
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 22 July 2020
Sec. Molecular and Cellular Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01231
This article is part of the Research Topic The Impact of Tumor Extracellular Matrix Cross-Talk on Cancer Hallmarks View all 22 articles
The extracellular matrix (ECM) is a complex network composed of a multitude of different macromolecules. ECM components typically provide a supportive structure to the tissue and engender positional information and crosstalk with neighboring cells in a dynamic reciprocal manner, thereby regulating tissue development and homeostasis. During tumor progression, tumor cells commonly modify and hijack the surrounding ECM to sustain anchorage-dependent growth and survival, guide migration, store pro-tumorigenic cell-derived molecules and present them to enhance receptor activation. Thereby, ECM potentially supports tumor progression at various steps from initiation, to local growth, invasion, and systemic dissemination and ECM-tumor cells interactions have long been considered promising targets for cancer therapy. Integrins represent key surface receptors for the tumor cell to sense and interact with the ECM. Yet, attempts to therapeutically impinge on these interactions using integrin inhibitors have failed to deliver anticipated results, and integrin inhibitors are still missing in the emerging arsenal of drugs for targeted therapies. This paradox situation should urge the field to reconsider the role of integrins in cancer and their targeting, but also to envisage alternative strategies. Here, we review the therapeutic targets implicated in tumor cell adhesion to the ECM, whose inhibitors are currently in clinical trials and may offer alternatives to integrin inhibition.
The extra-cellular matrix (ECM) is a dynamic niche continuously undergoing quantitative and qualitative remodeling by renewed synthesis and proteolytic modifications. During ECM remodeling, changes to its physical structure and organization occur, leading to a dysregulation in fiber composition, tissue architecture, and stiffness contributing to cancer progression and fibrosis (1). The cell can sense the surrounding ECM fibers by transmembrane surface molecules, such as integrins or other glycoproteins, acting as cellular mechano-chemical sensors. The relevance of the finely tuned integration and crosstalk between the ECM molecules, the cellular cytoskeleton, and the downstream signaling pathways, has been widely recognized and studied (2, 3). Their complex dynamic bi-directional interactions and mechano-transduction control have been associated to fundamental physiological processes such as branching tissues morphogenesis and angiogenesis during development and homeostasis. These interactions are also relevant to pathological conditions including cancer, from initial malignant transformation to the disruption of tissue polarity and promotion of invasiveness toward dissemination and metastasis development (4, 5). Integrins represent the key cell surface receptors for the cell to sense the ECM, triggering signaling pathways that determine cell fate and evolution toward a malignant phenotype and resistance to therapy (6, 7). Numerous experimental and preclinical studies conducted over the past decades highlighted the central role of integrins in affecting different steps of tumorigenesis, by controlling tumor cell adhesion, proliferation, migration, invasion, and survival (6). This made integrins appealing therapeutic targets leading to the development of integrin inhibitors and their clinical testing in cancer trials. Unfortunately and unexpectedly, integrin inhibitors failed to deliver any tangible therapeutic benefits for cancer patients (8–10). This failure may be due to the intrinsic complexity of integrin signaling that we still do not fully understand. But they also question the pharmacokinetic/pharmacodynamics properties of the integrin inhibitors developed, the integrin subunit and the associated biological process targeted, the preclinical models used as well as the design of the clinical trials performed (7, 8). Addressing those yet unanswered questions is likely to pave the road toward successful introduction of a novel generation of integrin inhibitors in clinical practice. In the meantime, long-ago discovered non-integrin ECM receptors as well as intra-cellular downstream effectors of the ECM-tumor cell crosstalk (signaling molecules) taking part in several key aspects of tumor progression, were largely neglected. Considering the clinical failure of integrin inhibitors, these ECM-tumor crosstalk targets are potential candidates that may be therapeutically exploited in alternative to integrin inhibitors. Here we review those currently tested in anti-cancer clinical trials, and portray their biology and activity in promoting tumor evolution.
CD44 is a non-kinase transmembrane glycoprotein expressed in various cancer types (11). CD44 extracellular domain contains binding sites for various ECM proteins such as collagen, laminin, and fibronectin (12, 13), while hyaluronic acid (HA) produced both by tumor cells and tumor stroma is the main and most specific CD44 ligand (14, 15) (Figure 1). CD44 functions are modulated by both glycosylation and alternative splicing (16–18). Unlike the standard CD44 (CD44s), variant CD44 isoforms (CD44v) contain exons with specific post-translational modifications allowing binding of tumor-promoting cytokines like osteopontin (OPN), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) (19–23). Upon HA binding, CD44 proteins change conformation, oligomerize, and redistribute in glycolipid-enriched domains (GEMs) at the cell membrane (24, 25). There, activated CD44 preferentially interacts with activated receptor tyrosine kinases (RTKs) (26), various adaptor proteins such as ankyrin or the ERM (ezrin, radixin, and meosin), ultimately leading to cytoskeletal changes (spectrin, F-actin) (27, 28), Src family kinases (SFK) members accumulation (29), and activation of downstream pathways, such as Rho-GTPases (30–33), PI3K/AKT, or Ras/MAPK (34, 35) (Figure 1). Since the seminal discovery of their role in metastasis (36), CD44s and CD44v have been implicated in various steps of tumor progression. In particular, HA-induced CD44 conformational changes and subsequent cytoskeletal modifications promote tumor cell migration, invasion, and epithelial-to-mesenchymal transition (EMT) (27, 28, 30, 37–45). In glioma cells, HA-CD44 interactions were shown to occur specifically at the leading edge of migrating cells upon regulation by activated protein kinase C (PKC) (46). Upon HA binding, various proteases cleave CD44 allowing dynamic cytoskeletal changes, filopodia formation and ultimately CD44-mediated migration (47–50). Recently, non-catalytic MMP-9–mediated activation of CD44 was shown to promote tumor cell amoeboid migration (51). Since mesenchymal migration is based on integrin—ECM interactions, it is tempting to hypothesize that CD44 may support migration plasticity and escape to integrin inhibition (52–54). Further along tumor progression, circulating tumor cells (CTC) need to extravasate at distant organs. CD44 expressed on CTC was shown to interact with the HA coat produced by endothelial cells and initiate the process of tumor cell extravasation (55), particularly to the bone marrow, as shown in various tumor models through in vitro studies (56, 57). Importantly, both Cathepsin K, a potent collagenase typically expressed by osteoclasts during osteolysis, and MMP-9 were reported to be induced upon HA-mediated CD44 activation in prostate and breast cancer cells, suggesting their role in the colonization of metastatic osteolytic prostate and/or breast cancer cells (58–60). CD44 alternative splicing was reported to promote lung colonization by metastatic cancer cells (61). Recent studies implicated HA-CD44 interaction in tumor cell resistance to chemotherapy, by inducing multi-drug resistance 1 gene (MDR1) expression (62), ABC drug transporters (63), ankyrin-induced drug fluxes (62), and tumor cell survival pathways like ErbB2 signaling and PI3K/AKT pathway (64). Alternatively, HA-CD44 interactions may provide chemo-resistance through decreased apoptosis/cell death pathways by inducing anti-apoptotic proteins like inhibitors of the apoptosis family members (IAPs) (65–68), reducing pro-apoptotic proteins (69) or modulating autophagy (70).
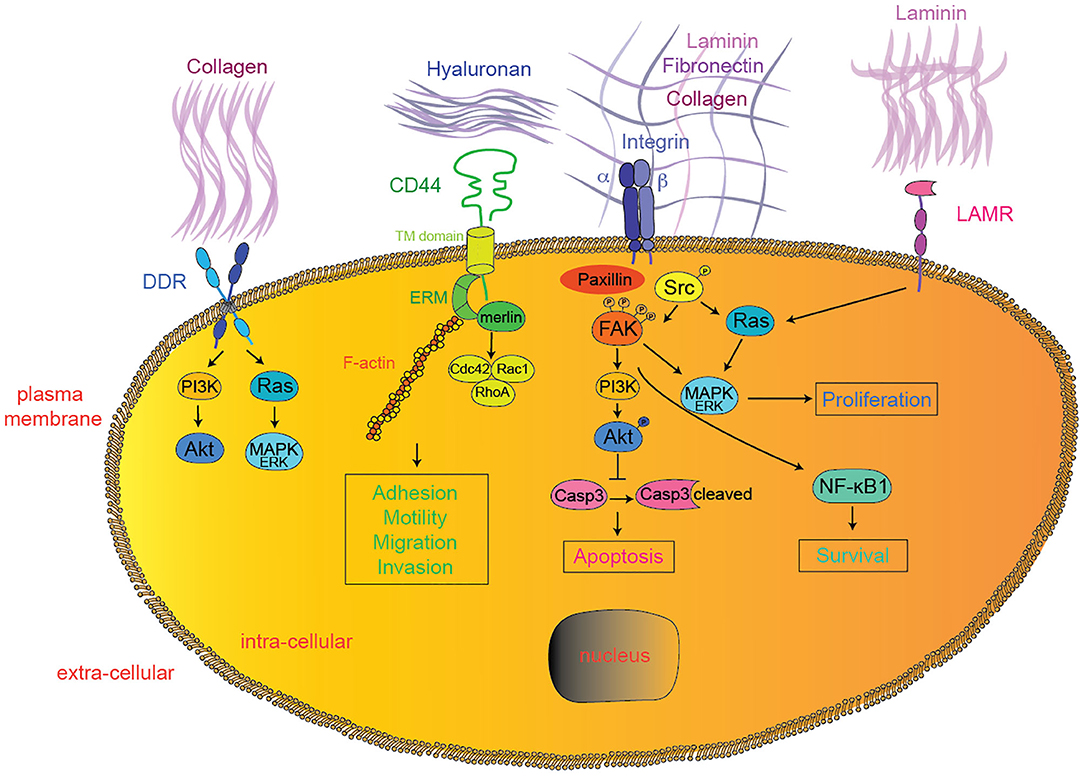
Figure 1. Extracellular matrix—tumor cell interactions. In addition to integrins, DDR, CD44, LAMRs, FAK, and SFK represent emerging therapeutic targets currently tested in clinical trials for solid tumors. Downstream effectors interactions were simplified for clarity reasons. DDR, discoidin domain receptor; LAMR, 36/67 kDa laminin receptors; FAK, focal adhesion kinase; PI3K, phosphoinositide-3-kinase; MAPK, mitogen-activated protein kinases; Casp3, caspase 3; NF-κB1, nuclear factor-kappa B1.
Altogether, CD44 is involved at multiple steps of tumor progression and its inhibition appears as a promising alternative for tumor-ECM targeting therapies. Low molecular mass HA, soluble CD44, CD44 blocking antibodies, CD44 blocking peptides/aptamers, CD44-targeting sh/siRNA or silibinin (a plant-derived inhibitor of CD44 expression) have all been used successfully to interfere with CD44 function in preclinical models of solid tumor progression (Table 1). The CD44-blocking antibody RO5429083 was tested in a phase I, dose-escalation clinical study in metastatic or locally advanced, CD44-positive malignant solid tumors (NCT01358903) as well as in a phase I clinical study, alone or in combination with cytarabine, for acute myelogenous leukemia (NCT01641250). Alternatively, CD44 targeting may serve to specifically deliver cytotoxic drugs or radioisotopes to tumor cells. Bivatuzumab-mertansine, a CD44v6-specific targeting antibody linked to the cytotoxic drug mertansine, was tested in phase I dose-escalation clinical studies for CD44v6-positive recurrent or metastatic breast cancers (NCT02254031, NCT02254005) and advanced squamous cell carcinoma of the head and neck (NCT02254044, NCT02254018). The 186Re-labeled bivatuzumab was tested in phase I biodistribution studies for non-small cell lung cancers (NCT02204059) and adenocarcinoma of the breast (NCT02204046). Although preliminary, these results encourage further clinical assessment of CD44-targeting therapies, either alone or in combination.
