- 1Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
- 2Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Department of Surgical Oncology and General Surgery, Ministry of Education, The First Affiliated Hospital of China Medical University, Shenyang, China
Background: Pancreatic ductal adenocarcinoma (PDAC) leads to the majority of cancer-related deaths due to its morbidity with similar mortality. Lack of effective prognostic biomarkers are the main reason for belated post-operative intervention of recurrence which causes high mortality. Numerous systematic reviews and meta-analyses have explored the prognostic value of biomarkers in PDAC so far. In this article, we performed an umbrella review analyzing these studies to provide an overview of associations between prognostic biomarkers and PDAC survival outcome and synthesized these results to guide better clinical practice.
Methods: Systematic reviews and meta-analyses investigating the associations between PDAC survival outcomes and prognostic biomarkers were acquired via the PubMed and Embase databases from inception till February 1, 2020. Associations supported by nominally statistically significant results were classified into strong, highly suggestive, suggestive, and weak based on several critical factors such as the statistical significance of summary estimates, the number of events, the estimate of the largest study included, interstudy heterogeneity, small-study effects, 95% predictive interval (PI), excess significance bias, and the results of credibility ceiling sensitivity analyses.
Results: We included 41 meta-analyses containing 63 associations between PDAC survival outcomes and prognostic biomarkers. Although, none was supported by strong evidence among these associations, an association between C-reactive protein to albumin ratio (CAR) and PDAC overall survival (OS) and an association between neutrophil-lymphocyte ratio (NLR) and PDAC OS were supported by highly suggestive evidence. Otherwise, the association between lactate dehydrogenase (LDH) and PDAC OS was supported by suggestive evidence. The remaining 60 associations were supported by weak or not suggestive evidence.
Conclusion: Associations between CAR or NLR and PDAC OS were supported by highly suggestive evidence. And the association between LDH and PDAC OS was supported by suggestive evidence. Although the methodological quality of the included systematic reviews and meta-analyses which were evaluated by AMSTAR2.0 is generally poor, the identification of the relatively robust prognostic biomarkers of PDAC may guide better post-operative intervention and follow-up to prolong patients' survival.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) leads to major cancer-related death due to its morbidity with similar mortality. The mortality remains unchanged and may even have increased during the last few decades (1). The current estimated 5-year overall survival (OS) of PDAC is <8% and PDAC ranks fourth among cancer-related deaths in the US both in men and women for consecutive years, and the 5-year OS reduces to a dismal 3% when distant metastases occurs (2–5). In China, PDAC ranks sixth among the most lethal cancers and an increasing number of younger patients have been subjected to this disease (6). To raise the rate of survival in PDAC patients, more financial costs have been made around the world (7, 8). Currently, surgical treatment is still the only radical therapy for PDAC (9, 10). Although adjuvant chemotherapy may prolong the survival of PDAC patients, the prognosis of PDAC patients remains poor due to the lack of early diagnostic methods and accurate recurrence and prognostic biomarkers (11–13).
Biomarkers are biological molecules or molecular combinations involved in tumor progression by modulating various signaling pathways which can be used in early diagnosis and prognostic evaluation for cancers (14, 15). Detecting efficient prognostic biomarkers preoperatively can ensure a correct and individual evaluation of the OS and recurrence potential. By doing this, clinical practitioners can perform specific follow-up strategies and timely intervention of possible recurrence to prolong patients' 5-year OS and decrease the mortality (16). Of note, the most frequently evaluated serum biomarker for prognostic evaluation after operation is carbohydrate antigen 19-9 (CA19-9) for PDAC (17, 18). It was reported that CA19-9 could precede radiological evidence for about 3–6 months predicting tumor recurrence (19). However, CA19-9 may not be so effective or accurate in prognostic evaluation. Other serum biomarkers have been proven to have better sensitivity and specificity than CA19-9 in PDAC but without widespread clinical application, such as circulating tumor DNA, miRNAs, lncRNAs, and even circRNAs (20–23). There have been numerous systematic reviews and meta-analyses focusing on prognostic biomarkers of PDAC so far. However, to the best of our knowledge, the results of these systematic reviews and meta-analyses have not been synthetically evaluated. To provide an overview of associations between PDAC survival outcomes and prognostic biomarkers, and to find robust prognostic biomarkers to guide clinical practice, an umbrella review analyzing currently available meta-analyses and systematic reviews and rating the evidence depending on the credibility of these associations was performed.
Methods
Literature Retrieval and Eligibility Criteria
A literature search of the PubMed and Embase databases was conducted for systematic reviews and meta-analyses investigating the associations between PDAC survival outcomes and prognostic biomarkers independently by two experienced researchers (YW and XZ) from inception till February 1, 2020. No filters regarding language or publication time were applied. The “related function” was used to include more articles. Then manual retrieval from the citations of included studies was also applied to supplement the potentially missing literature. The relevant search terms are presented below: (pancreatic cancer OR PDAC OR pancreatic ductal adenocarcinoma OR pancreatic carcinoma OR pancreatic neoplasm) AND (biomarkers OR prognosis OR prognostic biomarkers) AND (systematic reviews OR meta-analyses). The titles and abstracts of these studies were independently browsed by the same two experienced reviewers (YW and XZ). Then full texts of the potentially relevant ones were carefully read by both reviewers. The studies which met the inclusion criteria were finally included in the umbrella review.
Inclusion and Exclusion Criteria
The inclusive and exclusive criterion of the evaluated meta-analyses was shown below: (1) Studies including associations between prognostic biomarkers, rather than diagnostic or pharmacodynamic biomarkers, and PDAC survival outcomes such as overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), and cancer-specific survival (CSS) were included. (2) Studies investigating PDAC risk factors and genetic polymorphism and studies focusing on benign pancreatic lesions, such as solid pseudopapillary tumors of the pancreas, pancreatic cystic tumors, and intraductal papillary mucinous neoplasm were excluded. (3) Meta-analyses containing just one original study or not providing sufficient data were excluded in our present review. When two or more meta-analyses discussed the same association, we only included the meta-analyses with the latest or the largest primary studies.
