- Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Purpose: To investigate the efficacy of fertility-sparing treatment for young women with grade 2 presumed stage IA endometrioid endometrial adenocarcinoma (EEA).
Methods: We performed a retrospectively review of eight patients affected by grade 2 presumed stage IA endometrioid endometrial adenocarcinoma who underwent fertility-sparing treatment in the Peking Union Medical College Hospital between 2011 and 2018.
Results: The median age of patients was 26 years (range, 22–35 years). Complete response (CR) was found in seven of the eight cases. The median time to response was 3 months (range, 3–9 months). Among patients who achieved CR, three had recurrence and were treated with second-line fertility-sparing therapy. Two of the three recurrent patients achieved CR, and one patient subsequently conceived. Pregnancies and successful deliveries were achieved in two of four patients. The average follow-up period was 31 months (range, 21–77 months).
Conclusions: Fertility-sparing therapy is a feasible treatment option in patients with presumed stage IA, grade 2 endometrial cancer. Although our results are encouraging, they are based on very limited numbers, and patients should be informed the risk of tumor progression during treatment. Further evaluations are still required before recommending fertility-sparing therapy to endometrial cancer patients with more advanced disease in routine practice.
Introduction
Endometrial carcinoma (EC) is the most common gynecological malignancy in the world. It is typically diagnosed in post-menopausal women. The standard treatment for EC consists of hysterectomy and bilateral salpingo-oophorectomy with or without pelvic and para-aortic lymph node assessment (1). This treatment precludes future fertility and may thus be undesirable for women wishing to preserve their fertility.
Approximately 3–14% of endometrial cancer cases are diagnosed in women equal to or under 40 years of age who want to preserve their fertility (2, 3). The conservative treatment for young women with G1 EC and atypical hyperplasia (AH) who desire to conceive has been proved to be a safe and feasible therapy (4–8). Nevertheless, for women affected by grade 2 disease, there is limited literature regarding this issue, and the data correspondingly are limited. There has been no prospective trial on the effectiveness of fertility-sparing treatment in patients with grade 2 or more advanced disease. Therefore, the aim of this study was to investigate the efficacy of conservative management in patients with early-stage grade 2 endometrial cancer who desired to preserve their fertility.
Methods
The medical records of patients with grade 2 presumed stage IA endometrioid endometrial adenocarcinoma (EEA) who underwent fertility-sparing treatment at the Peking Union Medical College Hospital between 2011 and 2018 were retrospectively reviewed. The Institutional Review Board (IRB) of “the Ethics Committee of Peking Union Medical College Hospital” approved the use of patients' clinical data for this retrospective study. Patients must be informed that data about fertility-sparing treatment are not so robust to draw firm conclusion due to the limitation in sample size of available studies, and, most importantly, there is a risk of disease progression during treatment or after the initial response. All patients were informed that hormone therapy is not the standard treatment and requires serial dilation and curettage (D&C) or hysteroscopy during treatment period. Inclusion criteria were fertile age (40 years or younger), desire to preserve fertility, and histologically proven grade 2 EEA, tumor presumed to be International Federation of Gynecology and Obstetrics (FIGO) stage IA limited to the endometrium. The diagnosis was confirmed by endometrial biopsy under hysteroscopy. All of the histological slides were reviewed by at least two pathologists specializing in gynecological oncology. All patients were evaluated by pelvic examination, ultrasound scan/abdominal, and pelvic computed tomography/pelvic magnetic resonance imaging at baseline to confirm tumor limited into the endometrium and no evidence of lymph node involvement or extrauterine metastasis.
