- 1Tumor Etiology and Screening Department of Cancer Institute and General Surgery, The First Hospital of China Medical University, Shenyang, China
- 2Department of Oncology, PLA Cancer Center, General Hospital of Northern Theater Command, Shenyang, China
- 3Key Laboratory of Cancer Etiology and Prevention in Liaoning Education Department, The First Hospital of China Medical University, Shenyang, China
- 4Key Laboratory of GI Cancer Etiology and Prevention in Liaoning Province, The First Hospital of China Medical University, Shenyang, China
Objective: TNFAIP2 is a novel gene induced by TNF-α and participates in inflammatory reaction and tumor angiogenesis. This study aims to understand the correlation between TNFAIP2 gene polymorphism and prediction as well as prognosis of gastric cancer (GC) in a Chinese population.
Methods: One thousand two hundred seventy-nine cases were enrolled, including 640 GC and 639 non-cancer cases. The functional tagSNPs of the TNFAIP2 gene were screened by Haploview software and NIH Snpinfo website. Human whole-blood genomic DNA was extracted by phenol chloroform method and analyzed by KASP SNP typing and sequencing method. ELISA was used to determine the expression of TNFAIP2 protein in serum samples. The miRNAs bound to TNFAIP2 3′ UTR rs8126 were predicted by MirSNP and TargetScan database. SPSS 22.0 software was used for statistical analysis, and P < 0.05 showed statistical difference.
Results: Four functional TNFAIP2 tagSNPs were found by bioinformatics analysis. TNFAIP2 rs8126 T>C polymorphism increased GC risk, and the risk in TC genotype cases was higher than that in TT genotype cases (P = 0.001, OR = 1.557). In the dominant model, the TNFAIP2 rs8126 polymorphic carrier was 1.419 times higher (P = 0.007). TNFAIP2 rs710100 C>T polymorphism, TNFAIP2 rs3759571 G>A polymorphism, and TNFAIP2 rs3759573 A>G polymorphism were not correlated with GC risk. In the subgroup analysis, TNFAIP2 rs8126 TC genotype cases had a higher GC risk in male, aged 60 years or older, Helicobacter pylori-negative, non-smoking, and non-drinking. However, there was no correlation between TNFAIP2 SNPs and GC prognosis. The TNFAIP2 protein concentration in GC patients was significantly different from that in healthy persons (P = 0.029), but it was not associated with GC prognosis. The high or low expression of TNFAIP2 protein had no significant difference with gender, age, H. pylori infection, smoking, and drinking in GC patients. The serum TNFAIP2 protein expression in rs8126 TT genotype carriers was significantly higher than that in rs8126 CC genotype carriers (P < 0.001).
Conclusion: TNFAIP2 3′ UTR rs8126 T>C polymorphism was associated with GC risk in a Chinese population, especially in cases with males aged 60 years or older, H. pylori negative, non-smoking and non-drinking. Compared with healthy persons, serum TNFAIP2 protein expression was higher in Chinese GC patients, and TNFAIP2 3′ UTR rs8126 T>C polymorphism might affect TNFAIP2 protein expression.
Introduction
Gastric cancer (GC) is considered to be one of the most common malignant tumors in the world (1). It is usually asymptomatic or has mild symptoms in the early days but is prone to recurrence and metastasis due to tumor specificity and heterogeneity (2–4). In China, GC has become the second leading cause of cancer-related death, and the situation of disease prevention is extremely grim (5–7). So far, the pathogenesis of GC has not been completely clarified. Many etiological studies have found that some factors are closely related to GC, including environment, diet, microorganism, family inheritance, and physicochemical and genetic changes, especially specific oncogenes and tumor suppressor genes (8–10). In recent years, the Human Genome Atlas Project has provided a theoretical basis for exploring the correlation between genetic changes and malignant tumors. In nature, gene polymorphism is one of the most common forms of gene changes, and it can reflect the differences of biological activity between different individuals (11). The studies on gene polymorphism can lay an important foundation of molecular biology for revealing the mechanism of malignant tumors, and they have important roles in clarifying tumor susceptibility and predicting the development trend of tumors. Single nucleotide polymorphism (SNP), as the most common type of human genetic variation, is an important part of the research on gene polymorphism and can be used to explore the mechanism of tumor generation (12, 13).
Tumor necrosis factor alpha-induced protein 2 (TNFAIP2), also known as B94 and EXOC3L3, is a member of tumor necrosis factor alpha-induced proteins (TNFAIPs). It is located on human chromosome 14q32.32 and contains 14 exons, which has a genomic DNA span of 13.45 kDa and can encode a protein with 654 amino acids and a molecular weight of 72.6 kDa. TNFAIP2 interacts with EXOC1, EXOC2, EXOC4, EXOC7, and EXOC8 and participates in the formation and the development of human organs (14). It may also be involved in various biological processes such as angiogenesis, cell differentiation, bone marrow tissue generation, and spermatogenesis, and its main function is to regulate inflammation and angiogenesis (15). In in vitro studies, TNFAIP2 is believed to have differential expression during angiogenesis (16). In addition, TNFAIP2 also regulates the apoptosis of tumor cells and is considered to be a target gene for retinoic acid in acute promyelocytic leukemia (17). Previous studies have reported that functional TNFAIP2 SNPs, mainly located in the 3′ non-coding region (3′ UTR), may regulate gene expression by modifying the binding ability of miRNA to target genes and eventually lead to the differences in disease susceptibility. Recently, some studies have confirmed the relationship between TNFAIP2 SNPs and malignant tumors such as head and neck squamous cell carcinoma (SCCHN) and esophageal squamous cell carcinoma (ESCC), which is beneficial for screening high-risk groups and predicting outcomes of tumors (14, 15, 18, 19).
However, the correlation between TNFAIP2 gene polymorphism and prediction or prognosis of GC is rarely reported, especially in Asian or Chinese populations. At present, only one study from an American population reported that, compared with TT + TC genotype, the TNFAIP2 3′ UTR rs8126 CC genotype significantly increased GC risk, especially in the drinking population (14).
This study aims to understand the correlation between TNFAIP2 gene polymorphism and prediction or prognosis of GC in a Chinese population, explore the effect of TNFAIP2 gene polymorphism on the expression of TNFAIP2 protein, and attempt to provide a theoretical basis for molecular target prediction, disease diagnosis, and individualized treatment of GC.
Materials and Methods
Study Participants
This was a case–control study from multiple medical centers in Liaoning Province, northern China, and 640 patients with GC and 639 non-GC cases were enrolled between December 1997 and December 2013. The inclusion criteria included the following: all participants had a clear pathological diagnosis and typing by electronic gastroscopy. The exclusion criteria included the following: (A) The participants had a major organ dysfunction; (B) The participants had autoimmune diseases; (C) The participants had other malignant tumors; and (D) The participants had infectious diseases. The fasting venous blood and serum of all participants were isolated and saved under the condition of 20°C below zero. The epidemiological information and the clinicopathological parameters of the cases were recorded, and the GC patients were followed up by telephone every 6 months. The main follow-up contents were overall survival, and the deadline for data collection was June 30, 2017 (Figure 1). This study was approved by the ethics committee of the First Affiliated Hospital of China Medical University [No. (2015)77], and all participants had signed the informed consent.
