
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 30 June 2020
Sec. Thoracic Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01049
 Yanwei Zhang1†
Yanwei Zhang1† Beibei Sun2†
Beibei Sun2† Minjuan Hu1†
Minjuan Hu1† Yuqing Lou1
Yuqing Lou1 Jun Lu1
Jun Lu1 Xueyan Zhang1
Xueyan Zhang1 Huimin Wang1
Huimin Wang1 Jialin Qian1‡
Jialin Qian1‡ Tianqing Chu1‡
Tianqing Chu1‡ Baohui Han1*‡
Baohui Han1*‡Background: This study was performed to evaluate the value of inflammatory biomarkers in predicting the prognosis of early-stage (stage IA-IIB) lung adenocarcinoma.
Methods: Ten inflammatory biomarkers were tested with a Luminex bead-based assay in early-stage lung adenocarcinoma patients who underwent resection.
Results: A total of 152 early-stage lung adenocarcinoma patients were analyzed in this study. The mean patient age (SD) was 59.9 (9.4) years. In total, 58.6% of patients were females, and never smokers accounted for 84.0%. Lung adenocarcinoma patients with high CXCL9 levels had a 71% reduced risk of recurrence relative to patients with low CXCL9 levels (HR = 0.29, 95% CI: 0.13–0.64, p = 0.0021). After Bonferroni correction, CXCL9 remained significantly related to the risk of early-stage lung adenocarcinoma recurrence. Lung adenocarcinoma patients with high CXCL9 levels had an 80% reduced risk of death relative to patients with low CXCL9 levels (HR = 0.20, 95% CI: 0.05–0.78, p = 0.021), and those in the TCGA validation cohort were at a 29% reduced risk of death (HR = 0.71, 95% CI: 0.45–0.99, p = 0.044).
Conclusion: Our results demonstrate for the first time that the CXCL9 level is a protective factor for both disease-free survival (DFS) and overall survival (OS) in early-stage lung adenocarcinoma patients.
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers and is the leading cause of death from cancer worldwide (1). Adenocarcinoma is the most frequent subtype of NSCLC, and its incidence continues to increase (2–4). Lung cancer patients diagnosed in the early stages have a good prognosis, but approximately 30–55% of patients develop recurrence despite resection (5). Therefore, the identification of biomarkers able to predict which early-stage patients have a high risk for recurrence and death in subsequent years is urgently needed.
There is a growing body of evidence showing that chronic inflammation is a hallmark of lung carcinogenesis (6–8). Some broad investigations have also proven that inflammatory markers are associated with the risk of lung cancer (9–11). One of our previous studies evaluated the relationship between 10 inflammatory markers and early-stage lung adenocarcinoma risk (12), and the results showed that four inflammatory markers were significantly associated with the risk of early-stage lung adenocarcinoma: CXCL13 (C-X-C motif chemokine ligand 13), CCL22 (C-C motif chemokine ligand 22), CXCL9 (C-X-C motif chemokine ligand 9), and IL-10 (interleukin 10). After Bonferroni correction, only CXCL13 and CCL22 were found to be independently related to the risk of early-stage lung adenocarcinoma.
However, few studies have used a broad panel to evaluate the prognostic value of inflammatory markers in early-stage lung adenocarcinoma. To fill this gap, the present study therefore aimed to investigate the association between these 10 inflammatory markers and the prognosis of early-stage lung adenocarcinoma patients with a median follow-up time of 60.6 months. The 10 inflammatory biomarkers were measured in all study participants, including CRP (C-reactive protein), CCL22, CXCL13, CXCL9, TNFRII (Tumor necrosis factor receptor II), IL-1b (interleukin 1 beta), IL-6 (interleukin 6), IL-10, IFN-r (interferon-gamma), and TGF-a (transforming growth factor alpha), and they were previously reported to be associated with lung cancer (13–18).
Patients who were histologically confirmed as having lung adenocarcinoma by lung resection according to the classification criteria of the World Health Organization (19) at Shanghai Chest Hospital between September 2013 and March 2015 were included in the present study. The pathologic stage was determined according to the International Association for the Study of Lung Cancer (IASLC) tumor-node-metastasis (TNM) classification, 8th edition (20). The inclusion criteria were as follows: (1) patients with a TNM stage IA to IIB; (2) available serum samples before diagnosis; and (3) complete follow-up data. In contrast, patients were excluded if they were missing baseline data. All the enrolled patients signed informed consent documents. This study was approved by the ethics committee of Shanghai Chest Hospital.