Patients were scheduled to receive medroxyprogesterone acetate (MPA) 500 mg qd or megestrol acetate (MA) 160 mg bid orally on a daily basis or the combination of intramuscular injections of 3.75 mg gonadotropin-releasing hormone agonist (GnRHa) every 4 weeks plus levonorgestrel-intrauterine system (LNG-IUS) inserted. Response was assessed histologically every 3 months after the start of treatment. Complete blood counts and biochemistry panels along with renal function and liver profiles were performed every month, and serum CA125 and pelvic ultrasound were carried out every 3 months. Endometrial tissue sampling for diagnosis should be carried out by hysteroscopy and endometrial curettage or D&C. All histology was reported by two expert pathologists specialized in gynecological oncology. Pathological response to MPA treatment was categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR was defined as the absence of evidence of hyperplasia or carcinoma. PR was defined as histological regression or endometrial decidual change. SD was defined as the persistence of EC or AH. PD was defined as progression to a lesion of higher grade or clinically progressive disease including myometrial invasion, extrauterine disease, or lymph node metastasis (9). Evaluation was performed every 3 months via hysteroscopy; patients with PR or SD continued the same treatment for an additional 3 months or change the dose, whereas those with PD were immediately proposed to receive surgery. Patients with CR who desire to become pregnant were encouraged to conceive or referred to in vitro fertilization (IVF) immediately. Those with CR who opted to postpone pregnancy were prescribed oral contraceptives/progestin or LNG-IUS to prevent cancer recurrence.
Results
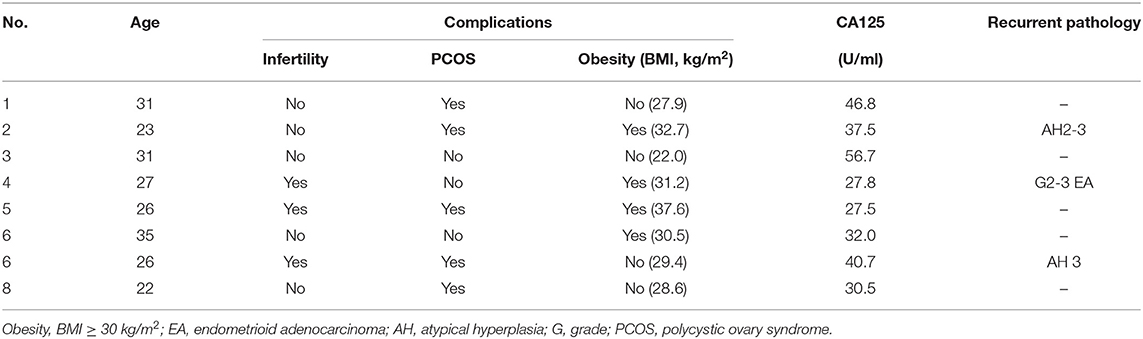
Between 2011 and 2018, there were eight patients included in this study (Table 1). The median age of patients was 26 years (range, 22–35 years). All patients desired to preserve fertility and accepted fertility-sparing treatment. Patients in the study were treated with MA or MPA or the combination of GnRHa plus LNG-IUS initially. Patients' treatment regimen, therapeutic response, recurrence, and oncologic and fertility outcomes are presented in Table 2.
Of the eight patients, seven (87.5%) achieved CR. The median time to CR was 3 months (range, 3–9 months). One patient (patient 2) changed therapeutic regimen in initial treatment due to no response to initial 3 months of progestin therapy. The only patient (patient 1) who failed to respond to initial 7 months of hormone treatment finally underwent hysterectomy with surgical staging. The post-operatively histopathological result showed FIGO stage IIIC1. This patient, who was treated with chemotherapy and radiotherapy after surgery, was alive and free of disease at last follow-up.
Three of the seven who achieved CR (patients 2, 4, and 7) developed recurrent disease. The time to recurrence was 17, 24, and 36 months, respectively. After recurrence with G2-3 endometrial carcinoma, patient 4 who insisted on receiving 4 months of MA failed to respond. She underwent hysterectomy with surgical staging. The final pathology of the uterus was FIGO stage IIIC1 grade 3 EC, and she received chemotherapy post-operatively and was free of disease at last follow-up. The remaining two (patients 2 and 7) who still desired fertility were treated with second round of fertility-sparing management and got CR again. Patient 2 with the recurrent pathology of AH2-3 were treated with the combination of intramuscular injections of GnRHa every 4 weeks combined with LNG-IUS. She achieved CR again after 3 months and is undergoing in vitro fertilization and embryo transfer (IVF-ET). Patient 7 delivered successfully after the first recurrence and relapsed again with pathology of AH3. She achieved CR at 6 months after a third round progestin treatment using MA 160 mg bid after the second recurrence. Among the eight patients, one patient (patient 6) underwent hysterectomy according to the patient's decision even though she showed CR.