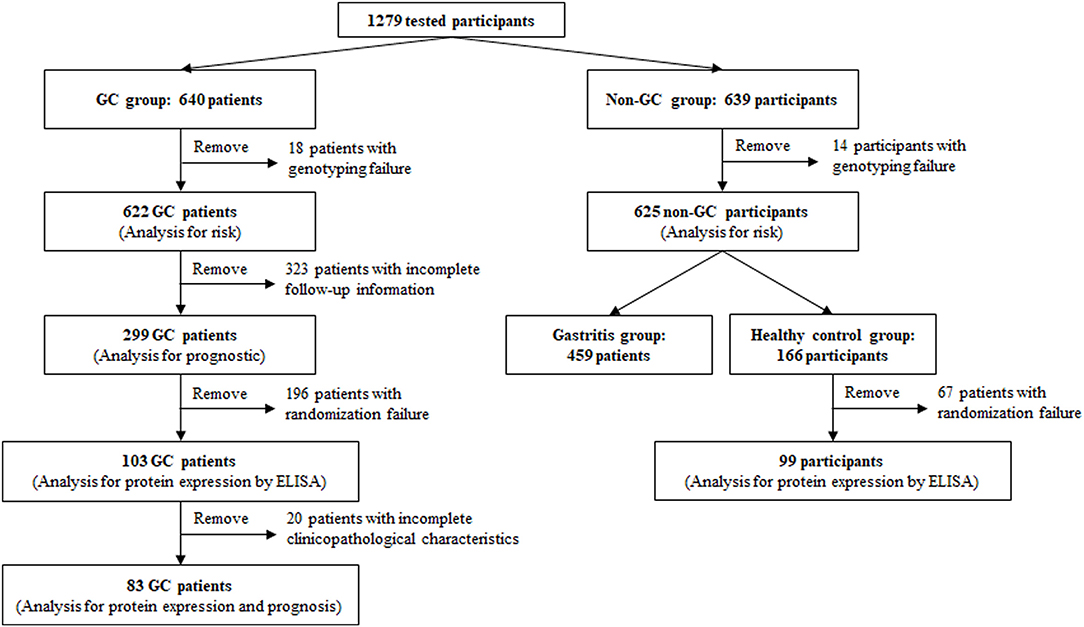
Figure 1. Participants' disposition. Human whole-blood genomic DNA tests were performed on 1,279 participants in this study, including 640 gastric cancer (GC) patients and 639 non-GC participants. Due to genotyping failure on some participants, the analysis of correlation between TNFAIP2 TagSNPs and GC risk was performed on 1,247 eligible participants, including 622 GC patients and 625 non-GC participants. Due to incomplete follow-up information, the analysis of correlation between TNFAIP2 TagSNPs and GC prognosis was performed on 299 GC patients. The analysis of TNFAIP2 protein expression and GC risk and prognosis was performed on 202 participants randomly selected from the GC group and the healthy control group, including 103 GC patients and 99 healthy persons. Due to incomplete clinicopathological characteristics, only 83 GC patients were enrolled in the analysis of correlation between serum TNFAIP2 protein expression and GC prognosis.
Functional TagSNP Selection
The functional tagSNPs of the TNFAIP2 gene were screened by Haploview software and NIH Snpinfo website (https://snpinfo.niehs.nih.gov/). The F-SNP website (http://compbio.cs.queensu.ca/F-SNP/) and the NIH Snpinfo website were used to predict the functional tagSNPs, respectively. The parameters were set as: Chinese Han population, minimum allele frequency >5%, and frequency distribution r2 > 0.8 (Supplementary Figures 1, 2).
Genotyping
Human whole-blood genomic DNA was extracted by phenol chloroform method and analyzed by KASP SNP typing and sequencing method. In the Sequenom MassARRAY platform (Sequenom, San Diego, CA, USA), SNP genotyping was performed by Bio Miao Biological Technology (Beijing, China). In addition, we randomly selected 10% of the samples for repeated analysis and found that the consistency rate of all the duplicated samples was 100%.
Detection of Serum TNFAIP2 Protein and H. pylori-IgG by ELISA
Enzyme-linked immunosorbent assay (ELISA) was used to determine the expression of the TNFAIP2 protein in the serum samples. Double-antibody sandwich method was used for ELISA, and the ELISA kit was purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. The absorbance (OD value) was measured by Multiskan Ascent (Thermo Labsystems, USA) at 450 nm, and the TNFAIP2 concentration was calculated by a standard curve. Serum H. pylori-IgG titer was also detected by ELISA (Helicobacter pylori IgG kit; Biohit, Helsinki, Finland), and the details were described in our published study (20).
Statistical Analysis
SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Firstly, we tested the normal distribution for units of measurement. If it conformed to the normal distribution, T-test could be used for statistical analysis. If it did not conform to the normal distribution, non-parametric test should be used for statistical analysis. The counting units were statistically analyzed by chi-square test. Multivariate logistic regression model was used to compare TNFAIP2 SNPs genotypes between the GC group and the non-GC group, and OR value and confidence interval (95% CI) were calculated to represent the relative risk. Logistic regression model was used to evaluate the interaction relationship between TNFAIP2 SNPs and H. pylori infection, smoking, and drinking. Adjusting for gender and age, a full-factor model was used to calculate the P-value of the interaction relationship between TNFAIP2 SNPs genotypes and H. pylori infection, smoking, and drinking. Cox proportional risk model was used for univariate and multivariate analysis to calculate the relationship between the clinical parameters and the prognosis of GC patients. P < 0.05 was considered as statistically significant.
Results
The Basic Characteristics of Study Participants
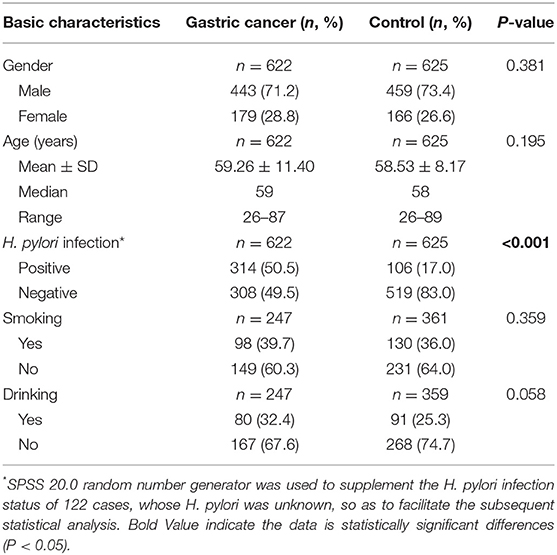
In this study, 1,247 qualified peripheral blood samples were analyzed for gene polymorphism, including 622 cases in the GC group and 625 cases in the non-GC group. Age and sex were matched in both groups. The mean age in the GC group and in the non-GC group was 59.26 ± 11.4 (26–87) and 58.53 ± 8.17 (26–89), respectively. The difference in H. pylori infection between the two groups was statistically significant (P < 0.001), but there were no significant differences in smoking and drinking (Table 1).
Functional TagSNPs Selected
Haploview software and NIH Snpinfo website were used to screen for functional tagSNPs, respectively. We found four functional TNFAIP2 SNPs and used them as candidate SNPs for further genotyping and statistical analysis, including miRNA binding sites (rs8126 and rs710100) and transcription factor binding sites (rs3759571 and rs3759573).