Data Extraction
Two investigators (YW and XZ) independently extracted the data from included meta-analyses and contradictions between the two reviewers were resolved through discussion with a third reviewer (JG). The name of first author, country, biomarker name, cases number, population size, and relative risk estimation, including risk ratio (RR), odds ratio (OR), and hazard ratio (HR), and the corresponding 95% confidential interval (CI) were retrieved from each of the included meta-analysis.
Quality Assessment
AMSTAR (A MeaSurement Tool to Assess Systematic Reviews) version 2.0 was used to evaluate the methodological quality of each of the included systematic reviews (24). AMSTAR is an important tool used in umbrella reviews to conduct methodological quality assessment of the included systematic reviews and meta-analyses. AMSTAR version 2.0 is an updated version of AMSTAR and contains 16 items to create a more comprehensive and rational evidence evaluation. According to the comprehensive evaluation of the 16 items, the methodological quality of the included studies can be graded as high, moderate, low, or critically low rather than obtaining an overall score.
Statistical Analysis
Random-effect models were used to evaluate the synthesized summary effects for the included meta-analyses. The summary RR estimates, the 95% CI, and the corresponding P-values were calculated. P < 0.05 was deemed as significant.
Cochran's Q-test and I2 statistic were applied to assess interstudy heterogeneity. And I2 > 50% indicates significant heterogeneity (25). Ninety-five percent prediction interval (95% PI), which predicts the likely effect in an individual setting rather than just an average effect across all included studies in a meta-analysis, was used to facilitate the application of the results to clinical practice (26, 27).
Small-study effects were assessed using Egger's regression asymmetry test. Small-study effects were considered detected when a P < 0.1 was reached (27).
Potential reporting selection biases or publication biases can be detected by the excess significance test. A Chi-square test with a two-tailed P < 0.1 as the statistical significance threshold was used to assess whether the actual observed number (O) was different from the expected number (E) (28). The E number which was the sum of the statistical power estimates of the component studies in the included meta-analyses expecting to be statistically significant was calculated using an algorithm of a non-central t distribution with the relative risk estimate of the largest study set as the plausible effect size. The excess significance test was considered positive in cases where both O>E and P < 0.1.
Credibility ceiling sensitivity analyses were performed to account for the inherent methodological limits of observational studies. The level of the credibility ceiling was set at 10% for the present study which indicates that the weight of an observational study in the summary effect is limited to 10% no matter how well the study was conducted (29).
Strength of Existing Evidence
Based on the results of a series of aforementioned analyses, the strength of the statistically significant (P < 0.05) associations between risk factors and risk of PDAC can be categorized into four levels. To reach the “strong evidence” level, the included meta-analysis were expected to show a P-value of random-effect model smaller than 10−6, include more than 1000 PDAC cases, the 95% PI should exclude the null value, have a smaller heterogeneity with I2 < 50%, have no evidence of small-study effect, and an excess significance bias and also survive the 10% credibility ceiling test. “Highly suggestive” evidences refer to associations with a P-value of random-effect model smaller than 10−6 and covering more than 1,000 cases. A “suggestive” association was required to reach a P < 10−3 and include more than 1,000 cases. The rest of the associations where P-value was statistically significant were graded as “weak” evidence (30, 31).
