
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 30 June 2020
Sec. Surgical Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01050
This article is part of the Research Topic Surgical Interventions in Gastric Cancers View all 9 articles
 Yun-Shi Huang1,2†
Yun-Shi Huang1,2† Xiao-Dong Chen1†
Xiao-Dong Chen1† Ming-Ming Shi2
Ming-Ming Shi2 Li-Bin Xu2
Li-Bin Xu2 Su-Jun Wang2
Su-Jun Wang2 Wei-Sheng Chen2
Wei-Sheng Chen2 Guan-Bao Zhu2*
Guan-Bao Zhu2* Wei-Teng Zhang1,2*
Wei-Teng Zhang1,2* Xian Shen1,2*
Xian Shen1,2*Objectives: The present study aimed to explore the association between spleen density and post-operative outcomes of patients after curative gastrectomy.
Methods: From June 2014 to December 2015, we conducted a retrospective study to analyze pertinent clinical data from gastric cancer patients who underwent gastrectomy at the First and the Second Affiliated Hospital of Wenzhou Medical University. Spleen density was determined via computed tomography scans. Univariate and multivariate analyses were performed to determine the risk factors associated with post-operative outcomes after gastric cancer surgery.
Results: Three hundred and ninety five patients were included, of whom 98 (24.8%) were defined as having a diffuse reduction of spleen density based on diagnostic cutoff values (spleen density ≤ 43.89 HU). Multivariate analysis revealed diffuse reduction of spleen density as an independent risk factor for post-operative complications and long-term overall survival.
Conclusions: Spleen density can predict severe postoperative complications and long-term overall survival in gastric cancer patients. As an imaging evaluation method, spleen density is a novel tool can be used in clinical as a prognostic predictor for patients with gastric cancer.
Gastric cancer remains the fifth most frequent malignancy and the third leading cause of cancer deaths globally (1). In China, 679,100 individuals were diagnosed with gastric cancer, and 498,000 gastric cancer-related deaths occurred in 2015 (2). Despite the development of multimodal treatments including surgery, traditional chemotherapy, and the implementation of neoadjuvant therapy, which can greatly improve the prognosis, gastric cancer is still a deadly disease with a poor clinical outcome (3). However, an accurate method to predict the prognosis of gastric cancer patients simply and effectively is not yet available.
The spleen is the largest peripheral immune organ and participates in the regulation of immune homoeostasis, but its role has been ignored among clinicians (4, 5). Diffuse reduction of spleen density (DROSD) is an imaging manifestation in the abdominal computed tomography (CT), which was originally reported in patients with acute pancreatitis (AP) (6). Meanwhile, in our long-term clinical practice, we also observed that this phenomenon existed in some gastric cancer patients. Interestingly, the post-operative prognosis of these patients was more serious. Some scholars found that AP patients with DROSD had more severe immune dysfunction than without (7). The host immune system is relevant to cancer development and progression (8, 9). Currently, there is a paucity of studies investigating the impact of DROSD in gastric cancer patients.
This study aimed to investigate whether DROSD, as determined by decreased CT values of the spleen, would predict post-operative outcomes in a cohort of patients after curative gastrectomy for gastric cancer.
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. From June 2014 to December 2015, only patients who underwent curative gastrectomy for gastric cancer were included, with the following criteria: (1) patients who underwent preoperative abdominal CT scans and had a serological examination within 1 month before surgery and (2) those who are willing to participate in this study and provide informed consent. The following patients were excluded: (1) patients with past histories of splenic diseases, (2) those who have hematological system disease such as lymphoma, (3) those who have severe preoperative infection, (4) those who have incomplete medical records, (5) those who underwent a palliative surgery, and (6) those who undergone neoadjuvant chemotherapy. We collected and analyzed the data from the remaining 395 GC patients who underwent radical gastrectomy in the First Affiliated Hospital of Wenzhou Medical University and the Second Affiliated Hospital of Wenzhou Medical University. The experimental flow chart is shown in Figure 1. All patients underwent conventional therapy following the Japanese Gastric Cancer Treatment Guidelines (10).
