- 1Department of Oncology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Basic Medical Sciences, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Pharmacy, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 4Department of Laboratory Medicine, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 5Department of Oncology, Baoshan Branch Hospital, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: Multiple primary malignancies (MPMs) refer to two or more primary malignant tumors in the same individual, the prevalence of which ranges from 0. 734 to 11.7%. The risk factors for MPMs vary and include both genetic and environmental causes. FANCA gene mutation might be a predisposition to the development of a second primary cancer. Here, we report a case in which a patient with a FANCA mutation developed thyroid papillary carcinoma and gastric adenocarcinoma.
Case Presentation: A 48-year-old woman was diagnosed with thyroid cancer underwent resection in 2006. In 2008, the patient developed gastric adenocarcinoma and underwent radical gastrectomy. Gastric cancer was completely remitted after radiochemotherapy, but metastasis developed, and she received immunotherapy. The patient died on October 27, 2019. Peripheral blood gene detection showed germline FANCA mutation.
Conclusions: Gene detection is of great importance in cancer patients, especially in those with MPMs. FANCA mutation is a predisposition to tumorigenesis that can increase the risk of developing MPMs. Patients with heterozygous FANCA gene mutations have poorer outcomes.
Introduction
Multiple primary malignancies (MPMs) refer to two or more primary malignant tumors in the same individual. The classic diagnostic criteria proposed by Warren and Gates in 1932 are as follows: (i) histologic confirmation of malignancy in both the primary and secondary tumor; (ii) the two malignancies must be anatomically separated by normal mucosa; and (iii) the possibility that the second primary malignancy is a metastasis from the first tumor must be excluded. However, there are two special conditions that should be underlined: (i) systemic cancers that could potentially involve many organs should be regarded as one, and (ii) cancers with different histology should be counted as multiple cancers, regardless of whether their sites are the same and whether the diagnoses are simultaneous (1). The prevalence of MPMs varies from 0.734 to 11.7% because of differences in geography and diagnostic approaches (2, 3). Epidemiological studies have shown that MPMs occur in the endometrium and ovary in 5% of women with endometrial cancer and in 10% of women with ovarian cancer, that the rate is 3.4% in patients with gastric cancer (GC), and that the other most common primary malignancy is colorectal cancer (20.4%) (4).
Multiple primary malignancies can be synchronous or metachronous, with diagnostic intervals of <6 months or more than 6 months, respectively. In patients with MPMs, different anticancer strategies should be applied according to the nature and stage of each tumor, making treatment challenging. In general, compared to synchronous MPM patients, metachronous MPM patients have a higher incidence and survival rate (5, 6), although curative surgery is applied more for synchronous tumors than for metachronous tumors (2).
The cooperation of both genetic and environmental factors results in cancer via the mechanisms of microsatellite instability and loss of heterozygosity. Patients with metachronous MPMs usually develop another tumor after the former one enters clinical remission, and their prognosis is poorer than that of patients with a single malignancy (7). The development of MPMs may be the result of germline mutations in cancer predisposition genes, of random chance, of radiotherapy or chemotherapy of the first tumor, or of environmental factors (8).
Herein, we report the case of a patient with a FANCA mutation who suffered from thyroid papillary carcinoma, gastric adenocarcinoma, and tumor metastasis of the ovary, posterior uterus, and gallbladder.
Case Presentation
A 48-year-old woman was admitted for gastric malignancy on April 16, 2019, with a medical history of thyroid papillary carcinoma. In 2006, the patient underwent right thyroid cancer resection with a pathological diagnosis of thyroid papillary carcinoma, and regular follow-up visits afterward all showed complete remission. Unfortunately, she was diagnosed with gastric malignancy in 2008. The patient received radical gastrectomy in our hospital, and the pathological diagnosis was poorly differentiated adenocarcinoma of the greater curvature (diffused invasive type, 4 × 3.6 × 2 cm), and the cancer tissue had invaded into the serosa layer as well as the nerve bundle. Tumor thrombi were seen in the lymphatic vessels, whereas lymph nodes were negative. The patient was treated with radiotherapy and chemotherapy (detailed therapeutic regimen, dosage, and side effects are not available), with no obvious signs of tumor recurrence at regular follow-up.