Results
Characteristics of the Included Systematic Reviews and Meta-Analyses
In total, 2259 records were extracted from the literature search and manual screening using the PubMed and Embase databases. A total of 2,083 records were excluded due to an irrelevant theme after browsing titles and abstracts from the acquired records. Finally, 41 of the remaining 176 studies which met the inclusion criteria were included in the present umbrella review after a full-text review (32–72). The search flowchart is shown in Figure 1. And Supplementary Table 1 displays the full list of 176 meta-analyses and the exclusive reasons for the 135 studies. A total of 63 different associations between PDAC survival outcomes and prognostic biomarkers were covered in the included 41 meta-analyses which contained more than 31,000 subjects and over 300 primary studies. Characteristics of the 63 associations in the included meta-analyses were presented in Table 1. Data of the included primary studies in the 41 meta-analyses were extracted, processed, and coded in order to conduct further analysis.
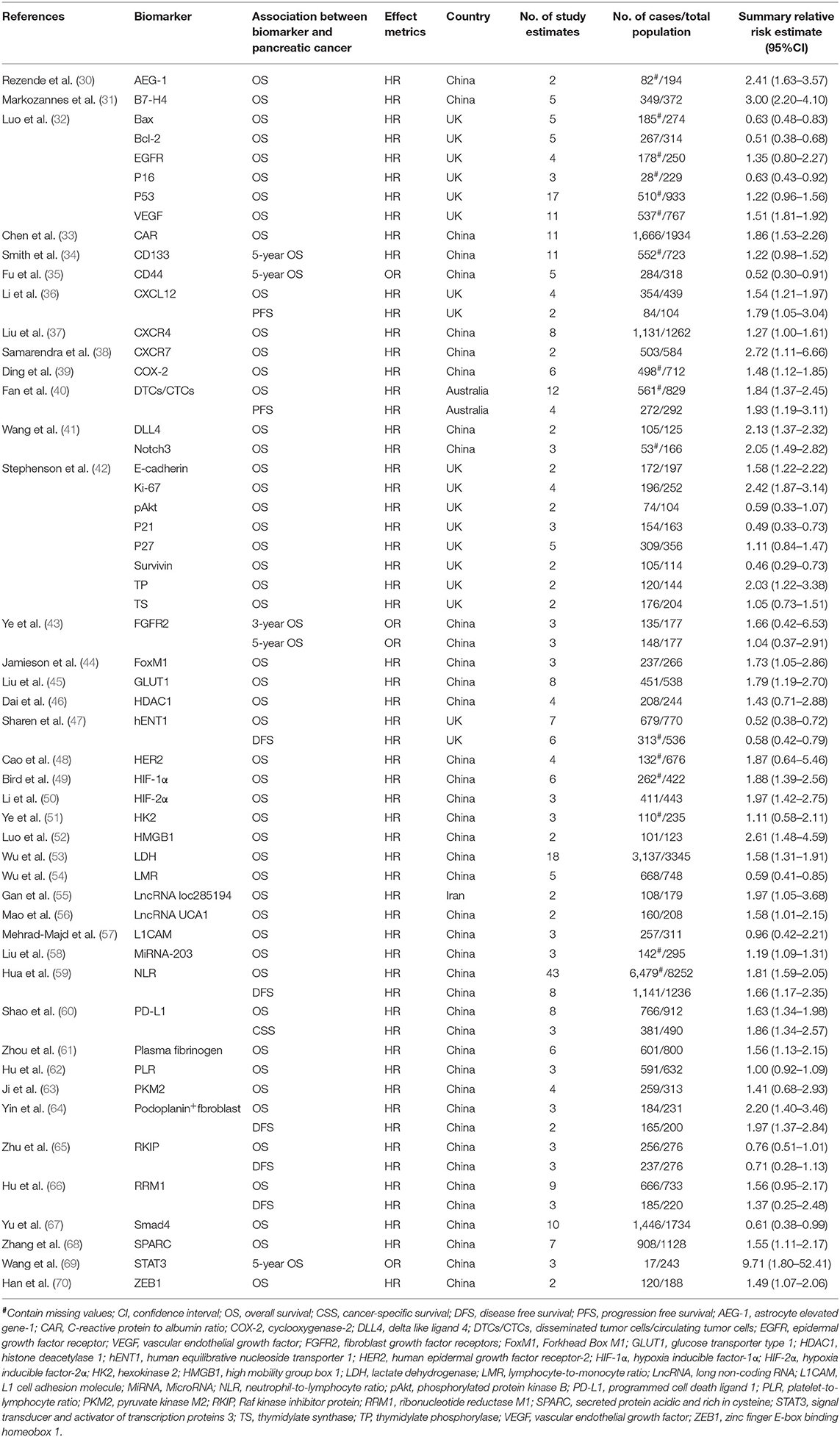
Table 1. Characteristics of the 63 associations in the included 41 systematic reviews and meta-analyses.
Quality Assessment Methodology Using AMSTAR 2.0
The 16-item AMSTAR 2.0 tool was recruited to assess the methodological quality of the 41 included meta-analyses. The results showed that qualities of all the included studies were considered as critically low. All of these meta-analyses had more than two critical flaws [usually in items 2 (41/41,100%), 7 (41/41,100%), and 13 (41/41,100%)] and several non-critical flaws [usually in items 3 (41/41,100%), 10 (41/41,100%), and 12 (41/41,100%)]. It is worth noting that studies with more than one critical flaw were considered as critically low-quality studies regardless of non-critical flaws. Considering the critically low quality of all the included systematic reviews, the results should be interpreted cautiously. The detailed results and rating criteria are shown in Supplementary Table 2.
Summary Effect Size
The quantitative syntheses of the 63 associations were conducted using a random-effect model to provide more conservative estimates. A total of 44 of the 63 associations in the included meta-analyses were statistically significant with P < 0.05, while the remaining 19 associations showing P > 0.05 (Table 2 and Supplementary Table 3). Of the statistically significant associations, four reached P < 10−6, namely, associations between C-reactive protein to albumin ratio (CAR), neutrophil-lymphocyte ratio (NLR), or B7-H4 and PDAC OS and the association between CD133 and 5-year OS of PDAC. And 20 other associations reached moderate statistical significance (P < 10−3). More than half of the associations that reached statistical significance reported an increased risk of mortality of PDAC, indicating a potential biomarker role in PDAC prognostic prediction. However, the remaining statistically significant associations reported a decreased risk of mortality of PDAC, such as B7-H4 and PDAC OS, and Bax and PDAC OS.
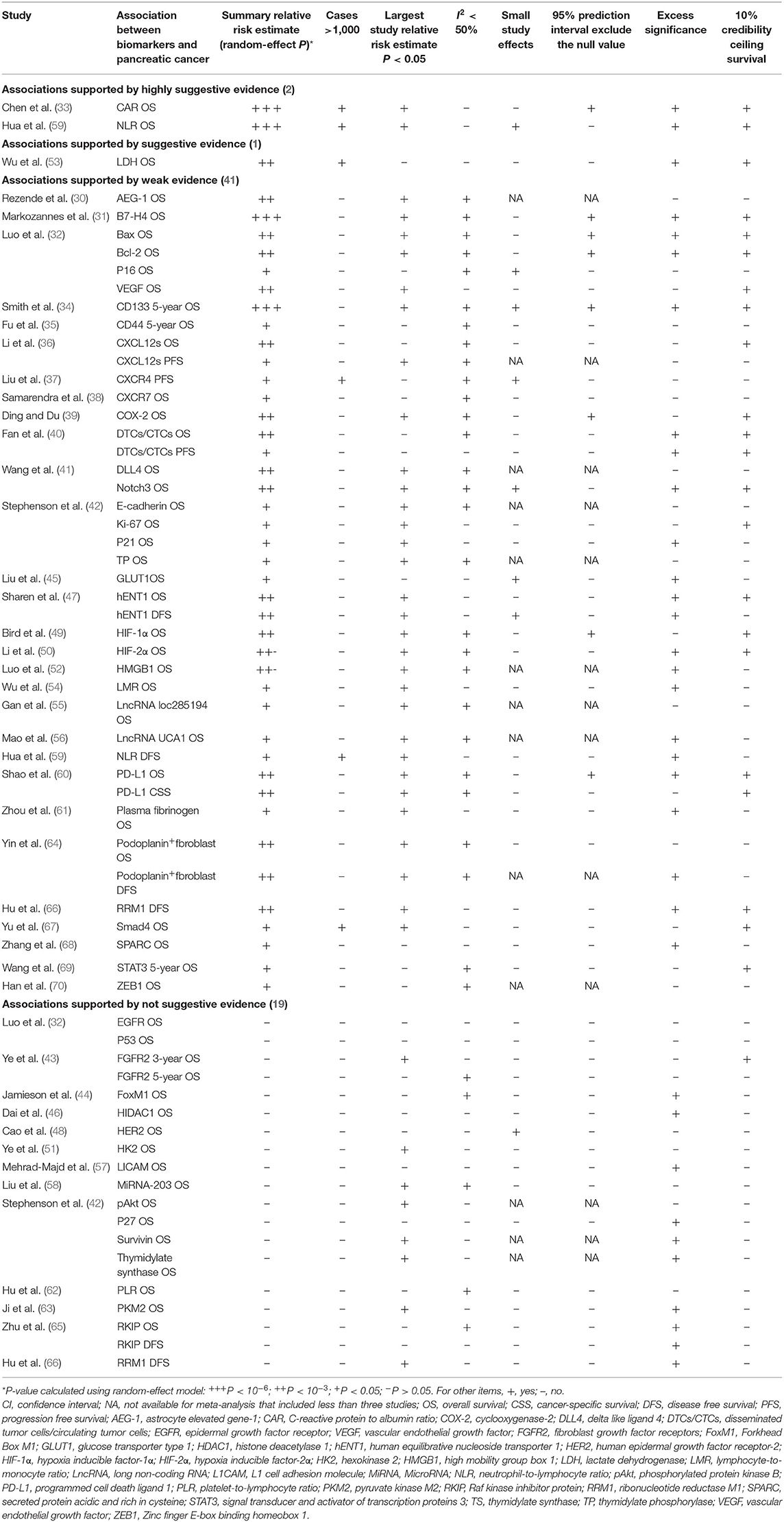
Table 2. Evidence-rating results based on the results of statistical analyses of the 63 associations.