The Correlation Between TNFAIP2 TagSNPs and GC Risk in General Population
A total of 1,247 samples were included to analyze the correlation between TNFAIP2 SNPs and GC risk. The wild and the mutant bases of SNPs were defined by searching the NCBI website. TNFAIP2 SNPs were classified by KASP SNP typing and sequencing as follows: wild type, heterozygous type, mutant type, dominant model, and recessive model. The differences of TNFAIP2 SNPs between the GC group and the non-GC group were compared, and the correlation between TNFAIP2 SNPs and GC risk was analyzed. The results showed that TNFAIP2 rs8126 T>C polymorphism was associated with GC risk in general populations, and the risk in TC genotype cases was higher than that in TT genotype cases (P = 0.001, OR = 1.557). In the dominant model, the GC risk in TNFAIP2 rs8126 polymorphic carriers was 1.419 times higher (P = 0.007). However, TNFAIP2 rs710100 C>T polymorphism, TNFAIP2 rs3759571 G>A polymorphism, and TNFAIP2 rs3759573 A>G polymorphism were not associated with GC risk. In particular, TNFAIP2 rs3759573 A>G polymorphism was not consistent with Hardy–Weinberg's genetic linkage balance (PHWE < 0.05) and was excluded in the subsequent analysis (Table 2).
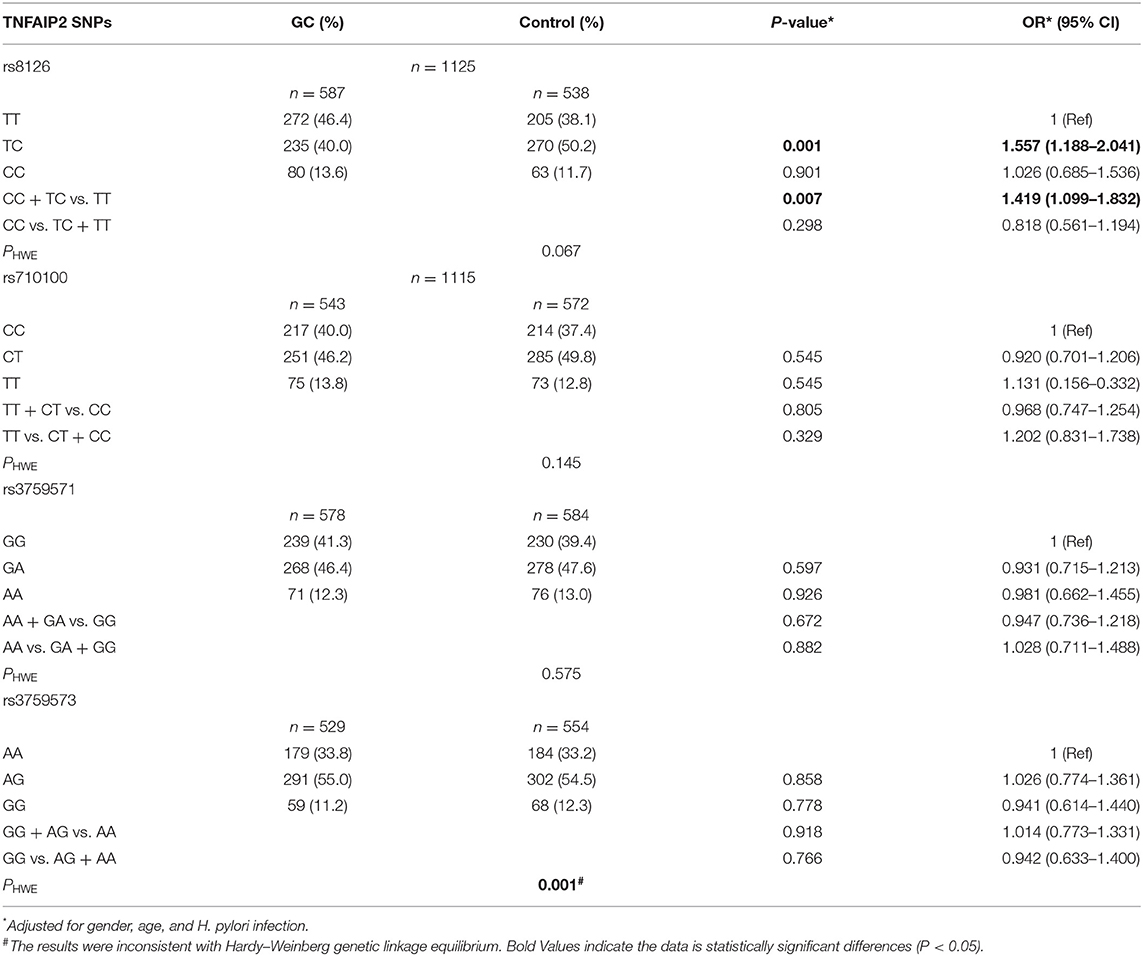
Table 2. The correlation between TNFAIP2 TagSNPs and gastric cancer (GC) risk in the general population.
The Correlation Between TNFAIP2 TagSNPs and GC Risk in Subgroup Population
In the subgroup analysis, we found that, in male subjects, TNFAIP2 rs8126 TC genotype cases were associated with a higher GC risk than TT genotype cases (P = 0.005, OR = 1.573), and GC risk was 1.443 times higher in TNFAIP2 rs8126 polymorphic carriers in the dominant model (P = 0.018). In subjects aged over 60 years, TNFAIP2 rs8126 TC genotype cases had a higher GC risk than TT genotype cases (P = 0.005, OR = 1.816), and GC risk was 1.693 times higher in TNFAIP2 rs8126 polymorphic carriers in the dominant model (P = 0.010). In subjects younger than 60 years old, TNFAIP2 rs8126 TC genotype cases had a higher GC risk than TT genotype cases (P = 0.049, OR = 1.440). In subjects without H. pylori infection, TNFAIP2 rs8126 TC genotype cases had a higher GC risk than TT genotype cases (P = 0.006, OR = 1.560), and GC risk was 1.440 times higher in TNFAIP2 rs8126 polymorphic carriers in the dominant model (P = 0.017). In non-smoking subjects, TNFAIP2 rs8126 TC genotype cases had a higher GC risk than TT genotype cases (P = 0.038, OR = 1.701), and GC risk was 1.643 times higher in TNFAIP2 rs8126 polymorphic carriers in the dominant model (P = 0.038). In non-drinking subjects, TNFAIP2 rs8126 TC genotype cases had a higher GC risk than TT genotype cases (P = 0.045, OR = 1.630) (Table 3).
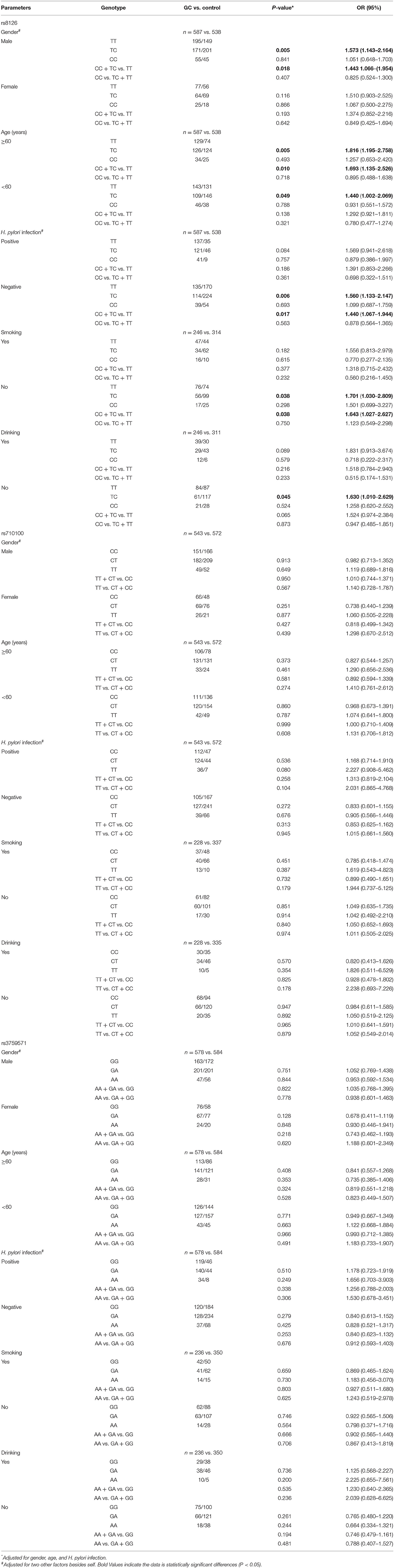
Table 3. The correlation between TNFAIP2 TagSNPs and gastric cancer (GC) risk in the subgroup population.