We prospectively collected and analyzed the following data in this study: (1) age; gender; body mass index (BMI); Nutritional Risk Screening 2002 (NRS 2002) scores; albumin and hemoglobin concentration; platelet/lymphocyte ratio (PLR); neutrophil/lymphocyte ratio (NLR); sarcopenia; Charlson Comorbidity Index; the American Society of Anaesthesiologists (ASA) grade; hypertension; diabetes mellitus; previous abdominal surgery; differentiation of tumors; tumor location, size and TNM stage (preoperative patients and disease characteristics aspect); (2) laparoscopy-assisted, type of resection, combined resection, and type of reconstruction (the operative details aspect); and (3) long-term overall survival (any causes of deaths), postoperative complications and readmission within 1 month after surgery (the postoperative outcomes aspect).
Spleen densities of patients with gastric cancer were measured at the Department of Radiology of the First Affiliated Hospital of Wenzhou Medical University using the same scanning parameters. In short, location conditions included 120 kV of the tube voltage, 50 mA of the tube current, 750 ms of tube circumrotation time, 5 mm of the layer thickness, and 5 mm of the layer spacing. Retrospective analysis of non-enhanced CT scan sequence images was performed by two investigators. As shown in Figure 2, considering that the spleen density is affected by its own hemoperfusion, spleen CT values (Hounsfield units, HU) were measured at the upper pole, hilum, and inferior pole levels using a dedicated processing system (version 3.0.11.3 BN17 32 bit; INFINITT Healthcare Co. Ltd.). Spleen density was defined as the average of the three measurements of spleen CT values. In order to control for systematic errors, re-averaging of the spleen density measurements by the two investigators was ultimately performed. The mean of spleen density in this cohort were 46.95 HU (interquartile range, 43.91–49.80 HU).
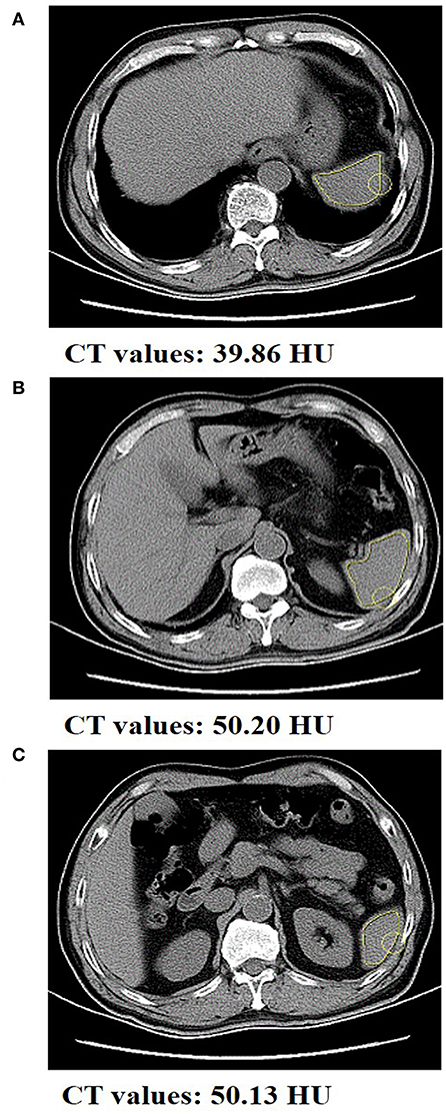
Figure 2. Abdominal CT scan of a gastric cancer patient with spleen density. The CT value of spleen at the (A) upper pole level, (B) the hilum level, and the (C) inferior pole level was 39.86, 50.20, and 50.13 HU, respectively.
The restrictive cubic splines by R Language was plotted to check whether spleen density is a linear risk structure. As shown in Supplementary Figure 1, the risk curve fluctuation obviously as spleen density gradually increases, considering the linear relationship is not very strong (P = 0.169). Therefore, categorical variable instead of continuous variable was used to analyze spleen density. To determine the spleen density cutoff values, with a mostly significant difference, we used optimum stratification to find the most significant P-value by means of log-rank chi-squared statistics (11). In the previous literature, this method has been presented to solve the threshold value of the continuous variable at which patients are best separated with respect to time to mortality (12). The cut-off values obtained by this method were used to classify patients into DROSD and non-DROSD.
All patients were required to come back and undergo the necessary examinations within the first month after surgery. After that, they were followed up every 3 months for further examinations as needed. Patients were contacted by phone and were scheduled to come back to the hospital to fulfill the follow-up program in the above time points. The follow-up program was consisted of a physical examination, laboratory tests, and ultrasonography and/or CT and/or endoscopy. Data on patient mortality, including time and cause of death, were obtained primarily through medical records, telephone follow-up and the local population database. Overall survival rate was determined as the proportion of all patients who survive after surgery for gastric cancer. Postoperative complication was recorded within a month after surgery, which was classified using the Clavien–Dindo (CD) Classification (13). Severe postoperative complications (SPCs) were defined as complications classified as Grade III or above. The date of the last follow-up was October 2018.