Heterogeneity
Of the 63 associations, there were 17 associations with moderate to high heterogeneity (I2 = 50–75%) and 13 associations with high heterogeneity (I2 > 75%). When we further evaluated the interstudy heterogeneity using 95% PI estimates, we found eight associations with the null value excluded (Table 2 and Supplementary Table 3).
Small-Study Effects
Among the 63 associations between PDAC survival outcomes and prognostic biomarkers, small-study effects were detected in eight associations [NLR OS, P16 OS, CD133 5-year OS, CXCR4 PFS, Notch3 OS, glucose transporter type 1(GLUT1) OS, human equilibrative nucleoside transporter 1 (hENT1) DFS, and Human epidermal growth factor receptor-2 (HER2) OS] according to the Egger's test (P < 0.1) as shown in Table 2 and Supplementary Table 3.
Excess Significance
Significant excess significance (O>E and P < 0.1) was shown in 35 of the 63 associations (Table 2 and Supplementary Table 3).
10% Credibility Ceiling
There were 37 of the 63 associations that survived the 10% credibility ceiling (P < 0.1) which included all associations graded as highly suggestive, or suggestive and most of the associations categorized as weak evidence. Table 2 and Supplementary Table 3 show the detailed information.
Robustness of Evidence
Among the 63 associations between PDAC survival outcomes and prognostic biomarkers, none was supported by strong evidence. However, associations between CAR or NLR and PDAC OS were supported by highly suggestive evidence. Besides, association between lactate dehydrogenase (LDH) and PDAC OS. The remaining 60 associations were supported by weak or not suggestive evidence. Detailed results of these analysis processes are shown in Supplementary Table 3.
Discussion
Principal Finding and Existing Evidence Interpretation
Both diagnostic and prognostic biomarkers are vital to PDAC treatment and can prolong the survival of PDAC patients. However, it is difficult to find valuable diagnostic biomarkers of PDAC due to its insidious onset and retroperitoneal localized lesions. Therefore, in this umbrella review, we mainly focused on prognostic biomarkers evaluation in order to guide clinical practice and the timely detection of early post-operative recurrence to prolong the survival of the patients (73).
To the best of our knowledge, the present umbrella review is the first to comprehensively collect the existing and available meta-analyses and assess the robustness of evidence to provide an overview of associations between PDAC survival outcomes and prognostic biomarkers. Generally, 41 meta-analyses containing 54 different prognostic biomarkers which consisted of both tissue biomarkers and serum biomarkers were included in this umbrella review. None of the associations were supported by strong evidence. However, two associations were supported by highly suggestive evidence, namely, an association between CAR and PDAC OS and an association between NLR and PDAC OS. Only one association was supported by suggestive evidence, an association between LDH and PDAC OS. And the remaining 60 associations were supported by either weak or not suggestive evidence. Nevertheless, the results should be cautiously interpreted given the relatively poor quality and small number of samples of the meta-analyses included in our umbrella review evaluated by AMSTAR2.0.
Comparison With Other Studies and Possible Explanations
In our study, some inflammation-based prognostic biomarkers acquired stronger evidence grading rather than tissue biomarkers. For example, associations between CAR or NLR and PDAC OS were supported by highly suggestive evidence. PDAC can be a deeline in its advanced stage, even at a local advanced stage, which can cause cachexia of patients leading to high mortality (74). Systemic inflammation response (SIR) seems to play an important role in cachexia, such as weight loss and functional decline (75, 76). Elevated C-reactive protein (CRP) is one of the most frequently used biomarkers indicating systemic inflammation and extreme hypoalbuminemia is common in advanced PDAC patients with refractory cachexia due to severe malnutrition. According to what we have mentioned above, preoperative level of CAR implies the systemic inflammation extent of patients, and a higher preoperative CAR level may relate to more severe cachexia and a poorer prognosis in patients. Actually, several studies have indicated the prognostic value of CAR in PDAC. A study by Ikuta et al. indicated that in 136 PDAC patients, preoperative CAR was an independent poor OS predictor using multivariate analysis. Moreover, the prognostic evaluation power of CAR was significantly higher than other inflammation-based factors such as NLR, platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) (77). Besides, the study by Arima et al. also confirmed this result through using CAR at day 14 after an operation (78). NLR is the other prognostic biomarker supported by highly suggestive evidence. In the process of SIR, the level of neutrophil increases to secrete various cytokines. As SIR mitigates, the level of NLR decreases which may explain why NLR can be used as a prognostic biomarker in PDAC patients. Several studies suggested that the prognostic accuracy of the combination of post-operative NLR and AJCC 8th edition is much better than that of the combination of the TNM staging system and AJCC 8th edition (79, 80). Moreover, a recent study unveiled that preoperative NLR may also be a useful prognostic biomarker in PDAC patients undergoing surgical resection (79).