Of the seven patients with complete remission, three patients desired to conceive immediately, and two patients had pregnancies and successfully delivered during the follow-up period.
The remaining patients (patient 5, 6, and 8) are all alive and free of disease. The median follow-up of the eight patients was 31 months (range, 21–77 months), with no disease-related death and no major adverse effects related to high-dose progestin.
Discussion
The incidence of endometrial cancer is increasing with lower average onset age, and most patients have not yet completed the reproductive function. Therefore, fertility-sparing treatment has received much attention during recent years. Since progesterone was first reported to be used in the fertility-sparing treatment of early endometrial cancer, researchers continue to validate that conservative treatment for young women with G1 EC and AH who desire to conceive is a safe and feasible therapy. Previous literature shows that oral progestin therapy can achieve CR rates ranging between 67 and 80% (4–8). Our recent meta-analysis found that among 445 grade 1 stage IA EC patients who administered oral progestin, the remission rate was 82.4% (10). The pregnancy rate was 28.8%, and the recurrence rate was 25% (10). The differentiation degree of EC is the most important indicator to predict stage and response to progestin treatment (11). Duska et al. (12) reviewed women with EC under the age of 40, and the results showed that only grade 1 EC could predict stage I disease among them. In addition, Thigpen et al. (13) demonstrated that in advanced or recurrent EC, the response rate to MPA was 37% for grade 1 tumors compared with 23% for grade 2 and 9% for grade 3 tumors. Looking through the current literature, there are few reported cases of progestin-based fertility-sparing therapy in young women with intramucosal G2-3 EC. According to the Mayo Clinic's experience, the risk of lymphatic spread in low-risk women (patients preoperatively diagnosis with G1 or G2, endometrioid histological type, and tumor diameter B2.0 cm) is <1% (14). Therefore, fertility-sparing treatment could be expanded to stage IA type I and G2 EC since they are generally considered to be “low-risk” population.
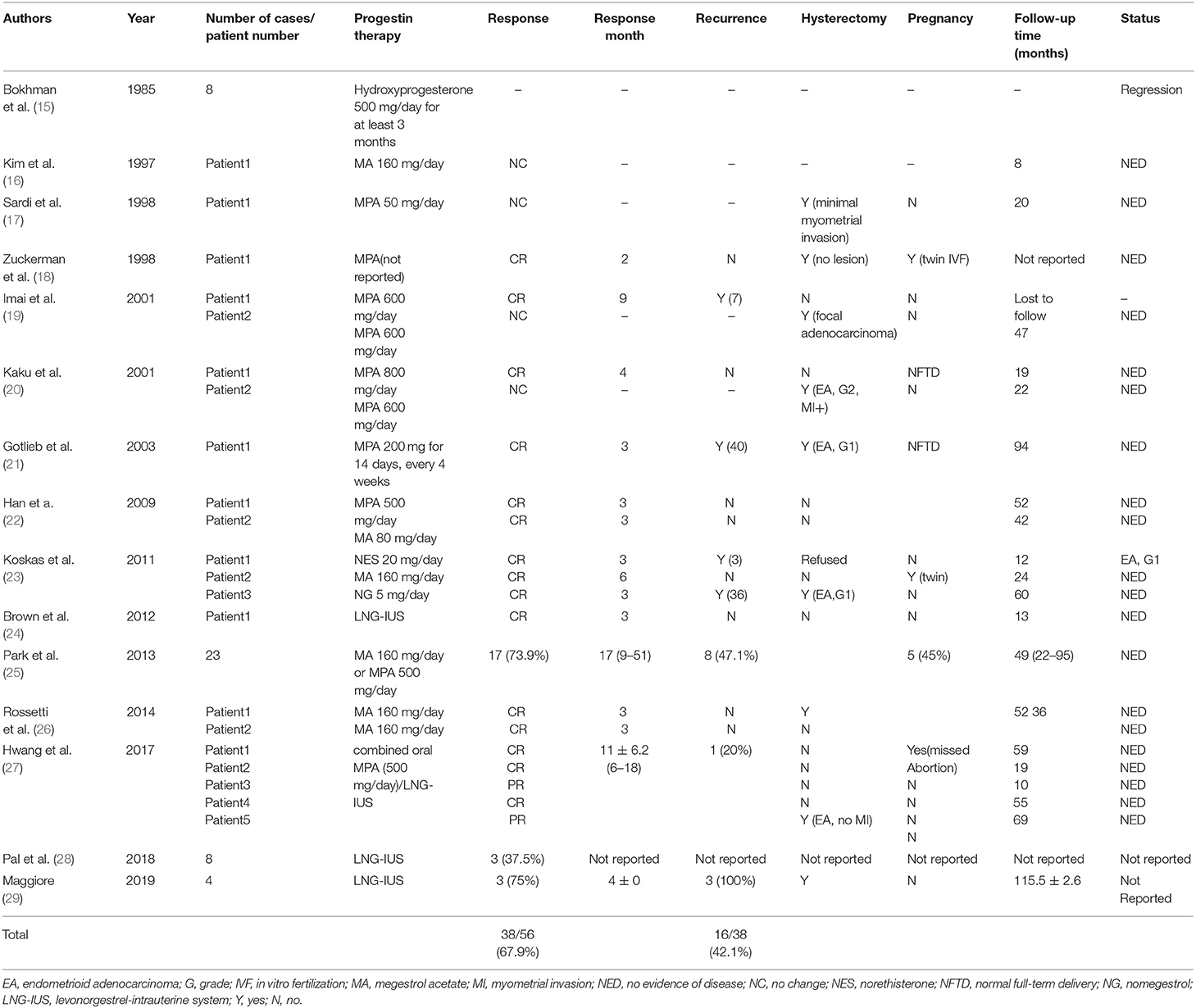