In this cohort, most of the patients were followed up by review of medical records and phone, and a small number of patients were contacted directly by phone. We eventually completed the follow-up work by retrieving data from the local population database for 29 patients who cannot be contacted by phone.
The Kolmogorov–Smirnov test was used to determine whether continuous data conform to normal distribution. Normal distribution, non-normal distribution, and categorical variables were presented as mean and standard deviation, median and interquartile range, and quantity and percentage, respectively. The Student's t-test was used to compare the data that conform to normal distribution. Mann–Whitney U-test was used to compare non-normally distributed data and Pearson chi-squared test or Fisher's exact test to compare categorical data. The outcome of this study was overall survival, calculated from the date of surgery to the date of death or last available follow-up. The Kaplan–Meier method was used to analyze the overall survival, and log-rank test was performed to compare the difference in survival between the subgroups. Multivariate Cox proportional hazards regression analysis was performed to determine the independent risk factors for long-term overall survival. Variables with P < 0.10 in a univariate analysis were included in the multivariate analysis, and variables with P < 0.05 were retained ultimately in the multivariate model.
All tests were two-tailed and were considered to be statistically significant when P < 0.05. All data were analyzed using the SPSS Statistics version 20.0 (IBM, Armonk, New York, USA).
From June 2014 to December 2015, a total of 395 patients met our criteria and were included for analysis. Demographic and clinical characteristics of patients with gastric cancer are represented in Table 1.
Cutoff value for DROSD associated with long-term overall survival was 43.89 HU. Using this cutoff value, 98 (24.8%) patients were found to have DROSD. As shown in Table 1, patients with DROSD had a lower preoperative hemoglobin level, higher NLR, hypertension, larger tumor size and poorer C–D Classification than those without DROSD (all P < 0.05).
As Table 2 shows, there were 157 postoperative events involving 93 patients (23.5%). Of them, 42 (10.6%) patients had grade IIIa or higher PCs. Pulmonary complications, which mainly included pulmonary infections and pleural effusions, and intra-abdominal infections were the most frequent PCs. Postoperative infection complications were included Grade II or above wound infection, pulmonary infections and intra-abdominal infections.
On univariate analysis, age (P = 0.004), NRS score (P = 0.057), Charlson comorbidity index (P = 0.037), diabetes mellitus (P = 0.071), combined resection (P = 0.002), laparoscopy-assisted (P = 0.010), and DROSD (P = 0.013) differed significantly (Table 3). Significant variables on univariate analysis were included in the multivariate logistic regression analysis. Age (OR = 2.459, P = 0.014), Combined resection (OR = 3.495, P = 0.004), laparoscopy-assisted (OR = 0.222, P = 0.044), and DROSD (OR = 2.390, P = 0.014) were independently associated with SPCs.
Based on above results, we also evaluate the relationship between DORSD and postoperative infection complications. On univariate analysis, hypoalbuminemia (P = 0.082), NLR (P = 0.002), charlson comorbidity index (P < 0.001), ASA grade (P = 0.031), tumor size (P = 0.003), TNM stages (P = 0.004), total gastrectomy (P = 0.006), combined resection (P = 0.063), laparoscopy-assisted (P = 0.004) and DROSD (P = 0.018) differed significantly (Table 4). On multivariate analysis, NLR (OR = 1.880, P = 0.040), charlson comorbidity index (OR = 2.457, P = 0.001), tumor size (OR = 2.105, P = 0.016) and laparoscopy-assisted (OR = 0.264, P = 0.031) were independently associated with postoperative infectious complications (all P < 0.05).