The Interaction Effects Between TNFAIP2 TagSNPs and Environmental Factors on GC Risk
The interaction effects between TNFAIP2 SNPs (rs8126, rs710100, and rs3759571) and environmental factors (H. pylori infection, smoking, and drinking) on GC risk were analyzed, and the results showed that there was no significant correlation between them (Pinteraction > 0.05; Table 4).
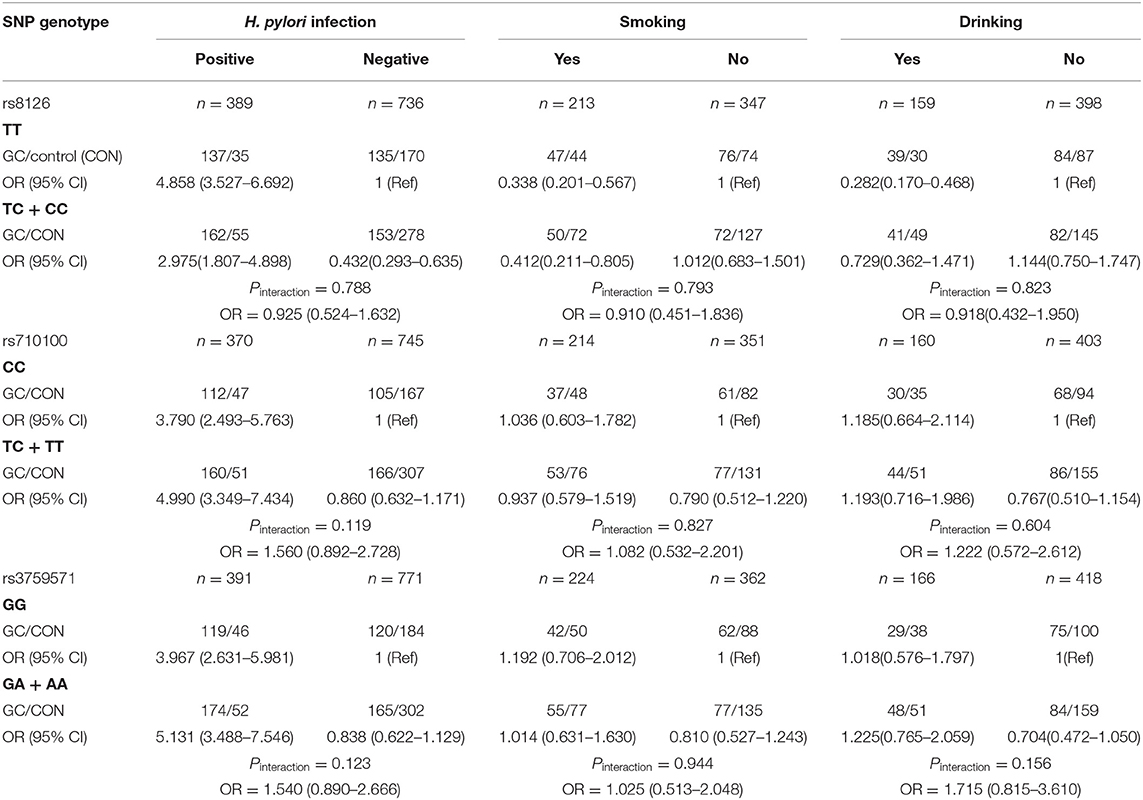
Table 4. The interaction effects between TNFAIP2 TagSNPs and environmental factors on gastric cancer (GC) risk.
The Correlation Between TNFAIP2 TagSNPs and GC Prognosis
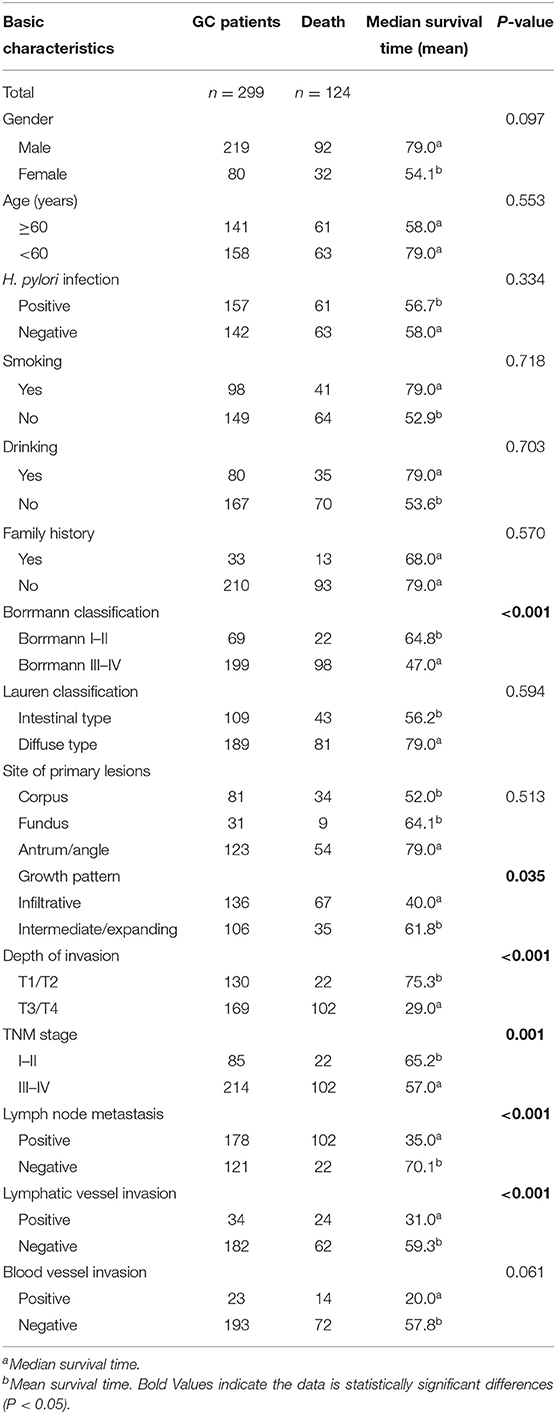
Prognostic analysis was performed in 299 GC patients who had complete survival follow-up data. We found that GC prognosis was correlated with Borrmann classification, depth of invasion, growth pattern, lymphatic vessel invasion, lymph node metastasis, and TNM stage (Table 5). Both univariate analysis and multivariate analysis showed no statistical differences between TNFAIP2 SNPs and GC prognosis (P > 0.05), suggesting that TNFAIP2 SNPs had nothing to do with GC prognosis in this group (Table 6). In the subgroup analysis, TNFAIP2 rs8126 polymorphism was stratified by gender, age, and H. pylori infection, and no correlation was found between TNFAIP2 rs8126 polymorphism and GC prognosis (P > 0.05) (Table 7).