DDR1 and DDR2 belong to the family of the transmembrane receptor tyrosine kinase (RTK) with an extracellular discoidin domain binding to collagen in its native triple-helical conformation (227, 228) (Figure 1). DDR1 and DDR2 bind to various collagen isoforms with different affinities. DDR1 typically binds to collagens I-VI and VIII, while DDR2 preferentially binds to collagens I-III and X (228–231). Upon collagen binding, DDRs cluster and get activated through auto-phosphorylation at multiple tyrosine residues within the cytosolic part of the protein (232, 233), leading to the recruitment of adaptor or signaling proteins like ShcA, SHP-2, SFKs, the proline-rich tyrosine kinase 2 (Pyk2), and the non-muscle myosin heavy chain (NMHC) IIA (234, 235). In cancer cells, DDR activation was reported to induce Ras/MAPK (236), PI3K/AKT (236), Notch (237), NF-κB (238), PKCα/JAK/Stat (239), and p130CS/JNK pathways (234), thereby participating in various steps of tumor progression (Figure 1). Both DDR1 and DDR2 were shown to promote tumor cell proliferation, survival (236, 238, 240, 241), and migration (242–245). Interestingly, EMT was reported to rely on the switch from DDR1 (epithelial) to DDR2 (mesenchymal) expression (246), although various reports implicate both DDR1 and DDR2 in EMT-mediated tumor cell invasion (234, 247). More recently, DDRs were implicated in the late stages of metastatic tumor progression (244, 248). Typically, DDR1 drives site-specific metastasis of lung cancer cells to bone (248). Additionally, the collagen-dependent interaction between Transmembrane 4 L6 Family Member 1 (TM4SF1) and DDR1 regulates dormancy vs. growth at the metastatic site (239). Finally, both DDR1 and DDR2 promote resistance to radio- and chemo-therapy in various preclinical models (94, 236–238, 249). However, despite these converging evidences implicating DDRs in tumor progression, one should consider that DDR-mediated effects are highly versatile and cell-dependent. For example, DDR1 was shown to either support or prevent integrin α2β1-mediated cell migration in different experimental models (234, 250, 251). Moreover, the dynamic regulation of DDR expression during tumor progression will determine the consequences of DDR inhibition (231). Thus, the complex regulation of DDR activity in tumor cells may stand for the controversy concerning their contribution to cancer progression (243, 248, 252–254) and affect the potential efficacy of DDR targeting in cancer. Still, the recent identification of activating mutations in the cytoplasmic signaling portions of DDR affecting intracellular signaling (240, 255–257) opens new perspectives in the identification of patients who might benefit the most from DDR inhibition.
DDR1 and DDR2 kinases are efficiently inhibited by multikinase inhibitors like ponatinib, imatinib, dasatinib, and nilotinib (258). Dasatinib, nilotinib, a DDR1 blocking antibody, the selective DDR1 inhibitors 7rh and DDR1-IN-1 and the selective allosteric DDR2 inhibitor WRG-28 were shown to efficiently prevent DDR-mediated tumor progression in preclinical models (Table 1). Driven by these encouraging results, dasatinib was tested in a phase II clinical trial for patients with advanced non-small cell lung cancers harboring a DDR2 mutation (NCT01514864). Unfortunately, it was abandoned because of lack of efficacy and slow enrollment. Currently, nilotinib is being assessed in a phase II clinical trial for malignant locally advanced or metastatic solid neoplasms presenting DDR1 or DDR2 mutations (NCT02029001). Importantly, non-canonical activation of DDR1 was shown to promote metastasis through tyrosine kinase-independent signaling in preclinical models (239), warranting cautious assessment of RTK inhibitors to target DDR. Further efforts should aim at the development of specific DDR1 and DDR2 inhibitors targeting canonical and non-canonical activation routes, the identification of the patients who may benefit the most from DDR inhibition and their use in combination therapies.
The 67 kDa (LAMR67) laminin receptor was first identified as a receptor for laminin 1 (259–261) (Figure 1). It is currently hypothesized that LAMR67 arises from post-translational modifications of the precursor 37 kDa laminin receptor (LAMR37), although the precise mechanisms (like sumoylation) are still to be resolved (262–264). LAMRs harbor multiple cellular localizations, as assessed by the wide range of cellular processes they are implicated in: ribosomal biogenesis (265), protein translation (266–268), pre-rRNA processing (269), cellular adhesion and migration (267, 270), invasion (271), cellular proliferation (272, 273), cytoskeletal modulation (267, 274), and chromatin and histone modifications (275). Both LAMR37 and LAMR67 were identified at the cell membrane where they potentially bind to laminins, associate with integrins (276, 277) and get phosphorylated (278, 279). Although the downstream signaling mechanisms are still unelucidated, various authors reported modifications of Ras/MAPK and JNK/p38 signaling upon laminin-binding to LAMRs (280), possibly through interactions with FAK and paxillin (267, 281) (Figure 1). Given their various implications in cellular regulation, it is not surprising to find elevated LAMR expression in various cancers (282–288) and their involvement in tumor cell growth, migration, invasion, and aggressiveness (266, 282, 289). Importantly, laminin 1—LAMR interaction was shown to be implicated in tumor cell adhesion (271, 290) and invasion (291, 292) and LAMR down-regulation was shown to promote tumor cell apoptosis (293–296). Whether this is mediated by laminin 1-dependent activation of LAMR remains unknown. Recent data suggest that LAMR interaction with FAK may depend on laminin 1—LAMR interaction and promote Ras/MAPK and/or PI3K/AKT-mediated survival (297, 298). However, LAMR was found to promote tumor progression through various laminin 1-independent manners, such as regulation of telomerases (299), reviewed in (300).
Despite various emerging strategies aimed to target LAMR (300), in vivo preclinical studies assessing the feasibility and efficiency of targeting LAMR are still scant. Both a LAMR37 blocking antibody and a small molecule inhibitor preventing laminin-LAMR interaction were shown to impede metastatic progression (Table 1). The green tea-derived epigallocatechin-3-gallate (EGCG) is a small molecule affecting a large number of cellular targets, including LAMR67 (301) and LAMR37 (302). EGCG is currently assessed in a phase I study for chemopreventive effect in patients with curative-intent resections of colorectal cancer (NCT02891538). Interestingly, the immunogenic LAMR tumor-associated antigen, referred as oncofoetal antigen immature laminin receptor protein (OFA-iLRP), has been successfully used as a tumor antigen for vaccine-based therapies in preclinical studies (Table 1). Cellular immunotherapy using autologous dendritic cell loaded with OFA-iLRP was tested in a phase I-II clinical study for metastatic breast cancers (NCT00879489). Altogether, LAMR targeting appears promising for cancer therapy, although major efforts should aim at the development of specific inhibitors and acquisition of stronger preclinical data prior to further clinical trial.
Focal adhesion kinase (FAK) is a cytoplasmic non-receptor protein tyrosine kinase. It is an important cell signaling hub highly phosphorylated upon integrin activation, and has long been recognized as promoting cancer cell migration, proliferation, and survival/chemoresistance through downstream activation of Rho-GEF, talin, cortactin, SFKs, PI3K/AKT, Ras/MAPK, or NF-κB pathways (303, 304) (Figure 1). More recent studies have described that besides its classical localization at the plasma membrane of tumor cells, FAK can also translocate to the nucleus and act as a transcription factor driving the expression of cytokines and chemokines favoring tumor immune evasion, independently of integrin signaling (305). In pancreatic cancer, FAK inhibition increases the immune infiltrate within the tumor environment, thereby sensitizing tumors to immune-checkpoint blockade (306). In addition, FAK inhibition also affect stromal cells. By targeting carcinoma-associated endothelial cells, FAK inhibition enhances vascular permeability, drug delivery, and overcomes chemo-resistance to DNA-damaging agents (307). Altogether, these data largely support the potential for therapeutic benefits of FAK inhibitors, used alone or in combination therapies, in the arsenal of anti-cancer strategies, illustrated by their success in various preclinical models (Table 1). FAK inhibition mostly relies on small molecule inhibitors working through various mechanisms: ATP competitive kinase inhibition (TAE-226, VS-4718, VS-6062, VS-6063, GSK-2256098, PF-573228), FAK scaffold inhibition (compounds 14, Y11, Y15, C4, INT2-31, M13, R2), or more recently ATP competitive non-kinase inhibition (BI853520) (Table 1). In combination, FAK inhibition was reported to improve the efficacy of chemotherapeutic agents (docetaxel, paclitaxel, temzolomide, 5-FU, gemcitabine, doxorubicin), targeted therapies (EGFR inhibitor, Src inhibitor, sunitinib, BRAF inhibitor, CXCR4 inhibitor, HDAC inhibitor), or immunotherapy (PD1 antagonists, T cell immunotherapy) (Table 1). Acceptable safety profiles were obtained in phase I clinical trials for VS-6062 (104, 308), GSK-2256098 (309–311), VS-6063 (312, 313), VS-4718 and BI853520 (314–316), with VS-6062, GSK-2256098, and VS-6063 showing stabilization of disease in patients with various advanced solid tumors. Both GSK-2256098, in combination with trametinib, and VS-6063, however, failed to show efficacy in phase II clinical trials for pancreatic adenocarcinoma and malignant mesothelioma, respectively [NCT02428270, (317)]. This unexpected failure may have been prevented by the stratification of the patients based on FAK amplification/activity in order to select for the best responders. VS-6063 is currently tested in multiple clinical trials: (i) a phase II clinical trial in a pre-operative setting for malignant mesothelioma (NCT02004028); (ii) a phase II clinical trial in association with the PD-1 inhibitor pembrolizumab for advanced solid tumors (NCT02758587, NCT03727880); (iii) a phase I clinical trial in association with the RAF/MEK inhibitor RO5126766 for advanced solid tumors (NCT03875820); (iv) a phase I clinical trial in association with the anti-PDL1 antibody avelumab for epithelial ovarian cancer (NCT02943317); (v) a phase I clinical trial in association with pembrolizumab and gemcitabine for advanced solid tumors (NCT02546531). The results of these ongoing clinical trials will be decisive to shape the future development of FAK inhibitors in clinical practice.
The SFK, composed of c-Src, Fyn, Yes, Lck, Lyn, Hck, Fgr, and Blk, are cytoplasmic non-receptor protein tyrosine kinases. Their prominent functions are mediated by their SH2 and SH3 domains interacting with various RTKs (such as EGF-R, HER2, IGF-R, HGF-R, and PDGF-R), thereby participating in integration and regulation of RTK signaling. But SFK also participate in ECM-mediated signaling. Through phosphorylation of FAK, SFK activation stabilizes focal adhesion complexes enhancing cell adhesion to the ECM (318) (Figure 1). Altogether, SFK are implicated in many steps of tumorigenesis, including proliferation, migration, invasion, survival in the circulation and at distant metastatic sites (319–324), achieved through modulation of various downstream effectors as PI3K/AKT, Ras/MAPK, or Stat3 (325, 326). Additionally, SFK activation confers therapeutic resistance to targeted RTK therapies (e.g., Trastuzumab/Herceptin for HER2), to hormone-receptor endocrine therapies (e.g., Tamoxifen for Estrogen Receptor), as well as to traditional chemo- and radiotherapies (327). Given their central role in tumor cell signaling and pleiotropic functions in cancer, SFK represent a promising target for anti-cancer therapies. SFK are currently most efficiently targeted using non-specific ATP-competitive multikinase inhibitors, such as dasatinib, bosutinib, saracatinib, ponatinib, and vandetanib, targeting many different tyrosine kinases (such as BCR-ABL, Kit, PDGFR, EGFR, RET, VEGFR) in addition to SFK members (328). With the exception of vandetanib, approved for the treatment of thyroid medullary carcinoma, dasatinib, ponatinib, and bosetanib have been approved by the FDA for hematological malignancies only, based on their BCR/Abl inhibitory capacity (328). In vivo preclinical data, however, suggest their potential efficacy in solid tumors as well, alone or in combination, although not necessarily through SFK inhibition (Table 1). Up to date, the results of phase II clinical trials with SKF inhibitors in monotherapy have been disappointing, as they showed only modest or no efficacy (326, 329). Such failure may be largely attributed to the current lack of biomarkers for the identification patients with aberrant SFK, the lack of specificity of SFK inhibitors, and the sometimes opposing effects of SFK members at various steps of tumor progression (330, 331). The interpretation of the numerous ongoing clinical trials (http://www.clinicaltrials.gov/) as well as the design of future successful clinical trials testing SFK inhibitors for solid tumors will largely depend on our capacity to overcome these important issues.