Follow-up was performed annually after discharge at the outpatient department of our hospital or through a telephone interview or post mail if the patient failed to show at the scheduled time. The main endpoint was disease-free survival (DFS), defined as the time from lung resection to the date of disease relapse or death from any cause, whichever occurred first. Patients who did not experience any event were censored at the date of the last follow-up in April 2019. The secondary endpoint was overall survival (OS), defined as the time from surgery until the date of death or the last follow-up, whichever occurred first.
The circulating levels of 10 inflammatory markers were measured in serum specimens collected at baseline (processed at 2,400–3,000 rpm for 15 min), and then the samples were frozen within 2 h of collection and stored at −80°C. Inflammatory markers were measured using a Luminex bead-based assay (12). Concentrations were calculated using a four- or five-parameter standard curve. All the samples were assayed in duplicate, and the detection results were averaged for analysis.
Data are summarized as the mean ± standard deviation (SD) for continuous variables and as counts and percentages for categorical variables. Their differences were compared by the Wilcoxon rank-sum test, Student's t-test, Pearson's X2 test or Fisher's exact test, where appropriate. A survival tree analysis was employed to determine the optimal cutoff point of each biomarker as described previously (21, 22) (STREE software, available at http://c2s2.yale.edu/software/stree/).
For inflammatory biomarkers, measurements below the lowest limit of detection (LLOD) were assigned a value of half the LLOD, as described previously (12, 13). Biomarkers in which the levels were over the LLOD in more than 25% of subjects were dichotomized by the optimal cutoff points determined by the survival tree analysis. Biomarkers in which the levels were over the LLOD in 10–25% of individuals were categorized as detectable and undetectable.
The impact of each biomarker on DFS or OS in lung adenocarcinoma patients was evaluated by a multivariate Cox proportional hazards model and is as expressed as the hazard ratio (HR) and its 95% confidence interval (CI) after controlling for age, sex, smoking status, TNM stage, and neoadjuvant therapy. The Kaplan–Meier (K–M) method and log-rank test were used to compare DFS and OS between patient groups defined by each dichotomized biomarker. Stratified analyses were performed separately according to age, sex, and smoking status. The findings were validated using mRNA expression and OS data from The Cancer Genome Atlas (TCGA) for lung adenocarcinoma (www.cbioportal.org; accessed July 2019).
All statistical analyses were completed with SPSS software version 22.0 for Windows (IBM SPSS, Inc., Chicago, IL, USA) unless indicated. Statistical significance was taken as a two-sided P < 0.05, and multiple testing was controlled by Bonferroni correction.
A total of 152 early-stage lung adenocarcinoma patients were ultimately analyzed in this study, and the patient demographics are shown in Table 1. The mean patient age (SD) was 59.9 (9.4) years. Females accounted for 58.6% of the total population, and 84.0% were never smokers. According to TNM stage, 71.7% of patients were at stage I, and 28.3% of patients were at stage II. The median values of the inflammatory markers were comparable between the recurrence and non-recurrence groups (all p > 0.05). The optimal cutoff points for CXCL9, CXCL13, CCL22, TNFRII, IL-6, and CRP were 7.8 pg/ml, 21.4 pg/ml, 77.7 pg/ml, 275.4 pg/ml, 14.1 pg/ml, and 888244.7 pg/ml, respectively. The median follow-up time was 60.6 months.
In the multivariate Cox analyses, only CXCL9 was significantly associated with DFS after adjusting for age, sex, smoking status, stage, and adjuvant therapy (Table 2). Lung adenocarcinoma patients with high CXCL9 levels had a 71% reduced risk of recurrence relative to patients with low CXCL9 levels (HR = 0.29, 95% CI: 0.13–0.64, p = 0.0021). K–M analysis showed that lung adenocarcinoma patients with high CXCL9 levels had a significantly longer median DFS time than those with low CXCL9 levels (log-rank test p = 0.014; Figure 1A). While other markers, such as CXCL13, CCL22, and IL-10, did not show significant differences in DFS of lung adenocarcinoma patients between high and low groups (Figures 1B–D). After Bonferroni correction, CXCL9 remained significant, with a significance level of 0.5% (0.05/10, here 10 refers to 10 biomarkers under study).