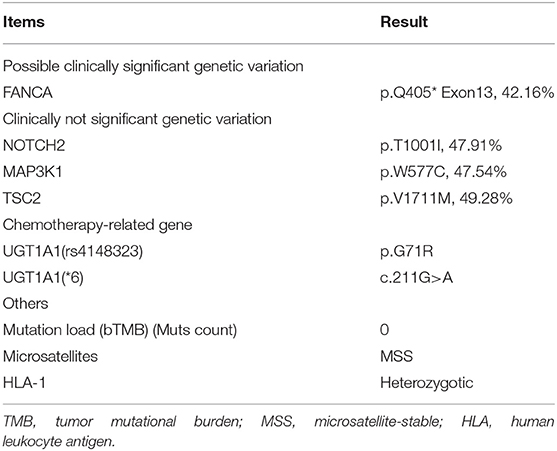
In January 2019, she developed gradually worsening upper abdominal pain, and then nausea and vomiting occurred. However, the results of gastroscopy and colonoscopy were normal. On April 4, positron emission tomography/computed tomography indicated probable tumor metastasis by detecting a solid mass in the bilateral accessory area (58 × 55 mm on the left side, 50 × 32 mm on the right side, maximum standard uptake value was 2.7–3.1), nodules in the posterior uterus and gallbladder fossa, and increased fluorodeoxyglucose (FDG) metabolism. Abdominal pelvic, peritoneal, and pleural effusions were also detected. There were nodules at the bottom of the gallbladder with increased FDG metabolism. FDG metabolism was increased in the residual stomach and ascending and descending colon. Next-generation sequencing suggested a germline FANCA mutation, microsatellite stability, mismatch repair, and low tumor mutational burden (Table 1). The tumor cells were PD-1 [–], PD-L1 [–], PMS-2 [+], MLH-1 [+], MSH-2 [+], and MSH-6 [+].
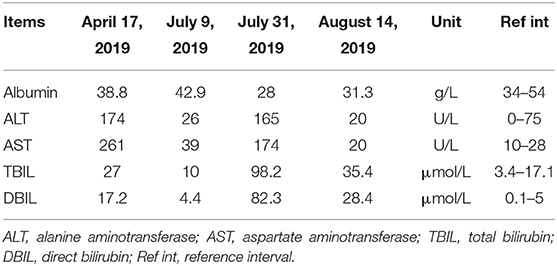
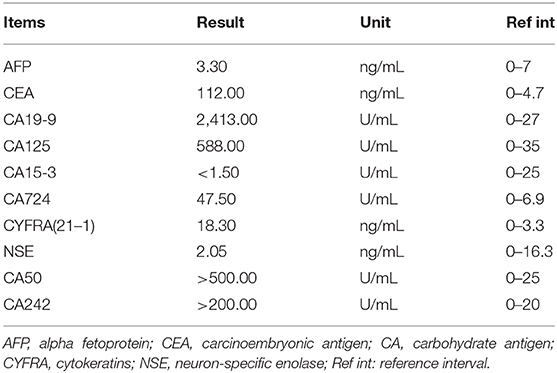
On admission, laboratory tests showed that the complete blood count, C-reactive protein level, renal function, and coagulation function were normal. However, the patient's hepatic function markedly declined, accompanied by elevated aminotransferase and bilirubin (April 17, 2019) (Table 2). Chest x-ray indicated increased lung texture with a small amount of pleural effusion on both sides. Ultrasound was used to position the ascites and showed a free anechoic zone in the abdominal cavity that was 40 mm in the deepest position and 15 mm from the body surface. The tumor marker results of ascites are listed in Table 3.
Then, three cycles of chemotherapy were successfully completed from April 19, 2019, to June 14, 2019 (240 mg paclitaxel d1 + 55 mg cisplatin d1–2). On July 9, 2019, the bone scan showed multiple bone metastases. The patient received a zoledronic acid injection (4 mg) and ascites drainage (drainage of yellow skin water). On July 17, 2019, an ovarian lesion biopsy revealed pathologically that poorly differentiated adenocarcinoma infiltration or metastasis could be seen in the right ovarian tissue, indicating gastric adenocarcinoma metastasis. On July 18, 2019, the second line of chemotherapy combined with immunotherapy was administered (100 mg nivolumab d1 + 240 mg irinotecan d1 + 500 mg leucovorin d1 + 500 mg fluorouracil fast drip, 2.75 g intravenous pump maintained for 46 h). After chemotherapy, the patient experienced nausea and vomiting, followed by skin and urine yellowing and accompanied by markedly elevated aminotransferase and bilirubin, decreased albumin (Table 2), and hypokalemia (2.83 mmol/L; reference range, 3.5–5.2 mmol/L) (July 31, 2019). The abdominal magnetic resonance imaging results showed hilar occupancy with intrahepatic bile duct dilatation upstream, with a high possibility of a tumor. The patient received cholangiography and percutaneous transhepatic cholangial drainage (PTCD) on August 5. After PTCD, the patient's aminotransferase and bilirubin levels substantially improved (August 14, 2019) (Table 2). Intestinal obstruction was considered, and the condition was relieved after gastrointestinal decompression. Supportive treatments were administered during hospitalization.