To date, there are 15 articles regarding the fertility-sparing treatment of stage IA, G2 EC in young patients (15–29) (Table 3). Taking together the data from the papers, 38 (67.9%) of 56 patients achieved CR, and 16 (42.1%) of those 38 CR cases later showed recurrent disease. Park et al. (25) conducted a multicenter retrospective cohort study to estimate the oncologic and pregnancy outcomes after oral MPA or MA treatment of patients with grade 2 or 3 stage IA EC. Among patients without myometrial invasion, the CR rate and the recurrence rate were 76.5% (13/17) and 23.1% (3/13), respectively. Our study demonstrated that the CR rate for G2 EC was 87.5% (7/8) with a median time to CR of 3 months, and the recurrence rate after complete remission was 42.8% (3/7). These results are comparable to those of patients with stage IA, grade 1 differentiation without myometrial invasion. Due to the limitations of retrospective study design and the small sample size, more prospective large-scale studies will have to await further.
Conservative treatment of endometrial adenocarcinoma traditionally involved oral progestin therapy; more recently, many reports have introduced LNG-IUS alone or combined with GnRHa or oral progestin as an alternative to oral systemic progestin alone for the treatment of women with EC and AH (24, 27–30). Brown et al. (24) in 2012 reported a case of an 18-year-old girl with grade 2 endometrioid adenocarcinoma, treated with LNG-IUS successfully. Hwang et al. (27) evaluated the efficacy of combined MPA/LNG-IUS treatment for grade 2 stage IA EC; the CR rate was 60% (3/5), and one patient (13.3%) who relapsed after 14 months achieving CR received combined therapy and achieved CR by 6 months again. A prospective observational study showed that for grade 1 EC, combined oral MPA/LNG-IUS treatment was more effective than oral progestin alone (31). The above findings suggested that, as a fertility-sparing option, compared with oral progestin alone, combined therapy may produce more favorable outcomes for grade 2 EC. In our study, two patients achieved CR by using GnRHa combined with LNG-IUS treatment, and one of them changed regimen from MA to the combination of GnRHa and LNG-IUS due to ineffective treatment. Our result suggested that the combination of GnRHa with LNG-IUS represents an effective therapeutic approach for patients with G2 EC.