The association between LDH and PDAC OS was supported by suggestive evidence in our umbrella review. LDH is a vital enzyme in glycolysis which can facilitate the conversion of pyruvate to lactate. And the serum LDH level is higher in patients than in healthy individuals which indicates a tumor-promoting role (81). Therefore, LDH may play an important role in tumor progression, especially in PDAC whose microenvironment is usually hypoxic. A meta-analysis showed that high level of glycolysis-related proteins including LDH5 were associated with shorter OS of various cancer patients including PDAC patients (82). Moreover, LDH combined with other biomarkers can also be prognostic in PDAC. For instance, the ratio of LDH to albumin (LAR) can be a prognostic indicator for unresectable PDAC patients. Besides, the combination of LAR, CEA, and the TNM staging system can improve prognosis predictive power compared with the TNM staging system alone (83).
Several studies have revealed the prognostic role of CA19-9 in PDAC. Palmquist et al. indicated that patients with a high level of CA19-9, interleukin-6, and human cartilage glycoprotein-40 had a shorter OS than patients with lower levels (84). Besides, the study by Gu et al. analyzed 109 Chinese PDAC patients and indicated that the preoperative level of CA19-9 was independently correlated with PDAC OS (85). However, the role of CA19-9 in PDAC post-operative recurrence and prognosis has not been systematically reviewed (86). As far as we know, no systematic reviews or meta-analyses discussed the prognostic value of CA19-9 in PDAC patients. And it is the reason why CA19-9 was not included in the present study. Therefore, the potential of CA19-9 as a potential prognostic biomarker should be comprehensively explored in future studies.
Limitations
The present umbrella review is the first to make a comprehensive survey about the associations between PDAC survival outcomes and prognostic biomarkers. However, several limitations still exist in our study. Firstly, the methodological quality of the included systematic reviews is generally poor, thus the interpretation of the results in this umbrella review should be questioned. It may be the reason why no strong evidence exists in our study. Besides, some prognostic biomarkers of PDAC including CA19-9 were not comprehensively evaluated by systematic reviews or meta-analyses. Therefore, these biomarkers were not included in this study. Secondly, most of the eligible meta-analyses include <10 studies, which weakened the power of the statistical tests to identify small-study effects and excess significance. Thirdly, diagnostic biomarkers of PDAC were not evaluated in our study which should be further assessed in the future. Fourthly, more than half of the included meta-analyses were conducted in one country (China) rather than evenly distributed around the world which may cause some bias and be unrepresentative.
Conclusion
The associations between CAR or NLR and PDAC OS were supported by highly suggestive evidence. And the association between LDH and PDAC OS was supported by suggestive evidence. However, these results should be cautiously interpreted due to the relatively inferior methodological quality of the included meta-analyses evaluated by AMSTAR2.0.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
JG and YW conceived and designed the study. YW and XZ performed the literature search, and acquired and collated the data, which were analyzed by LZ, JL, BJ and CL. JG was guarantor, and attests that all listed authors meet authorship criteria and that no authors meeting the criteria have been omitted. All authors drafted and critically revised the manuscript for important intellectual content, and gave final approval of the version to be published and contributed to the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81972324), the China Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant No. 2016-I2M-3-019), and the non-profit Central Research Institute Fund of Chinese of Academy of Medical Sciences (Grant No. 2018PT32014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01466/full#supplementary-material
References
1. Lucas AL, Malvezzi M, Carioli G, Negri E, La Vecchia C, Boffetta P, et al. Global trends in pancreatic cancer mortality from 1980 through 2013 and predictions for 2017. Clin Gastroenterol Hepatol. (2016) 14:1452–62. doi: 10.1016/j.cgh.2016.05.034
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
6. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
7. Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. (2019) 156:254–72. doi: 10.1053/j.gastro.2018.08.063
8. Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, et al. A systematic review of the burden of pancreatic cancer in Europe: real-world impact on survival, quality of life and costs. J Gastrointest Cancer. (2015) 46:201–11. doi: 10.1007/s12029-015-9724-1
9. Kamarajah SK, Bundred JR, Marc OS, Jiao LR, Hilal MA, Manas DM, et al. A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB. (2019) 22:329–39. doi: 10.1016/j.hpb.2019.09.016
10. Wu WM, Jin G, Wang CY, Miao Y, Wang HZ, Lou WH, et al. Pancreatic surgery Study Group of Chinese Society of Surgery of Chinese Medical Association. The current surgical treatment of pancreatic cancer in China: a national wide cross-sectional study. J Pancreatol. (2019) 2:16–21. doi: 10.1097/JP9.0000000000000012
11. Maeda S, Unno M, Yu J. Adjuvant and neoadjuvant therapy for pancreatic cancer. J Pancreatol. (2019) 2:100–6. doi: 10.1097/JP9.0000000000000028
12. Dimastromatteo J, Houghton JL, Lewis JS, Kelly KA. Challenges of pancreatic cancer. Cancer J. (2015) 21:188–93. doi: 10.1097/PPO.0000000000000109
13. Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. (2016) 2016:8962321. doi: 10.1155/2016/8962321
14. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69:89–95. doi: 10.1067/mcp.2001.113989
15. Füzéry AK, Levin J, Chan MM, Chan DW. Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin Proteomics. (2013) 10:13. doi: 10.1186/1559-0275-10-13
16. Daamen LA, Groot VP, Intven MPW, Besselink MG, Busch OR, Koerkamp BG. Postoperative surveillance of pancreatic cancer patients. Eur J Surg Oncol. (2019) 45:1770–7. doi: 10.1016/j.ejso.2019.05.031
17. Distler M, Pilarsky E, Kersting S, Grutzmann R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas-a retrospective tumor marker prognostic study. Int J Surg. (2013) 11:1067.e72. doi: 10.1016/j.ijsu.2013.10.005
18. Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. (2006) 70:255–64. doi: 10.1159/0000948885
19. Li J, Li Z, Kan H, Sun Z, Xing J, Cheng Y, et al. CA19-9 elevation as an indication to start salvage treatment in surveillance after pancreatic cancer resection. Pancreatology. (2019) 19:302–6. doi: 10.1016/j.pan.2019.01.023
20. Cheng H, Luo G, Jin K, Fan Z, Huang Q, Gong Y, et al. Kras mutation correlating with circulating regulatory T cells predicts the prognosis of advanced pancreatic cancer patients. Cancer Med. (2020) 9:2153–9. doi: 10.1002/cam4.2895
21. Huang J, Liu J, Chen-Xiao K, Zhang X, Lee WN, Go VL, et al. Advance in microRNA as a potential biomarker for early detection of pancreatic cancer. Biomark Res. (2016) 4:20. doi: 10.1186/s40364-016-0074-3
22. Han T, Hu H, Zhuo M, Wang L, Cui JJ, Jiao F, et al. Long non-coding RNA: an emerging paradigm of pancreatic cancer. Curr Mol Med. (2016) 16:702–9. doi: 10.2174/1566524016666160927095812
23. Wang YZ, An Y, Li BQ, Lu J, Guo JC. Research progress on circularRNAs in pancreatic cancer: emerging but promising. Cancer Biol Ther. (2019) 20:1163–71. doi: 10.1080/15384047.2019.1617563
24. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358: j4008. doi: 10.1136/bmj.j4008
25. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. (2007) 335:914–6. doi: 10.1136/bmj.39343.408449.80
26. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. doi: 10.1136/bmj.d549
27. Patsopoulos NA, Evangelou E, Ioannidis JP. Heterogeneous views on heterogeneity. Int J Epidemiol. (2009) 38:1740–2. doi: 10.1093/ije/dyn235
28. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
29. Salanti G, Ioannidis JP. Synthesis of observational studies should consider credibility ceilings. J Clin Epidemiol. (2009) 62:115–22. doi: 10.1016/j.jclinepi.2008.05.014
30. Rezende LFM, Sá TH, Markozannes G, Rey-López JP, Lee IM, Tsilidis KK, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. (2018) 52:826–33. doi: 10.1136/bjsports-2017-098391
31. Markozannes G, Tzoulaki I, Karli D, Evangelou E, Ntzani E, Gunter MJ, et al. Diet, body size, physical activity and risk of prostate cancer: an umbrella review of the evidence. Eur J Cancer. (2016) 69:61–9. doi: 10.1016/j.ejca.2016.09.026
32. Luo Y, Zhang X, Tan Z, Wu P, Xiang X, Dang Y, et al. Astrocyte elevated gene-1 as a novel clinicopathological and prognostic biomarker for gastrointestinal cancers: a meta-analysis with 2999 patients. PLoS ONE. (2015) 10: e0145659. doi: 10.1371/journal.pone.0145659
33. Chen X, Tao L, Yuan C, Xiu D. The prognostic value of B7-H4 in pancreatic cancer: systematic review and meta-analysis. Medicine. (2018) 97:e0088. doi: 10.1097/MD.0000000000010088
34. Smith RA, Tang J, Tudur-Smith C, Neoptolemos JP, Ghaneh P. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer. (2011) 104:1440–51. doi: 10.1038/bjc.2011.110
35. Fu YJ, Li KZ, Bai JH, Liang ZQ. C-reactive protein/albumin ratio is a prognostic indicator in Asians with pancreatic cancers: a meta-analysis. Medicine. (2019) 98:e18219. doi: 10.1097/MD.0000000000018219
36. Li X, Zhao H, Gu J, Zheng L. Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis. Int J Clin Exp Pathol. (2015) 8:12084–92.
37. Liu Y, Wu T, Lu D, Zhen J, Zhang L. CD44 overexpression related to lymph node metastasis and poor prognosis of pancreatic cancer. Int J Biol Markers. (2018) 33:308–13. doi: 10.1177/1724600817746951
38. Samarendra H, Jones K, Petrinic T, Silva MA, Reddy S, Soonawalla Z, et al. A meta-analysis of CXCL12 expression for cancer prognosis. Br J Cancer. (2017) 117:124–35. doi: 10.1038/bjc.2017.134
39. Ding Y, Du Y. Clinicopathological significance and prognostic role of chemokine receptor CXCR4 expression in pancreatic ductal adenocarcinoma, a meta-analysis and literature review. Int J Surg. (2019) 65:32–8. doi: 10.1016/j.ijsu.2019.03.009
40. Fan H, Wang W, Yan J, Xiao L, Yang L. Prognostic significance of CXCR7 in cancer patients: a meta-analysis. Cancer Cell Int. (2018) 18:212. doi: 10.1186/s12935-018-0702-0
41. Wang D, Guo XZ, Li HY, Zhao JJ, Shao XD, Wu CY. Prognostic significance of cyclooxygenase-2 protein in pancreatic cancer: a meta-analysis. Tumour Biol. (2014) 35:10301–7. doi: 10.1007/s13277-014-2260-y
42. Stephenson D, Nahm C, Chua T, Gill A, Mittal A, de Reuver P, et al. Circulating and disseminated tumor cells in pancreatic cancer and their role in patient prognosis: a systematic review and meta-analysis. Oncotarget. (2017) 8:107223–36. doi: 10.18632/oncotarget.19928
43. Ye J, Wen J, Ning Y, Li Y. Higher notch expression implies poor survival in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Pancreatology. (2018) 18:954–61. doi: 10.1016/j.pan.2018.09.014
44. Jamieson NB, Carter CR, McKay CJ, Oien KA. Tissue biomarkers for prognosis in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Clin Cancer Res. (2011) 17:3316–31. doi: 10.1158/1078-0432.CCR-10-3284
45. Liu G, Xiong D, Xiao R, Huang Z. Prognostic role of fibroblast growth factor receptor 2 in human solid tumors: a systematic review and meta-analysis. Tumour Biol. (2017) 39. doi: 10.1177/1010428317707424. [Epub ahead of print].