On October 16, 2019, the patient was discharged with a diagnosis of stage IV gastric body carcinoma rTxNxM1 (bilateral ovaries, bone, liver, abdomen). The patient passed away on October 27, 2019. The flow chart of treatment in this patient is presented in Supplementary Figure 1.
Discussion
Multiple primary malignancies are relatively rare in cancer patients, but the frequency of MPM occurrence has increased gradually. Many factors contribute to this increase. For example, the longer average lifetime has led to the larger elderly population, and in the general population, the development of inspection methods plays a large role (5). Additionally, the improvement of therapeutic approaches, as well as regular follow-up visits, greatly increases survival, making it possible for the development of a second primary tumor (9). Patients with metachronous MPMs usually develop another tumor after the former enters clinical remission. In general, these patients have a poorer prognosis than those with a single malignancy (7) but have a higher incidence (5) and a better prognosis (6) than synchronous MPM patients. In particular, for GC, those with MPMs have a shorter lifespan than those without MPMs (10). The treatment of MPMs is challenging because of the different nature of each primary tumor (6). One study found that, in the case of synchronous multiple primary lung cancers, surgical treatment might be a good choice (11), and most studies stress that the staging of each malignancy is the most significant factor in the decision of treatment options for MPM patients (9).
In terms of risk factors, MPMs are closely associated with male sex, old age, and the time of diagnosis of the first cancer (12). In young patients, MPMs can be attributed to genetic predisposition and the treatment of primary cancer. In adult patients, although the risk of MPMs is largely correlated with the age at diagnosis, germline mutations in cancer predisposition genes still play a significant role (8, 13). Individuals with certain germline mutations and corresponding cancer syndromes are always at high risk (14). It has been reported that most of the genes that are associated with cancer have germline mutations involved in DNA repair, and FANCA is one of these genes (15). Others are MLH1, BRCA1, BRCA2, MUTYH, ATM, PMS2, MSH6, and BAP1.
Germline FANCA mutation is the most frequent mutation in patients with Fanconi anemia (FA), an autosomal recessive inherited disease that is caused by homozygous mutation of the genes encoding Fanconi complementary group of proteins (FANCA-FANCU) (16) but is less reported in patients with solid tumors. According to The Cancer Genome Atlas database, the prevalence of somatic mutations in FANCA genes is ~3% for stomach cancer. Recently, the close relationship between alterations in FA pathways and tumorigenesis has been revealed. Hierarchical clustering analysis showed that the DNA damage repair (DDR) pathways, especially the FA pathway, were deranged across all subtypes of GC (17). The association between FANCA gene variations and breast and ovarian cancers has also been confirmed (18, 19), and the other cancers most reported to be associated with FANCA mutations are GC (17), prostate cancer (20) and colorectal cancer (21). There have been no reports on thyroid cancer yet.
Heterozygous FANCA gene mutations do not cause the FA phenotype but significantly increase cancer susceptibility in a sporadic manner (22). The major cellular function of the FA pathway is to maintain the stability of the genome during DNA replication and the damage repair process (17). Therefore, FANCA mutations will increase secondary mutations caused by DNA mismatch and the subsequent malignant transformation of normal cells, which can explain the association between FANCA mutations and the high risk of tumorigenesis, as well as, correspondingly, the association between germline mutations and an increased risk of MPM development.
The treatment of FANCA mutation carriers with malignancies should be individualized (23). A study showed that FANCA expression was associated with platinum hypersensitivity both in vitro and in patient-derived xenografts (24). FANCA belongs to the DDR gene, and this genetic status indicates that FA patients are more sensitive to chemotherapy, with better therapeutic efficacy but a higher rate of complications (7). On the other hand, FANCA overexpression is the major mechanism leading to cellular radioresistance, with the acceleration of DNA repair and improvement in repair fidelity, with pATM trafficking, p53/p21-axis overactivation, and so on, being the main candidate mechanism (25). Moreover, FA is associated with more than one-half of reports on adverse reactions to radiotherapy (most reported FA patients died within months of exposure) (23). Thus, cancer patients with FANCA mutations are probably hypersensitive to chemotherapy but resistant to radiotherapy. There have been no reports of treatment options for other cancers with FANCA mutations other than for breast, ovarian, and prostate cancers. Additionally, because of the rarity of studies, there are currently no Food and Drug Administration/National Medical Products Administration–approved targeting agents for FANCA gene biomarkers, and an optimum method for the prediction of radiosensitivity and the best parameter has not been found (23). For the above reasons, treatment strategies for FANCA mutation carriers depend on the specific cancer types, with consideration of predisposed MPM risk and radiotherapy-related adverse reactions.