Most of the patients who underwent hysterectomy after conservative treatment failure were confirmed to be early clinical stage, well-differentiated endometrioid adenocarcinoma, indicating the disease was less likely to progress during hormone therapy. However, in our study, one patient who failed to respond to 7 months of hormone treatment underwent staging surgery, and the final pathology suggested stage IIIC1 EC. According to the European Society of Gynecological Oncology recommendation, we should consider it unsuitable for patients with persistent or progressive disease after 6 months of progestin treatment to continue conservative treatment (32). As for this patient, the disease was stable in the first 6 months treatment, and she insist on trying another cycle of treatment. Unfortunately, during the seventh month of treatment, she experienced disease progression. Another patient with recurrence receiving 4 months of MA and suffering no change of lesions underwent definitive surgery with a diagnosis of stage IIIC1 EC. Although these two patients are alive and free of disease at last follow-up, we should be aware of the risk of disease progression during fertility-sparing treatment. It should be considered that poorly differentiated EC patients who received fertility-sparing treatment be counseled on the need for close follow-up, as well as the dangers of delaying hysterectomy.
There are few papers focusing on the pregnancy outcome of fertility-sparing treatment in patients with intramucosal G2-3 EC (18, 20, 21, 23, 25, 27). In the present series, although only 69.2% of patients who achieve CR attempted to conceive during the study, the pregnancy and live birth rates were 37% and 33%, respectively. While in our study, the overall live birth rate was 66.7%. It should be recommended that patients achieving CR should consult a reproductive endocrinologist to maximize the possibility of a live birth and minimize the time between diagnosis and definitive EC treatment.
Limitations
Although our study is a single-center retrospective study with a limited number of cases, we believe it can provide useful clinical data to support the feasibility of conservative treatment in patients with stage IA G2 EC. Meanwhile, it is necessary to conduct a regional or international randomized clinical trial that focused only on fertility-sparing treatment in patients with stage IA G2 EC in order to clarify the oncologic and fertility outcomes. The fertility-preserving treatment for early EC should be performed on the basis of the following requirements: first of all, a good prognosis after fertility-sparing therapy (exceeding 95% in 5-year survival rate); second, in terms of overall survival rate, fertility-sparing therapy should be not inferior to standard surgical treatment. At present, G2 intramucosal endometrial cancer is a low-risk type that does not require adjuvant treatment after primary surgery. However, the long-term prognosis is not clear because of the limited number of cases. The follow-up time of this center is limited, and long-term follow-up of these patients will also be performed to verify long-term survival.
Conclusions
Based on our own encouraging results, fertility-sparing treatment can be a feasible and effective option for patients with grade 2 presumed stage IA EC without myometrial invasion who desire to preserve their fertility. However, conservative management in this population should still be considered with caution, and patients need to be monitored more closely so that fertility-sparing treatment can be terminated in time when patients failed to respond to treatment.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This retrospective study had obtained approval of the Institutional Ethical Committee of Peking Union Medical College Hospital. All patients had signed an informed consent.
Author Contributions
MY, JY, DC, and KS: conceived and designed the study. MY, JY, DC, and XH: data acquisition. YW, MY, ZY, and XZ: analyzed the data. MY: wrote the original draft. YW, MY, JY, DC, and KS: wrote, reviewed, and edit. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-2017-I2M-1-002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all of the faculty, nurses, and staff at Department of Obstetrics & Gynecology in Peking Union Medical College Hospital for the excellent care they provide patients. The authors also sincerely thank all the patients and their family members for their contribution to this research effort.