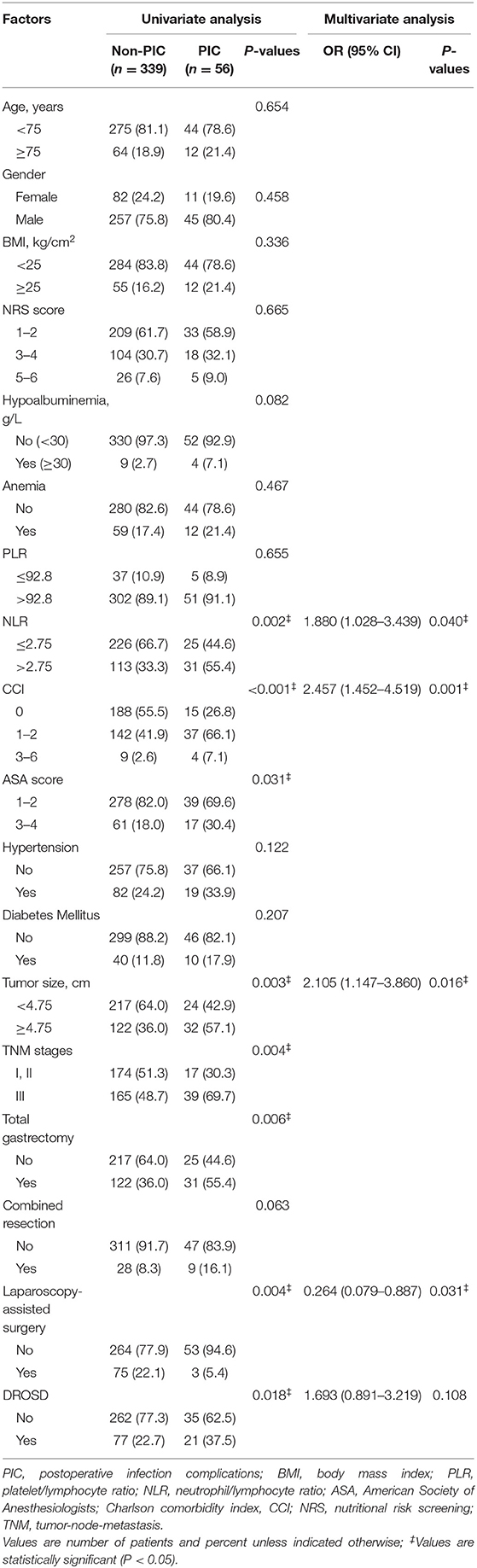
Table 4. Univariate1 and multivariate analysis associated with post-operative infection complications.
We excluded 4 patients who died within 1 month after surgery in order to better study the relationship between DROSD and OS. The remaining 391 patients were included in the analysis. The median follow-up duration was 39.2 months (range, 18.3–45.5 months). At the last follow-up, 151 (38.6%) patients died.
As shown in Figure 3, patients with DROSD had a poorer OS rate than those without DROSD (P < 0.001). The 1- and 3-year overall survival rates were 76.0 and 45.8%, respectively, for patients with DROSD, and were 87.8 and 66.4%, respectively, for those without DROSD. The median OS was shorter in patients with DROSD than in those without DROSD (28.9 vs. 51.7 months; P < 0.001; Figure 3).
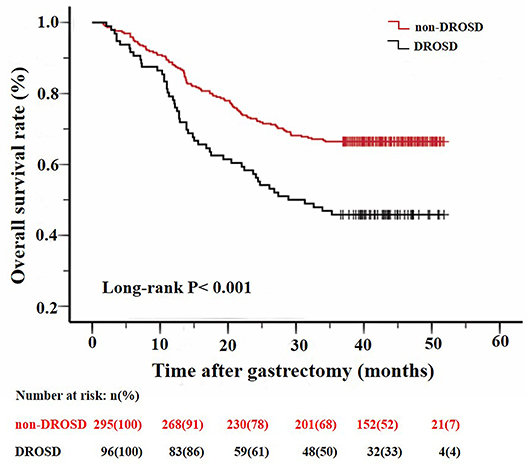
Figure 3. Kaplan–Meier survival curves for overall survival in patients with and in those without DROSD. Two curves were compared using log-rank test.
We further assessed the prognostic value of DROSD in different TNM stage groups. The results revealed that preoperative spleen density was a prognostic indicator in patients with stage II (P = 0.014; Figure 4A) and stage III (P = 0.017; Figure 4C) gastric cancer. However, for patients with stage I gastric cancer, no significant association of spleen density with OS was identified (P = 0.560; Figure 4B).