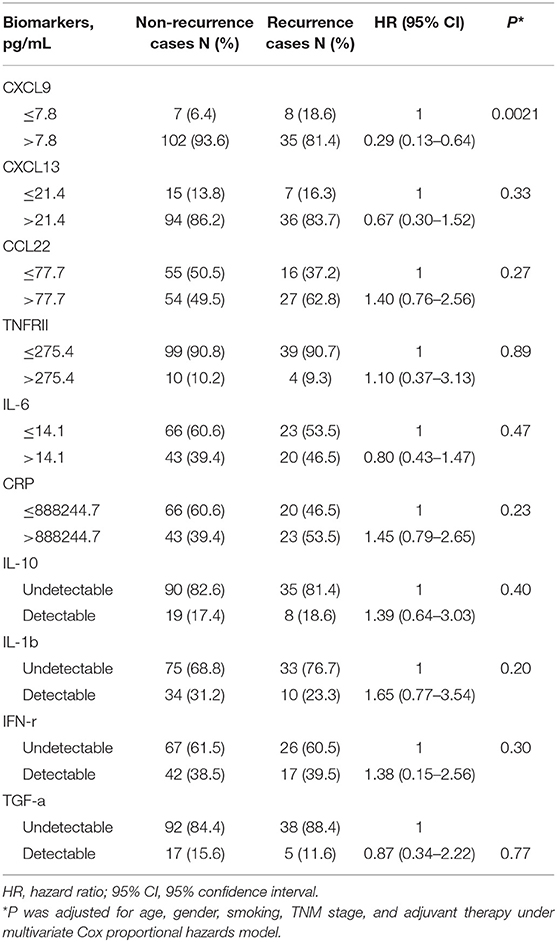
Table 2. Risk prediction of inflammation biomarkers for disease-free survival (DFS) of early stage lung adenocarcinoma.
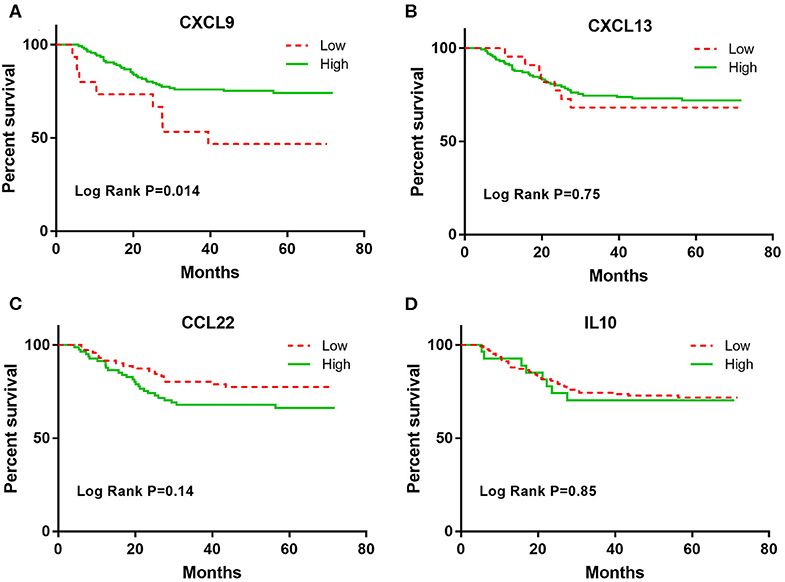
Figure 1. The Kaplan-Meier curves for the DFS among early stage lung adenocarcinoma patients according to CXCL9, CXCL13, CCL22, and IL-10 levels. (A) CXCL9, (B) CXCL13, (C) CCL22, and (D) IL10.
The association of CXCL9 with DFS in lung adenocarcinoma patients was stratified by age, sex, and smoking status (Table 3). Elevated CXCL9 levels were found to predict a good prognosis in never smokers, females, and both young and old lung adenocarcinoma patients. The K-M analysis showed that increased CXCL9 levels were still significantly corelated with longer DFS in never smokers and young lung adenocarcinoma patients (Figures 2A,B).