References
1. Wang Y, Yang JX. Fertility-preserving treatment in women with early endometrial cancer: the Chinese experience. Cancer Manag Res. (2018) 10:6803–13. doi: 10.2147/CMAR.S188087
2. Crissman JD, Azoury RS, Barnes AE, Schellhas HF. Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol. (1981) 57:699–704.
3. Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. (1984) 64:417–20. doi: 10.3109/00016348509155159
4. Kesterson J, Fanning J. Fertility-sparing treatment of endometrial cancer: options, outcomes and pitfalls. J Gynecol Oncol. (2012) 23:120–4. doi: 10.3802/jgo.2012.23.2.120
5. Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. (2004) 92:263–70. doi: 10.1016/j.ygyno.2011.11.043
6. Serkan E, Ali A. Fertility-sparing therapy in young women with endometrial cancer: 2010 update. Int J Gynecol Cancer. (2010) 20:1170–87. doi: 10.1111/IGC.0b013e3181e94f5a
7. Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. (2004) 95:133–8. doi: 10.1016/j.ygyno.2004.06.045
8. Tangjitgamol S, Manusirivithaya S, Hanprasertpong J. Fertility-sparing in endometrial cancer. Gynecol Obstet Invest. (2009) 67:250–68. doi: 10.1159/000209324
9. Wang Y, Yu M, Yang JX, Cao DY, Yuan Zhen, Zhou HM, et al. Prolonged conservative treatment in patients with recurrent endometrial cancer after primary fertility-sparing therapy: 15-year experience. Int J Clin Oncol. (2019) 24:712–20. doi: 10.1007/s10147-019-01404-2
10. Qin Y, Yu Z, Yang J, Cao D, Shen K. Oral progestin treatment for early-stage endometrial cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. (2016) 26:1081–91. doi: 10.1097/IGC.0000000000000723
11. Rodolakis A, Biliatis I, Morice P, Reed N, Denschlag D. European Society of gynecological oncology task force for fertility preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer. (2015) 25:1258–65. doi: 10.1097/IGC.0000000000000493
12. Duska LR, Garrett A, Bo RR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. (2001) 83:388–93. doi: 10.1006/gyno.2001.6434
13. Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Given FT. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the gynecologic oncology group. J Clin Oncol. (1999) 17:1736–44. doi: 10.1200/JCO.1999.17.6.1736
14. AlHilli MM, Mariani A, Bakkum-Gamez JN, Dowdy SC, Weaver AL, Peethambaram PP. Risk-scoring models for individualized prediction of overall survival in low-grade and high-grade endometrial cancer. Gynecol Oncol. (2014) 133:485–93. doi: 10.1016/j.ygyno.2014.03.567
15. Bokhman JV Chepick OF Volkova AT Vishnevsky AS. Can primary endometrial carcinoma stage I be cured without surgery and radiation therapy? Gynecol Oncol. (1985) 20:139–55. doi: 10.1016/0090-8258(85)90135-0
16. Kim YB, Holschneider CH, Ghosh K, Nieberg RK, Montz FJ. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer. (1997) 79:320–7. doi: 10.1002/SICI1097-0142199701159:2<320::AID-CNCR15>3.0.CO;2-2
17. Sardi J, Anchezar Henry JP, Paniceres G, Gomez Rueda N, Vighi S. Primary hormonal treatment for early endometrial carcinoma. Eur J Gynaecol Oncol. (1998) 19:565–8.
18. Zuckerman B, Lavie O, Neuman M, Rabinowitz R, Ben-Chetrit A, Voss E, et al. Endometrial carcinoma stage I-grade II. Conservative treatment followed by a healthy twin pregnancy. Int J Gynecol Cancer. (1998) 8:172–4. doi: 10.1046/j.1525-1438.1998.97100.x
19. Imai M, Jobo T, Sato R, Kawaguchi M, Kuramoto H. Medroxyprogesterone acetate therapy for patients with adenocarcinoma of the endometrium who wish to preserve the uterus-usefulness and limitations. Eur J Gynaecol Oncol. (2001) 22:217–20.
20. Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. (2001) 167:39–48. doi: 10.1016/S0304-3835(01)00462-1
21. Gotlieb WH, Beiner ME, Shalmon B, Korach Y, Segal Y, Zmira N, et al. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. (2003) 102:718–25. doi: 10.1097/00006250-200310000-00013
22. Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, Lim JY, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. (2009) 19:1068–73. doi: 10.1111/IGC.0b013e3181aae1fb
23. Koskas M, Yazbeck C, Walker F, Clouqueur E, Agostini A, Ruat S, et al. Fertility-sparing management of grade 2 and 3 endometrial adenocarcinomas. Anticancer Res. (2011) 31:3047–9.
24. Brown AJ, Westin SN, Broaddus RR, Schmeler K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet Gynecol. (2012) 119(2 Pt 2):423–6. doi: 10.1097/AOG.0b013e318234d97c
25. Park JY, Kim DY, Kim TJ, Kim JW, Kim JH, Kim YM, et al. Hormonal therapy for women with stage IA endometrial cancer of all grades. Obstet Gynecol. (2013) 122:7–14. doi: 10.1097/AOG.0b013e3182964ce3
26. Rossetti D, Bogani G, Carnelli M, Vitale SG, Grosso G, Frigerio L. Efficacy of IVF following conservative management of endometrial cancer. Gynecol Endocrinol. (2014) 30:280–1. doi: 10.3109/09513590.2014.892065
27. Hwang JY, Kim DH, Bae HS, Kim ML, Jung YW, Yun BS, et al. Combined oral medroxyprogesterone/levonorgestrel-intrauterine system treatment for women with grade 2 stage IA endometrial cancer. Int J Gynecol Cancer. (2017) 27:738–42. doi: 10.1097/IGC.0000000000000927
28. Pal N, Broaddus RR, Urbauer DL, Balakrishnan N, Milbourne A, Schmeler KM, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet Gynecol. (2018) 131:109–16. doi: 10.1097/AOG.0000000000002390
29. Leone Roberti Maggiore U, Martinelli F, Dondi G, Bogani G, Chiappa V, Evangelista MT, et al. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: a retrospective study. J Gynecol Oncol. (2019) 30:e57. doi: 10.3802/jgo.2019.30.e57
30. Zhou HM, Cao DY, Yang JX, Shen K, Lang JH. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer. (2017) 27:1178–82. doi: 10.1097/IGC.0000000000001008
31. Kim MK, Seong SJ, Kim YS, Song T, Kim ML, Yoon BS, et al. Combined medroxyprogesterone acetate/levonorgestrel–intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. (2013) 209:358.e1–4. doi: 10.1016/j.ajog.2013.06.031
32. Rodolakis A, Biliatis I, Morice P, Reed N, Mangler M, Kesic V, et al. European society of gynecological oncology task force for fertility preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer. (2015) 25:1258–65. doi: 10.1097/IGC.0000000000000493
Keywords: fertility-sparing treatment, grade 2 endometrioid endometrial cancer, hormonal therapy, LNG-IUS, GnRH-a
Citation: Yu M, Wang Y, Yuan Z, Zong X, Huo X, Cao D, Yang J and Shen K (2020) Fertility-Sparing Treatment in Young Patients With Grade 2 Presumed Stage IA Endometrioid Endometrial Adenocarcinoma. Front. Oncol. 10:1437. doi: 10.3389/fonc.2020.01437
Received: 08 December 2019; Accepted: 07 July 2020;
Published: 25 August 2020.
Edited by:
Fabio Martinelli, Istituto Nazionale dei Tumori (IRCCS), ItalyReviewed by:
Salvatore Giovanni Vitale, University of Messina, ItalyXiaojun Chen, Fudan University, China
Copyright © 2020 Yu, Wang, Yuan, Zong, Huo, Cao, Yang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-yan Cao, Y2FvZG9uZ3lhbjIwMTdAMTYzLmNvbQ==
 Mei Yu
Mei Yu Yao Wang
Yao Wang Dong-yan Cao
Dong-yan Cao