Despite huge expectations based on preclinical studies, integrin inhibitors failed to deliver anticipated results and have not entered the clinical practice yet. Understanding and surmounting the pitfalls of integrin inhibition will be crucial to further sustain the targeting of tumor cell–ECM interactions as an anticancer strategy. Yet, other long-time discovered molecules at the interface between tumor cell and ECM as CD44, DDR, LAMR, FAK, and SFK, are emerging as alternative therapeutic targets in clinical trials. Alike integrin inhibitors, their therapeutic relevance will depend on the specificity and pharmacokinetic/dynamic properties of the inhibitors developed, on the adequacy of the preclinical models used for validation, on the biological process targeted, on the biomarkers used for the identification of best responders and on the combination strategies applied in clinical trials. Importantly, our growing knowledge of the biology of ECM—tumor cell interactions will be instrumental in overcoming these important pitfalls and extend the arsenal of clinically valuable inhibitors targeting the ECM—tumor cells crosstalk in the near future.
GL wrote the review and edited the manuscript. CR edited the manuscript. FK planned the outline, wrote the review, and edited the manuscript. All authors read and approved the submitted version of the manuscript.
Work in our laboratories was supported by the Swiss National Science Foundation grants PZ00P3_185926 (to FK) and 31003A_179248 (to CR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. (2012) 196:395–406. doi: 10.1083/jcb.201102147
2. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. (2009) 326:1216–9. doi: 10.1126/science.1176009
3. Hynes RO. Stretching the boundaries of extracellular matrix research. Nat Rev Mol Cell Biol. (2014) 15:761–3. doi: 10.1038/nrm3908
4. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. (2014) 15:786–801. doi: 10.1038/nrm3904
5. Ringer P, Colo G, Fassler R, Grashoff C. Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biol. (2017) 64:6–16. doi: 10.1016/j.matbio.2017.03.004
6. Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. (2019) 35:347–67. doi: 10.1016/j.ccell.2019.01.007
7. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. (2018) 18:533–48. doi: 10.1038/s41568-018-0038-z
8. Alday-Parejo B, Stupp R, Ruegg C. Are integrins still practicable targets for anti-cancer therapy? Cancers. (2019) 11:978. doi: 10.3390/cancers11070978
9. Kapp TG, Rechenmacher F, Sobahi TR, Kessler H. Integrin modulators: a patent review. Expert Opin Ther Pat. (2013) 23:1273–95. doi: 10.1517/13543776.2013.818133
10. Vicente-Manzanares M, Sanchez-Madrid F. Targeting the integrin interactome in human disease. Curr Opin Cell Biol. (2018) 55:17–23. doi: 10.1016/j.ceb.2018.05.010
11. Yin T, Wang G, He S, Liu Q, Sun J, Wang Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell Immunol. (2016) 300:41–5. doi: 10.1016/j.cellimm.2015.11.009
12. Ishii S, Ford R, Thomas P, Nachman A, Steele G Jr, Jessup JM. CD44 participates in the adhesion of human colorectal carcinoma cells to laminin and type IV collagen. Surg Oncol. (1993) 2:255–64. doi: 10.1016/0960-7404(93)90015-Q
13. Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. (1992) 116:817–25. doi: 10.1083/jcb.116.3.817
14. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. (1990) 61:1303–13. doi: 10.1016/0092-8674(90)90694-A
15. Banerji S, Wright AJ, Noble M, Mahoney DJ, Campbell ID, Day AJ, et al. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol. (2007) 14:234–9. doi: 10.1038/nsmb1201
16. Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. (1989) 56:1057–62. doi: 10.1016/0092-8674(89)90638-7
17. Goldstein LA, Zhou DF, Picker LJ, Minty CN, Bargatze RF, Ding JF, et al. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. (1989) 56:1063–72. doi: 10.1016/0092-8674(89)90639-9
18. Idzerda RL, Carter WG, Nottenburg C, Wayner EA, Gallatin WM, St John T. Isolation and DNA sequence of a cDNA clone encoding a lymphocyte adhesion receptor for high endothelium. Proc Natl Acad Sci USA. (1989) 86:4659–63. doi: 10.1073/pnas.86.12.4659
19. Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B, et al. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. (1995) 128:687–98. doi: 10.1083/jcb.128.4.687
20. Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. (2009) 114:5236–44. doi: 10.1182/blood-2009-04-219204
21. Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. (2014) 14:342–56. doi: 10.1016/j.stem.2014.01.009
22. Megaptche AP, Erb U, Buchler MW, Zoller M. CD44v10, osteopontin and lymphoma growth retardation by a CD44v10-specific antibody. Immunol Cell Biol. (2014) 92:709–20. doi: 10.1038/icb.2014.47
23. Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. (1996) 271:509–12. doi: 10.1126/science.271.5248.509
24. Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. (1993) 54:271–335. doi: 10.1016/S0065-2776(08)60537-4
25. Liu D, Sy MS. Phorbol myristate acetate stimulates the dimerization of CD44 involving a cysteine in the transmembrane domain. J Immunol. (1997) 159:2702–11.
26. Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. (2006) 281:34936–41. doi: 10.1074/jbc.C600138200
27. Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. (2010) 11:276–87. doi: 10.1038/nrm2866
28. Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. (1994) 126:1099–109. doi: 10.1083/jcb.126.4.1099
29. Foger N, Marhaba R, Zoller M. Involvement of CD44 in cytoskeleton rearrangement and raft reorganization in T cells. J Cell Sci. (2001) 114:1169–78.
30. Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. (2001) 276:48679–92. doi: 10.1074/jbc.M106759200
31. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. (2003) 4:33–45. doi: 10.1038/nrm1004
32. Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. (2008) 18:251–9. doi: 10.1016/j.semcancer.2008.03.007
33. Bourguignon LY, Singleton PA, Zhu H, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem. (2003) 278:29420–34. doi: 10.1074/jbc.M301885200
34. Orian-Rousseau V, Morrison H, Matzke A, Kastilan T, Pace G, Herrlich P, et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. (2007) 18:76–83. doi: 10.1091/mbc.e06-08-0674
35. Weber GF. Molecular mechanisms of metastasis. Cancer Lett. (2008) 270:181–90. doi: 10.1016/j.canlet.2008.04.030
36. Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. (1991) 65:13–24. doi: 10.1016/0092-8674(91)90403-L
37. Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. (2002) 277:39703–12. doi: 10.1074/jbc.M204320200
38. Bourguignon LY, Wong G, Earle CA, Xia W. Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFkappaB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton. (2011) 68:671–93. doi: 10.1002/cm.20544
39. Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem. (2010) 285:36721–35. doi: 10.1074/jbc.M110.162305
40. Zhao S, Chen C, Chang K, Karnad A, Jagirdar J, Kumar AP, et al. CD44 expression level and isoform contributes to pancreatic cancer cell plasticity, invasiveness, and response to therapy. Clin Cancer Res. (2016) 22:5592–604. doi: 10.1158/1078-0432.CCR-15-3115
41. Bellerby R, Smith C, Kyme S, Gee J, Gunthert U, Green A, et al. Overexpression of specific CD44 isoforms is associated with aggressive cell features in acquired endocrine resistance. Front Oncol. (2016) 6:145. doi: 10.3389/fonc.2016.00145
42. Cho SH, Park YS, Kim HJ, Kim CH, Lim SW, Huh JW, et al. CD44 enhances the epithelial-mesenchymal transition in association with colon cancer invasion. Int J Oncol. (2012) 41:211–8. doi: 10.3892/ijo.2012.1453
43. Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. (2011) 121:1064–74. doi: 10.1172/JCI44540
44. Ni J, Cozzi PJ, Hao JL, Beretov J, Chang L, Duan W, et al. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate. (2014) 74:602–17. doi: 10.1002/pros.22775
45. Thomas L, Byers HR, Vink J, Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. (1992) 118:971–7. doi: 10.1083/jcb.118.4.971
46. Lamontagne CA, Grandbois M. PKC-induced stiffening of hyaluronan/CD44 linkage; local force measurements on glioma cells. Exp Cell Res. (2008) 314:227–36. doi: 10.1016/j.yexcr.2007.07.013
47. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. (2004) 95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x
48. Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, et al. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. (2004) 165:893–902. doi: 10.1083/jcb.200310024
49. Nakamura H, Suenaga N, Taniwaki K, Matsuki H, Yonezawa K, Fujii M, et al. Constitutive and induced CD44 shedding by ADAM-like proteases and membrane-type 1 matrix metalloproteinase. Cancer Res. (2004) 64:876–82. doi: 10.1158/0008-5472.CAN-03-3502
50. Sugahara KN, Hirata T, Tanaka T, Ogino S, Takeda M, Terasawa H, et al. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. (2008) 68:7191–9. doi: 10.1158/0008-5472.CAN-07-6198
51. Orgaz JL, Pandya P, Dalmeida R, Karagiannis P, Sanchez-Laorden B, Viros A, et al. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat Commun. (2014) 5:4255. doi: 10.1038/ncomms5255
52. Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. (2011) 147:992–1009. doi: 10.1016/j.cell.2011.11.016
53. Te Boekhorst V, Preziosi L, Friedl P. Plasticity of cell migration in vivo and in silico. Annu Rev Cell Dev Biol. (2016) 32:491–526. doi: 10.1146/annurev-cellbio-111315-125201
54. Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. (2010) 339:83–92. doi: 10.1007/s00441-009-0892-9
55. Siegelman MH, Stanescu D, Estess P. The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion. J Clin Invest. (2000) 105:683–91. doi: 10.1172/JCI8692
56. Okado T, Hawley RG. Adhesion molecules involved in the binding of murine myeloma cells to bone marrow stromal elements. Int J Cancer. (1995) 63:823–30. doi: 10.1002/ijc.2910630613
57. Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. (2004) 64:5702–11. doi: 10.1158/0008-5472.CAN-04-0389
58. Lark MW, Stroup GB, James IE, Dodds RA, Hwang SM, Blake SM, et al. A potent small molecule, nonpeptide inhibitor of cathepsin K (SB 331750) prevents bone matrix resorption in the ovariectomized rat. Bone. (2002) 30:746–53. doi: 10.1016/S8756-3282(02)00675-0
59. Corey E, Brown LG, Quinn JE, Poot M, Roudier MP, Higano CS, et al. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res. (2003) 9:295–306.
60. Littlewood-Evans AJ, Bilbe G, Bowler WB, Farley D, Wlodarski B, Kokubo T, et al. The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res. (1997) 57:5386–90.
61. Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. (2012) 3:883. doi: 10.1038/ncomms1892
62. Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. (2008) 283:17635–51. doi: 10.1074/jbc.M800109200
63. Ricciardelli C, Ween MP, Lokman NA, Tan IA, Pyragius CE, Oehler MK. Chemotherapy-induced hyaluronan production: a novel chemoresistance mechanism in ovarian cancer. BMC Cancer. (2013) 13:476. doi: 10.1186/1471-2407-13-476
64. Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. (2003) 278:25285–8. doi: 10.1074/jbc.C300173200
65. Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. (2012) 31:149–60. doi: 10.1038/onc.2011.222
66. Chen L, Bourguignon LY. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer. (2014) 13:52. doi: 10.1186/1476-4598-13-52
67. Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. (2012) 287:32800–24. doi: 10.1074/jbc.M111.308528
68. Fedorchenko O, Stiefelhagen M, Peer-Zada AA, Barthel R, Mayer P, Eckei L, et al. CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood. (2013) 121:4126–36. doi: 10.1182/blood-2012-11-466250
69. Park YS, Huh JW, Lee JH, Kim HR. shRNA against CD44 inhibits cell proliferation, invasion and migration, and promotes apoptosis of colon carcinoma cells. Oncol Rep. (2012) 27:339–46. doi: 10.3892/ijo.2016.3801
70. Lv L, Liu HG, Dong SY, Yang F, Wang QX, Guo GL, et al. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumour Biol. (2016) 37:8811–24. doi: 10.1007/s13277-015-4755-6
71. Slomiany MG, Dai L, Tolliver LB, Grass GD, Zeng Y, Toole BP. Inhibition of functional hyaluronan-CD44 interactions in CD133-positive primary human ovarian carcinoma cells by small hyaluronan oligosaccharides. Clin Cancer Res. (2009) 15:7593–601. doi: 10.1158/1078-0432.CCR-09-2317
72. Ween MP, Hummitzsch K, Rodgers RJ, Oehler MK, Ricciardelli C. Versican induces a pro-metastatic ovarian cancer cell behavior which can be inhibited by small hyaluronan oligosaccharides. Clin Exp Metastasis. (2011) 28:113–25. doi: 10.1007/s10585-010-9363-7
73. Slomiany MG, Dai L, Bomar PA, Knackstedt TJ, Kranc DA, Tolliver L, et al. Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. (2009) 69:4992–8. doi: 10.1158/0008-5472.CAN-09-0143
74. Ahrens T, Sleeman JP, Schempp CM, Howells N, Hofmann M, Ponta H, et al. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. (2001) 20:3399–408. doi: 10.1038/sj.onc.1204435
75. Xu XM, Chen Y, Chen J, Yang S, Gao F, Underhill CB, et al. A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res. (2003) 63:5685–90.
76. Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. (1997) 186:1985–96. doi: 10.1084/jem.186.12.1985
77. Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. (2002) 277:38013–20. doi: 10.1074/jbc.M202404200
78. Li L, Hao X, Qin J, Tang W, He F, Smith A, et al. Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology. (2014) 146:1108–18. doi: 10.1053/j.gastro.2013.12.035
79. Weigand S, Herting F, Maisel D, Nopora A, Voss E, Schaab C, et al. Global quantitative phosphoproteome analysis of human tumor xenografts treated with a CD44 antagonist. Cancer Res. (2012) 72:4329–39. doi: 10.1158/0008-5472.CAN-12-0136
80. Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, et al. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. (1993) 177:443–55. doi: 10.1084/jem.177.2.443
81. Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. (2004) 64:4810–6. doi: 10.1158/0008-5472.CAN-04-0129
82. Misra S, Hascall VC, De Giovanni C, Markwald RR, Ghatak S. Delivery of CD44 shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ MICE. J Biol Chem. (2009) 284:12432–46. doi: 10.1074/jbc.M806772200
83. Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. (2013) 288:16085–97. doi: 10.1074/jbc.M112.445551
84. Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. (2010) 70:2455–64. doi: 10.1158/0008-5472.CAN-09-2505
85. Zheng J, Zhao S, Yu X, Huang S, Liu HY. Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics. (2017) 7:1373–88. doi: 10.7150/thno.17826
86. Nambiar D, Prajapati V, Agarwal R, Singh RP. In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer Lett. (2013) 334:109–17. doi: 10.1016/j.canlet.2012.09.004
87. Zhong X, Zhang W, Sun T. DDR1 promotes breast tumor growth by suppressing antitumor immunity. Oncol Rep. (2019) 42:2844–54. doi: 10.3892/or.2019.7338
88. Jin H, Ham IH, Oh HJ, Bae CA, Lee D, Kim YB, et al. Inhibition of discoidin domain receptor 1 prevents stroma-induced peritoneal metastasis in gastric carcinoma. Mol Cancer Res. (2018) 16:1590–600. doi: 10.1158/1541-7786.MCR-17-0710
89. Aguilera KY, Huang H, Du W, Hagopian MM, Wang Z, Hinz S, et al. Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma. Mol Cancer Ther. (2017) 16:2473–85. doi: 10.1158/1535-7163.MCT-16-0834
90. Grither WR, Longmore GD. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc Natl Acad Sci USA. (2018) 115:E7786–94. doi: 10.1073/pnas.1805020115
91. Ambrogio C, Gomez-Lopez G, Falcone M, Vidal A, Nadal E, Crosetto N, et al. Combined inhibition of DDR1 and notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. (2016) 22:270–7. doi: 10.1038/nm.4041
92. Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V, et al. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. (2018) 10:e7918. doi: 10.15252/emmm.201707918
93. Lu QP, Chen WD, Peng JR, Xu YD, Cai Q, Feng GK, et al. Antitumor activity of 7RH, a discoidin domain receptor 1 inhibitor, alone or in combination with dasatinib exhibits antitumor effects in nasopharyngeal carcinoma cells. Oncol Lett. (2016) 12:3598–608. doi: 10.3892/ol.2016.5088
94. Vehlow A, Klapproth E, Jin S, Hannen R, Hauswald M, Bartsch JW, et al. Interaction of discoidin domain receptor 1 with a 14-3-3-Beclin-1-akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. (2019) 26:3672–83.e7. doi: 10.1016/j.celrep.2019.02.096
95. Xu C, Buczkowski KA, Zhang Y, Asahina H, Beauchamp EM, Terai H, et al. NSCLC driven by DDR2 mutation is sensitive to dasatinib and JQ1 combination therapy. Mol Cancer Ther. (2015) 14:2382–9. doi: 10.1158/1535-7163.MCT-15-0077
96. Kim DG, Lee JY, Kwon NH, Fang P, Zhang Q, Wang J, et al. Chemical inhibition of prometastatic lysyl-tRNA synthetase-laminin receptor interaction. Nat Chem Biol. (2014) 10:29–34. doi: 10.1038/nchembio.1381
97. Narumi K, Inoue A, Tanaka M, Isemura M, Shimo-Oka T, Abe T, et al. Inhibition of experimental metastasis of human fibrosarcoma cells by anti-recombinant 37-kDa laminin binding protein antibody. Jpn J Cancer Res. (1999) 90:425–31. doi: 10.1111/j.1349-7006.1999.tb00765.x
98. McClintock SD, Warner RL, Ali S, Chekuri A, Dame MK, Attili D, et al. Monoclonal antibodies specific for oncofetal antigen–immature laminin receptor protein: effects on tumor growth and spread in two murine models. Cancer Biol Ther. (2015) 16:724–32. doi: 10.1080/15384047.2015.1026484
99. Barsoum AL, Liu B, Rohrer JW, Coggin JH Jr, Tucker JA, Pannell LK, et al. Production, safety and antitumor efficacy of recombinant oncofetal antigen/immature laminin receptor protein. Biomaterials. (2009) 30:3091–9. doi: 10.1016/j.biomaterials.2009.02.022
100. Rohrer JW, Barsoum AL, Coggin JH Jr. Identification of oncofetal antigen/immature laminin receptor protein epitopes that activate BALB/c mouse OFA/iLRP-specific effector and regulatory T cell clones. J Immunol. (2006). 176:2844–56. doi: 10.4049/jimmunol.176.5.2844
101. Hauck CR, Hsia DA, Puente XS, Cheresh DA, Schlaepfer DD. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. (2002) 21:6289–302. doi: 10.1093/emboj/cdf631
102. Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. (2006) 25:5969–84. doi: 10.1038/sj.onc.1209588
103. Liu TJ, LaFortune T, Honda T, Ohmori O, Hatakeyama S, Meyer T, et al. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther. (2007) 6:1357–67. doi: 10.1158/1535-7163.MCT-06-0476
104. Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. (2008) 68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155
105. Stokes JB, Adair SJ, Slack-Davis JK, Walters DM, Tilghman RW, Hershey ED, et al. Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther. (2011) 10:2135–45. doi: 10.1158/1535-7163.MCT-11-0261
106. Jeong K, Murphy JM, Rodriguez YAR, Kim JS, Ahn EE, Lim SS. FAK inhibition reduces metastasis of alpha4 integrin-expressing melanoma to lymph nodes by targeting lymphatic VCAM-1 expression. Biochem Biophys Res Commun. (2019) 509:1034–40. doi: 10.1016/j.bbrc.2019.01.050
107. Kuonen F, Surbeck I, Sarin KY, Dontenwill M, Ruegg C, Gilliet M, et al. TGFbeta, fibronectin and integrin alpha5beta1 promote invasion in basal cell carcinoma. J Invest Dermatol. (2018) 138:2432–42. doi: 10.1016/j.jid.2018.04.029
108. Tanjoni I, Walsh C, Uryu S, Tomar A, Nam JO, Mielgo A, et al. PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biol Ther. (2010) 9:764–77. doi: 10.4161/cbt.9.10.11434
109. Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. (2010) 9:778–90. doi: 10.4161/cbt.9.10.11433
110. Kang Y, Hu W, Ivan C, Dalton HJ, Miyake T, Pecot CV, et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. (2013) 105:1485–95. doi: 10.1093/jnci/djt210
111. Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. (2008) 51:7405–16. doi: 10.1021/jm800483v
112. Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. (2009) 8:2435–43. doi: 10.4161/cc.8.15.9145
113. Heffler M, Golubovskaya VM, Dunn KM, Cance W. Focal adhesion kinase autophosphorylation inhibition decreases colon cancer cell growth and enhances the efficacy of chemotherapy. Cancer Biol Ther. (2013) 14:761–72. doi: 10.4161/cbt.25185
114. Golubovskaya VM, Figel S, Ho BT, Johnson CP, Yemma M, Huang G, et al. A small molecule focal adhesion kinase (FAK) inhibitor, targeting Y397 site: 1-(2-hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo [3.3.1.1(3,7)]decane; bromide effectively inhibits FAK autophosphorylation activity and decreases cancer cell viability, clonogenicity and tumor growth in vivo. Carcinogenesis. (2012) 33:1004–13. doi: 10.1093/carcin/bgs120
115. Kurenova EV, Hunt DL, He D, Magis AT, Ostrov DA, Cance WG. Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. J Med Chem. (2009) 52:4716–24. doi: 10.1021/jm900159g
116. Kurenova E, Liao J, He DH, Hunt D, Yemma M, Bshara W, et al. The FAK scaffold inhibitor C4 disrupts FAK-VEGFR-3 signaling and inhibits pancreatic cancer growth. Oncotarget. (2013) 4:1632–46. doi: 10.18632/oncotarget.1365
117. Stewart JE, Ma X, Megison M, Nabers H, Cance WG, Kurenova EV, et al. Inhibition of FAK and VEGFR-3 binding decreases tumorigenicity in neuroblastoma. Mol Carcinog. (2015) 54:9–23. doi: 10.1002/mc.22070
118. Ucar DA, Kurenova E, Garrett TJ, Cance WG, Nyberg C, Cox A, et al. Disruption of the protein interaction between FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle. (2012) 11:3250–9. doi: 10.4161/cc.21611
119. Ucar DA, Magis AT, He DH, Lawrence NJ, Sebti SM, Kurenova E, et al. Inhibiting the interaction of cMET and IGF-1R with FAK effectively reduces growth of pancreatic cancer cells in vitro and in vivo. Anticancer Agents Med Chem. (2013) 13:595–602. doi: 10.2174/1871520611313040009
120. Golubovskaya VM, Palma NL, Zheng M, Ho B, Magis A, Ostrov D, et al. A small-molecule inhibitor, 5'-O-tritylthymidine, targets FAK and Mdm-2 interaction, and blocks breast and colon tumorigenesis in vivo. Anticancer Agents Med Chem. (2013) 13:532–45. doi: 10.2174/1871520611313040002
121. Golubovskaya VM, Ho B, Zheng M, Magis A, Ostrov D, Morrison C, et al. Disruption of focal adhesion kinase and p53 interaction with small molecule compound R2 reactivated p53 and blocked tumor growth. BMC Cancer. (2013) 13:342. doi: 10.1186/1471-2407-13-342
122. Tiede S, Meyer-Schaller N, Kalathur RKR, Ivanek R, Fagiani E, Schmassmann P, et al. The FAK inhibitor BI 853520 exerts anti-tumor effects in breast cancer. Oncogenesis. (2018) 7:73. doi: 10.1038/s41389-018-0083-1
123. Laszlo V, Valko Z, Ozsvar J, Kovacs I, Garay T, Hoda MA, et al. The FAK inhibitor BI 853520 inhibits spheroid formation and orthotopic tumor growth in malignant pleural mesothelioma. J Mol Med. (2019) 97:231–42. doi: 10.1007/s00109-018-1725-7
124. Moritake H, Saito Y, Sawa D, Sameshima N, Yamada A, Kinoshita M, et al. TAE226, a dual inhibitor of focal adhesion kinase and insulin-like growth factor-I receptor, is effective for Ewing sarcoma. Cancer Med. (2019) 8:7809–21. doi: 10.1002/cam4.2647
125. Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. (2007) 67:10976–83. doi: 10.1158/0008-5472.CAN-07-2667
126. Bagi CM, Christensen J, Cohen DP, Roberts WG, Wilkie D, Swanson T, et al. Sunitinib and PF-562,271 (FAK/Pyk2 inhibitor) effectively block growth and recovery of human hepatocellular carcinoma in a rat xenograft model. Cancer Biol Ther. (2009) 8:856–65. doi: 10.4161/cbt.8.9.8246
127. Chen G, Gao C, Gao X, Zhang DH, Kuan SF, Burns TF, et al. Wnt/beta-Catenin pathway activation mediates adaptive resistance to BRAF inhibition in colorectal cancer. Mol Cancer Ther. (2018) 17:806–13. doi: 10.1158/1535-7163.MCT-17-0561
128. Golubovskaya VM, Huang G, Ho B, Yemma M, Morrison CD, Lee J, et al. Pharmacologic blockade of FAK autophosphorylation decreases human glioblastoma tumor growth and synergizes with temozolomide. Mol Cancer Ther. (2013) 12:162–72. doi: 10.1158/1535-7163.MCT-12-0701
129. Dragoj M, Bankovic J, Sereti E, Stojanov SJ, Dimas K, Pesic M, et al. Anti-invasive effects of CXCR4 and FAK inhibitors in non-small cell lung carcinomas with mutually inactivated p53 and PTEN tumor suppressors. Invest New Drugs. (2017) 35:718–32. doi: 10.1007/s10637-017-0494-4