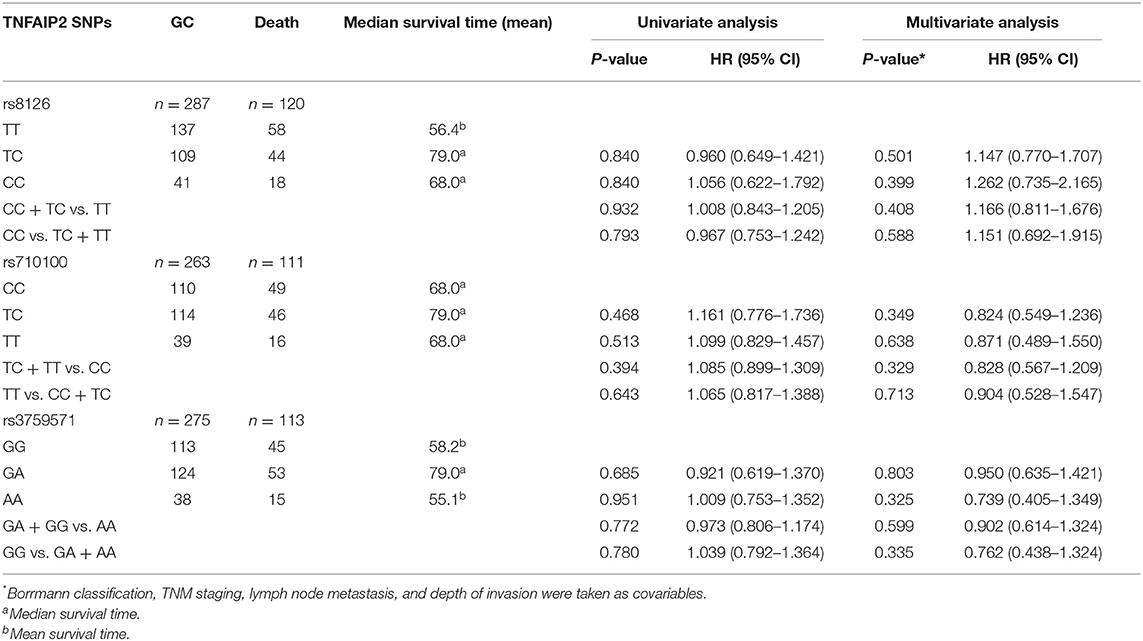
Table 6. The correlation between TNFAIP2 SNPs and gastric cancer (GC) prognosis in the general analysis.
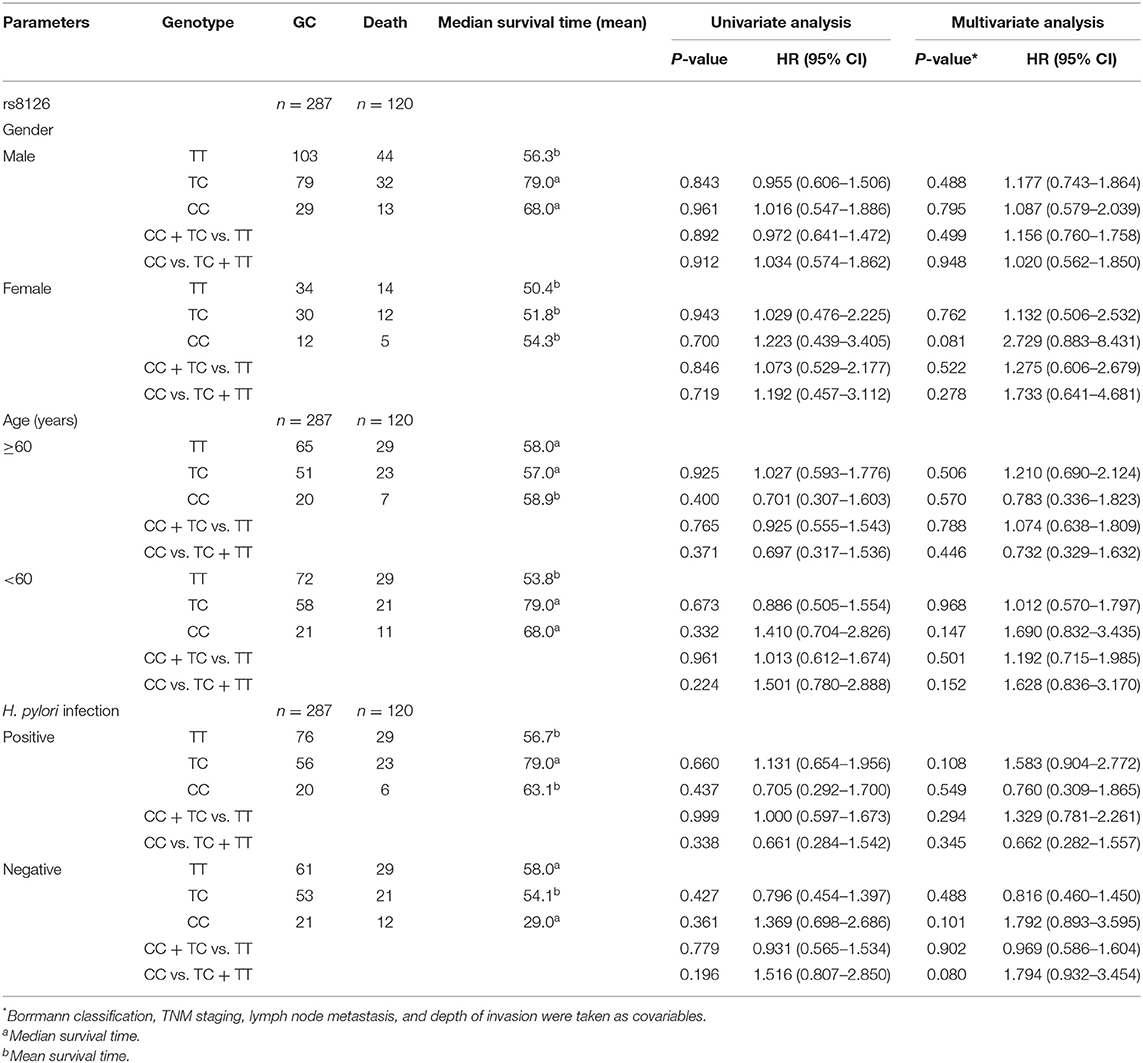
Table 7. The correlation between TNFAIP2 rs8126 polymorphism and gastric cancer (GC) prognosis in the subgroup analysis.
Serum TNFAIP2 Protein Expression Between GC Patients and Healthy Persons
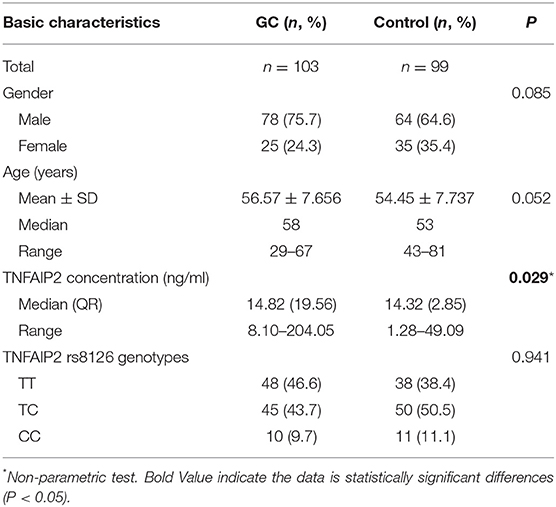
ELISA was performed on 202 serum samples randomly selected from the GC group and the healthy control group, including 103 GC patients and 99 healthy persons. There was no statistical difference in age, gender, and TNFAIP2 rs8126 genotypes between the two groups. The average age of the GC group and the healthy control group was 56.57 ± 7.656 (29–67) years old and 54.45 ± 7.737 (43–81) years old, respectively. The TNFAIP2 protein concentration in GC patients was significantly different from that in healthy persons (P = 0.029; Table 8).