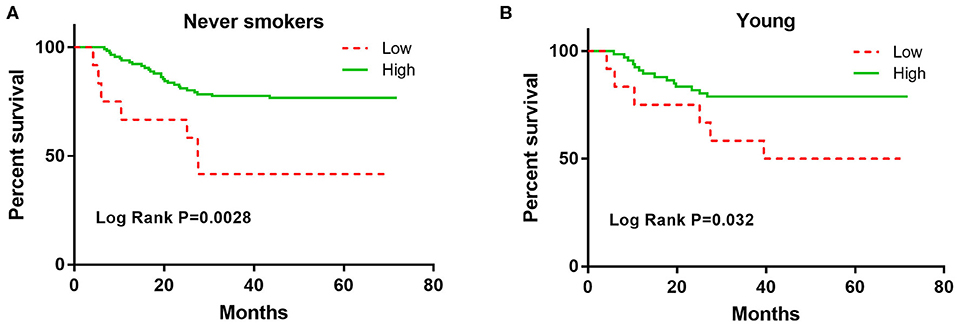
Figure 2. The Kaplan-Meier curves for the DFS among never smokers and young early stage lung adenocarcinoma patients according to CXCL9. (A) never smokers, (B) young.
Lung adenocarcinoma patients with high CXCL9 levels had an 80% reduced risk of death (HR = 0.20, 95% CI: 0.05–0.78, p = 0.021) relative to patients with low CXCL9 levels (Table 4), although K–M analysis did not reach statistical significance (log-rank test p = 0.082; Supplement Figure 1).
After analysis of the TCGA database, 492 lung adenocarcinoma patients were enrolled in the validation cohort, and their characteristics are summarized in Supplement Table 1. Patients with high CXCL9 levels in the validation cohort had a 29% reduced risk of death (HR = 0.71, 95% CI: 0.45–0.99, p = 0.044; Table 4).
To the best of our knowledge, this is the first study that evaluated multiple inflammatory biomarkers and prognosis for early-stage lung adenocarcinoma in a Chinese population. The key finding of the present study was that CXCL9, a chemokine, was associated with both the DFS and OS of early-stage lung adenocarcinoma patients.
CXCL9, a ligand of CXCR3, is secreted by various cell types, including immune cells (23–25) and non-immune cells (26, 27). CXCL9 plays a controversial role in tumor development and pathogenesis (28–30) and exhibits both positive and negative prognostic values for different tumor types (31). For example, Lieber et al. (32) found that ovarian carcinoma patients with high CXCL9 levels had significantly longer relapse-free survival (RFS) than patients with low CXCL9 levels. In contrast, Chang et al. (33) found that high serum CXCL9 levels predicted poor DFS and OS in oral squamous cell carcinoma (OSCC) patients. In a recent study (34), CXCL9 was also found to be related to the efficacy of PD-1 immunotherapy. Chow et al. (34) revealed the role of CXCL9 in recruiting intratumoral CXCR3+ CD8+ T cells and its use as a potential biomarker of the response to immunotherapy.
Regarding lung cancer, Addison et al. found high levels of CXCL9 in ninety NSCLC tissues and that CXCL9 could inhibit tumor-derived angiogenesis (35). However, a study conducted by Kowalczuk et al. (36) found that CXCL9 expression was low in NSCLC tumor tissues but not related to either DFS or OS. Moreover, Nakanishi et al. (37) indicated that CXCL9 was highly upregulated in tumor tissues, but no significant correlation between the CXCL9 level and DFS was observed. Bodelon et al. (38) investigated the correlation between 77 inflammatory markers and the prognosis of lung cancer, and CXCL9 levels were also not found to be related with long survival of NSCLC patients. In our previous study (12), a high CXCL9 level was found to be related to a reduced risk of lung adenocarcinoma. However, the association was non-significant after Bonferroni correction. In the present study, high CXCL9 levels were found to be related to prolonged DFS and OS in early-stage lung adenocarcinoma patients. Nevertheless, the underlying biological mechanisms of this correlation are required to better define the prognosis and likelihood of a therapeutic response in lung adenocarcinoma patients.
Our findings demonstrate for the first time that the CXCL9 level is as a protective factor for both the DFS and OS of early-stage lung adenocarcinoma patients. The application of CXCL9 as a predictive and prognostic biomarker of early-stage lung adenocarcinoma patients will be investigated in the future with large, well-designed, multicenter cohort studies, along with in vitro and in vivo functional experiments.
All datasets generated for this study are included in the article/Supplementary Material.
This study was approved by the Ethics Committee of Shanghai Chest hospital, and all participants provided written informed consent.