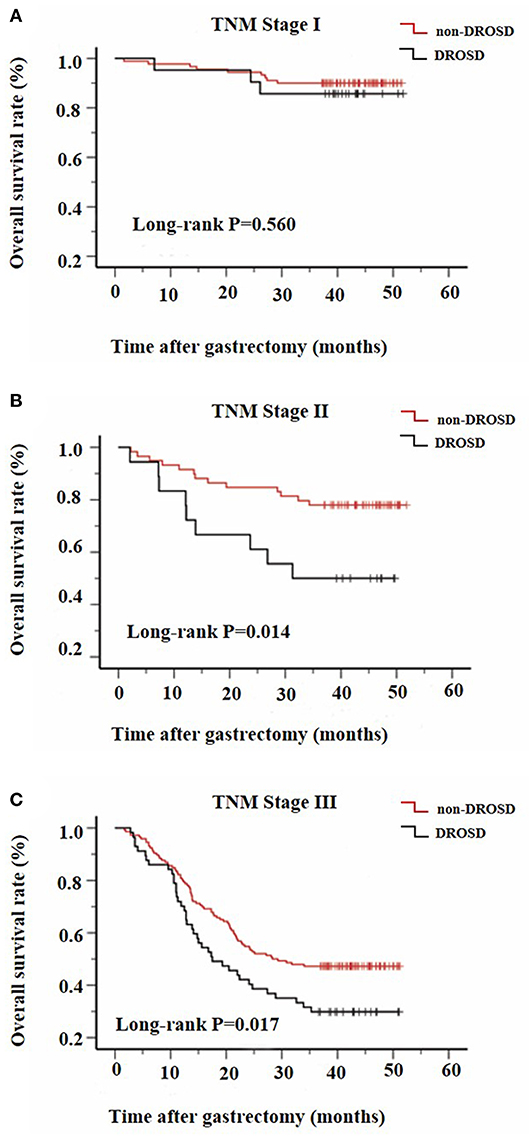
Figure 4. Kaplan–Meier survival curves for overall survival in patients with and in those without DROSD under adjusted TNM stage. Overall survival of patients with (A) TNM stage I; (B) TNM stage II; and (C) TNM stage III gastric cancer.
As shown in Table 5, in a univariate analysis, age (P < 0.0001), NRS 2002 scores (P < 0.0001), hypoalbuminemia (P = 0.001), NLR (P = 0.008), Charlson Comorbidity Index (P < 0.0001), ASA grade (P = 0.057), hypertension (P = 0.012), differentiation of tumor (P < 0.0001), tumor location (P < 0.0001), tumor size (P < 0.0001), TNM stage (P < 0.0001), total gastrectomy (P < 0.0001), combined resection (P < 0.0001), laparoscopy-assisted surgery (P = 0.02), type of reconstruction (P < 0.0001), C–D Classification (P < 0.0001), readmission within 30 days (P < 0.016), and DROSD (P < 0.0001) were significant prognostic factors.
Significant variables in a univariate analysis were included in multivariate Cox regression analysis. We found that age (HR = 1.782, P = 0.003), Charlson Comorbidity Index of 3–6 (HR = 3.210, P = 0.001), diffuse gastric cancer (HR = 3.470, P = 0.001), advanced TNM stage (P < 0.0001), total gastric resection (HR = 2.433, P < 0.0001), readmission within 30 days (HR = 2.078, P = 0.008), and DROSD (HR = 1.568, P = 0.011) were independently associated with a lower OS rate.
The topic of post-operative outcomes after curative gastrectomy is of great concern for both surgeons and patients (14, 15). To study the related factors affecting prognosis of gastric cancer and early identification of patients with a poor prognosis will be particularly meaningful (16). There are many indicators to predict the prognosis of gastric cancer (17, 18). The downside of TNM stage, a classic indicator of prognosis for gastric cancer, was determined postoperatively (19). Currently, lack of efficient means to early predict postoperative outcomes of patients has been considered as one of the obstacles for improve prognosis of gastric cancer.
In contrast, as an imaging evaluation method, the measurement of spleen density by CT is compatible with daily clinical practice because it is well-visualized, cost-effective, and can easily be diagnosed preoperatively. CT of the abdomen is currently the primary means of staging for gastric cancer and is widely available in clinical practice (20). However, the value of abdominal CT has not been fully reflected. Spleen density is a novel tool that can be used in clinical practices as a prognostic predictor for patients with gastric cancer. In the present study, we reported that the incidence rate of DROSD was up to 24.8% in the patient cohort. Furthermore, DROSD was identified as an independent risk factor for post-operative complications (OR = 2.390, P = 0.014) and long-term OS (HR = 1.568, P = 0.011) in gastric cancer. Through this paper, we hope that patients with poor prognosis can be screened early according to spleen density.