130. Dawson JC, Serrels B, Byron A, Muir MT, Makda A, Garcia-Munoz A, et al. A synergistic anti-cancer FAK and HDAC inhibitor combination discovered by a novel chemical-genetic high-content phenotypic screen. Mol Cancer Ther. (2019) 19:637–49. doi: 10.1101/590802
131. Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. (2016) 22:851–60. doi: 10.1038/nm.4123
132. Lin HM, Lee BY, Castillo L, Spielman C, Grogan J, Yeung NK, et al. Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate. (2018) 78:308–17. doi: 10.1002/pros.23476
133. Byeon Y, Lee JW, Choi WS, Won JE, Kim GH, Kim MG, et al. CD44-Targeting PLGA nanoparticles incorporating paclitaxel and FAK siRNA Overcome chemoresistance in epithelial ovarian cancer. Cancer Res. (2018) 78:6247–56. doi: 10.1158/0008-5472.CAN-17-3871
134. Bieerkehazhi S, Chen Z, Zhao Y, Yu Y, Zhang H, Vasudevan SA, et al. Novel Src/Abl tyrosine kinase inhibitor bosutinib suppresses neuroblastoma growth via inhibiting Src/Abl signaling. Oncotarget. (2017) 8:1469–80. doi: 10.18632/oncotarget.13643
135. Kim WG, Guigon CJ, Fozzatti L, Park JW, Lu C, Willingham MC, et al. SKI-606, an Src inhibitor, reduces tumor growth, invasion, and distant metastasis in a mouse model of thyroid cancer. Clin Cancer Res. (2012) 18:1281–90. doi: 10.1158/1078-0432.CCR-11-2892
136. Rabbani SA, Valentino ML, Arakelian A, Ali S, Boschelli F. SKI-606 (Bosutinib) blocks prostate cancer invasion, growth, and metastasis in vitro and in vivo through regulation of genes involved in cancer growth and skeletal metastasis. Mol Cancer Ther. (2010) 9:1147–57. doi: 10.1158/1535-7163.MCT-09-0962
137. Messersmith WA, Rajeshkumar NV, Tan AC, Wang XF, Diesl V, Choe SE, et al. Efficacy and pharmacodynamic effects of bosutinib (SKI-606), a Src/Abl inhibitor, in freshly generated human pancreas cancer xenografts. Mol Cancer Ther. (2009) 8:1484–93. doi: 10.1158/1535-7163.MCT-09-0075
138. Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. (2007) 67:1580–8. doi: 10.1158/0008-5472.CAN-06-2027
139. Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, et al. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. (2005) 65:5358–64. doi: 10.1158/0008-5472.CAN-04-2484
140. Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. (2008) 68:3323–33. doi: 10.1158/0008-5472.CAN-07-2997
141. Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. (2006) 168:962–72. doi: 10.2353/ajpath.2006.050570
142. Scott AJ, Song EK, Bagby S, Purkey A, McCarter M, Gajdos C, et al. Evaluation of the efficacy of dasatinib, a Src/Abl inhibitor, in colorectal cancer cell lines and explant mouse model. PLoS ONE. (2017) 12:e0187173. doi: 10.1371/journal.pone.0187173
143. Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, et al. Antitumor effects and biomarkers of activity of AZD0530, a src inhibitor, in pancreatic cancer. Clin Cancer Res. (2009) 15:4138–46. doi: 10.1158/1078-0432.CCR-08-3021
144. Yang JC, Ok JH, Busby JE, Borowsky AD, Kung HJ, Evans CP. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. (2009) 69:151–60. doi: 10.1158/0008-5472.CAN-08-0442
145. Lang L, Shay C, Xiong Y, Thakkar P, Chemmalakuzhy R, Wang X, et al. Combating head and neck cancer metastases by targeting Src using multifunctional nanoparticle-based saracatinib. J Hematol Oncol. (2018) 11:85. doi: 10.1186/s13045-018-0623-3
146. Xiong J, Wu JS, Mao SS, Yu XN, Huang XX. Effect of saracatinib on pulmonary metastases from hepatocellular carcinoma. Oncol Rep. (2016) 36:1483–90. doi: 10.3892/or.2016.4968
147. Yamaguchi H, Takanashi M, Yoshida N, Ito Y, Kamata R, Fukami K, et al. Saracatinib impairs the peritoneal dissemination of diffuse-type gastric carcinoma cells resistant to Met and fibroblast growth factor receptor inhibitors. Cancer Sci. (2014) 105:528–36. doi: 10.1111/cas.12387
148. Cavalloni G, Peraldo-Neia C, Sarotto I, Gammaitoni L, Migliardi G, Soster M, et al. Antitumor activity of Src inhibitor saracatinib (AZD-0530) in preclinical models of biliary tract carcinomas. Mol Cancer Ther. (2012) 11:1528–38. doi: 10.1158/1535-7163.MCT-11-1020
149. Dong M, Rice L, Lepler S, Pampo C, Siemann DW. Impact of the Src inhibitor saracatinib on the metastatic phenotype of a fibrosarcoma (KHT) tumor model. Anticancer Res. (2010) 30:4405–13.
150. Arcaroli JJ, Touban BM, Tan AC, Varella-Garcia M, Powell RW, Eckhardt SG, et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin Cancer Res. (2010) 16:4165–77. doi: 10.1158/1078-0432.CCR-10-0066
151. Bertotti A, Bracco C, Girolami F, Torti D, Gastaldi S, Galimi F, et al. Inhibition of Src impairs the growth of met-addicted gastric tumors. Clin Cancer Res. (2010) 16:3933–43. doi: 10.1158/1078-0432.CCR-10-0106
152. Serrels B, Serrels A, Mason SM, Baldeschi C, Ashton GH, Canel M, et al. A novel Src kinase inhibitor reduces tumour formation in a skin carcinogenesis model. Carcinogenesis. (2009) 30:249–57. doi: 10.1093/carcin/bgn278
153. Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. (2008) 27:6365–75. doi: 10.1038/onc.2008.250
154. Zhang J, Zhou Q, Gao G, Wang Y, Fang Z, Li G, et al. The effects of ponatinib, a multi-targeted tyrosine kinase inhibitor, against human U87 malignant glioblastoma cells. Onco Targets Ther. (2014) 7:2013–9. doi: 10.2147/OTT.S67556
155. Laramy JK, Kim M, Gupta SK, Parrish KE, Zhang S, Bakken KK, et al. Heterogeneous binding and central nervous system distribution of the multitargeted kinase inhibitor ponatinib restrict orthotopic efficacy in a patient-derived xenograft model of glioblastoma. J Pharmacol Exp Ther. (2017) 363:136–47. doi: 10.1124/jpet.117.243477
156. Whittle SB, Patel K, Zhang L, Woodfield SE, Du M, Smith V, et al. The novel kinase inhibitor ponatinib is an effective anti-angiogenic agent against neuroblastoma. Invest New Drugs. (2016) 34:685–92. doi: 10.1007/s10637-016-0387-y
157. Gozgit JM, Wong MJ, Moran L, Wardwell S, Mohemmad QK, Narasimhan NI, et al. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther. (2012) 11:690–9. doi: 10.1158/1535-7163.MCT-11-0450
158. Li SQ, Cheuk AT, Shern JF, Song YK, Hurd L, Liao H, et al. Targeting wild-type and mutationally activated FGFR4 in rhabdomyosarcoma with the inhibitor ponatinib (AP24534). PLoS ONE. (2013) 8:e76551. doi: 10.1371/journal.pone.0076551
159. Garner AP, Gozgit JM, Anjum R, Vodala S, Schrock A, Zhou T, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res. (2014) 20:5745–55. doi: 10.1158/1078-0432.CCR-14-1397
160. Li L, Yu J, Jiao S, Wang W, Zhang F, Sun S. Vandetanib (ZD6474) induces antiangiogenesis through mTOR-HIF-1 alpha-VEGF signaling axis in breast cancer cells. Onco Targets Ther. (2018) 11:8543–53. doi: 10.2147/OTT.S175578
161. Ferrari SM, Bocci G, Di Desidero T, Ruffilli I, Elia G, Ragusa F, et al. Vandetanib has antineoplastic activity in anaplastic thyroid cancer, in vitro and in vivo. Oncol Rep. (2018) 39:2306–14. doi: 10.3892/or.2018.6305
162. Wang X, Qiu Y, Yu Q, Li H, Chen X, Li M, et al. Enhanced glioma therapy by synergistic inhibition of autophagy and tyrosine kinase activity. Int J Pharm. (2018) 536:1–10. doi: 10.1016/j.ijpharm.2017.09.007
163. Cascone T, Xu L, Lin HY, Liu W, Tran HT, Liu Y, et al. The HGF/c-MET pathway is a driver and biomarker of VEGFR-inhibitor resistance and vascular remodeling in non-small cell lung cancer. Clin Cancer Res. (2017) 23:5489–501. doi: 10.1158/1078-0432.CCR-16-3216
164. Starenki D, Hong SK, Wu PK, Park JI. Vandetanib and cabozantinib potentiate mitochondria-targeted agents to suppress medullary thyroid carcinoma cells. Cancer Biol Ther. (2017) 18:473–83. doi: 10.1080/15384047.2017.1323594
165. De Andrade JP, Park JM, Gu VW, Woodfield GW, Kulak MV, Lorenzen AW, et al. EGFR is regulated by TFAP2C in luminal breast cancer and is a target for vandetanib. Mol Cancer Ther. (2016) 15:503–11. doi: 10.1158/1535-7163.MCT-15-0548-T
166. Hatem R, Labiod D, Chateau-Joubert S, de Plater L, El Botty R, Vacher S, et al. Vandetanib as a potential new treatment for estrogen receptor-negative breast cancers. Int J Cancer. (2016) 138:2510–21. doi: 10.1002/ijc.29974
167. Saito M, Ishigame T, Tsuta K, Kumamoto K, Imai T, Kohno T. A mouse model of KIF5B-RET fusion-dependent lung tumorigenesis. Carcinogenesis. (2014) 35:2452–6. doi: 10.1093/carcin/bgu158
168. Wunderlich A, Khoruzhyk M, Roth S, Ramaswamy A, Greene BH, Doll D, et al. Pretherapeutic drug evaluation by tumor xenografting in anaplastic thyroid cancer. J Surg Res. (2013) 185:676–83. doi: 10.1016/j.jss.2013.06.017
169. Takeda H, Takigawa N, Ohashi K, Minami D, Kataoka I, Ichihara E, et al. Vandetanib is effective in EGFR-mutant lung cancer cells with PTEN deficiency. Exp Cell Res. (2013) 319:417–23. doi: 10.1016/j.yexcr.2012.12.018
170. Inoue K, Torimura T, Nakamura T, Iwamoto H, Masuda H, Abe M, et al. Vandetanib, an inhibitor of VEGF receptor-2 and EGF receptor, suppresses tumor development and improves prognosis of liver cancer in mice. Clin Cancer Res. (2012) 18:3924–33. doi: 10.1158/1078-0432.CCR-11-2041
171. Guerin O, Etienne-Grimaldi MC, Monteverde M, Sudaka A, Brunstein MC, Formento P, et al. Contrasted effects of the multitarget TKi vandetanib on docetaxel-sensitive and docetaxel-resistant prostate cancer cell lines. Urol Oncol. (2013) 31:1567–75. doi: 10.1016/j.urolonc.2012.03.003
172. Klein JD, Christopoulos A, Ahn SM, Gooding WE, Grandis JR, Kim S. Antitumor effect of vandetanib through EGFR inhibition in head and neck squamous cell carcinoma. Head Neck. (2012) 34:1269–76. doi: 10.1002/hed.21917
173. Navis AC, Hamans BC, Claes A, Heerschap A, Jeuken JW, Wesseling P, et al. Effects of targeting the VEGF and PDGF pathways in diffuse orthotopic glioma models. J Pathol. (2011) 223:626–34. doi: 10.1002/path.2836
174. Gule MK, Chen Y, Sano D, Frederick MJ, Zhou G, Zhao M, et al. Targeted therapy of VEGFR2 and EGFR significantly inhibits growth of anaplastic thyroid cancer in an orthotopic murine model. Clin Cancer Res. (2011) 17:2281–91. doi: 10.1158/1078-0432.CCR-10-2762
175. Wachsberger PR, Lawrence YR, Liu Y, Daroczi B, Xu X, Dicker AP. Epidermal growth factor receptor expression modulates antitumor efficacy of vandetanib or cediranib combined with radiotherapy in human glioblastoma xenografts. Int J Radiat Oncol Biol Phys. (2012) 82:483–91. doi: 10.1016/j.ijrobp.2010.09.019
176. Sano D, Fooshee DR, Zhao M, Andrews GA, Frederick MJ, Galer C, et al. Targeted molecular therapy of head and neck squamous cell carcinoma with the tyrosine kinase inhibitor vandetanib in a mouse model. Head Neck. (2011) 33:349–58. doi: 10.1002/hed.21455
177. Ichihara E, Ohashi K, Takigawa N, Osawa M, Ogino A, Tanimoto M, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res. (2009) 69:5091–8. doi: 10.1158/0008-5472.CAN-08-4204
178. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. (2009) 15:3484–94. doi: 10.1158/1078-0432.CCR-08-2904
179. Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. (2002) 62:4645−55.