BH, TC, and YZ contributed to the conception and design of the research. BS and MH contributed to the experiment. YZ, JQ, YL, and JL contributed to the analysis and interpretation of the data. XZ and HW contributed to the clinical data collections. YZ and JQ wrote the first draft of manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the following sources: National Science Foundation of China (No. 81802265), Shanghai Science and Technology Committee Program (19QA1408000), and Shanghai Xuhui District municipal health commission, (No. XHLHGG201806).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research has been selected as a Poster Presentation in 21th International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer (Sep 7, 2019) (39).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01049/full#supplementary-material
Supplement Figure 1. The Kaplan-Meier curves for the OS among early stage lung adenocarcinoma patients according to CXCL9.
Supplement Table 1. Baseline characteristics of TCGA validation cohort patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83:584–94. doi: 10.1016/S0025-6196(11)60735-0
3. Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. (2014) 84:13–22. doi: 10.1016/j.lungcan.2014.01.009
4. Nakamura H, Saji H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today. (2014) 44:1004–12. doi: 10.1007/s00595-013-0636-z
5. Carnio S, Novello S, Papotti M, Loiacono M, Scagliotti GV. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: tumor based approaches including gene signatures. Transl Lung Cancer Res. (2013) 2:372–81. doi: 10.3978/j.issn.2218-6751.2013.10.05
6. Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. (2008) 14:1023–4. doi: 10.1038/nm1008-1023
7. O'Callaghan DS, O'Donnell D, O'Connell F, O'Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. (2010) 5:2024–36. doi: 10.1097/JTO.0b013e3181f387e4
8. Nader CP, Cidem A, Verrills NM, Ammit AJ. Protein phosphatase 2A (PP2A): a key phosphatase in the progression of chronic obstructive pulmonary disease (COPD) to lung cancer. Respir Res. (2019) 20:222. doi: 10.1186/s12931-019-1192-x
9. Shiels MS, Shu XO, Chaturvedi AK, Gao YT, Xiang YB, Cai Q, et al. A prospective study of immune and inflammation markers and risk of lung cancer among female never smokers in Shanghai. Carcinogenesis. (2017) 38:1004–10. doi: 10.1093/carcin/bgx075
10. Mouronte-Roibas C, Leiro-Fernandez V, Ruano-Ravina A, Ramos-Hernandez C, Casado-Rey P, Botana-Rial M, et al. Predictive value of a series of inflammatory markers in COPD for lung cancer diagnosis: a case-control study. Respir Res. (2019) 20:198. doi: 10.1186/s12931-019-1155-2
11. Shiels MS, Katki HA, Hildesheim A, Pfeiffer RM, Engels EA, Williams M, et al. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J Natl Cancer Inst. (2015) 107:djv199. doi: 10.1093/jnci/djv199
12. Zhang Y, Yu K, Hu S, Lou Y, Liu C, Xu J, et al. MDC and BLC are independently associated with the significant risk of early stage lung adenocarcinoma. Oncotarget. (2016) 7:83051–9. doi: 10.18632/oncotarget.13031
13. Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. (2013) 105:1871–80. doi: 10.1093/jnci/djt309
14. Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. (2010) 28:2719–26. doi: 10.1200/JCO.2009.27.0454
15. Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. (2005) 14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316
16. Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. (2011) 103:1112–22. doi: 10.1093/jnci/djr216
17. Bigbee WL, Gopalakrishnan V, Weissfeld JL, Wilson DO, Dacic S, Lokshin AE, et al. A multiplexed serum biomarker immunoassay panel discriminates clinical lung cancer patients from high-risk individuals found to be cancer-free by CT screening. J Thorac Oncol. (2012) 7:698–708. doi: 10.1097/JTO.0b013e31824ab6b0
18. Lan X, Lan T, Faxiang Q. Interleukin-10 promoter polymorphism and susceptibility to lung cancer: a systematic review and meta-analysis. Int J Clin Exp Med. (2015) 8:15317–28. Availabe online at: https://ijcem.com/files/ijcem0012910.pdf
19. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
20. Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC lung cancer staging project: proposals for the revisions of the t descriptors in the forthcoming eighth edition of the tnm classification for lung cancer. J Thorac Oncol. (2015) 10:990–1003. doi: 10.1097/JTO.0000000000000559