This is the first time we reported that DROSD has a negative impact on short- and long-term prognosis for patient after radical gastrectomy. The mechanisms by which DROSD confers increased risk of poor prognosis are still unclear, but the following reasons can be hypothesized. First, spleen is an abdominal parenchymal organ of the body (21). AP patients with DROSD have downregulated immune function, and DROSD patients had more severe lymphocytes decreased than non-DROSD patients (7). It is worth noting that immune function were associated with favorable prognosis for gastric cancer patients (22–24). Meantime, we also found that DROSD patients have a higher NLR level (P < 0.05). NLR is an indicator of systemic inflammatory response (25). The higher NLR level can enhance the occurrence of inflammatory cytokine cascades (26) and negatively affect the immune system, which can partially explain the negative impact of DROSD on post-operative outcomes. Whether gastric cancer patients with DROSD have also experienced the function disorder of immunity, which lead to the patient having short- and long-term differences still requires further experimental verification. Second, it has been reported that splenic volume increase is a surrogate marker of inflammation cells accumulation and associated with worse long-term survival (27, 28). As mentioned in the previous literature, the reduction of spleen density in acute severe pancreatitis rats was related to the increase of spleen volume (7). There may be a correlation between the spleen density and the volume in human, which leads to spleen density associated with poor long-term prognosis. Third, some scholars have speculated that DROSD is caused by spleen fat infiltration (6). Does obesity affect the prognosis of cancer patients? Visceral fat area, as an evaluation index of obesity, can evaluate operative difficulties and is reportedly associated with post-operative complications (29, 30). However, BMI has little to do with long-term prognosis in the present study (P > 0.05). Even the previous literature has pointed out that obesity is a protective factor for the long-term prognosis of cancer patients (31–33).
Spleen density is susceptible to many factors, such as hemoperfusion. The mechanism for the DROSD is not fully expounded. It has been reported that spleen density reduction is associated with lipid metabolism (6). Some animal experiments show that DROSD is not related to lipid deposition but hemoperfusion (7). The rich blood flow of spleen can change physical density, thereby affecting its density value on CT (34, 35). This is consistent with the result in Table 1, that is, DROSD is associated with hypertension and preoperative hemoglobin concentration (P < 0.05). Whether DROSD is related to lipid metabolism or hemoperfusion needs to be further studied in animal and human studies. Meantime, we also noticed that NLR and others, as possible confounding factors of spleen density, can affecting the reliability of conclusion. Therefore, factors such as NLR and hypoalbuminemia were included in the multivariate analysis, and the results showed that DROSD was an independent risk factor that affected the post-operative outcome of gastric cancer patients.
We found that old age, charlson comorbidity index of 3–6, diffuse gastric cancer, advanced TNM stage, total gastric resection, and readmission within 30 days were associated with poor prognosis in patients with gastric cancer. The survival difference in the elderly patients and young patients can be partially explained by the dissimilarity in treatment (36). Liu et al. (37) found that the OS rate after distal gastrectomy for distal gastric cancer patients was higher than that after total gastrectomy. Moreover, some literature also pointed out that Charlson Comorbidity Index, diffuse gastric cancer, and readmission were also associated with poor long-term survival (38–40).
Similar to our research result, tumor staging is closely related to long-term prognosis of cancer (17). To objectively evaluate the impact of spleen density on the OS, we stratified the patients according to their TNM stage. The results showed that DROSD group has a significantly poorer OS than the non-DROSD group, under TNM stage II and III (P < 0.05). However, for patients with TNM stage I, the difference was not significant, although there was a trend toward worse OS in the DROSD patients. Since patients with TNM stage I generally have a longer postoperative survival time, we propose that a longer follow-up period is needed to further research the effect of DROSD on long-term postoperative survival.
Our study had several potential limitations that should not be ignored. First, the data of this study were obtained only from double hospital, and a bias may exist due to the lack of multicenter validation of the research conclusions. Second, even we assigned two investigators to measure the spleen density together, the artificial measurement errors still exist.
DROSD is an independently risk factor for severe postoperative complications and long-term overall survival in gastric cancer patients. As an imaging evaluation method, spleen density is a novel tool can be used in clinical as a prognostic predictor for patients with gastric cancer.
The datasets generated for this study are available on request to the corresponding author.
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and the Second Affiliated Hospital of Wenzhou Medical University.