46. Dai J, Yang L, Wang J, Xiao Y, Ruan Q. Prognostic value of FOXM1 in patients with malignant solid tumor: a meta-analysis and system review. Dis Markers. (2015) 2015:352478. doi: 10.1155/2015/352478
47. Sharen G, Peng Y, Cheng H, Liu Y, Shi Y, Zhao J. Prognostic value of GLUT-1 expression in pancreatic cancer: results from 538 patients. Oncotarget. (2017) 8:19760–7. doi: 10.18632/oncotarget.15035
48. Cao LL, Yue Z, Liu L, Pei L, Yin Y, Qin L, et al. The expression of histone deacetylase HDAC1 correlates with the progression and prognosis of gastrointestinal malignancy. Oncotarget. (2017) 8:39241–53. doi: 10.18632/oncotarget.16843
49. Bird NT, Elmasry M, Jones R, Psarelli E, Dodd J, Malik H, et al. Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br J Surg. (2017) 104:328–36. doi: 10.1002/bjs.10482
50. Li X, Zhao H, Gu J, Zheng L. Prognostic role of HER2 amplification based on fluorescence in situ hybridization (FISH) in pancreatic ductal adenocarcinoma (PDAC): a meta-analysis. World J Surg Oncol. (2016) 14:38. doi: 10.1186/s12957-016-0792-x
51. Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD, Liang TB. Hypoxia-inducible factor 1α expression and its clinical significance in pancreatic cancer: a meta-analysis. Pancreatology. (2014) 14:391–7. doi: 10.1016/j.pan.2014.06.008
52. Luo D, Liu H, Lin D, Lian K, Ren H. The clinicopathological and prognostic value of hypoxia-inducible factor-2α in cancer patients: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2019) 28:857–66. doi: 10.1158/1055-9965.EPI-18-0881
53. Wu J, Hu L, Wu F, Zou L, He T. Poor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysis. Oncotarget. (2017) 8:32332–44. doi: 10.18632/oncotarget.15974
54. Wu T, Zhang W, Yang G, Li H, Chen Q, Song R, et al. HMGB1 overexpression as a prognostic factor for survival in cancer: a meta-analysis and systematic review. Oncotarget. (2016) 7:50417–27. doi: 10.18632/oncotarget.10413
55. Gan J, Wang W, Yang Z, Pan J, Zheng L, Yin L. Prognostic value of pretreatment serum lactate dehydrogenase level in pancreatic cancer patients: a meta-analysis of 18 observational studies. Medicine. (2018) 97:e13151. doi: 10.1097/MD.0000000000013151
56. Mao Y, Chen D, Duan S, Zhao Y, Wu C, Zhu F, et al. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: a meta-analysis. Cancer Cell Int. (2018) 18:201. doi: 10.1186/s12935-018-0698-5
57. Mehrad-Majd H, Ravanshad S, Moradi A, Khansalar N, Sheikhi M, Akhtari J. Decreased expression of lncRNA loc285194 as an independent prognostic marker in cancer: a systematic review and meta-analysis. Pathol Res Pract. (2019) 215:152426. doi: 10.1016/j.prp.2019.04.018
58. Liu FT, Dong Q, Gao H, Zhu ZM. The prognostic significance of UCA1 for predicting clinical outcome in patients with digestive system malignancies. Oncotarget. (2017) 8:40620–32. doi: 10.18632/oncotarget.16534
59. Hua T, Liu S, Xin X, Jin Z, Liu Q, Chi S, et al. Prognostic significance of L1 cell adhesion molecule in cancer patients: a systematic review and meta-analysis. Oncotarget. (2016) 7:85196–207. doi: 10.18632/oncotarget.13236
60. Shao Y, Gu W, Ning Z, Song X, Pei H, Jiang J. Evaluating the prognostic value of microRNA-203 in solid tumors based on a meta-analysis and the cancer genome atlas (TCGA) datasets. Cell Physiol Biochem. (2017) 41:1468–80. doi: 10.1159/000470649
61. Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta. (2018) 479:181–9. doi: 10.1016/j.cca.2018.01.024
62. Hu Y, Chen W, Yan Z, Ma J, Zhu F, Huo J. Prognostic value of PD-L1 expression in patients with pancreatic cancer: a PRISMA-compliant meta-analysis. Medicine. (2019) 98: e14006. doi: 10.1097/MD.0000000000014006
63. Ji R, Ren Q, Bai S, Wang Y, Zhou Y. Prognostic significance of pretreatment plasma fibrinogen in patients with hepatocellular and pancreatic carcinomas: a meta-analysis. Medicine. (2018) 97:e10824. doi: 10.1097/MD.0000000000010824
64. Yin X, Wu L, Yang H, Yang H. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: a systematic review and meta-analysis. Medicine. (2019) 98:e17475. doi: 10.1097/MD.0000000000017475
65. Zhu H, Luo H, Zhu X, Hu X, Zheng L, Zhu X. Pyruvate kinase M2 (PKM2) expression correlates with prognosis in solid cancers: a meta-analysis. Oncotarget. (2017) 8:1628–40. doi: 10.18632/oncotarget.13703
66. Hu G, Wang S, Xu F, Ding Q, Chen W, Zhong K, et al. Tumor-infiltrating podoplanin+ fibroblasts predict worse outcome in solid tumors. Cell Physiol Biochem. (2018) 51:1041–50. doi: 10.1159/000495484
67. Yu M, Wang Q, Ding JW, Yang Z, Xie C, Lu NH. Association between raf kinase inhibitor protein loss and prognosis in cancers of the digestive system: a meta-analysis. Cancer Biomark. (2014) 14:389–400. doi: 10.3233/CBM-140410