180. McCarty MF, Wey J, Stoeltzing O, Liu W, Fan F, Bucana C, et al. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther. (2004) 3:1041–8.
181. Conrad C, Ischenko I, Kohl G, Wiegand U, Guba M, Yezhelyev M, et al. Antiangiogenic and antitumor activity of a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor ZD6474 in a metastatic human pancreatic tumor model. Anticancer Drugs. (2007) 18:569–79. doi: 10.1097/CAD.0b013e3280147d13
182. Drevs J, Konerding MA, Wolloscheck T, Wedge SR, Ryan AJ, Ogilvie DJ, et al. The VEGF receptor tyrosine kinase inhibitor, ZD6474, inhibits angiogenesis and affects microvascular architecture within an orthotopically implanted renal cell carcinoma. Angiogenesis. (2004) 7:347–54. doi: 10.1007/s10456-005-1394-3
183. Wu W, Onn A, Isobe T, Itasaka S, Langley RR, Shitani T, et al. Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther. (2007) 6:471–83. doi: 10.1158/1535-7163.MCT-06-0416
184. Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. (2004) 10:784–93. doi: 10.1158/1078-0432.CCR-1100-03
185. Taguchi F, Koh Y, Koizumi F, Tamura T, Saijo N, Nishio K. Anticancer effects of ZD6474, a VEGF receptor tyrosine kinase inhibitor, in gefitinib (“Iressa”)-sensitive and resistant xenograft models. Cancer Sci. (2004) 95:984–9. doi: 10.1111/j.1349-7006.2004.tb03187.x
186. Dunn EF, Iida M, Myers RA, Campbell DA, Hintz KA, Armstrong EA, et al. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. (2011) 30:561–74. doi: 10.1038/onc.2010.430
187. Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. (2011) 17:483–93. doi: 10.1158/1078-0432.CCR-10-1670
188. Berndsen RH, Swier N, van Beijnum JR, Nowak-Sliwinska P. Colorectal cancer growth retardation through induction of apoptosis, using an optimized synergistic cocktail of axitinib, erlotinib, and dasatinib. Cancers. (2019) 11:1878. doi: 10.3390/cancers11121878
189. Seoane S, Montero JC, Ocana A, Pandiella A. Effect of multikinase inhibitors on caspase-independent cell death and DNA damage in HER2-overexpressing breast cancer cells. J Natl Cancer Inst. (2010) 102:1432–46. doi: 10.1093/jnci/djq315
190. Rao G, Kim IK, Conforti F, Liu J, Zhang YW, Giaccone G. Dasatinib sensitises KRAS-mutant cancer cells to mitogen-activated protein kinase kinase inhibitor via inhibition of TAZ activity. Eur J Cancer. (2018) 99:37–48. doi: 10.1016/j.ejca.2018.05.013
191. Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C, et al. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS ONE. (2013) 8:e56505. doi: 10.1371/journal.pone.0056505
192. Walker S, Wankell M, Ho V, White R, Deo N, Devine C, et al. Targeting mTOR and Src restricts hepatocellular carcinoma growth in a novel murine liver cancer model. PLoS ONE. (2019) 14:e0212860. doi: 10.1371/journal.pone.0212860
193. Tian J, Raffa FA, Dai M, Moamer A, Khadang B, Hachim IY, et al. Dasatinib sensitises triple negative breast cancer cells to chemotherapy by targeting breast cancer stem cells. Br J Cancer. (2018) 119:1495–507. doi: 10.1038/s41416-018-0287-3
194. Xiao J, Xu M, Hou T, Huang Y, Yang C, Li J. Dasatinib enhances antitumor activity of paclitaxel in ovarian cancer through Src signaling. Mol Med Rep. (2015) 12:3249–56. doi: 10.3892/mmr.2015.3784
195. Levitt JM, Yamashita H, Jian W, Lerner SP, Sonpavde G. Dasatinib is preclinically active against Src-overexpressing human transitional cell carcinoma of the urothelium with activated Src signaling. Mol Cancer Ther. (2010) 9:1128–35. doi: 10.1158/1535-7163.MCT-10-0096
196. Perez M, Lucena-Cacace A, Marin-Gomez LM, Padillo-Ruiz J, Robles-Frias MJ, Saez C, et al. Dasatinib, a Src inhibitor, sensitizes liver metastatic colorectal carcinoma to oxaliplatin in tumors with high levels of phospho-Src. Oncotarget. (2016) 7:33111–24. doi: 10.18632/oncotarget.8880
197. Vallo S, Michaelis M, Gust KM, Black PC, Rothweiler F, Kvasnicka HM, et al. Dasatinib enhances tumor growth in gemcitabine-resistant orthotopic bladder cancer xenografts. BMC Res Notes. (2016) 9:454. doi: 10.1186/s13104-016-2256-3
198. Zeng F, Ju RJ, Liu L, Xie HJ, Mu LM, Lu WL. Efficacy in treating lung metastasis of invasive breast cancer with functional vincristine plus dasatinib liposomes. Pharmacology. (2018) 101:43–53. doi: 10.1159/000480737
199. Young AI, Law AM, Castillo L, Chong S, Cullen HD, Koehler M, et al. MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Res. (2016) 18:125. doi: 10.1186/s13058-016-0781-6
200. Teng Y, Cai Y, Pi W, Gao L, Shay C. Augmentation of the anticancer activity of CYT997 in human prostate cancer by inhibiting Src activity. J Hematol Oncol. (2017) 10:118. doi: 10.1186/s13045-017-0485-0
201. Balkhi HM, Haq E, Gul T, Sana S. Anti-glioma effects of caffeic acid phenethyl ester and dasatinib combination therapy in an in vivo rat glioma model. Anticancer Agents Med Chem. (2018) 18:1729–35. doi: 10.2174/1871520618666180515144835
202. Song N, Guo H, Ren J, Hao S, Wang X. Synergistic anti-tumor effects of dasatinib and dendritic cell vaccine on metastatic breast cancer in a mouse model. Oncol Lett. (2018) 15:6831–8. doi: 10.3892/ol.2018.8188
203. Yu GT, Mao L, Wu L, Deng WW, Bu LL, Liu JF, et al. Inhibition of SRC family kinases facilitates anti-CTLA4 immunotherapy in head and neck squamous cell carcinoma. Cell Mol Life Sci. (2018) 75:4223–34. doi: 10.1007/s00018-018-2863-3
204. Formisano L, D'Amato V, Servetto A, Brillante S, Raimondo L, Di Mauro C, et al. Src inhibitors act through different mechanisms in non-small cell lung cancer models depending on EGFR and RAS mutational status. Oncotarget. (2015) 6:26090–103. doi: 10.18632/oncotarget.4636
205. Fuse MA, Plati SK, Burns SS, Dinh CT, Bracho O, Yan D, et al. Combination therapy with c-met and Src inhibitors induces caspase-dependent apoptosis of merlin-deficient schwann cells and suppresses growth of schwannoma cells. Mol Cancer Ther. (2017) 16:2387–98. doi: 10.1158/1535-7163.MCT-17-0417
206. Lang L, Shay C, Zhao X, Xiong Y, Wang X, Teng Y. Simultaneously inactivating Src and AKT by saracatinib/capivasertib co-delivery nanoparticles to improve the efficacy of anti-Src therapy in head and neck squamous cell carcinoma. J Hematol Oncol. (2019) 12:132. doi: 10.1186/s13045-019-0827-1
207. Wang L, Yu X, Dong J, Meng Y, Yang Y, Wang H, et al. Combined SRC inhibitor saracatinib and anti-ErbB2 antibody H2-18 produces a synergistic antitumor effect on trastuzumab-resistant breast cancer. Biochem Biophys Res Commun. (2016) 479:563–70. doi: 10.1016/j.bbrc.2016.09.111
208. Han S, Meng Y, Tong Q, Li G, Zhang X, Chen Y, et al. The ErbB2-targeting antibody trastuzumab and the small-molecule SRC inhibitor saracatinib synergistically inhibit ErbB2-overexpressing gastric cancer. MAbs. (2014) 6:403–8. doi: 10.4161/mabs.27443
209. Chen Y, Guggisberg N, Jorda M, Gonzalez-Angulo A, Hennessy B, Mills GB, et al. Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin Cancer Res. (2009) 15:3396–405. doi: 10.1158/1078-0432.CCR-08-3127
210. Simpkins F, Hevia-Paez P, Sun J, Ullmer W, Gilbert CA, da Silva T, et al. Src inhibition with saracatinib reverses fulvestrant resistance in ER-positive ovarian cancer models in vitro and in vivo. Clin Cancer Res. (2012) 18:5911–23. doi: 10.1158/1078-0432.CCR-12-1257
211. Chen Y, Alvarez EA, Azzam D, Wander SA, Guggisberg N, Jorda M, et al. Combined Src and ER blockade impairs human breast cancer proliferation in vitro and in vivo. Breast Cancer Res Treat. (2011) 128:69–78. doi: 10.1007/s10549-010-1024-7
212. Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. (2013) 12:16–26. doi: 10.1158/1535-7163.MCT-12-0109
213. Liu J, Wu J, Zhou L, Pan C, Zhou Y, Du W, et al. ZD6474, a new treatment strategy for human osteosarcoma, and its potential synergistic effect with celecoxib. Oncotarget. (2015) 6:21341–52. doi: 10.18632/oncotarget.4179
214. Spanheimer PM, Park JM, Askeland RW, Kulak MV, Woodfield GW, De Andrade JP, et al. Inhibition of RET increases the efficacy of antiestrogen and is a novel treatment strategy for luminal breast cancer. Clin Cancer Res. (2014) 20:2115–25. doi: 10.1158/1078-0432.CCR-13-2221
215. Cesca M, Frapolli R, Berndt A, Scarlato V, Richter P, Kosmehl H, et al. The effects of vandetanib on paclitaxel tumor distribution and antitumor activity in a xenograft model of human ovarian carcinoma. Neoplasia. (2009) 11:1155–64. doi: 10.1593/neo.09866
216. Ciardiello F, Caputo R, Damiano V, Caputo R, Troiani T, Vitagliano D, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. (2003) 9:1546–56.