All authors contributed in drafting the manuscript and revising it critically. Furthermore, they were involved in the following tasks. Y-SH planned and designed the study and directed its implementation. X-DC drafted the protocol. M-MS obtained statutory and ethics approvals. L-BX contributed to data acquisition. S-JW conducted statistical analyses. W-SC had access to all raw data. XS did the data preparation and quality control. W-TZ and G-BZ wrote and revised the manuscript. All authors read and approved the final manuscript prior to submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01050/full#supplementary-material
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, et al. Morbidity and mortality associated with gastrectomy for gastric Cancer. Ann Surg Oncol. (2014) 21:3008–14. doi: 10.1245/s10434-014-3664-z
4. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. (2005) 5:606–16. doi: 10.1038/nri1669
5. Vancauwenberghe T, Snoeckx A, Vanbeckevoort D, Dymarkowski S, Vanhoenacker FM. Imaging of the spleen: what the clinician needs to know. Singapore Med J. (2015) 56:133–44. doi: 10.11622/smedj.2015040
6. Jiang XY, Bian J, Zhang CZ, Wang SS, Nie TM, Zhang L. Transient reduction of spleen density in acute pancreatitis: case reports and literature review. J Comput Assist Tomogr. (2014) 38:568–70. doi: 10.1097/RCT.0000000000000082
7. Shao G, Zhou Y, Song Z, Jiang M, Wang X, Jin X, et al. The diffuse reduction in spleen density: an indicator of severe acute pancreatitis? Biosci Rep. (2017) 37:BSR20160418. doi: 10.1042/BSR20160418
8. Prestwich RJ, Errington F, Hatfield P, Merrick AE, Ilett EJ, Selby PJ, et al. The immune system–is it relevant to cancer development, progression and treatment? Clin Oncol. (2008) 20:101–12. doi: 10.1016/j.clon.2007.10.011
9. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
10. Japanese gastric cancer treatment guidelines 2014. (ver. 4). Gastric Cancer. (2017) 20:1–19. doi: 10.1007/s10120-016-0622-4
11. Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS. Finding Optimal Cutpoints for Continuous Covariates With Binary and Time-to-Event Outcomes. Technical Report Series #79. Mayo Foundation (2006).
12. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine. (2016) 95:e3164. doi: 10.1097/MD.0000000000003164
13. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
14. Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, et al. Medical management of gastric cancer: a 2017 update. Cancer Med. (2018) 7:123–33. doi: 10.1002/cam4.1274
15. Kim CY, Nam BH, Cho GS, Hyung WJ, Kim MC, Lee HJ, et al. Learning curve for gastric cancer surgery based on actual survival. Gastric Cancer. (2016) 19:631–8. doi: 10.1007/s10120-015-0477-0
16. Moehler M, Galle PR, Gockel I, Junginger T, Schmidberger H. The multidisciplinary management of gastrointestinal cancer. Multimodal treatment of gastric cancer. Best Pract Res Clin Gastroenterol. (2007) 21:965–81. doi: 10.1016/j.bpg.2007.10.003
17. Ye J, Ren Y, Wei Z, Hou X, Dai W, Cai S, et al. External validation of a modified 8th AJCC TNM system for advanced gastric cancer: Long-term results in southern China. Surg Oncol. (2018) 27:146–53. doi: 10.1016/j.suronc.2018.02.009
18. Lu J, Chen Y, Liu Y, Ding J, Piao Z, Liu W. Clinical significance of prognostic score based on age, tumor size, and grade in gastric cancer after gastrectomy. Cancer Manag Res. (2018) 10:4279–86. doi: 10.2147/CMAR.S171663
19. Wittekind C. The development of the TNM classification of gastric cancer. Pathol Int. (2015) 65:399–403. doi: 10.1111/pin.12306
20. Kim JW, Shin SS, Heo SH, Choi YD, Lim HS, Park YK, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. (2012) 22:654–62. doi: 10.1007/s00330-011-2283-3
21. Sinwar PD. Overwhelming post splenectomy infection syndrome - review study. Int J Surg. (2014) 12:1314–6. doi: 10.1016/j.ijsu.2014.11.005
22. Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. (2016) 42:1176–82. doi: 10.1016/j.ejso.2016.05.029