The Correlation Between Serum TNFAIP2 Protein Expression and Clinicopathological Parameters in GC Patients
According to median TNFAIP2 protein concentration, 103 GC patients were divided into high-expression group and low-expression group, and the correlation between TNFAIP2 protein expression and clinicopathological parameters in GC patients was analyzed. We found that a high or a low expression of TNFAIP2 protein had no significant difference with gender, age, H. pylori infection, smoking, and drinking (Table 9).
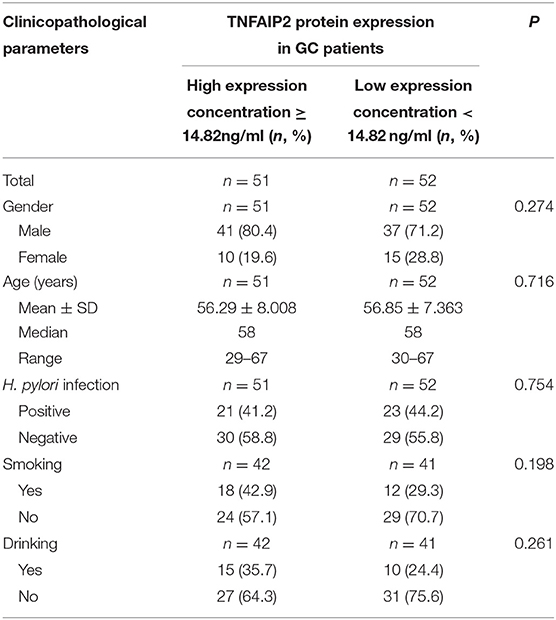
Table 9. The correlation between serum TNFAIP2 protein expression and clinicopathological parameters in gastric cancer (GC) patients.
The Correlation Between Serum TNFAIP2 Protein Expression and GC Prognosis
A total of 83 cases with complete clinical data and survival data were selected from 103 GC patients. The basic characteristics of the patients included gender, age, H. pylori infection, smoking, drinking, family history, Borrmann classification, Lauren classification, site of primary lesions, growth pattern, depth of invasion, TNM stage, and lymph node metastasis. We found significant differences in depth of invasion (P < 0.001) and lymph node metastasis (P = 0.002; Table 10). According to serum TNFAIP2 protein concentration, the univariate analysis showed that TNFAIP2 protein expression was not significantly correlated with GC prognosis (P = 0.798; hazard ratio, HR = 1.090). The multivariate analysis with depth of invasion and lymph node metastasis as covariables confirmed that there was no significant difference in GC prognosis between the two groups (P = 0.339; HR = 1.387). The results suggested that serum TNFAIP2 protein expression was not associated with the prognosis of GC patients in this group (Table 11).
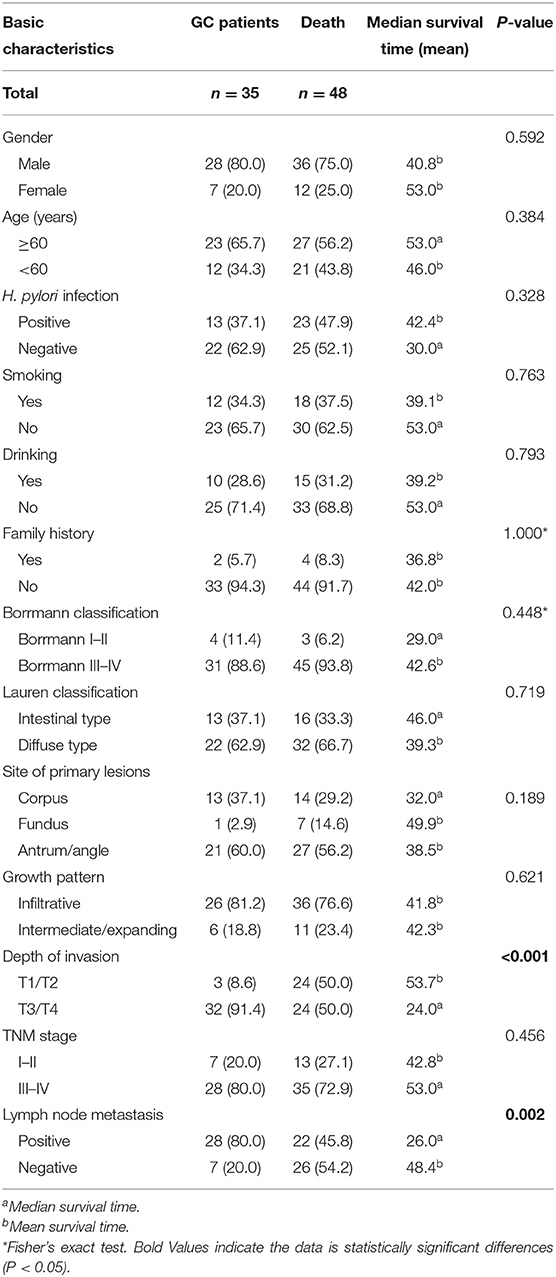
Table 10. The correlation between basic characteristics and survival in gastric cancer (GC) patients.
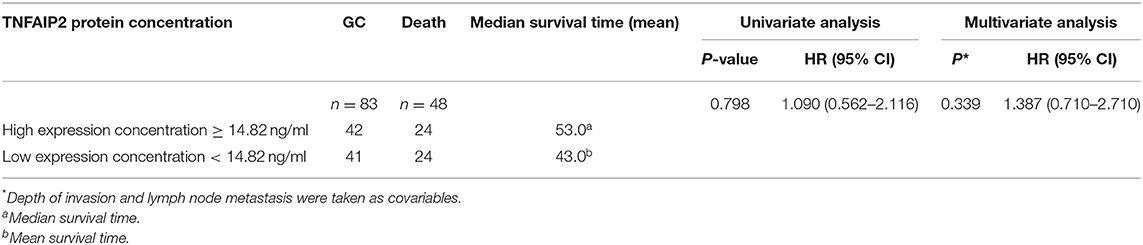
Table 11. The correlation between serum TNFAIP2 protein expression and gastric cancer (GC) prognosis.
The Correlation Between TNFAIP2 3′ UTR rs8126 T>C Polymorphism and TNFAIP2 Protein Expression
The correlation between TNFAIP2 3′ UTR rs8126 T>C polymorphism and TNFAIP2 protein expression was analyzed by different polymorphism genotypes in 103 GC patients, and we found that TNFAIP2 protein expression in rs8126 TT genotype carriers was significantly higher than that in rs8126 CC genotype carriers (P < 0.001) (Table 12).