217. Li C, Yang C, Wei G. Vandetanib inhibits cisplatinresistant neuroblastoma tumor growth and invasion. Oncol Rep. (2018) 39:1757–64. doi: 10.3892/or.2018.6255
218. Troiani T, Lockerbie O, Morrow M, Ciardiello F, Eckhardt SG. Sequence-dependent inhibition of human colon cancer cell growth and of prosurvival pathways by oxaliplatin in combination with ZD6474 (Zactima), an inhibitor of VEGFR and EGFR tyrosine kinases. Mol Cancer Ther. (2006) 5:1883–94. doi: 10.1158/1535-7163.MCT-06-0055
219. Klinge CM. Inhibition of non-small-cell lung cancer growth by combined fulvestrant and vandetanib. Future Oncol. (2012) 8:529–33. doi: 10.2217/fon.12.42
220. Siegfried JM, Gubish CT, Rothstein ME, Henry C, Stabile LP. Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol. (2012) 7:485–95. doi: 10.1097/JTO.0b013e31824177ea
221. Jo MY, Kim YG, Kim Y, Lee SJ, Kim MH, Joo KM, et al. Combined therapy of temozolomide and ZD6474 (vandetanib) effectively reduces glioblastoma tumor volume through anti-angiogenic and anti-proliferative mechanisms. Mol Med Rep. (2012) 6:88–92. doi: 10.3892/mmr.2012.868
222. Hoang T, Huang S, Armstrong E, Eickhoff JC, Harari PM. Augmentation of radiation response with the vascular targeting agent ZD6126. Int J Radiat Oncol Biol Phys. (2006) 64:1458–65. doi: 10.1016/j.ijrobp.2005.11.017
223. Bianco C, Giovannetti E, Ciardiello F, Mey V, Nannizzi S, Tortora G, et al. Synergistic antitumor activity of ZD6474, an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, with gemcitabine and ionizing radiation against pancreatic cancer. Clin Cancer Res. (2006) 12:7099–107. doi: 10.1158/1078-0432.CCR-06-0833
224. Wachsberger P, Burd R, Ryan A, Daskalakis C, Dicker AP. Combination of vandetanib, radiotherapy, and irinotecan in the LoVo human colorectal cancer xenograft model. Int J Radiat Oncol Biol Phys. (2009) 75:854–61. doi: 10.1016/j.ijrobp.2009.06.016
225. Sano D, Matsumoto F, Valdecanas DR, Zhao M, Molkentine DP, Takahashi Y, et al. Vandetanib restores head and neck squamous cell carcinoma cells' sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res. (2011) 17:1815–27. doi: 10.1158/1078-0432.CCR-10-2120
226. Crescenzi M, Persano L, Esposito G, Zulato E, Borsi L, Balza E, et al. Vandetanib improves anti-tumor effects of L19mTNFalpha in xenograft models of esophageal cancer. Clin Cancer Res. (2011) 17:447–58. doi: 10.1158/1078-0432.CCR-10-1420
227. Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. (2011) 27:265–90. doi: 10.1146/annurev-cellbio-092910-154013
228. Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. (1997) 1:13–23. doi: 10.1016/S1097-2765(00)80003-9
229. Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. (1997) 1:25–34. doi: 10.1016/S1097-2765(00)80004-0
230. Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. (2006) 25:355–64. doi: 10.1016/j.matbio.2006.05.006
231. Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. (2012) 31:295–321. doi: 10.1007/s10555-012-9346-z
232. Juskaite V, Corcoran DS, Leitinger B. Collagen induces activation of DDR1 through lateral dimer association and phosphorylation between dimers. Elife. (2017) 6:e25716. doi: 10.7554/eLife.25716
233. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. (2010) 141:1117–34. doi: 10.1016/j.cell.2010.06.011
234. Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. (2008) 180:1277–89. doi: 10.1083/jcb.200708137
235. Dejmek J, Dib K, Jonsson M, Andersson T. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. (2003) 103:344–51. doi: 10.1002/ijc.10752
236. Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. (2003) 22:1289–301. doi: 10.1093/emboj/cdg129
237. Kim HG, Hwang SY, Aaronson SA, Mandinova A, Lee SW. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem. (2011) 286:17672–81. doi: 10.1074/jbc.M111.236612
238. Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-kappaB pathway activation. Cancer Res. (2006) 66:8123–30. doi: 10.1158/0008-5472.CAN-06-1215
239. Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. (2016) 166:47–62. doi: 10.1016/j.cell.2016.06.009
240. Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. (2011) 1:78–89. doi: 10.1158/2159-8274.CD-11-0005
241. Badaoui M, Mimsy-Julienne C, Saby C, Van Gulick L, Peretti M, Jeannesson P, et al. Collagen type 1 promotes survival of human breast cancer cells by overexpressing Kv10.1 potassium and Orai1 calcium channels through DDR1-dependent pathway. Oncotarget. (2018) 9:24653–71. doi: 10.18632/oncotarget.19065
242. Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neuro-Oncol. (2006) 76:239–48. doi: 10.1007/s11060-005-6874-1
243. Neuhaus B, Buhren S, Bock B, Alves F, Vogel WF, Kiefer F. Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell Mol Life Sci. (2011) 68:3757–70. doi: 10.1007/s00018-011-0676-8
244. Badiola I, Villace P, Basaldua I, Olaso E. Downregulation of discoidin domain receptor 2 in A375 human melanoma cells reduces its experimental liver metastasis ability. Oncol Rep. (2011) 26:971–8. doi: 10.3892/or.2011.1356
245. Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, et al. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. (2005) 11:520–8.
246. Maeyama M, Koga H, Selvendiran K, Yanagimoto C, Hanada S, Taniguchi E, et al. Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer. (2008) 113:2823–31. doi: 10.1002/cncr.23900
247. Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biol. (2011) 30:243–7. doi: 10.1016/j.matbio.2011.03.007
248. Valencia K, Ormazabal C, Zandueta C, Luis-Ravelo D, Anton I, Pajares MJ, et al. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. (2012) 18:969–80. doi: 10.1158/1078-0432.CCR-11-1686
249. Vehlow A, Cordes N. DDR1 (discoidin domain receptor tyrosine kinase 1) drives glioblastoma therapy resistance by modulating autophagy. Autophagy. (2019) 15:1487–8. doi: 10.1080/15548627.2019.1618540
250. Wang CZ, Su HW, Hsu YC, Shen MR, Tang MJ. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol Biol Cell. (2006) 17:2839–52. doi: 10.1091/mbc.e05-11-1068
251. Yeh YC, Wang CZ, Tang MJ. Discoidin domain receptor 1 activation suppresses alpha2beta1 integrin-dependent cell spreading through inhibition of Cdc42 activity. J Cell Physiol. (2009) 218:146–56. doi: 10.1002/jcp.21578
252. Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. (2007) 96:808–14. doi: 10.1038/sj.bjc.6603614
253. Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, et al. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. (2010) 24:311–9. doi: 10.3892/or_00000861
254. Barker KT, Martindale JE, Mitchell PJ, Kamalati T, Page MJ, Phippard DJ, et al. Expression patterns of the novel receptor-like tyrosine kinase, DDR, in human breast tumours. Oncogene. (1995) 10:569–75.
255. Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G, et al. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. (2005) 65:7591–5. doi: 10.1158/0008-5472.CAN-05-1855
256. Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. (2008) 455:1069–75. doi: 10.1038/nature07423
257. Jing H, Song J, Zheng J. Discoidin domain receptor 1: new star in cancer-targeted therapy and its complex role in breast carcinoma. Oncol Lett. (2018) 15:3403–8. doi: 10.3892/ol.2018.7795
258. Kothiwale S, Borza CM, Lowe EW Jr, Pozzi A, Meiler J. Discoidin domain receptor 1 (DDR1) kinase as target for structure-based drug discovery. Drug Discov Today. (2015) 20:255–61. doi: 10.1016/j.drudis.2014.09.025
259. Lesot H, Kuhl U, Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. (1983) 2:861–5. doi: 10.1002/j.1460-2075.1983.tb01514.x
260. Malinoff HL, Wicha MS. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. (1983) 96:1475–9. doi: 10.1083/jcb.96.5.1475
261. Rao NC, Barsky SH, Terranova VP, Liotta LA. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. (1983) 111:804–8. doi: 10.1016/0006-291X(83)91370-0
262. Rao CN, Castronovo V, Schmitt MC, Wewer UM, Claysmith AP, Liotta LA, et al. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. (1989) 28:7476–86. doi: 10.1021/bi00444a047
263. Ren J, Gao X, Jin C, Zhu M, Wang X, Shaw A, et al. Systematic study of protein sumoylation: Development of a site-specific predictor of SUMOsp 2.0. Proteomics. (2009) 9:3409–12. doi: 10.1002/pmic.200800646
264. DiGiacomo V, Meruelo D. Looking into laminin receptor: critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol Rev Camb Philos Soc. (2016) 91:288–310. doi: 10.1111/brv.12170
265. Ford CL, Randal-Whitis L, Ellis SR. Yeast proteins related to the p40/laminin receptor precursor are required for 20S ribosomal RNA processing and the maturation of 40S ribosomal subunits. Cancer Res. (1999) 59:704–10.
266. Scheiman J, Tseng JC, Zheng Y, Meruelo D. Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol Ther. (2010) 18:63–74. doi: 10.1038/mt.2009.199
267. Venticinque L, Jamieson KV, Meruelo D. Interactions between laminin receptor and the cytoskeleton during translation and cell motility. PLoS ONE. (2011) 6:e15895. doi: 10.1371/journal.pone.0015895
268. O'Donohue MF, Choesmel V, Faubladier M, Fichant G, Gleizes PE. Functional dichotomy of ribosomal proteins during the synthesis of mammalian 40S ribosomal subunits. J Cell Biol. (2010) 190:853–66. doi: 10.1083/jcb.201005117
269. Venticinque L, Meruelo D. Comprehensive proteomic analysis of nonintegrin laminin receptor interacting proteins. J Proteome Res. (2012) 11:4863–72. doi: 10.1021/pr300307h
270. Poon SL, Klausen C, Hammond GL, Leung PC. 37-kDa laminin receptor precursor mediates GnRH-II-induced MMP-2 expression and invasiveness in ovarian cancer cells. Mol Endocrinol. (2011). 25:327–38. doi: 10.1210/me.2010-0334
271. Omar A, Reusch U, Knackmuss S, Little M, Weiss SF. Anti-LRP/LR-specific antibody IgG1-iS18 significantly reduces adhesion and invasion of metastatic lung, cervix, colon and prostate cancer cells. J Mol Biol. (2012) 419:102–9. doi: 10.1016/j.jmb.2012.02.035
272. Satoh K, Narumi K, Abe T, Sakai T, Kikuchi T, Tanaka M, et al. Diminution of 37-kDa laminin binding protein expression reduces tumour formation of murine lung cancer cells. Br J Cancer. (1999) 80:1115–22. doi: 10.1038/sj.bjc.6690474
273. Scheiman J, Jamieson KV, Ziello J, Tseng JC, Meruelo D. Extraribosomal functions associated with the C terminus of the 37/67 kDa laminin receptor are required for maintaining cell viability. Cell Death Dis. (2010) 1:e42. doi: 10.1038/cddis.2010.19
274. Guirguis R, Margulies I, Taraboletti G, Schiffmann E, Liotta L. Cytokine-induced pseudopodial protrusion is coupled to tumour cell migration. Nature. (1987) 329:261–3. doi: 10.1038/329261a0
275. Kinoshita K, Kaneda Y, Sato M, Saeki Y, Wataya-Kaneda M, Hoffmann A. LBP-p40 binds DNA tightly through associations with histones H2A, H2B, and H4. Biochem Biophys Res Commun. (1998) 253:277–82. doi: 10.1006/bbrc.1998.9699
276. Ardini E, Tagliabue E, Magnifico A, Buto S, Castronovo V, Colnaghi MI, et al. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the alpha6beta4 integrin. J Biol Chem. (1997) 272:2342–5. doi: 10.1074/jbc.272.4.2342
277. Romanov V, Sobel ME, pinto da Silva P, Menard S, Castronovo V. Cell localization and redistribution of the 67 kD laminin receptor and alpha 6 beta 1 integrin subunits in response to laminin stimulation: an immunogold electron microscopy study. Cell Adhes Commun. (1994) 2:201–9. doi: 10.3109/15419069409004438
278. Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. (2005) 23:94–101. doi: 10.1038/nbt1046
279. Davis CM, Papadopoulos V, Jia MC, Yamada Y, Kleinman HK, Dym M. Identification and partial characterization of laminin binding proteins in immature rat sertoli cells. Exp Cell Res. (1991) 193:262–73. doi: 10.1016/0014-4827(91)90095-C
280. Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells: the role of the M(r) 67,000 laminin receptor. Cancer Res. (2004) 64:3572–9. doi: 10.1158/0008-5472.CAN-03-3424
281. Kim KJ, Chung JW, Kim KS. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J Biol Chem. (2005). 280:1360–8. doi: 10.1074/jbc.M410176200
282. Berno V, Porrini D, Castiglioni F, Campiglio M, Casalini P, Pupa SM, et al. The 67 kDa laminin receptor increases tumor aggressiveness by remodeling laminin-1. Endocr Relat Cancer. (2005) 12:393–406. doi: 10.1677/erc.1.00870
283. Carbone A, Gloghini A, Colombatti A, Castronovo V, Menard S. Expression of the monomeric 67-kd laminin-binding protein in human lymphomas as defined by MLuC5 monoclonal antibody and paraffin section immunohistochemistry. Hum Pathol. (1995) 26:541–6. doi: 10.1016/0046-8177(95)90251-1
284. Colnaghi MI. The simultaneous expression of c-erbB-2 oncoprotein and laminin receptor on primary breast tumors has a predicting potential analogous to that of the lymph node status. Adv Exp Med Biol. (1994) 353:149–54. doi: 10.1007/978-1-4615-2443-4_14
285. Martignone S, Menard S, Bufalino R, Cascinelli N, Pellegrini R, Tagliabue E, et al. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J Natl Cancer Inst. (1993) 85:398–402. doi: 10.1093/jnci/85.5.398
286. Sanjuan X, Fernandez PL, Miquel R, Munoz J, Castronovo V, Menard S, et al. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J Pathol. (1996) 179:376–80. doi: 10.1002/(SICI)1096-9896(199608)179:4<376::AID-PATH591>3.0.CO;2-V
287. Tagliabue E, Pastorino U. Re: killing of laminin receptor-positive human lung cancers by tumor-infiltrating lymphocytes bearing gamma delta + T-cell receptors. J Natl Cancer Inst. (1996) 88:1241–2. doi: 10.1093/jnci/88.17.1241
288. Viacava P, Naccarato AG, Collecchi P, Menard S, Castronovo V, Bevilacqua G. The spectrum of 67-kD laminin receptor expression in breast carcinoma progression. J Pathol. (1997) 182:36–44. doi: 10.1002/(SICI)1096-9896(199705)182:1<36::AID-PATH802>3.0.CO;2-W
289. Wewer UM, Taraboletti G, Sobel ME, Albrechtsen R, Liotta LA. Role of laminin receptor in tumor cell migration. Cancer Res. (1987) 47:5691–8.
