191932
54. Dittmar JO, Kratochwil C, Dittmar A, Welzel T, Habermehl D, Rieken S, et al. First intraindividual comparison of contrast-enhanced MRI, FET- and DOTATOC- PET in patients with intracranial meningiomas. Radiat Oncol. (2017) 12:169. doi: 10.1186/s13014-017-0913-x
55. Grzbiela H, Tarnawski R, D’Amico A, Sta̧pór-Fudzińska M. The use of 68Ga-DOTA-(Tyr3)-octreotate PET/CT for improved target definition in radiotherapy treatment planning of meningiomas – a case report. Curr Radiopharm. (2015) 8:45–8. doi: 10.2174/1874471008666150316222923
56. Galldiks N, Albert NL, Sommerauer M, Grosu AL, Ganswindt U, Law I, et al. PET imaging in patients with meningioma-report of the RANO/PET group. Neuro Oncol. (2017) 19:1576–87. doi: 10.1093/neuonc/nox112
57. Golemi A, Ambrosini A, Cecchi P, Ruiu A, Chondrogiannis S, Farsad M, et al. (68)Ga-DOTANOC PET/CT detection of multiple extracranial localizations in a patient with anaplastic meningioma. Rev Esp Med Nucl Imagen Mol. (2015) 34:258–60. doi: 10.1016/j.remn.2015.03.003
58. Klingenstein A, Haug AR, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. (2015) 34:16–22. doi: 10.3109/01676830.2014.959185
59. Al Feghali KA, Yeboa DN, Chasen B, Gule MK, Johnson JM, Chung C. The use of (68)Ga-DOTATATE PET/CT in the non-invasive diagnosis of optic nerve sheath meningioma: a case report. Front Oncol. (2018) 8:454. doi: 10.3389/fonc.2018.00454
60. Klutmann S, Bohuslavizki KH, Brenner W, Behnke A, Tietje N, Kröger S, et al. Somatostatin receptor scintigraphy in postsurgical follow-up examinations of meningioma. J Nucl Med. (1998) 39:1913–7.
61. Klutmann S, Bohuslavizki KH, Tietje N, Kröger S, Behnke A, Brenner W, et al. Clinical value of 24-hour delayed imaging in somatostatin receptor scintigraphy for meningioma. J Nucl Med. (1999) 40:1246–51.
62. Hildebrandt G, Scheidhauer K, Luyken C, Schicha H, Klug N, Dahms P, et al. High sensitivity of the in vivo detection of somatostatin receptors by 111indium (DTPA-octreotide)-scintigraphy in meningioma patients. Acta Neurochir (Wien). (1994) 126:63–71. doi: 10.1007/bf01476412
63. Bohuslavizki KH, Brenner W, Braunsdorf WE, Behnke A, Tinnemeyer S, Hugo HH, et al. Somatostatin receptor scintigraphy in the differential diagnosis of meningioma. Nucl Med Commun. (1996) 17:302–10. doi: 10.1097/00006231-199604000-00157
64. Luyken C, Hildebrandt G, Krisch B, Scheidhauer K, Klug N. Clinical relevance of somatostatin receptor scintigraphy in patients with skull base tumours. Acta Neurochir Suppl. (1996) 65:102–4. doi: 10.1007/978-3-7091-9450-8_28
65. Maini CL, Sciuto R, Tofani A, Ferraironi A, Carapella CM, Occhipinti E, et al. Somatostatin receptor imaging in CNS tumours using 111In-octreotide. Nucl Med Commun. (1995) 16:756–66. doi: 10.1097/00006231-199509000-00006
66. Nathoo N, Ugokwe K, Chang AS, Li L, Ross J, Suh JH, et al. The role of 111indium-octreotide brain scintigraphy in the diagnosis of cranial, dural-based meningiomas. J Neurooncol. (2007) 81:167–74. doi: 10.1007/s11060-006-9210-5
67. Mokhtarzadeh A, Maltry A, McClelland C. Waiting to deliver a final diagnosis. Surv Ophthalmol. (2017) 62:583–6. doi: 10.1016/j.survophthal.2017.01.006