In conclusion, in cancer patients with a diagnosis of MPM, gene detection is of great clinical significance, especially in cases of germline mutations, which increase the risk of MPM. FANCA mutation detection might be suggested in the first case of malignancy because it is a predisposition to tumorigenesis. Cancer patients with FANCA gene mutations are associated with a high risk of complications from chemoradiotherapy despite sensitivity to chemotherapy, and more clinical samples are required to further verify these findings. Therefore, supervision after therapy is extremely important in this fragile population.
Ethics Statement
Written informed consent was obtained from the patient's next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
QX, L-YZ, and Y-DY: data collection and manuscript writing. YL and Y-SD: data collection. X-YX: project development, data collection, and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the young scientist project of the National Natural Science Foundation of China (81702843).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01199/full#supplementary-material
References
1. Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine. (2017) 96:e6799. doi: 10.1097/MD.0000000000006799
2. Gokyer A, Kostek O, Hacioglu MB, Erdogan B, Kodaz H, Turkmen E, et al. Clinical features of the patient with multiple primary tumors: single center experience. North Clin Istanb. (2017) 4:43–51. doi: 10.14744/nci.2017.67044
3. Muto Y, Suzuki K, Kato T, Ichida K, Takayama Y, Fukui T, et al. Multiple primary malignancies of six organs in a Japanese male patient: a case report. Mol Clin Oncol. (2019) 10:511–5. doi: 10.3892/mco.2019.1819
4. Adeyanju MA, Ilori AA. Multiple primary tumors. Niger J Clin Pract. (2017) 20:1346–9. doi: 10.4103/njcp.njcp_432_16
5. Jiao F, Yao LJ, Zhou J, Hu H, Wang LW. Clinical features of multiple primary malignancies: a retrospective analysis of 72 Chinese patients. Asian Pac J Cancer Prev. (2014) 15:331–4. doi: 10.7314/apjcp.2014.15.1.331
6. Zhang Y, Ge Y, Wu X, Liu S. Clinical treatment of advanced synchronous triple primary malignancies: comprehensive treatment based on targeted therapy. Onco Targets Ther. (2019) 12:2421–30. doi: 10.2147/OTT.S200625
7. Wang L, Wang H, Wang T, Liu J, Chen W, Wang Y, et al. Analysis of polymorphisms in genes associated with the FA/BRCA pathway in three patients with multiple primary malignant neoplasms. Artif Cells Nanomed Biotechnol. (2019) 47:1101–12. doi: 10.1080/21691401.2019.1575846
8. Villacis RAR, Basso TR, Canto LM, Pinheiro M, Santiago KM, Giacomazzi J, et al. Rare germline alterations in cancer-related genes associated with the risk of multiple primary tumor development. J Mol Med. (2017) 95:523–33. doi: 10.1007/s00109-017-1507-7
9. Kim SH, Park BS, Kim HS, Kim JH. Synchronous quintuple primary gastrointestinal tract malignancies: case report. World J Gastroenterol. (2017) 23:173–7. doi: 10.3748/wjg.v23.i1.173
10. Kim C, Chon H, Kang B, Kim K, Jeung HC, Chung H, et al. Prediction of metachronous multiple primary cancers following the curative resection of gastric cancer. BMC Cancer. (2013) 13:394. doi: 10.1186/1471-2407-13-394
11. Peng Y, Ren W, Wang H, Li M, Feng Z, Peng Z. Surgical treatment is an effective approach for patients with synchronous multiple primary lung cancers. J Cancer Res Ther. (2017) 13:702–6. doi: 10.4103/jcrt.JCRT_140_17
12. Kozawa E, Sugiura H, Tsukushi S, Urakawa H, Arai E, Futamura N, et al. Multiple primary malignancies in elderly patients with high-grade soft tissue sarcoma. Int J Clin Oncol. (2014) 19:384–90. doi: 10.1007/s10147-013-0543-8
13. Schon K, Tischkowitz M. Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Res Treat. (2018) 167:417–23. doi: 10.1007/s10549-017-4531-y