290. Khumalo T, Reusch U, Knackmuss S, Little M, Veale RB, Weiss SF. Adhesion and invasion of breast and oesophageal cancer cells are impeded by anti-LRP/LR-specific antibody IgG1-iS18. PLoS ONE. (2013) 8:e66297. doi: 10.1371/journal.pone.0066297
291. Rebelo TM, Chetty CJ, Ferreira E, Weiss SF. Anti-LRP/LR-specific antibody IgG1-iS18 impedes adhesion and invasion of pancreatic cancer and neuroblastoma cells. BMC Cancer. (2016) 16:917. doi: 10.1186/s12885-016-2953-2
292. Chetty C, Khumalo T, Da Costa Dias B, Reusch U, Knackmuss S, Little M, et al. Anti-LRP/LR specific antibody IgG1-iS18 impedes adhesion and invasion of liver cancer cells. PLoS ONE. (2014) 9:e96268. doi: 10.1371/journal.pone.0096268
293. Rebelo TM, Vania L, Ferreira E, Weiss SFT. siRNA - mediated LRP/LR knock-down reduces cellular viability of malignant melanoma cells through the activation of apoptotic caspases. Exp Cell Res. (2018) 368:1–12. doi: 10.1016/j.yexcr.2018.04.003
294. Chetty CJ, Ferreira E, Jovanovic K, Weiss SFT. Knockdown of LRP/LR induces apoptosis in pancreatic cancer and neuroblastoma cells through activation of caspases. Exp Cell Res. (2017) 360:264–72. doi: 10.1016/j.yexcr.2017.09.016
295. Khumalo T, Ferreira E, Jovanovic K, Veale RB, Weiss SF. Knockdown of LRP/LR Induces apoptosis in breast and oesophageal cancer cells. PLoS ONE. (2015) 10:e0139584. doi: 10.1371/journal.pone.0139584
296. Vania L, Rebelo TM, Ferreira E, Weiss SFT. Knock-down of LRP/LR promotes apoptosis in early and late stage colorectal carcinoma cells via caspase activation. BMC Cancer. (2018) 18:602. doi: 10.1186/s12885-018-4531-2
297. Lu CL, Xu J, Yao HJ, Luo KL, Li JM, Wu T, et al. Inhibition of human 67-kDa laminin receptor sensitizes multidrug resistance colon cancer cell line SW480 for apoptosis induction. Tumour Biol. (2016) 37:1319–25. doi: 10.1007/s13277-015-3873-5
298. Sun L, Liu L, Liu X, Wang Y, Li M, Yao L, et al. MGr1-Ag/37LRP induces cell adhesion-mediated drug resistance through FAK/PI3K and MAPK pathway in gastric cancer. Cancer Sci. (2014) 105:651–9. doi: 10.1111/cas.12414
299. Naidoo K, Malindisa ST, Otgaar TC, Bernert M, Da Costa Dias B, Ferreira E, et al. Knock-Down of the 37kDa/67kDa Laminin Receptor LRP/LR impedes telomerase activity. PLoS ONE. (2015) 10:e0141618. doi: 10.1371/journal.pone.0141618
300. Vania L, Morris G, Otgaar TC, Bignoux MJ, Bernert M, Burns J, et al. Patented therapeutic approaches targeting LRP/LR for cancer treatment. Expert Opin Ther Pat. (2019) 29:987–1009. doi: 10.1080/13543776.2019.1693543
301. Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. (2004) 11:380–1. doi: 10.1038/nsmb743
302. Zidane N, Ould-Abeih MB, Petit-Topin I, Bedouelle H. The folded and disordered domains of human ribosomal protein SA have both idiosyncratic and shared functions as membrane receptors. Biosci Rep. (2012) 33:113–24. doi: 10.1042/BSR20120103
303. Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. (2014) 14:598–610. doi: 10.1038/nrc3792
304. Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, et al. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol. (2015) 17:1412–21. doi: 10.1038/ncb3250
305. Zhou J, Yi Q, Tang L. The roles of nuclear focal adhesion kinase (FAK) on cancer: a focused review. J Exp Clin Cancer Res. (2019) 38:250. doi: 10.1186/s13046-019-1265-1
306. Golubovskaya VM. Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci. (2014) 19:687–706. doi: 10.2741/4236
307. Roy-Luzarraga M, Hodivala-Dilke K. Molecular pathways: endothelial cell FAK-a target for cancer treatment. Clin Cancer Res. (2016) 22:3718–24. doi: 10.1158/1078-0432.CCR-14-2021
308. Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol. (2012) 30:1527–33. doi: 10.1200/JCO.2011.38.9346
309. Mak G, Soria JC, Blagden SP, Plummer R, Fleming RA, Nebot N, et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br J Cancer. (2019) 120:975–81. doi: 10.1038/s41416-019-0452-3
310. Soria JC, Gan HK, Blagden SP, Plummer R, Arkenau HT, Ranson M, et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol. (2016) 27:2268–74. doi: 10.1093/annonc/mdw427
311. Brown NF, Williams M, Arkenau HT, Fleming RA, Tolson J, Yan L, et al. A study of the focal adhesion kinase inhibitor GSK2256098 in patients with recurrent glioblastoma with evaluation of tumor penetration of [11C]GSK2256098. Neuro Oncol. (2018) 20:1634–42. doi: 10.1093/neuonc/noy078
312. Jones SF, Siu LL, Bendell JC, Cleary JM, Razak AR, Infante JR, et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs. (2015) 33:1100–7. doi: 10.1007/s10637-015-0282-y
313. Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, et al. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. (2016) 77:997–1003. doi: 10.1007/s00280-016-3010-1
314. Doi T, Yang JC, Shitara K, Naito Y, Cheng AL, Sarashina A, et al. Phase I study of the focal adhesion kinase inhibitor BI 853520 in Japanese and Taiwanese patients with advanced or metastatic solid tumors. Target Oncol. (2019) 14:57–65. doi: 10.1007/s11523-019-00620-0
315. de Jonge MJA, Steeghs N, Lolkema MP, Hotte SJ, Hirte HW, van der Biessen DAJ, et al. Phase I study of BI 853520, an inhibitor of focal adhesion kinase, in patients with advanced or metastatic nonhematologic malignancies. Target Oncol. (2019) 14:43–55. doi: 10.1007/s11523-018-00617-1
316. Verheijen RB, van der Biessen DAJ, Hotte SJ, Siu LL, Spreafico A, de Jonge MJA, et al. Randomized, open-label, crossover studies evaluating the effect of food and liquid formulation on the pharmacokinetics of the novel focal adhesion kinase (FAK) inhibitor BI 853520. Target Oncol. (2019) 14:67–74. doi: 10.1007/s11523-018-00618-0
317. Fennell DA, Baas P, Taylor P, Nowak AK, Gilligan D, Nakano T, et al. Maintenance defactinib versus placebo after first-line chemotherapy in patients with merlin-stratified pleural mesothelioma: COMMAND-A double-blind, randomized, phase II study. J Clin Oncol. (2019) 37:790–8. doi: 10.1200/JCO.2018.79.0543
318. Brunton VG, Frame MC. Src and focal adhesion kinase as therapeutic targets in cancer. Curr Opin Pharmacol. (2008) 8:427–32. doi: 10.1016/j.coph.2008.06.012
319. Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. (2011) 19:372–86. doi: 10.1016/j.ccr.2011.01.036
320. Pichot CS, Hartig SM, Xia L, Arvanitis C, Monisvais D, Lee FY, et al. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. (2009) 101:38–47. doi: 10.1038/sj.bjc.6605101
321. Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. (2009) 16:67–78. doi: 10.1016/j.ccr.2009.05.017
322. Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. (2010) 70:5706–16. doi: 10.1158/0008-5472.CAN-09-2356
323. Sakuma Y, Tsunezumi J, Nakamura Y, Yoshihara M, Matsukuma S, Koizume S, et al. ABT-263, a Bcl-2 inhibitor, enhances the susceptibility of lung adenocarcinoma cells treated with Src inhibitors to anoikis. Oncol Rep. (2011) 25:661–7. doi: 10.3892/or.2010.1123
324. Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. (2011) 19:401–15. doi: 10.1016/j.ccr.2011.01.018
325. Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. (2009) 15:1163–9. doi: 10.1038/nm.2009
326. Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. (2012) 33:122–8. doi: 10.1016/j.tips.2011.11.002
327. Kanda R, Kawahara A, Watari K, Murakami Y, Sonoda K, Maeda M, et al. Erlotinib resistance in lung cancer cells mediated by integrin beta1/Src/Akt-driven bypass signaling. Cancer Res. (2013) 73:6243–53. doi: 10.1158/0008-5472.CAN-12-4502
328. Roskoski R Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. (2015). 94:9–25. doi: 10.1016/j.phrs.2015.01.003
329. Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. (2010) 16:3526–32. doi: 10.1158/1078-0432.CCR-09-1834
330. Elias D, Vever H, Laenkholm AV, Gjerstorff MF, Yde CW, Lykkesfeldt AE, et al. Gene expression profiling identifies FYN as an important molecule in tamoxifen resistance and a predictor of early recurrence in patients treated with endocrine therapy. Oncogene. (2015) 34:1919–27. doi: 10.1038/onc.2014.138
Keywords: extracellular matrix, tumor, progression, crosstalk, clinical perspectives
Citation: Lorusso G, Rüegg C and Kuonen F (2020) Targeting the Extra-Cellular Matrix—Tumor Cell Crosstalk for Anti-Cancer Therapy: Emerging Alternatives to Integrin Inhibitors. Front. Oncol. 10:1231. doi: 10.3389/fonc.2020.01231
Received: 10 January 2020; Accepted: 16 June 2020;
Published: 22 July 2020.
Edited by:
Nils Cordes, Technische Universität Dresden, GermanyReviewed by:
Elisa Giannoni, University of Florence, ItalyCopyright © 2020 Lorusso, Rüegg and Kuonen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: François Kuonen, ZnJhbmNvaXMua3VvbmVuQGNodXYuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.