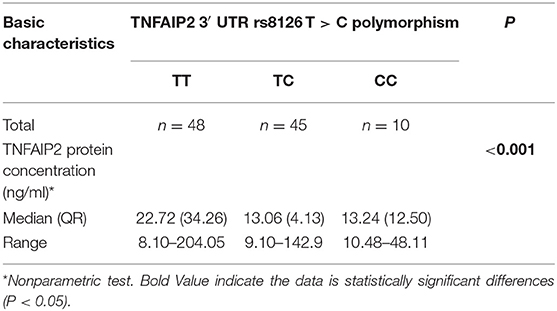
Table 12. The correlation between TNFAIP2 3′ UTR rs8126 T > C polymorphism and TNFAIP2 protein expression.
Discussion
TNFAIP2 is a novel gene induced by TNF-α and can regulate inflammatory and tumor angiogenesis (21). In recent years, studies have found that SNPs in mRNA 3′ UTR may impact the miRNA-mediated expression and regulation of oncogenes and tumor suppressors and confirmed that TNFAIP2 3′ UTR SNPs are correlated with risk of multiple malignancies, especially that TNFAIP2 3′ UTR rs8126 polymorphism may affect TNFAIP2 expression in GC, SCCHN, and ESCC by disturbing the binding of miR-184 with TNFAIP2 mRNA (14, 18, 19). However, only one study reports the correlation between TNFAIP2 SNPs and GC risk in the American population (14), and the correlation between TNFAIP2 SNPs and GC prognosis has not been reported until now, especially in Asian or Chinese populations.
This is the first study about TNFAIP2 SNPs in Chinese Han population, and this explored the correlation between TNFAIP2 SNPs and prediction as well as the prognosis of GC in a large sample population and its effect on TNFAIP2 protein expression. By analyzing TNFAIPS SNP genotyping of 1,247 samples, we found that the GC risk in TNFAIP2 rs8126 TC genotype cases was higher than that in TT genotype cases (P = 0.001, OR = 1.557), and the GC risk in polymorphic carriers of TNFAIP2 rs8126 was increased to 1.419 times in the dominant model (P = 0.007). These results were consistent with the American study and confirmed the correlation between TNFAIP2 rs8126 polymorphism and GC risk (14). In the subgroup analysis, we found that cases with TNFAIP2 rs8126 TC genotype had a higher GC risk in males, aged 60 years or older, H. pylori negative, non-smoking, and non-drinking. These results suggested that TNFAIP2 rs8126 T>C polymorphism was an important factor in predicting GC risk, and it is beneficial to the discovery and the diagnosis of early gastric cancer.
This study is the first to report the interaction effects between H. pylori infection and TNFAIP2 SNPs on GC risk. H. pylori infection is currently considered to be one of the environmental factors closely related to the risk and prognosis of GC (22, 23). Clarifying the interaction effects between TNFAIP2 SNPs and H. pylori infection is conducive to revealing the influence of key environmental factors on GC risk. Our results showed that there was no interaction between H. pylori infection and TNFAIP2 SNPs (rs8126, rs710100, and rs3759571) (Pinteraction > 0.05), suggesting that the interaction effects between H. pylori infection and TNFAIP2 SNPs could not affect GC risk in this group, and no other similar results had been reported so far. In addition, we analyzed the interaction effects between smoking and drinking and TNFAIP2 SNPs on GC risk and found that there was no interaction between smoking and drinking and TNFAIP2 SNPs on GC risk (Pinteraction > 0.05). This result was different from that of the American population (14), which may be related to differences in race, dietary habits and diet, and type and content of alcohol between Chinese and Americans.
This study also revealed the correlation between TNFAIP2 SNPs and GC prognosis in a Chinese population for the first time. Both univariate and multivariate analyses in the general population and in the subgroup suggested that TNFAIP2 rs8126 T>C polymorphism, TNFAIP2 rs3759571 G>A polymorphism, and TNFAIP2 rs3759573 A>G polymorphism were not related to GC prognosis. These results were not entirely consistent with those reported in other tumors. For example, TNFAIP2 was an independent prognostic factor for nasopharyngeal carcinoma (24) and TNFAIP2 3′ UTR rs8126 may shorten the survival time of patients with septic shock (16).
At the same time, the serum of 202 participants was tested by ELISA to explore differences in TNFAIP2 protein expression between GC patients and healthy persons. We found that the TNFAIP2 protein concentration in GC patients was significantly higher than that in healthy persons, suggesting that the TNFAIP2 protein may be more highly expressed in GC patients. However, the clinicopathological parameters such as gender, age, H. pylori infection, smoking, and drinking in GC patients did not affect serum TNFAIP2 protein expression. In addition, we analyzed the correlation between basic characteristics and survival in GC patients and found that GC patients with T1/T2 invasion depth and no lymph node metastasis had a better prognosis, but both the univariate analysis and the multivariate analysis showed that TNFAIP2 protein expression was not significantly correlated with GC prognosis, suggesting that serum TNFAIP2 protein expression was not associated with GC prognosis.
In the last part, we revealed the correlation between TNFAIP2 3′ UTR rs8126 T>C polymorphism and TNFAIP2 protein expression. As far as we know, 3′ UTR consisted of cis-/trans elements and may affect mRNA translation, stability, and subcellular localization. In malignant tumors, the reprogramming of 3′ UTRs mainly included cleavage, polyadenylation, chromosomal rearrangements, hormone-regulated 3′ UTR processing, point mutations, and polymorphisms (25). Therefore, abnormal gene expression caused by reprogramming nucleotides in 3'UTRs might be one of the important factors leading to the occurrence and the progression of tumors. rs8126 was located in the 3′ UTR of the TNFAIP2 gene sequence. A previous study showed that the rs8126 genetic variant was significantly associated with increased ESCC risk in a Chinese population (19). In this paper, our results showed that the serum TNFAIP2 protein expression in rs8126 TT genotype carriers was significantly higher than that in rs8126 CC genotype carriers, and it was suggested that TNFAIP2 3′ UTR rs8126 T>C polymorphism could affect serum TNFAIP2 protein expression. Our data also validated the previous hypothesis that functional genetic variants in 3′ UTR of gene might influence miRNA-mediated expression and regulation of mRNA.
As far as we know, this study has the largest sample size about TNFAIP2 SNPs in a Chinese Han population until now, and the study is the first to reveal the correlation between TNFAIP2 SNPs and GC risk, prognosis, and related risk factors in Chinese people. In addition, this is the first report on the correlation between serum TNFAIP2 protein expression and GC risk and prognosis. However, there are some limitations in this paper. For example, due to the lack of statistical data on previous treatment history, therapeutic effect, concomitant diseases, and other prognostic factors, these might affect the reliability of partial results, and the above results needed to be verified by further studies.
To sum up, TNFAIP2 3′ UTR rs8126 T>C polymorphism is associated with GC risk in a Chinese population, especially in cases with males, aged 60 years or older, H. pylori-negative, non-smoking, and non-drinking. However, there was no correlation between TNFAIP2 SNPs and GC prognosis. Compared with healthy persons, serum TNFAIP2 protein expression was higher in GC patients, but it was not associated with GC prognosis. In addition, TNFAIP2 3′ UTR rs8126 T>C polymorphism might affect serum TNFAIP2 protein expression, and the mechanism remains to be further explored.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: dbSNP (https://www.ncbi.nlm.nih.gov/snp/—ss2137544092, ss3984446983, ss3984446984, and ss3984446985).
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Science Research Ethics Committee of the First Affiliated Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YY and FG: conceived and designed the experiments. FG: performed the experiments. FG, QX, ZL, H-XD, Z-DZ, and L-PS: collected the samples and analyzed the data. YY: contributed reagents, materials, and analysis tools. FG and YY: wrote and revised the paper. All authors: read and approved the final manuscript.