14. Pilie PG, Johnson AM, Hanson KL, Dayno ME, Kapron AL, Stoffel EM, et al. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer. (2017) 123:3925–32. doi: 10.1002/cncr.30817
15. Lin PC, Yeh YM, Wu PY, Hsu KF, Chang JY, Shen MR. Germline susceptibility variants impact clinical outcome and therapeutic strategies for stage III colorectal cancer. Sci Rep. (2019) 9:3931. doi: 10.1038/s41598-019-40571-0
16. Wilkes DC, Sailer V, Xue H, Cheng H, Collins CC, Gleave M, et al. A germline FANCA alteration that is associated with increased sensitivity to DNA damaging agents. Cold Spring Harb Mol Case Stud. (2017) 3:a001487. doi: 10.1101/mcs.a001487
17. Huang JP, Lin J, Tzen CY, Huang WY, Tsai CC, Chen CJ, et al. FANCA D1359Y mutation in a patient with gastric polyposis and cancer susceptibility: a case report and review of literature. World J Gastroenterol. (2018) 24:4412–8. doi: 10.3748/wjg.v24.i38.4412
18. Abbasi S, Rasouli M. A rare FANCA gene variation as a breast cancer susceptibility allele in an Iranian population. Mol Med Rep. (2017) 15:3983–8. doi: 10.3892/mmr.2017.6489
19. Foretova L, Navratilova M, Svoboda M, Vasickova P, Stahlova EH, Hazova J, et al. Recommendations for preventive care for women with rare genetic cause of breast and ovarian cancer. Klin Onkol. (2019) 32:6–13. doi: 10.14735/amko2019S6
20. Hongo H, Kosaka T, Aimono E, Nishihara H, Oya M. Aggressive prostate cancer with somatic loss of the homologous recombination repair gene FANCA: a case report. Diagn Pathol. (2020) 15:5. doi: 10.1186/s13000-019-0916-z
21. Zhunussova G, Afonin G, Abdikerim S, Jumanov A, Perfilyeva A, Kaidarova D, et al. Mutation Spectrum of Cancer-Associated Genes in Patients With Early Onset of Colorectal Cancer. Front Oncol. (2019) 9:673. doi: 10.3389/fonc.2019.00673
22. Chen H, Zhang S, Wu Z. Fanconi anemia pathway defects in inherited and sporadic cancers. Transl Pediatr. (2014) 3:300–4. doi: 10.3978/j.issn.2224-4336.2014.07.05
23. Sirak I, Sinkorova Z, Senkerikova M, Spacek J, Laco J, Vosmikova H, et al. Hypersensitivity to chemoradiation in FANCA carrier with cervical carcinoma-A case report and review of the literature. Rep Pract Oncol Radiother. (2015) 20:309–15. doi: 10.1016/j.rpor.2014.11.006
24. Beltran H, Eng K, Mosquera JM, Sigaras A, Romanel A, Rennert H, et al. Whole-Exome Sequencing of Metastatic Cancer and Biomarkers of Treatment Response. JAMA Oncol. (2015) 1:466–74. doi: 10.1001/jamaoncol.2015.1313
25. Hess J, Unger K, Orth M, Schotz U, Schuttrumpf L, Zangen V, et al. Genomic amplification of Fanconi anemia complementation group A (FancA) in head and neck squamous cell carcinoma (HNSCC): cellular mechanisms of radioresistance and clinical relevance. Cancer Lett. (2017) 386:87–99. doi: 10.1016/j.canlet.2016.11.014
Keywords: multiple primary malignancy, gastric cancer, thyroid cancer, FANCA gene, case report, literature review
Citation: Xia Q, Zhao L-Y, Yan Y-D, Liao Y, Di Y-S and Xiao X-Y (2020) A Multiple Primary Malignancy Patient With FANCA Gene Mutation: A Case Report and Literature Review. Front. Oncol. 10:1199. doi: 10.3389/fonc.2020.01199
Received: 27 February 2020; Accepted: 12 June 2020;
Published: 31 July 2020.
Edited by:
Zhaohui Huang, Affiliated Hospital of Jiangnan University, ChinaReviewed by:
Xiangning Meng, Harbin Medical University, ChinaGuifang Xu, Nanjing Drum Tower Hospital, China
Copyright © 2020 Xia, Zhao, Yan, Liao, Di and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu-Ying Xiao, eGlhb3hpdXlpbmcyMDAyJiN4MDAwNDA7MTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qing Xia1†
Qing Xia1† Yi-Dan Yan
Yi-Dan Yan Yuan Liao
Yuan Liao Xiu-Ying Xiao
Xiu-Ying Xiao