68. Nussbaum-Hermassi L, Ahle G, Zaenker C, Duca C, Namer IJ. Optic nerve sheath meningioma detected by single- photon emission computed tomography/computed tomography somatostatin receptor scintigraphy: a case report. J Med Case Rep. (2016) 10:96. doi: 10.1186/s13256-016-0885-8
69. Chandra P, Purandare N, Shah S, Agrawal A, Rangarajan V. Somatostatin receptor SPECT/CT using (99m)Tc labeled HYNIC-TOC Aids in diagnosis of primary optic nerve sheath meningioma. Indian J Nucl Med. (2017) 32:63–5. doi: 10.4103/0972-3919.198487
70. Wang S, Yang W, Deng J, Zhang J, Ma F, Wang J. Correlation between 99mTc-HYNIC-octreotide SPECT/CT somatostatin receptor scintigraphy and pathological grading of meningioma. J Neurooncol. (2013) 113:519–26. doi: 10.1007/s11060-013-1146-y
71. Yano S, Kuratsu J. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. (2006) 105:538–43. doi: 10.3171/jns.2006.105.4.538
72. Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. (2013) 13:337. doi: 10.1007/s11910-013-0337-4
73. Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. (2010) 113:1036–42. doi: 10.3171/2010.3.Jns091966
74. Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. (2007) 69:969–73. doi: 10.1212/01.wnl.0000271382.62776.b7
75. Alexiou GA, Markoula S, Gogou P, Kyritsis AP. Genetic and molecular alterations in meningiomas. Clin Neurol Neurosurg. (2011) 113:261–7. doi: 10.1016/j.clineuro.2010.12.007
76. De Menis E, Tulipano G, Villa S, Billeci D, Bonfanti C, Pollara P, et al. Development of a meningioma in a patient with acromegaly during octreotide treatment: are there any causal relationships? J Endocrinol Invest. (2003) 26:359–63. doi: 10.1007/bf03345185
77. Kunert-Radek J, Stepien H, Radek A, Pawlikowski M. Somatostatin suppression of meningioma cell proliferation in vitro. Acta Neurol Scand. (1987) 75:434–6. doi: 10.1111/j.1600-0404.1987.tb05474.x
78. Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. (2004) 15:966–73. doi: 10.1093/annonc/mdh216
79. Melmed S. New therapeutic agents for acromegaly. Nat Rev Endocrinol. (2016) 12:90–8. doi: 10.1038/nrendo.2015.196
80. Graillon T, Defilles C, Mohamed A, Lisbonis C, Germanetti AL, Chinot O, et al. Combined treatment by octreotide and everolimus: octreotide enhances inhibitory effect of everolimus in aggressive meningiomas. J Neurooncol. (2015) 124:33–43. doi: 10.1007/s11060-015-1812-3
81. Graillon T, Romano D, Defilles C, Lisbonis C, Saveanu A, Figarella-Branger D, et al. Pasireotide is more effective than octreotide, alone or combined with everolimus on human meningioma in vitro. Oncotarget. (2017) 8:55361–73. doi: 10.18632/oncotarget.19517
82. Simó M, Argyriou AA, Macià M, Plans G, Majós C, Vidal N, et al. Recurrent high-grade meningioma: a phase II trial with somatostatin analogue therapy. Cancer Chemother Pharmacol. (2014) 73:919–23. doi: 10.1007/s00280-014-2422-z
83. Ferjoux G, Lopez F, Esteve JP, Ferrand A, Vivier E, Vely F, et al. Critical role of Src and SHP-2 in sst2 somatostatin receptor-mediated activation of SHP-1 and inhibition of cell proliferation. Mol Biol Cell. (2003) 14:3911–28. doi: 10.1091/mbc.e03-02-0069
84. Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. (2008) 286:230–7. doi: 10.1016/j.mce.2008.02.002
85. Bousquet C, Guillermet-Guibert J, Saint-Laurent N, Archer-Lahlou E, Lopez F, Fanjul M, et al. Direct binding of p85 to sst2 somatostatin receptor reveals a novel mechanism for inhibiting PI3K pathway. EMBO J. (2006) 25:3943–54. doi: 10.1038/sj.emboj.7601279
86. Hasskarl J, Kaufmann M, Schmid HA. Somatostatin receptors in non-neuroendocrine malignancies: the potential role of somatostatin analogs in solid tumors. Future Oncol. (2011) 7:895–913. doi: 10.2217/fon.11.66
87. Kvols LK, Woltering EA. Role of somatostatin analogs in the clinical management of non-neuroendocrine solid tumors. Anticancer Drugs. (2006) 17:601–8. doi: 10.1097/01.cad.0000210335.95828.ed
88. Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg. (1999) 91:93–9. doi: 10.3171/jns.1999.91.1.0093
89. Ferone D, van Hagen PM, Semino C, Dalm VA, Barreca A, Colao A, et al. Somatostatin receptor distribution and function in immune system. Dig Liver Dis. (2004) 36(Suppl. 1):S68–77. doi: 10.1016/j.dld.2003.11.020
90. Rammo R, Rock A, Transou A, Raghunathan A, Rock J. Anaplastic meningioma: octreotide therapy for a case of recurrent and progressive intracranial disease. J Neurosurg. (2016) 124:496–500. doi: 10.3171/2015.1.Jns142260
91. Ortolá Buigues A, Crespo Hernández I, Jorquera Moya M, Díaz Pérez J. Unresectable recurrent multiple meningioma: a case report with radiological response to somatostatin analogues. Case Rep Oncol. (2016) 9:520–5. doi: 10.1159/000448212
92. García-Luna PP, Relimpio F, Pumar A, Pereira JL, Leal-Cerro A, Trujillo F, et al. Clinical use of octreotide in unresectable meningiomas. A report of three cases. J Neurosurg Sci. (1993) 37:237–41.
93. Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, et al. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. (2011) 13:530–5. doi: 10.1093/neuonc/nor044
94. Schulz C, Mathieu R, Kunz U, Mauer UM. Treatment of unresectable skull base meningiomas with somatostatin analogs. Neurosurg Focus. (2011) 30:E11. doi: 10.3171/2011.1.Focus111
95. Norden AD, Ligon KL, Hammond SN, Muzikansky A, Reardon DA, Kaley TJ, et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology. (2015) 84:280–6. doi: 10.1212/wnl.0000000000001153
96. Fougner SL, Bollerslev J, Latif F, Hald JK, Lund T, Ramm-Pettersen J, et al. Low levels of raf kinase inhibitory protein in growth hormone-secreting pituitary adenomas correlate with poor response to octreotide treatment. J Clin Endocrinol Metab. (2008) 93:1211–6. doi: 10.1210/jc.2007-2272
97. Oriola J, Lucas T, Halperin I, Mora M, Perales MJ, Alvarez-Escolá C, et al. Germline mutations of AIP gene in somatotropinomas resistant to somatostatin analogues. Eur J Endocrinol. (2013) 168:9–13. doi: 10.1530/eje-12-0457
98. Laudicella R, Albano D, Annunziata S, Calabrò D, Argiroffi G, Abenavoli E, et al. Theragnostic use of radiolabelled dota-peptides in meningioma: from clinical demand to future applications. Cancers (Basel). (2019) 11:1412. doi: 10.3390/cancers11101412
99. van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, et al. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. (2006) 47: 1599–606.