23. Kanda M, Mizuno A, Tanaka C, Kobayashi D, Fujiwara M, Iwata N, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine. (2016) 95:e3781. doi: 10.1097/MD.0000000000003781
24. Sakimura C, Tanaka H, Okuno T, Hiramatsu S, Muguruma K, Hirakawa K, et al. B cells in tertiary lymphoid structures are associated with favorable prognosis in gastric cancer. J Surg Res. (2017) 215:74–82. doi: 10.1016/j.jss.2017.03.033
25. Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci Rep. (2017) 7:16717. doi: 10.1038/s41598-017-16955-5
26. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. (2018) 44:607–12. doi: 10.1016/j.ejso.2018.02.003
27. Takeishi K, Kawanaka H, Itoh S, Harimoto N, Ikegami T, Yoshizumi T, et al. Impact of splenic volume and splenectomy on prognosis of hepatocellular carcinoma within Milan criteria after curative hepatectomy. World J Surg. (2018) 42:1120–8. doi: 10.1007/s00268-017-4232-z
28. Hodgson A, Wier EM, Fu K, Sun X, Wan F. Ultrasound imaging of splenomegaly as a proxy to monitor colon tumor development in Apc(min716/+) mice. Cancer Med. (2016) 5:2469–76. doi: 10.1002/cam4.842
29. Chen HK, Zhu GW, Huang YJ, Zheng W, Yang SG, Ye JX. Impact of body mass index on short-term outcomes of laparoscopic gastrectomy in Asian patients: a meta-analysis. World J Clin Cases. (2018) 6:985–94. doi: 10.12998/wjcc.v6.i15.985
30. Liu Y, Guo D, Niu Z, Wang Y, Fu G, Zhou Y, et al. Prediction of the risk of laparoscopy-assisted gastrectomy by comparing visceral fat area and body mass index. Gastroenterol Res Pract. (2018) 2018:1359626. doi: 10.1155/2018/1359626
31. Glatz T, Kulemann B, Kuvendjiska J, Fichtner-Feigl S, Hoeppner J. Short-term and long-term outcomes of oesophagogastric surgery for cancer in obese and normal weight patients. ANZ J Surg. (2020) 90:277–82. doi: 10.1111/ans.15612
32. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol. (2019) 45:160–6. doi: 10.1016/j.ejso.2018.09.012
33. Fattouh M, Chang GY, Ow TJ, Shifteh K, Rosenblatt G, Patel VM, et al. Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck. (2018) 41:707–14. doi: 10.1002/hed.25420
34. Mull RT. Mass estimates by computed tomography: physical density from CT numbers. Am J Roentgenol. (1984) 143:1101–4. doi: 10.2214/ajr.143.5.1101
35. Okuma H, Gonoi W, Ishida M, Shirota G, Kanno S, Shintani Y, et al. Comparison of volume and attenuation of the spleen between postmortem and antemortem computed tomography. Int J Legal Med. (2016) 130:1081–7. doi: 10.1007/s00414-016-1337-0
36. Nelen SD, Verhoeven RHA, Lemmens V, de Wilt JHW, Bosscha K. Increasing survival gap between young and elderly gastric cancer patients. Gastric Cancer. (2017) 20:919–28. doi: 10.1007/s10120-017-0708-7
37. Liu Z, Feng F, Guo M, Liu S, Zheng G, Xu G, et al. Distal gastrectomy versus total gastrectomy for distal gastric cancer. Medicine. (2017) 96:e6003. doi: 10.1097/MD.0000000000006003
38. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of comorbidities on survival in gastric, colorectal, and lung cancer patients. J Epidemiol. (2018) 29:110–5. doi: 10.2188/jea.JE20170241
39. Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, et al. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med. (2013) 11:58. doi: 10.1186/1479-5876-11-58
Keywords: gastric cancer, spleen density, computed tomography, post-operative outcomes, prognostic roles
Citation: Huang Y-S, Chen X-D, Shi M-M, Xu L-B, Wang S-J, Chen W-S, Zhu G-B, Zhang W-T and Shen X (2020) Diffuse Reduction of Spleen Density Is an Independent Predictor of Post-Operative Outcomes After Curative Gastrectomy in Gastric Cancer: A Multi-Center Study. Front. Oncol. 10:1050. doi: 10.3389/fonc.2020.01050
Received: 14 November 2019; Accepted: 27 May 2020;
Published: 30 June 2020.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Robert J. Canter, University of California, Davis, United StatesCopyright © 2020 Huang, Chen, Shi, Xu, Wang, Chen, Zhu, Zhang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian Shen, c2hlbnhpYW4xMTIwQDEyNi5jb20=; Wei-Teng Zhang, anl6d3Q1NDVAMTI2LmNvbQ==; Guan-Bao Zhu, d21jemdiQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.