Funding
This study was funded partly by grants from the National Key R&D Program of China (Grant #2018YFC1311600).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01127/full#supplementary-material
Supplementary Figure 1. Linkage disequilibrium diagram on TNFAIP2 tagSNPs by Haploview software. The tagSNPs of the TNFAIP2 gene were screened by Haploview software and F-SNP website was used to predict the function of tagSNPs. The parameters were set as Chinese Han population; minimum allele frequency >5%; frequency distribution r2 > 0.8. This linkage disequilibrium diagram showed that rs2234130, rs710100, rs146514706, and rs1132339 were tagSNPs of the TNFAIP2 gene, and the alleles of rs2234130 included rs8126, rs3759571, rs3759573, rs2234130, rs749206, rs4369588, rs2234143, rs8176365, rs2234131, rs2403128, rs944000, rs1887940, rs2234133, rs4283165, and rs11160713.
Supplementary Figure 2. Prediction diagram on TNFAIP2 tagSNPs by the NIH Snpinfo website. The functional tagSNPs of the TNFAIP2 gene were predicted by the NIH Snpinfo website. The parameters were set as Chinese Han population; minimum allele frequency >5%; frequency distribution r2 > 0.8. This prediction diagram showed that rs1887940 and rs710100 were tagSNPs of the TNFAIP2 gene, and the alleles of rs1887940 included rs8126, rs1887940, rs2234130, and rs749206.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. (2018) 103:356–87. doi: 10.1016/j.ejca.2018.07.005
3. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018). 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
4. Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. (2017) 19:36. doi: 10.1007/s11894-017-0575-8
5. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
6. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. (2019) 39:22. doi: 10.1186/s40880-019-0368-6
7. Yang L, Zheng R, Wang N, Yuan Y, Liu S, Li H, et al. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. (2018) 30:291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01
8. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. (2014) 23:700–13. doi: 10.1158/1055-9965.EPI-13-1057
9. Russo A, Li P, Strong VE. Differences in the multimodal treatment of gastric cancer: East versus west. J Surg Oncol. (2017) 115:603–14. doi: 10.1002/jso.24517
10. Yoshida T, Yamaguchi T, Maekawa S, Takano S, Kuno T, Tanaka K, et al. Identification of early genetic changes in well-differentiated intramucosal gastric carcinoma by target deep sequencing. Gastric Cancer. (2019) 22:742–50. doi: 10.1007/s10120-019-00926-y
11. Zou J, Wu D, Li T, Wang X, Liu Y, Tan S. Association of PD-L1 gene rs4143815 C>G polymorphism and human cancer susceptibility: a systematic review and meta-analysis. Pathol Res Pract. (2019) 215:229–34. doi: 10.1016/j.prp.2018.12.002
12. Shaw V, Bullock K, Greenhalf W. Single-nucleotide polymorphism to associate cancer risk. Methods Mol Biol. (2016) 1381:93–110. doi: 10.1007/978-1-4939-3204-7_6
13. Laytragoon-Lewin N, Cederblad L, Andersson BÅ, Olin M, Nilsson M, Rutqvist LE, et al. Single-nucleotide polymorphisms and cancer risk, tumor recurrence, or survival of head and neck cancer patients. Oncology. (2017) 92:161–9. doi: 10.1159/000452278
14. Xu Y, Ma H, Yu H, Liu Z, Wang LE, Tan D, et al. The miR-184 binding-site rs8126 T>C polymorphism in TNFAIP2 is associated with risk of gastric cancer. PLoS One. (2013) 8:e64973. doi: 10.1371/journal.pone.0064973
15. Jia L, Zhou Z, Liang H, Wu J, Shi P, Li F, et al. KLF5 promotes breast cancer proliferation, migration and invasion in part by upregulating the transcription of TNFAIP2. Oncogene. (2016) 35:2040–51. doi: 10.1038/onc.2015.263
16. Thair SA, Topchiy E, Boyd JH, Cirstea M, Wang C, Nakada TA, et al. TNFAIP2 Inhibits Early TNFα-induced NF-κB signaling and decreases survival in septic shock patients. J Innate Immun. (2016) 8:57–66. doi: 10.1159/000437330
17. Rusiniak ME, Yu M, Ross DT, Tolhurst EC, Slack JL. Identification of B94 (TNFAIP2) as a potential retinoic acid target gene in acute promyelocytic leukemia. Cancer Res. (2000) 60:1824–9.
18. Liu Z, Wei S, Ma H, Zhao M, Myers JN, Weber RS, et al. A functional variant at the miR-184 binding site in TNFAIP2 and risk of squamous cell carcinoma of the head and neck. Carcinogenesis. (2011) 32:1668–74. doi: 10.1093/carcin/bgr209
19. Zhang J, Yu H, Zhang Y, Zhang X, Zheng G, Gao Y, et al. A functional TNFAIP2 3'-UTR rs8126 genetic polymorphism contributes to risk of esophageal squamous cell carcinoma. PLoS One. (2014) 9:e109318. doi: 10.1371/journal.pone.0109318
20. Gong YH, Sun LP, Jin SG, Yuan Y. Comparative study of serology and histology based detection of Helicobacter pylori infections: a large population-based study of 7,241 subjects from China. Eur J Clin Microbiol Infect Dis. (2010) 29:907–11. doi: 10.1007/s10096-010-0944-9
21. Jia L, Shi Y, Wen Y, Li W, Feng J, Chen C. The roles of TNFAIP2 in cancers and infectious diseases. J Cell Mol Med. (2018) 22:5188–95. doi: 10.1111/jcmm.13822
22. Alfarouk KO, Bashir AHH, Aljarbou AN, Ramadan AM, Muddathir AK, AlHoufie STS, et al. The possible role of Helicobacter pylori in gastric cancer and its management. Front Oncol. (2019) 9:75. doi: 10.3389/fonc.2019.00075
23. Huang J, Hang JJ, Qin XR, Huang J, Wang XY. Interaction of H. pylori with toll-like receptor 2-196 to−174 ins/del polymorphism is associated with gastric cancer susceptibility in southern China. Int J Clin Oncol. (2019) 24:494–500. doi: 10.1007/s10147-018-1379-z
24. Chen LC, Chen CC, Liang Y, Tsang NM, Chang YS, Hsueh C, et al. A novel role for TNFAIP2: its correlation with invasion and metastasis in nasopharyngeal carcinoma. Mod Pathol. (2011) 24:175–84. doi: 10.1038/modpathol.2010.193
Keywords: gastric cancer, TNFAIP2, SNP, prediction, prognosis
Citation: Guo F, Xu Q, Lv Z, Ding H-X, Sun L-P, Zheng Z-D and Yuan Y (2020) Correlation Between TNFAIP2 Gene Polymorphism and Prediction/Prognosis for Gastric Cancer and Its Effect on TNFAIP2 Protein Expression. Front. Oncol. 10:1127. doi: 10.3389/fonc.2020.01127
Received: 14 December 2019; Accepted: 04 June 2020;
Published: 24 July 2020.
Edited by:
Bin Li, Jinan University, ChinaReviewed by:
Pim Johan Koelink, Amsterdam University Medical Center, NetherlandsManpreet Kaur, Guru Nanak Dev University, India
Copyright © 2020 Guo, Xu, Lv, Ding, Sun, Zheng and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Yuan, yuanyuan@cmu.edu.cn
 Fang Guo
Fang Guo Qian Xu1,3,4
Qian Xu1,3,4 Yuan Yuan
Yuan Yuan