21. Zhang Y, Xu J, Lou Y, Hu S, Yu K, Li R, et al. Pretreatment direct bilirubin and total cholesterol are significant predictors of overall survival in advanced non-small-cell lung cancer patients with EGFR mutations. Int J Cancer. (2017) 140:1645–52. doi: 10.1002/ijc.30581
22. Zhang Y, Roth JA, Yu H, Ye Y, Xie K, Zhao H, et al. A 5-microRNA signature identified from serum microRNA profiling predicts survival in patients with advanced stage non-small cell lung cancer. Carcinogenesis. (2019) 40:643–50. doi: 10.1093/carcin/bgy132
23. Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, et al. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. (2003) 102:1959–65. doi: 10.1182/blood-2002-12-3945
24. Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. (2008) 68:5972–8. doi: 10.1158/0008-5472.CAN-07-6818
25. Ikeda A, Aoki N, Kido M, Iwamoto S, Nishiura H, Maruoka R, et al. Progression of autoimmune hepatitis is mediated by IL-18-producing dendritic cells and hepatic CXCL9 expression in mice. Hepatology. (2014) 60:224–36. doi: 10.1002/hep.27087
26. Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. (2009) 136:705–14. doi: 10.1053/j.gastro.2008.10.020
27. Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. (2008) 267:226–44. doi: 10.1016/j.canlet.2008.04.050
28. Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, et al. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. (2006) 291:C34–9. doi: 10.1152/ajpcell.00441.2005
29. Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer. (2013) 132:276–87. doi: 10.1002/ijc.27670
30. Pinto S, Martinez-Romero A, O'Connor JE, Gil-Benso R, San-Miguel T, Terradez L, et al. Intracellular coexpression of CXC- and CC- chemokine receptors and their ligands in human melanoma cell lines and dynamic variations after xenotransplantation. BMC Cancer. (2014) 14:118. doi: 10.1186/1471-2407-14-118
31. Ding Q, Lu P, Xia Y, Ding S, Fan Y, Li X, et al. CXCL9: evidence and contradictions for its role in tumor progression. Cancer Med. (2016) 5:3246–59. doi: 10.1002/cam4.934
32. Lieber S, Reinartz S, Raifer H, Finkernagel F, Dreyer T, Bronger H, et al. Prognosis of ovarian cancer is associated with effector memory CD8(+) T cell accumulation in ascites, CXCL9 levels and activation-triggered signal transduction in T cells. Oncoimmunology. (2018) 7:e1424672. doi: 10.1080/2162402X.2018.1424672
33. Chang KP, Wu CC, Fang KH, Tsai CY, Chang YL, Liu SC, et al. Serum levels of chemokine (C-X-C motif) ligand 9 (CXCL9) are associated with tumor progression and treatment outcome in patients with oral cavity squamous cell carcinoma. Oral Oncol. (2013) 49:802–7. doi: 10.1016/j.oraloncology.2013.05.006
34. Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity. (2019) 50:1498–512.e5. doi: 10.1016/j.immuni.2019.04.010
35. Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, et al. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther. (2000) 11:247–61. doi: 10.1089/10430340050015996
36. Kowalczuk O, Burzykowski T, Niklinska WE, Kozlowski M, Chyczewski L, Niklinski J. CXCL5 as a potential novel prognostic factor in early stage non-small cell lung cancer: results of a study of expression levels of 23 genes. Tumour Biol. (2014) 35:4619–28. doi: 10.1007/s13277-014-1605-x
37. Nakanishi T, Imaizumi K, Hasegawa Y, Kawabe T, Hashimoto N, Okamoto M, et al. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol Immunother. (2006) 55:1320–9. doi: 10.1007/s00262-006-0133-y
38. Bodelon C, Polley MY, Kemp TJ, Pesatori AC, McShane LM, Caporaso NE, et al. Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann Oncol. (2013) 24:2073–9. doi: 10.1093/annonc/mdt175
Keywords: CXCL9, inflammation, early stage, lung adenocarcinoma, prognosis
Citation: Zhang Y, Sun B, Hu M, Lou Y, Lu J, Zhang X, Wang H, Qian J, Chu T and Han B (2020) CXCL9 as a Prognostic Inflammatory Marker in Early-Stage Lung Adenocarcinoma Patients. Front. Oncol. 10:1049. doi: 10.3389/fonc.2020.01049
Received: 10 February 2020; Accepted: 27 May 2020;
Published: 30 June 2020.
Edited by:
Stephen V. Liu, Georgetown University Medical Center, United StatesCopyright © 2020 Zhang, Sun, Hu, Lou, Lu, Zhang, Wang, Qian, Chu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baohui Han, eGt5eWhhbkBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.