100. Seystahl K, Stoecklein V, Schüller U, Rushing E, Nicolas G, Schäfer N, et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. (2016) 18:1538–47. doi: 10.1093/neuonc/now060
101. Gerster-Gilliéron K, Forrer F, Maecke H, Mueller-Brand J, Merlo A, Cordier D. 90Y-DOTATOC as a therapeutic option for complex recurrent or progressive meningiomas. J Nucl Med. (2015) 56:1748–51. doi: 10.2967/jnumed.115.155853
102. Marincek N, Radojewski P, Dumont RA, Brunner P, Müller-Brand J, Maecke HR, et al. Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med. (2015) 56:171–6. doi: 10.2967/jnumed.114.147256
103. Bartolomei M, Bodei L, De Cicco C, Grana CM, Cremonesi M, Botteri E, et al. Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging. (2009) 36:1407–16. doi: 10.1007/s00259-009-1115-z
104. Minutoli F, Amato E, Sindoni A, Cardile D, Conti A, Herberg A, et al. Peptide receptor radionuclide therapy in patients with inoperable meningiomas: our experience and review of the literature. Cancer Biother Radiopharm. (2014) 29:193–9. doi: 10.1089/cbr.2013.1599
105. Kreissl MC, Hänscheid H, Löhr M, Verburg FA, Schiller M, Lassmann M, et al. Combination of peptide receptor radionuclide therapy with fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Radiat Oncol. (2012) 7:99. doi: 10.1186/1748-717x-7-99
106. Bushnell D, Menda Y, O’Dorisio T, Madsen M, Miller S, Carlisle T, et al. Effects of intravenous amino acid administration with Y-90 DOTA-Phe1-Tyr3-Octreotide (SMT487[OctreoTher) treatment. Cancer Biother Radiopharm. (2004) 19:35–41. doi: 10.1089/108497804773391658
107. Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. (2003) 30:9–15. doi: 10.1007/s00259-002-0982-3
108. Hasseleid BF, Meling TR, Rønning P, Scheie D, Helseth E. Surgery for convexity meningioma: simpson Grade I resection as the goal: clinical article. J Neurosurg. (2012) 117:999–1006. doi: 10.3171/2012.9.Jns12294
109. Pravdenkova S, Al-Mefty O, Sawyer J, Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. (2006) 105:163–73. doi: 10.3171/jns.2006.105.2.163
110. Gabeau-Lacet D, Aghi M, Betensky RA, Barker FG, Loeffler JS, Louis DN. Bone involvement predicts poor outcome in atypical meningioma. J Neurosurg. (2009) 111:464–71. doi: 10.3171/2009.2.Jns08877
111. Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. (1997) 86:113–20. doi: 10.3171/jns.1997.86.1.0113
Keywords: meningioma, SSTR2, somatostatin, somatostatin analogs, diagnosis, treatment, prognosis
Citation: Wu W, Zhou Y, Wang Y, Liu L, Lou J, Deng Y, Zhao P and Shao A (2020) Clinical Significance of Somatostatin Receptor (SSTR) 2 in Meningioma. Front. Oncol. 10:1633. doi: 10.3389/fonc.2020.01633
Received: 09 May 2020; Accepted: 27 July 2020;
Published: 03 September 2020.
Edited by:
Hailiang Tang, Huashan Hospital Affiliated to Fudan University, ChinaReviewed by:
Chao Zhang, Qilu Hospital of Shandong University, ChinaUjendra Kumar, The University of British Columbia, Canada
Copyright © 2020 Wu, Zhou, Wang, Liu, Lou, Deng, Zhao and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongchuan Deng, ZHljMDAxQHpqdS5lZHUuY24=; Peng Zhao, emhhb3BAemp1LmVkdS5jbg==; Anwen Shao, MjExMTgxMTZAemp1LmVkdS5jbg==; YW53ZW5zaGFvQHNpbmEuY29t
†These authors have contributed equally to this work
 Wei Wu
Wei Wu Yunxiang Zhou
Yunxiang Zhou Yali Wang2†
Yali Wang2† Peng Zhao
Peng Zhao Anwen Shao
Anwen Shao