68. Zhang X, Jin FS, Zhang LG, Chen RX, Zhao JH, Wang YN, et al. Predictive and prognostic roles of ribonucleotide reductase M1 in patients with pancreatic cancer treated with gemcitabine: a meta-analysis. Asian Pac J Cancer Prev. (2013) 14:4261-5
69. Wang JD, Jin K, Chen XY, Lv JQ, Ji KW. Clinicopathological significance of SMAD4 loss in pancreatic ductal adenocarcinomas: a systematic review and meta-analysis. Oncotarget. (2017) 8:16704–11. doi: 10.18632/oncotarget.14335
70. Han W, Cao F, Chen MB, Lu RZ, Wang HB, Yu M, et al. Prognostic value of SPARC in patients with pancreatic cancer: a systematic review and meta-analysis. PLoS ONE. (2016) 11: e0145803. doi: 10.1371/journal.pone.0145803
71. Wu P, Wu D, Zhao L, Huang L, Shen G, Huang J, et al. Prognostic role of STAT3 in solid tumors: a systematic review and meta-analysis. Oncotarget. (2016) 7:19863–83. doi: 10.18632/oncotarget.7887
72. Chen H, Lu W, Huang C, Ding K, Xia D, Wu Y, et al. Prognostic significance of ZEB1 and ZEB2 in digestive cancers: a cohort-based analysis and secondary analysis. Oncotarget. (2017) 8:31435–48. doi: 10.18632/oncotarget.15634
73. Kalisvaart M, Broadhurst D, Marcon F, et al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: systematic review and a single-centre retrospective study. HPB. (2020). doi: 10.1016/j.hpb.2020.01.005. [Epub ahead of print].
74. Nosacka RL, Delitto AE, Delitto D, Patel R, Judge SM, Trevino JG, et al. Distinct cachexia profiles in response to human pancreatic tumours in mouse limb and respiratory muscle. J Cachexia Sarcopenia Muscle. (2020) 11:820–37. doi: 10.1002/jcsm.12550
75. Gulen ST, Karadag F, Karul AB, Kilicarslan N, Ceylan E, Kuman NK, et al. Adipokines and systemic inflammation in weight-losing lung cancer patients. Lung. (2012) 190:327–32. doi: 10.1007/s00408-011-9364-6
76. Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. (2014) 110:1409–12. doi: 10.1038/bjc.2014.90
77. Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol. (2019) 15:e109–14. doi: 10.1111/ajco.13123
78. Arima K, Yamashita YI, Hashimoto D, Nakagawa S, Umezaki N, Yamao T, et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg. (2018) 216:111–5. doi: 10.1016/j.amjsurg.2017.08.016
79. Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Validation and clinical usefulness of pre- and postoperative systemic inflammatory parameters as prognostic markers in patients with potentially resectable pancreatic cancer. Pancreatology. (2019) 4:239–246. doi: 10.1016/j.pan.2019.12.004
80. Pu N, Yin H, Zhao G, Nuerxiati A, Wang D, Xu X, et al. Independent effect of postoperative neutrophil-to-lymphocyte ratio on the survival of pancreatic ductal adenocarcinoma with open distal pancreatosplenectomy and its nomogram-based prediction. J Cancer. (2019) 10:5935–43. doi: 10.7150/jca.35856
81. Markert CL. Lactate dehydrogenase isozymes: dissociation and recombination of subunits. Science. (1963) 140:1329–30. doi: 10.1126/science.140.3573.1329
82. Yu M, Chen S, Hong W, Gu Y, Huang B, Lin Y, et al. Prognostic role of glycolysis for cancer outcome: evidence from 86 studies. J Cancer Res Clin Oncol. (2019) 145:967–99. doi: 10.1007/s00432-019-02847-w
83. Gao S, Wu M, Chen Y, Lou W, Zhou G, Li J, et al. Lactic dehydrogenase to albumin ratio in prediction of unresectable pancreatic cancer with intervention chemotherapy. Future Oncol. (2018) 14:1377–86. doi: 10.2217/fon-2017-0556
84. Palmquist C, Dehlendorff C, Calatayud D, Hansen CP, Hasselby JP, Johansen JS. Prediction of unresectability and prognosis in patients undergoing surgery on suspicion of pancreatic cancer using carbohydrate antigen 19-9, interleukin 6, and YKL-40. Pancreas. (2020) 49:53–61. doi: 10.1097/MPA.0000000000001466
85. Gu X, Zhou R, Li C, Liu R, Zhao Z, Gao Y, et al. Preoperative maximum standardized uptake value and carbohydrate antigen 19-9 were independent predictors of pathological stages and overall survival in Chinese patients with pancreatic duct adenocarcinoma. BMC Cancer. (2019) 19:456. doi: 10.1186/s12885-019-5691-4
Keywords: pancreatic ductal adenocarcinoma, biomarkers, umbrella review, prognosis, survival
Citation: Wang Y, Zhong X, Zhou L, Lu J, Jiang B, Liu C and Guo J (2020) Prognostic Biomarkers for Pancreatic Ductal Adenocarcinoma: An Umbrella Review. Front. Oncol. 10:1466. doi: 10.3389/fonc.2020.01466
Received: 27 February 2020; Accepted: 09 July 2020;
Published: 17 September 2020.
Edited by:
Nelson Shu-Sang Yee, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Pierpaolo Correale, Azienda Ospedaliera “Bianchi-Melacrino-Morelli”, ItalyKamini Singh, Cornell University, United States
Copyright © 2020 Wang, Zhong, Zhou, Lu, Jiang, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junchao Guo, gjcpumch@163.com
†These authors have contributed equally to this work
 Yizhi Wang1†
Yizhi Wang1† Xi Zhong
Xi Zhong Bolun Jiang
Bolun Jiang Chengxi Liu
Chengxi Liu Junchao Guo
Junchao Guo